Abstract
Recent studies show that the non-opioid peptides, galanin (GAL) and orexin (OX), are similar to the opioid enkephalin (ENK) in being stimulated by dietary fat and also in enhancing the consumption of a high-fat diet (HFD). This suggests that, when a HFD is provided, these non-opioids may stimulate the opioid system to promote excess consumption of this diet. Using single- and double-labeling immunohistochemistry, the present study sought to identify possible neuroanatomical substrates for this close relationship. Focusing on the hypothalamic paraventricular nucleus (PVN), and particularly its anterior (aPVN), middle (mPVN) and posterior (pPVN) parts, the experiments examined whether GAL itself or the receptors for GAL and OX are stimulated by a HFD in the same areas and possibly the same neurons as ENK. Compared to animals fed a standard chow diet, rats consuming a HFD exhibited an increased density of medial parvocellular neurons immunoreactive (IR) for GAL in the mPVN and aPVN and for ENK in the mPVN and pPVN, distinguishing the mPVN as an area where both peptides were affected. While showing little evidence for GAL and ENK colocalization with a chow diet, double-labeling studies in HFD-fed rats revealed significant colocalization specifically in medial parvocellular neurons of the mPVN. Immediately posterior to this site, further analyses revealed a similar relationship between the OX 2 receptor (OX2R) and ENK in HFD-treated animals. While increasing the density of neurons immunoreactive for OX2R as well as for the GAL 1 receptor but not OX 1 receptor, HFD consumption increased the colocalization only of OX2R and ENK, specifically in the medial parvocellular neurons of the pPVN. These changes in HFD-fed rats, showing GAL and OX2R to colocalize with ENK exclusively in neurons of the medial parvocellular mPVN and pPVN, respectively, suggest possible neural substrates through which the non-opioid peptides may functionally interact with ENK when exposed to a HFD.
Keywords: galanin, enkephalin, orexin, paraventricular, hypothalamus, fat
Consumption of a high fat diet (HFD) is known to induce cellular changes within neurons of the brain. Many of these changes have been detected specifically in the feeding-related nuclei of the hypothalamus, including the paraventricular nucleus (PVN) and perifornical lateral hypothalamus (PFLH) (Akabayashi et al., 1994; Wortley et al., 2003). These nuclei, in turn, act through different orexigenic peptides, including the opioid enkephalin (ENK) and the non-opioids, galanin (GAL) and orexin (OX), to control neurons in their projection areas and ultimately affect behavior (Leibowitz et al., 1998; Taslimi et al., 2011).
Neurons expressing the ENK peptides, met- and leu-ENK, are dense in the PVN, and are more concentrated in the medial parvocellular area than the lateral magnocellular area of this nucleus (Ceccatelli et al., 1989; Meister et al., 1990b). This opioid is known to have an important role in feeding behavior, most notably in relation to dietary fat. Hypothalamic injection of ENK agonists increase food consumption (McLean and Hoebel, 1983; Naleid et al., 2007) while preferentially stimulating the ingestion of a HFD (Naleid et al., 2007), and the endogenous expression of ENK in the PVN is significantly stimulated by intake of a HFD (Chang et al., 2004; Chang et al., 2007b). The non-opioid peptides, GAL and OX, are found to be very similar to ENK in their relation to fat consumption. As with ENK, hypothalamic as well as ventricular injections of GAL and OX are effective in stimulating feeding (Kyrkouli et al., 1986; Crawley et al., 1990; Dube et al., 1999; Edwards et al., 1999), specifically increasing HFD consumption (Tempel et al., 1988; Clegg et al., 2002; Nagase et al., 2002), and the endogenous expression of GAL in the PVN and OX in the PFLH is, in turn, stimulated by a HFD (Odorizzi et al., 1999; Wortley et al., 2003; Chang et al., 2004). Thus, all three orexigenic peptides, both opioid and non-opioid, appear to function within a positive feedback circuit to promote excess consumption of a fat-rich diet.
With these similarities, the question arises as to whether these non-opioid and opioid peptide systems function closely and possibly interact in this process of controlling intake of a fat-rich diet. This relationship is suggested by the finding that opioid receptor antagonists can block the feeding-stimulatory effects of GAL and OX (Barton et al., 1996; Sweet et al., 2004), in addition to those of ENK itself (Arjune et al., 1991), indicating that these non-opioid peptides act in part through their effects on the opioid system. This conclusion is further supported by the recent finding that central injection of GAL or OX can stimulate endogenous expression of ENK in the PVN (Karatayev et al., 2009). In addition, one study using microarray technology has identified preproENK as one of only three molecules specifically down-regulated in the brain by a mutation of the OX 2 receptor (OX2R) in narcoleptic dogs (Lindberg et al., 2007). Together, these results indicate that the non-opioid peptides in the PVN, both GAL-expressing neurons and OX innervation to the nucleus, may be important regulators of local ENK, functioning through this opioid to stimulate feeding and mediating upstream the stimulatory effect of a HFD on endogenous ENK.
With a dense population of both GAL- and ENK-expressing neurons in the PVN, it is possible that these two peptides may coexist and interact through a local circuit within this nucleus. Whereas there is little evidence for the colocalization of GAL and ENK in the PVN under basal conditions (Meister et al., 1990b), these peptides are found to coexist in magnocellular neurons after hypophysectomy (Meister et al., 1990b) or an osmotic challenge (Meister et al., 1990a). Thus, under certain conditions, these two peptides could function closely in a local circuit involving effects of GAL on the synthesis or release of ENK. There is evidence that GAL in the PVN, due to its dense concentration in dendrites as well as axons and perinuclear area, may act in this manner to affect other neurotransmitters (Landry and Hokfelt, 1998; Palkovits, 2002). Specifically, GAL may affect ENK through its receptors, GalR1 or GalR2, which are found to exist in the PVN (Gustafson et al., 1996; Nichol et al., 1999). Whereas both receptors have been implicated in high-fat feeding (Gorbatyuk and Hokfelt, 1998; Erhuma et al., 2007; Zorrilla et al., 2007), the GalR1 is more highly concentrated than GalR2 in the PVN (O’Donnell et al., 1999) and may have a more significant role, with deletion of the GalR1 gene found to reduce HFD intake over several weeks (Zorrilla et al., 2007) and its expression in the PVN stimulated by an antagonist of fat metabolism (Gorbatyuk and Hokfelt, 1998). Analyses of how GAL or its receptors in the PVN relate to ENK at the neuronal level, particularly under HFD conditions, may elucidate how these peptide systems interact to cause their behavioral effects.
The non-opioid peptide OX in the PFLH may also interact with ENK, perhaps through its receptors that may exist on ENK-expressing neurons in the PVN. The OX neurons in the PFLH are known to project to the PVN (Peyron et al., 1998), where OX receptors have been identified (Marcus et al., 2001; Backberg et al., 2002). Behavioral studies suggest that the OX2R has a more direct role in controlling food intake and arousal, whereas the orexin 1 receptor (OX1R) is more involved in reward (Kodadek and Cai, 2010). Also, anatomical studies have shown OX2R to be localized more in parvocellular neurons (Marcus et al., 2001) similar to ENK, whereas OX1R is more on magnocellular neurons (Backberg et al., 2002). Together, this evidence suggests that it may be specifically the OX2R that, in response to OX stimulation, interacts more closely with the PVN enkephalinergic system to stimulate high-fat feeding.
Given the similarities between GAL, OX and ENK as they relate to dietary fat and their close anatomical association within the PVN, this study sought to characterize, at the neuronal level, how the non-opioid systems may relate to and interact with the opioid ENK in response to a HFD. Single- and double-labeling immunohistochemistry was used to determine whether the stimulatory effect of dietary fat on GAL and ENK occurs in the same location within the PVN, possibly involving neurons that colocalize these two peptides, and also whether GAL and OX receptors in the PVN are stimulated by a HFD and show evidence of colocalization with ENK in neurons of this nucleus.
2. EXPERIMENTAL PROCEDURES
2.1. Subjects
Adult, male Sprague-Dawley rats (N=48; Charles River Breeding Labs, Kingston, NY), weighing approximately 300 g at the onset of experiments, were individually housed (22°C, with lights off at 1:00 p.m. for 12 hr) in a fully accredited American Association for the Accreditation of Laboratory Animal Care facility. All animals were given one week to acclimate to lab conditions, during which time they were maintained ad libitum on laboratory chow (LabDiet Rodent Chow 5001, St. Louis, MO) and water. All procedures were approved by the Rockefeller University Animal Care and Use Committee and were in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and their suffering.
2.2. Diets
The control diet used in this report (4.1 kcal/g gross energy) was standard laboratory chow (LabDiet Rodent Chow 5001, St. Louis, MO), which consisted of 13% fat, 58% carbohydrate, and 29% protein. The high-fat diet has been described in detail in previous publications (Dourmashkin et al., 2006; Leibowitz et al., 2006). This diet (5.2 kcal/g) had 50% fat composed of 75% lard (Armour Star, Peoria, IL) and 25% soybean oil (Crisco, Orrville, OH), 25% carbohydrate from 30% dextrin (ICN Pharmaceuticals, Costa Mesa, CA), 30% cornstarch (ICN Pharmaceuticals), and 40% sucrose (Domino Foods Inc., Yonkers, NY), and 25% protein from casein (Bio-Serv, Frenchtown, NJ) and 0.03% L-cysteine hydrochloride (ICN Pharmaceuticals). It was supplemented with minerals (USP XIV Salt Mixture Briggs; ICN Pharmaceuticals) and vitamins (Vitamin Diet Fortification Mixture; ICN Pharmaceuticals). Both diets were presented in round glass jars in the home cage.
2.3. Test procedures
After the rats were acclimated for 3 days to their respective diets (N=24/group) with a daily 30-kcal meal for 2 hours starting at dark onset, they were then given ad libitum access for 5 days to either chow or HFD. Daily measurements of body weight revealed no significant difference between the diet groups at the beginning or end of this 5-day period, with the chow rats weighing 323±8 g prior to ad libitum diet access and 344±10 g following the 5-day access and the HFD rats weighing 323±4 g prior to ad libitum access and 358±6 g following 5-day access (not significant (ns) vs chow at both time points). Also, while diet intake was significantly greater for HFD compared to chow rats on the first day of access (133±8 vs 92±6 kcal, p<0.01), intake was no longer different by the fifth day (97±4 vs 104±6 kcal, ns). Thus, despite consumption of different diets for several days, the two groups of animals were similar in body weight and caloric intake by the time of surgery.
At the end of this 5-day period, the animals early in the dark cycle were anesthetized with 20 mg/kg xylazine and 100 mg/kg ketamine (i.p.) and injected bilaterally in the lateral ventricles (−0.92 mm anterior to bregma, ±1.4 mm lateral to midsagittal sinus, and 3.3 mm ventral to the surface of the level skull) with the mitosis inhibitor, colchicine (280 μg in 14 μl NaCl; Sigma, St. Louis, MO), in order to improve peptide and receptor visualization. Following surgery, they were placed on a heating pad until fully awake. Once awake, rats were returned to their home cages and allowed to consume their respective diets for an additional 2 days prior to sacrifice. During this time, although not quantified, visual inspection confirmed that diet intake was greatly reduced in both groups. Rats that did not survive for these 2 additional days were removed from the experiment. At sacrifice, rats were deeply anesthetized with Nembutal (50 mg/kg i.p.) and perfused transcardially with 200 ml of 0.9% NaCl followed by 400 ml of 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. Brains were then immediately removed, post-fixed in 4% phosphate-buffered solution overnight at 4°C, and then transferred to a 20% sucrose solution where they were stored at 4°C for 5 days.
2.4. Immunofluorescence histochemistry
Brains were processed in 30 μm free-floating coronal sections for immunofluorescence histochemistry to label GAL, ENK, GalR1, OX1R or OX2R. The GalR2 was not examined due to the lack of available antisera specific to this receptor subtype (Hawes et al., 2005; Lu and Bartfai, 2009). Five to seven brains were used per antibody or combination of antibodies, with sections from each brain separated and reacted in different antibody solutions. Thus, every third section was used for a single reaction. Briefly, sections were blocked in 5% normal serum containing 0.5% tritonX-100 phosphate-buffered saline (PBS) for one hour, then incubated in primary antiserum overnight. After a 30 minute rinse in PBS, the sections were incubated in the properly conjugated secondary antibody for 2 hours. Double-labeled sections were incubated in the 2 primary or secondary antibodies simultaneously. For example, sections processed for double-labeling of GAL and ENK were first incubated in guinea-pig anti-GAL and mouse anti-ENK and then in Cy3-conjugated donkey anti-guinea pig and FITC-conjugated donkey anti-mouse antibodies. Sections labeled for GalR1 received two additional incubations, described below. Between each step, sections were washed three times, for 10 min each, in PBS. Finally, sections were mounted and coverslipped with Vectashield mounting medium (Vector Inc., Burlingame, CA). The immunofluorescent image was examined using a Zeiss fluorescence microscope with Meta Vue software (Molecular Devices, Downington, PA) and, when necessary, followed-up by an LSM 510 laser confocal microscope (Zeiss, Oberkocken, Germany) with LSM 510 v. 3.2 software (Zeiss, Oberkocken, Germany).
The primary antibodies used were: guinea-pig anti-GAL 1:500 (Peninsula Laboratories, Torrance, CA); monoclonal mouse anti-ENK 1:200 (Millipore, Temecula, CA), polyclonal rabbit anti-GalR1 1:100 (Novus Biologicals, St. Charles, MO), rabbit anti-OX1R 1:500 (Imgenex, San Diego, CA) and rabbit anti-OX2R 1:200 (Enzo Life Sciences, Plymouth Meeting, PA). The specificity of these antibodies has been thoroughly demonstrated in previous publications (Cuello et al., 1984; Meister et al., 1990b; Sakurai et al., 1998; Hervieu et al., 2001; Pham et al., 2002). The secondary antibodies used were: GAL: Cy3-conjugated donkey anti-guinea pig 1:200 (Jackson ImmunoResearch Laboratories, West Grove, PA); ENK: FITC-conjugated donkey anti-mouse 1:100 (Jackson); OX1R and OX2R: Cy3-conjugated donkey anti-rabbit 1:200 (Millipore). For GalR1, the secondary antibody was biotinylated goat anti-rabbit 1:200 (Vector Laboratories, Burlingame, CA), which was followed by a 1 hour incubation in streptavidin-horseradish peroxidase 1:100 (PerkinElmer, Waltham, MA), then a 5 minute incubation in Cy3-conjugated tyramine (PerkinElmer) and finished by four 5 minute washes in TNT buffer prior to mounting.
Control sections processed with the supernatant of denatured antisera, which had been boiled for 10 min, cooled on ice and then centrifuged for 5 min, showed no immunofluorescence. To minimize variations in staining between the chow and HFD groups, all conditions including the number of sections reacted in a certain concentration and volume of antibody were kept the same during processing. To optimize staining, pilot experiments were run to determine antibody concentrations and incubation times that would lead the immunoreactivity of each peptide and receptor to be at the top of the linear area, close to the plateau.
2.5. Data analysis
Sections containing the PVN were defined as anterior (aPVN, −0.84 to −1.32 mm), middle (mPVN, −1.44 to −1.92 mm), or posterior (pPVN, −2.04 to −2.28 mm), depending on their relative position from bregma, as defined by Paxinos and Watson (2005). Parvocellular and magnocellular cells were determined by their relative positions within the PVN as defined by Paxinos and Watson (2005), with the parvocellular region existing medially and magnocellular region laterally. A total of 13-15 sections from each brain were analyzed for each antibody or combination of antibodies. Immunofluorescence was quantified by an evaluator blind to the experimental conditions using Image-Pro Plus software (Version 4.5, Media Cybernetics Inc., Silver Spring, MD), as described in detail in our previous publication (Chang et al., 2008), and was reported as density (objects/μm2). Each area of interest was outlined and analyzed. The population density was used to determine the density of cells expressing the protein or proteins of interest in each area. Since control sections revealed no immunofluorescence, all cells with visible fluorescence and a diameter of at least 4 μm were counted as immunostained. Cells with non-neuronal morphology were excluded from analysis. Before measurements, a threshold for each area was established. Using the selected sections, this threshold was set by matching the number of objects counted by the software in a defined area with the number of objects counted manually in that same area. The average value for each area in each animal was then used for statistical analysis. For soma size analysis, the diameter of 100-106 sample neurons immunostained for ENK, GAL, OX2R or their combination was measured across various areas within the PVN by tracing the neurons using Image-Pro Plus software (Version 4.5, Media Cybernetics Inc., Silver Spring, MD).
Data are expressed as mean ± SEM. In each experiment, a direct comparison between the values for a pair of groups was made using an unpaired Student’s t-test. The criterion for statistical significance was p<0.05.
3. RESULTS
3.1. Experiment 1: Effect of HFD on single-labeling of GAL and ENK neurons in the PVN
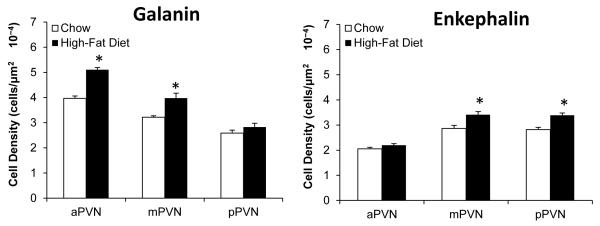
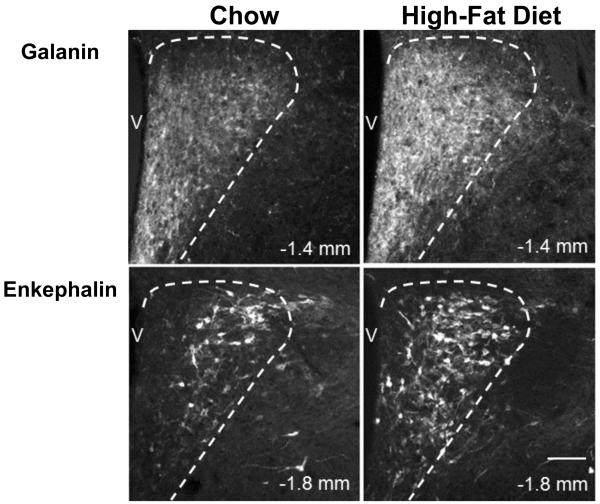
Building on prior studies showing HFD consumption to stimulate the expression of both GAL and ENK expression in the PVN (see Section 1), this experiment used single-labeling immunohistochemistry, to provide a more precise anatomical analysis of this effect and determine the extent of overlap and type of specific GAL and ENK neurons that are responsive to fat. As previously described (Ceccatelli et al., 1989), the GAL-immunoreactive (IR) neurons in the chow-fed rats (n=6) were dense in the medial region of the aPVN (−0.84 to −1.32 mm) and mPVN (−1.44 to −1.92 mm), where the neurons were invariably small in size (4-10 μm) and surrounded by a heavy GAL fiber innervation, but they were also seen in the lateral area of the mPVN and pPVN (−2.04 to −2.28 mm), where GAL immunoreactivity was evident in large, magnocellular neurons (18-35 μm). This contrasts with the ENK-IR neurons in the chow-fed rats (n=6), which while sparse in the aPVN were concentrated in the medial part of the mPVN and pPVN, where they were predominantly of the parvocellular type that were both small (4-10 μm, 65%) and medium (10-18 μm, 35%) in size. Compared to chow-fed rats, the rats consuming the HFD exhibited a significant increase in the density of these GAL- and ENK-IR neurons, in a site- and cell-specific manner (Fig. 1), with the strongest effect observed in the medial parvocellular region of the PVN as illustrated in the photomicrographs (Fig. 2). For GAL (n=6), this change induced by the HFD occurred in the aPVN (+29) and mPVN (+24%), where it was seen only in small neurons (4-10 μm), and it was not evident in the magnocellular neurons of the lateral PVN (+9%). For ENK (n=6), the HFD-induced increase in labeled neurons was strongest in the medial region of the mPVN (+20%) and pPVN (+19%), while not evident in the aPVN (+7%), and it occurred in both small and medium-size parvocellular neurons. These results provide further information on the GAL- and ENK-IR neurons sensitive to a HFD, showing them to be most concentrated and to anatomically overlap in the medial area of the mPVN and to be parvocellular in nature, small in size for GAL but both small and medium in size for ENK.
Fig. 1.
Galanin and enkephalin immunoreactivity is differentially enhanced after high-fat diet intake in parvocellular neurons of the anterior paraventricular nucleus (aPVN), middle paraventricular nucleus (mPVN) and posterior paraventricular nucleus (pPVN). Data are mean ± S.E.M., *p<0.05 vs. chow.
Fig. 2.
Photomicrographs show increased immunoreactivity in parvocellular galanin and enkephalin neurons of the paraventricular nucleus after high-fat diet intake. V: third ventricle. 10X magnification. Scale bar in bottom right panel = 50 μm. Dashed line represents border between parvocellular paraventricular nucleus and medial preoptic area (galanin images) or parvocellular paraventricular nucleus and magnocellular paraventricular nucleus (enkephalin images).
3.2. Experiment 2: Effect of HFD on double-labeling of GAL and ENK in the PVN
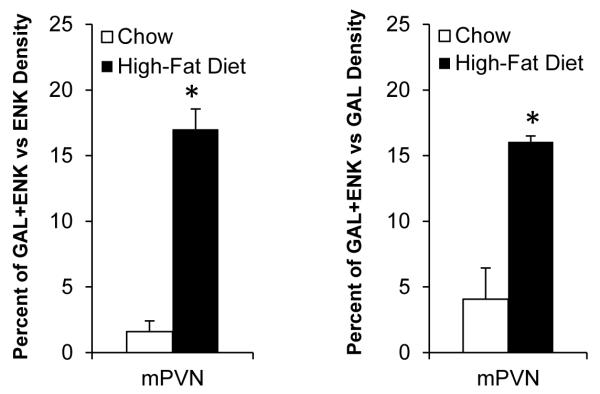
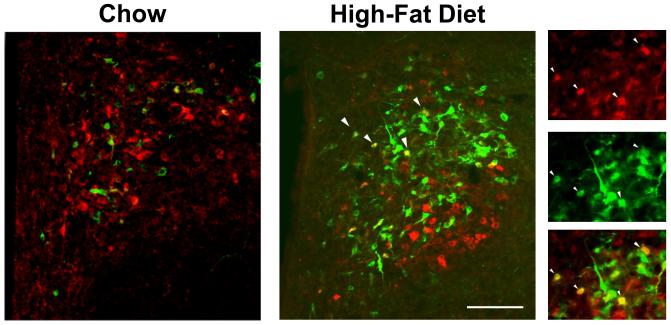
With the evidence of Experiment 1 showing the HFD to stimulate both GAL and ENK in the same type of neurons and same site of the medial mPVN, this experiment examined the possibility that this diet can stimulate these two peptides in the same neurons, causing significant colocalization. In the chow-fed rats (n=5), double-labeling immunohistochemistry revealed little colocalization of GAL and ENK throughout the PVN, whether in the parvocellular neurons of the medial PVN or the magnocellular neurons of the lateral PVN. Whereas the lateral magnocellular neurons also showed no colocalization in HFD-fed rats (n=5), exposure to this diet caused a significant increase in the density of GAL/ENK co-labeled neurons in the medial parvocellular region, whether expressed as percent of total ENK neurons (from 2% to 17%) or of total GAL neurons (from 4% to 16%) (Fig. 3 and Table 1). These co-labeled neurons, as illustrated in Fig. 4, were concentrated specifically at the level of the mPVN, not seen in the aPVN or pPVN, and they were invariably small in nature (4-10 μm, 88%) typical of the GAL neurons, with the medium-size ENK neurons exhibiting very little colocalization (12%) (Table 2). Thus, the stimulatory effect of dietary fat on GAL and ENK in parvocellular neurons, shown with single labeling to overlap specifically in the medial area of the mPVN, actually occurs in some of the same small neurons within this region.
Fig. 3.
Galanin and enkephalin colocalization is increased in parvocellular neurons of the middle paraventricular nucleus (mPVN) after high-fat diet intake. Data are mean ± S.E.M., *p<0.05 vs. chow.
Table 1.
In Experiment 2, the density of galanin (GAL) and enkephalin (ENK) single-labeled as well as double-labeled neurons was increased in the parvocellular region of the middle paraventricular nucleus after high-fat diet intake.
| Peptide(s) | Chow (cells/μm2 × 10−4) |
High-Fat Diet (cells/μm2 × 10−4) |
|---|---|---|
| GAL | 1.18 ± 0.18 | 3.88 ± 0.28* |
| ENK | 3.13 ± 0.19 | 3.74 ± 0.17* |
| GAL+ENK | 0.05 ± 0.03 | 0.62 ± 0.04* |
Data are mean ± S.E.M.
p<0.05 vs. chow.
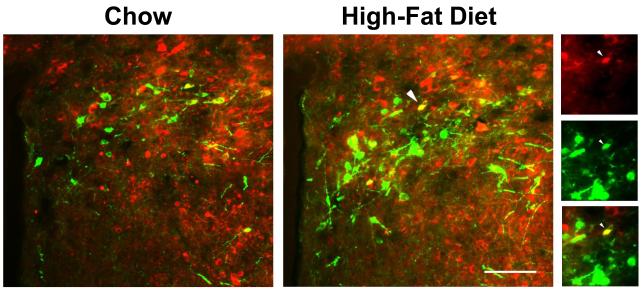
Fig. 4.
Photomicrographs show increased colocalization of galanin and enkephalin in the middle paraventricular nucleus after high-fat diet intake. Red neurons contain galanin, green neurons contain enkephalin, and yellow neurons contain both galanin and enkephalin. Right panel illustrates individual and merged immunofluorescent images taken after high-fat diet intake. White arrows indicate examples of neurons with colocalization, which can be seen in both the main image and right panel. Main images: 20X magnification; right panel: 40X magnification. Scale bar in main image = 50 μm.
Table 2.
In Experiment 2, colocalization of galanin (GAL) and enkephalin (ENK) after high-fat diet intake occurred predominantly in small neurons within the middle paraventricular nucleus.
| Soma Size | Diameter (μm) | % of Total GAL+ENK Neurons |
|---|---|---|
| Small | 4 - 10 | 87.7% |
| Medium | 10 - 18 | 12.3% |
| Large | 18 - 35 | 0.0% |
3.3. Experiment 3: Effect of a HFD on GAL and OX receptors in the PVN
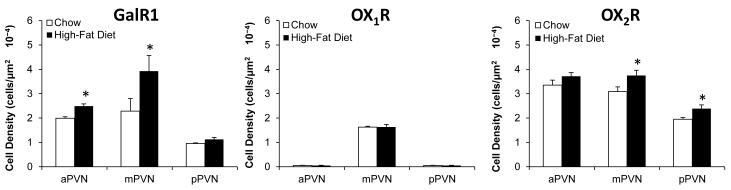
In addition to this HFD-induced colocalization of GAL peptide with ENK in medial parvocellular neurons of the mPVN, exposure to a HFD may also affect the receptors for GAL and OX, which could then contribute to the activation of ENK neurons in the PVN. Using single-labeling immunohistochemistry, this experiment tested whether GalR1 or the two OX receptors, OX1R and OX2R, are stimulated in the PVN by consumption of a HFD and whether this effect occurs in the same area where ENK is also stimulated by this diet. In both chow- and HFD-fed rats, examination of the GalR1-IR and OX1R-IR neurons revealed anatomical patterns that were somewhat different from that described for ENK-IR neurons (see Experiment 1, Section 3.1). In chow-fed rats (n=6), the GalR1-IR neurons were detected in both the parvocellular and magnocellular areas of the PVN as previously reported (Mitchell et al., 1997), and in HFD-fed rats (n=6), their density was increased in both the aPVN (+25%) and mPVN (+72%), but not the pPVN (+18%) (Fig. 5). The OX1R-IR neurons, which in chow-fed rats (n=5) were more highly concentrated in the magnocellular area of the lateral PVN as described (Backberg et al., 2002), exhibited no change in HFD-fed rats (n=5) in any area of the PVN where it was detected. In contrast to these two receptors, analyses of the OX2R-IR neurons revealed patterns that were similar to ENK. In chow-fed rats (n=7) as previously described (Marcus et al., 2001), this receptor was most highly concentrated in the medial parvocellular region of the PVN (Fig. 6), existing somewhat laterally on parvocellular neurons that were both small (4-10 μm, 46%) and medium (10-18 μm, 54%) in size, as seen for the ENK (Fig. 2). In contrast to OX1R, the density of these medial OX2R-IR soma was significantly stimulated by consumption of the HFD (n=7) (Fig. 5), as illustrated in the photomicrographs (Fig. 6). This effect occurred to an equal extent in the mPVN and pPVN (+22%), while not in the aPVN (+11%), and it was evident predominantly in parvocellular neurons that were medium in size. These results distinguish these medium-size parvocellular neurons in the mPVN and pPVN as primary sites where OX2R is similar to ENK in being stimulated by a HFD.
Fig. 5.
Galanin and orexin receptor immunoreactivity is differentially enhanced after high-fat diet intake in parvocellular neurons of the anterior paraventricular nucleus (aPVN), middle paraventricular nucleus (mPVN) and posterior paraventricular nucleus (pPVN). Data are mean ± S.E.M., *p<0.05 vs. chow.
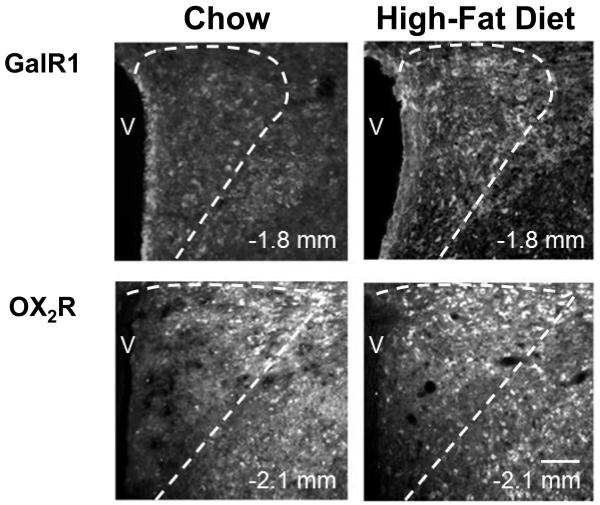
Fig. 6.
Photomicrographs show increased immunoreactivity in parvocellular galanin receptor 1 and orexin receptor 2 neurons of the paraventricular nucleus after high-fat diet intake. V: third ventricle. Scale bar in bottom right panel = 50 μm. Dashed line represents border between parvocellular paraventricular nucleus and magnocellular paraventricular nucleus (galanin receptor 1 images) or parvocellular paraventricular nucleus and subparaventricular zone (orexin receptor 2 images). 10X magnification.
3.4. Experiment 4: Effect of HFD on double-labeling of GAL and OX receptors with ENK in the PVN
With some anatomical overlap of the HFD-induced changes in GalR1-IR and OX2R-IR cells and those in ENK-IR neurons, this double-labeling experiment examined the possibility that, when exposed to a HFD, these specific receptors, possibly in contrast to OX1R, colocalize and perhaps interact with ENK in the medial parvocellular region of the PVN. In HFD- as well as chow-fed rats, this experiment failed to reveal any colocalization of GalR1 (n=6/group) or OX1R (n=7/group) with ENK in PVN neurons, suggesting that these receptor subtypes have little direct role in regulating levels of ENK in this nucleus. However, as compared to the chow-fed rats (n=6) which showed a low level of OX2R colocalization with ENK, the rats consuming a HFD (n=6) exhibited a significant increase in the density of OX2R/ENK co-labeled neurons in the PVN, whether expressed as percent of total ENK neurons (from 5% to 10%) or of total OX2R-labeled neurons (from 5% to 15%), and this change was detected specifically at the level of the pPVN (Fig. 7 and Table 3), but not the aPVN or mPVN. This increase in co-staining for OX2R and ENK in the pPVN occurred predominantly in medium-size neurons (81%), rather than small (7%) or large (12%) neurons (Fig. 8 and Table 4). These findings show the colocalization of OX receptors and ENK to be both receptor- and region-specific, suggesting that OX projections from the PFLH may act through OX2R in the pPVN to modulate ENK levels.
Fig. 7.
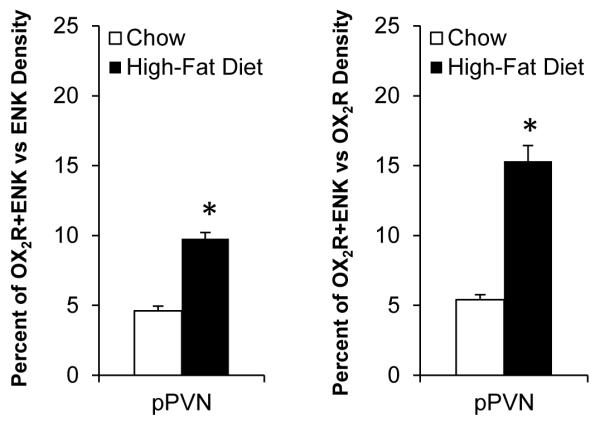
Orexin receptor 2 and enkephalin colocalization is increased in parvocellular neurons of the posterior paraventricular nucleus (pPVN) after high-fat diet intake. Data are mean ± S.E.M., *p<0.05 vs. chow.
Table 3.
In Experiment 4, the density of orexin 2 receptor (OX2R) and enkephalin (ENK) single-labeled as well as double-labeled neurons was increased in the parvocellular region of the posterior paraventricular nucleus after high-fat diet intake.
| Peptide(s) | Chow (cells/μm2 × 10−4) |
High-Fat Diet (cells/μm2 × 10−4) |
|---|---|---|
| OX2R | 1.85 ± 0.10 | 2.15 ± 0.06* |
| ENK | 2.20 ± 0.16 | 3.33 ± 0.20* |
| OX2R+ENK | 0.10 ± 0.01 | 0.33 ± 0.03* |
Data are mean±S.E.M.
p<0.05 vs. chow.
Fig. 8.
Photomicrographs show increased colocalization of orexin receptor 2 and enkephalin in the posterior paraventricular nucleus after high-fat diet intake. Red neurons contain orexin receptor 2, green neurons contain enkephalin, and yellow neurons contain both orexin receptor 2 and enkephalin. Right panel illustrates individual and merged immunofluorescent images taken after high-fat diet intake. White arrow indicates the neuron with colocalization that is seen in both the main image and right panel. Main images: 20X magnification; right panel: 40X magnification. Scale bar in main image = 50 μm.
Table 4.
In Experiment 4, colocalization of the orexin 2 receptor (OX2R) and enkephalin (ENK) after high-fat diet intake occurred predominantly in medium-size neurons within the posterior paraventricular nucleus.
| Soma Size | Diameter (μm) | % of Total OX2R+ENK Neurons |
|---|---|---|
| Small | 4 - 10 | 6.6% |
| Medium | 10 - 18 | 81.1% |
| Large | 18 - 35 | 12.3% |
4. DISCUSSION
Building on other studies describing the stimulatory effect of a HFD on the expression of GAL, OX and ENK in the hypothalamus, our experiments with peptide immunohistochemistry provide more detailed, anatomical information that help us to understand how these peptide systems, in response to a HFD, may interact at the neuronal level, possibly to further stimulate feeding behavior. These neuronal changes in distinct areas of the PVN suggest possible mechanisms of cellular regulation, with 1) GAL and ENK colocalizing for possible neurotransmitter co-release from the mPVN, 2) GAL increasing ENK levels by acting presynaptically in the mPVN, and 3) OX acting via the OX2R to increase ENK levels in the pPVN.
4.1. Anatomical distribution of GAL, ENK and the GAL and OX receptors as affected by a HFD
With single-labeling immunohistochemistry in rats injected with colchicine, the results of the present study show that, within the PVN, neurons expressing the peptides or peptide receptors exhibit a fair degree of regional overlap, giving hints as to their possible relationship in this nucleus. In the chow-fed rats, the results generally confirmed published reports on their expression and levels throughout the PVN. Specifically, immunoreactivity for the ENK peptides, met- and leu-ENK, was most concentrated in the medial parvocellular neurons of the mPVN and pPVN, as previously shown (Ceccatelli et al., 1989; Meister et al., 1990b), whereas immunoreactivity for the GAL peptide was dense in both the medial parvocellular neurons of the aPVN and mPVN and the lateral magnocellular neurons of the mPVN and pPVN, as described (Ch’ng et al., 1985; Ceccatelli et al., 1989; Merchenthaler et al., 1993). Further, as reported (Mitchell et al., 1997; Marcus et al., 2001), the OX2R and GALR1 were also fairly dense in the PVN, with the former restricted more to the medial parvocellular part of this nucleus and the latter distributed throughout the medial and lateral parts, while the OX1R was fairly sparse and seen only in the lateral part of the mPVN, apparently on magnocellular neurons as described (Backberg et al., 2002).
In the HFD-fed rats, the present analyses of these peptides and receptors provide new insights into their possible relationship to dietary fat, with rats exposed to this diet exhibiting stimulatory effects that were region-, neuron- and receptor-specific. With no change in immunoreactivity for OX1R, they showed an increased density of neurons immunoreactive for ENK, GAL, GalR1 and OX2R, in areas where they are normally expressed under chow conditions. For GAL-IR neurons, this change was evident in small neurons of the medial aPVN and mPVN, where previous studies have shown GAL gene expression and peptide levels to be increased by a chronic HFD (Leibowitz et al., 1998; Dourmashkin et al., 2005), and for ENK-IR neurons, it was detected in both small and medium-size neurons in the medial area of the mPVN and pPVN, where preproENK gene expression is increased by injection of a lipid emulsion or voluntary consumption of a HFD (Chang et al., 2004; Chang et al., 2007b). As for the receptors, the HFD effect on GalR1-IR neurons was evident in parvocellular neurons of the aPVN and mPVN, consistent with evidence showing PVN GalR1 mRNA to be affected by a fatty acid anti-metabolite (Gorbatyuk and Hokfelt, 1998), and the effect on OX2R-IR neurons was observed in a subset of medial parvocellular neurons in both the pPVN and mPVN, the first evidence for a stimulatory effect of a HFD on this specific receptor. These findings, showing a HFD to stimulate the peptides and their receptors in the same area where ENK is affected and in different size neurons in discrete areas, suggest that they may interact closely with ENK, even within the same neurons, but that these effects have separate downstream targets.
Since colchicine is not only a mitosis inhibitor but also a neurotoxin, it is possible that this treatment induced peptide or receptor expression in regions where it does not normally exist. For example, GAL is known to be upregulated in neuronal tissue after nerve injury (Hobson et al., 2010), and GAL-immunoreactive neurons are visualized predominantly in the magnocellular PVN of colchicine-untreated animals (Okere and Waterhouse, 2003) while evident in the parvocellular as well as magnocellular PVN of colchicine-treated animals (Ceccatelli et al., 1989). It should be noted, however, that neurons expressing GAL mRNA can be seen in the parvocellular PVN using in situ hybridization without colchicine (Leibowitz et al., 1998), demonstrating that GAL-containing soma do exist in this region under basal conditions. Further, colchicine treatment is also necessary to reveal the presence of ENK soma in the parvocellular PVN (Meister et al., 1990b) and this peptide has not been linked to neuronal damage. Thus, while necessary to reveal the soma and terminals of both GAL and ENK, colchicine was unlikely to induce their localization in regions where they did not already exist and function.
4.2. Colocalization of GAL and ENK in rats on a HFD
The evidence of the present study, consistent with published reports (Meister et al., 1990b), indicates that GAL and ENK in the PVN under standard lab chow conditions do not normally co-exist or that they colocalize at very low levels below the threshold of detection. After exposure to a HFD, however, these peptides are found to exhibit significant colocalization, in a specific subset of neurons in a distinct area of the PVN. This colocalization is evident predominantly in parvocellular neurons of the smallest type, rather than in medium-size parvocellular neurons where ENK alone is stimulated or in large magnocellular neurons where GAL but not ENK is normally detected. Moreover, it is evident only in the medial area at the mPVN level, rather than in the aPVN where only GAL-IR neurons are stimulated by the HFD or in the pPVN where only ENK-IR neurons are affected. This stimulation of GAL and ENK in the same subpopulation of neurons signifies a cellular change that may have functional consequences. The coexistence of peptides in PVN neurons, while common (Ceccatelli et al., 1989), does not necessarily reflect similar actions for the peptides, as some stimuli through different signal transduction pathways may produce differential changes in gene expression (Meister, 1993; De Mota et al., 2004; Jiang et al., 2004). Also, even if similarly affected by a specific stimulus, the co-expression of two peptides may (Cox et al., 1994) or may not (De Mota et al., 2004) lead to increased co-release of both together. In the specific case of GAL and ENK in the PVN, these peptides are found to respond and function very similarly, participating in a number of the same effects and behaviors. For example, both peptides are stimulated by, and they themselves stimulate, the consumption of fat (Tempel et al., 1988; Akabayashi et al., 1994; Chang et al., 2007b; Naleid et al., 2007) or ethanol (Leibowitz et al., 2003; Rada et al., 2004; Chang et al., 2007a; Barson et al., 2010), and they also play similar roles in stress (Ceccatelli et al., 1989) and inflammation (Ait-Ali et al., 2004). Thus, the colocalization of GAL and ENK in the PVN, induced by a HFD in a specific subset of small parvocellular neurons, suggests that they may co-exist and be co-released, possibly from the same secretory vesicles, to affect the same targets and potentiate each other’s actions.
The cause of this increased colocalization of GAL and ENK induced by a HFD may result, in part, from the ability of GAL to act presynaptically to directly stimulate ENK levels. With PVN injection of GAL found to stimulate ENK expression specifically in the medial part of this nucleus (Karatayev et al., 2009), it is noteworthy that GAL is highly concentrated in dendrites of the medial parvocellular neurons (Okere and Waterhouse, 2003), and its release in this area may function through GAL receptors to activate local somato-dendritic circuitry (Landry et al., 2005). Our immunohistochemical studies confirm that GAL is concentrated in fibers more than soma, while the reverse is true for ENK (Fig. 2), and thus HFD-induced stimulation of GAL levels in neurons of the medial PVN may increase GAL release onto its somato-dendritic receptors that possibly exist on ENK-containing neurons. Alternatively, GAL could be released from afferents arising from other hypothalamic or extra-hypothalamic nuclei, including the arcuate nucleus, dorsomedial nucleus, central nucleus of the amygdala or locus coeruleus, which also contain GAL neurons (Melander et al., 1986) and project to the parvocellular PVN (Sawchenko and Swanson, 1983; Cunningham and Sawchenko, 1988). Whereas GalR1, the most abundant GAL receptor in the PVN, is coupled to inhibitory Gi proteins (Lang et al., 2007), this receptor subtype was not found here to colocalize with ENK, in either chow- or HFD-fed rats. Thus, in stimulating ENK expression, GAL via GalR1 may act indirectly, perhaps through inhibition of the dense network of γ-aminobutyric acid-containing neurons that are present (Meister et al., 1988; Sakaue et al., 1988) and tonically active (Tasker and Dudek, 1993; Han et al., 2002) throughout the PVN. Alternatively, it may act via GalR2, which is coupled to stimulatory G-proteins (Mitchell et al., 1997; Lang et al., 2007) and expresses mRNA in a select subpopulation of parvocellular neurons in the PVN (O’Donnell et al., 1999) that may also express ENK. Examination of this possibility must await the availability of antisera specific to this receptor subtype (Hawes et al., 2005; Lu and Bartfai, 2009). Together, these analyses provide evidence for possible substrates in the mPVN that, in response to a HFD, mediate direct as well as indirect effects of the non-opioid GAL on the opioid peptide ENK and suggest that GAL itself may play a role in mediating the stimulatory effect of HFD consumption on PVN ENK.
4.3. Colocalization of OX2R and ENK in rats on HFD
Many studies of OX and its receptors have implicated this peptide system in the consumption of a fat-rich diet (see Section 1). Whereas the double-labeling experiments in chow-fed rats revealed little colocalization of the OX receptors with ENK in neurons of the PVN, exposure to the HFD significantly increased the density of neurons double-labeled for OX2R-IR and ENK-IR, which were predominantly medium in size and located specifically in the medial parvocellular region of the pPVN. This coexistence of OX2R and ENK in a subset of neurons suggests a possible role for this receptor subtype in stimulating the expression or synthesis of ENK. The consumption of a HFD is found to stimulate the gene expression and peptide levels of OX in the PFLH, which sends neuronal projections to the PVN (Peyron et al., 1998; Chang et al., 2004; Qi et al., 2009), and direct injection of OX-A or OX-B into the PVN increases feeding behavior through activation of the OX receptors (Dube et al., 1999; Zhu et al., 2002). Similarly, PVN injection of OX-A, which binds to the OX2R as well as OX1R (Sakurai et al., 1998), increases ENK mRNA in the medial PVN (Karatayev et al., 2009). It is noteworthy that OX2R is an excitatory receptor coupled to PKC and cAMP activation (Sakurai et al., 1998), the very same factors involved in stimulating ENK expression (Borsook and Hyman, 1995; Hook et al., 2008). This supports the idea that HFD-induced activation of OX and its OX2R can directly enhance the expression and levels of ENK. Our studies identify a specific subset of neurons, specifically medium size cells in the pPVN, which colocalize OX2R and ENK and possibly mediate this close relationship between OX and the opioid peptide.
4.4. Consequences of colocalization of GAL or OX2R with ENK in PVN
With the peptides GAL and OX, similar to ENK, each strongly implicated in consummatory behavior, the question that arises from these studies is the function that their HFD-induced co-expression has in controlling the ingestion of this diet. A role for PVN ENK in stimulating intake of fat is supported by evidence that rats prone to overconsuming a fat-rich diet have higher expression of ENK in this nucleus, even when a HFD is not present (Chang et al., 2010), and PVN injection of the μ-opioid agonist and ENK analogue DAMGO preferentially increases intake of a fat-rich diet (Naleid et al., 2007). The possibility that both GAL and OX exert their effects, in part, by activating ENK in the PVN is supported by evidence that pretreatment with an opioid receptor antagonist attenuates the feeding-stimulatory effects of these non-opioid peptides (Dube et al., 1999; Sweet et al., 2004) and that hypothalamically injected GAL and OX both stimulate the expression of ENK in the medial PVN (Karatayev et al., 2009). The evidence of the present study suggests that OX and GAL may directly increase ENK levels in medial parvocellular neurons of the PVN, with this effect occurring in a subset of small GAL-IR neurons in the mPVN and of medium-size OX2R-IR neurons in the pPVN. While the precise projections of these specific PVN areas have not been fully elucidated, there is evidence that parvocellular cells in these regions do, in fact, have distinct projections sites in the brain (Kirchgessner et al., 1988; Hallbeck and Blomqvist, 1999). Thus, whereas GAL and OX do not exert their effects solely through ENK, the increased ENK produced by these non-opioid peptides, in response to HFD intake, may affect different downstream nuclei and functions.
5. CONCLUSIONS
The present study addressed the question of how non-opioid peptides might interact with the opioid ENK in the PVN, given the similarities between these peptides as they relate to dietary fat. The results show that consumption of a diet rich in fat stimulates levels of GAL and OX2R along with ENK in the same subset of neurons, and even within the same neurons, in the medial parvocellular regions of mPVN and pPVN. We hypothesize that this colocalization on a HFD is due, in part, to the activation of the opioid system by the non-opioid peptides and also that ENK may mediate the functional effects of OX and GAL. This expands the positive feedback circuits, described separately for GAL, OX and ENK as they relate directly to dietary fat, and it incorporates ENK as an important downstream target of the non-opioid peptides that further stimulates fat intake. With this sequence of events in the positive feedback cycle, ENK as it induces the overconsumption of fatty foods may contribute to addictive-like behaviors.
Research Highlights.
Fat increases galanin and enkephalin in the same area of paraventricular nucleus
Fat increases galanin 1 and orexin 2 receptor levels in the paraventricular nucleus
Enkephalin and galanin colocalize in the paraventricular nucleus after fat intake
Enkephalin and orexin 2 receptor colocalize in the paraventricular nucleus with fat
Galanin and orexin may act through enkephalin to exert their effects on fat intake
Acknowledgements
This research was supported by USPHS Grant DA21518. We extend thanks to Olga Karatayev for her expert advice on the preparation of this manuscript.
Abbreviations
- aPVN
anterior paraventricular nucleus
- ENK
enkephalin
- GAL
galanin
- GalR1
galanin 1 receptor
- GalR2
galanin 2 receptor
- HFD
high-fat diet
- IR
immunoreactive
- mPVN
middle paraventricular nucleus
- ns
not significant
- OX
orexin
- OX1R
orexin 1 receptor
- OX2R
orexin 2 receptor
- PBS
phosphate-buffered saline
- PFLH
perifornical lateral hypothalamus
- pPVN
posterior paraventricular nucleus
- PVN
paraventricular nucleus of the hypothalamus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ait-Ali D, Turquier V, Grumolato L, Yon L, Jourdain M, Alexandre D, Eiden LE, Vaudry H, Anouar Y. The proinflammatory cytokines tumor necrosis factor-alpha and interleukin-1 stimulate neuropeptide gene transcription and secretion in adrenochromaffin cells via activation of extracellularly regulated kinase 1/2 and p38 protein kinases, and activator protein-1 transcription factors. Mol Endocrinol (Baltimore, MD) 2004;18:1721–1739. doi: 10.1210/me.2003-0129. [DOI] [PubMed] [Google Scholar]
- Akabayashi A, Koenig JI, Watanabe Y, Alexander JT, Leibowitz SF. Galanin-containing neurons in the paraventricular nucleus: a neurochemical marker for fat ingestion and body weight gain. Proc Natl Acad Sci U S A. 1994;91:10375–10379. doi: 10.1073/pnas.91.22.10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arjune D, Bowen WD, Bodnar RJ. Ingestive behavior following central [D-Ala2, Leu5, Cys6]-enkephalin (DALCE), a short-acting agonist and long-acting antagonist at the delta opioid receptor. Pharmacol Biochem Behav. 1991;39:429–436. doi: 10.1016/0091-3057(91)90203-e. [DOI] [PubMed] [Google Scholar]
- Backberg M, Hervieu G, Wilson S, Meister B. Orexin receptor-1 (OX-R1) immunoreactivity in chemically identified neurons of the hypothalamus: focus on orexin targets involved in control of food and water intake. Eur J Neurosci. 2002;15:315–328. doi: 10.1046/j.0953-816x.2001.01859.x. [DOI] [PubMed] [Google Scholar]
- Barson JR, Carr AJ, Soun JE, Sobhani NC, Rada P, Leibowitz SF, Hoebel BG. Opioids in the hypothalamic paraventricular nucleus stimulate ethanol intake. Alcohol Clin Exp Res. 2010;34:214–222. doi: 10.1111/j.1530-0277.2009.01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton C, York DA, Bray GA. Opioid receptor subtype control of galanin-induced feeding. Peptides. 1996;17:237–240. doi: 10.1016/0196-9781(95)02103-5. [DOI] [PubMed] [Google Scholar]
- Borsook D, Hyman SE. Proenkephalin gene regulation in the neuroendocrine hypothalamus: a model of gene regulation in the CNS. Am J Physiol. 1995;269:E393–408. doi: 10.1152/ajpendo.1995.269.3.E393. [DOI] [PubMed] [Google Scholar]
- Ceccatelli S, Eriksson M, Hokfelt T. Distribution and coexistence of corticotropin-releasing factor-, neurotensin-, enkephalin-, cholecystokinin-, galanin- and vasoactive intestinal polypeptide/peptide histidine isoleucine-like peptides in the parvocellular part of the paraventricular nucleus. Neuroendocrinology. 1989;49:309–323. doi: 10.1159/000125133. [DOI] [PubMed] [Google Scholar]
- Ch’ng JL, Christofides ND, Anand P, Gibson SJ, Allen YS, Su HC, Tatemoto K, Morrison JF, Polak JM, Bloom SR. Distribution of galanin immunoreactivity in the central nervous system and the responses of galanin-containing neuronal pathways to injury. Neuroscience. 1985;16:343–354. doi: 10.1016/0306-4522(85)90007-7. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Gaysinskaya V, Karatayev O, Leibowitz SF. Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J Neurosci. 2008;28:12107–12119. doi: 10.1523/JNEUROSCI.2642-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Ahsan R, Avena NM, Lee C, Lewis MJ, Hoebel BG, Leibowitz SF. Effect of ethanol on hypothalamic opioid peptides, enkephalin, and dynorphin: relationship with circulating triglycerides. Alcohol Clin Exp Res. 2007a;31:249–259. doi: 10.1111/j.1530-0277.2006.00312.x. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Ahsan R, Gaysinskaya V, Marwil Z, Leibowitz SF. Dietary fat stimulates endogenous enkephalin and dynorphin in the paraventricular nucleus: role of circulating triglycerides. Am J Physiol. 2007b;292:E561–570. doi: 10.1152/ajpendo.00087.2006. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Barson JR, Chang SY, Leibowitz SF. Increased enkephalin in brain of rats prone to overconsuming a fat-rich diet. Physiol Behav. 2010;101:360–369. doi: 10.1016/j.physbeh.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Davydova Z, Leibowitz SF. Circulating triglycerides impact on orexigenic peptides and neuronal activity in hypothalamus. Endocrinology. 2004;145:3904–3912. doi: 10.1210/en.2003-1582. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Air EL, Woods SC, Seeley RJ. Eating elicited by orexin-A, but not melanin-concentrating hormone, is opioid mediated. Endocrinology. 2002;143:2995–3000. doi: 10.1210/endo.143.8.8977. [DOI] [PubMed] [Google Scholar]
- Cox HM, Rudolph A, Gschmeissner S. Ultrastructural co-localization of neuropeptide Y and vasoactive intestinal polypeptide in neurosecretory vesicles of submucous neurons in the rat jejunum. Neuroscience. 1994;59:469–476. doi: 10.1016/0306-4522(94)90610-6. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Austin MC, Fiske SM, Martin B, Consolo S, Berthold M, Langel U, Fisone G, Bartfai T. Activity of centrally administered galanin fragments on stimulation of feeding behavior and on galanin receptor binding in the rat hypothalamus. J Neurosci. 1990;10:3695–3700. doi: 10.1523/JNEUROSCI.10-11-03695.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuello AC, Milstein C, Couture R, Wright B, Priestley JV, Jarvis J. Characterization and immunocytochemical application of monoclonal antibodies against enkephalins. J Histochem Cytochem. 1984;32:947–957. doi: 10.1177/32.9.6086744. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Jr., Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol. 1988;274:60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- De Mota N, Reaux-Le Goazigo A, El Messari S, Chartrel N, Roesch D, Dujardin C, Kordon C, Vaudry H, Moos F, Llorens-Cortes C. Apelin, a potent diuretic neuropeptide counteracting vasopressin actions through inhibition of vasopressin neuron activity and vasopressin release. Proc Natl Acad Sci U S A. 2004;101:10464–10469. doi: 10.1073/pnas.0403518101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourmashkin JT, Chang GQ, Gayles EC, Hill JO, Fried SK, Julien C, Leibowitz SF. Different forms of obesity as a function of diet composition. Int J Obes. 2005;29:1368–1378. doi: 10.1038/sj.ijo.0803017. [DOI] [PubMed] [Google Scholar]
- Dourmashkin JT, Chang GQ, Hill JO, Gayles EC, Fried SK, Leibowitz SF. Model for predicting and phenotyping at normal weight the long-term propensity for obesity in Sprague-Dawley rats. Physiol Behav. 2006;87:666–678. doi: 10.1016/j.physbeh.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Dube MG, Kalra SP, Kalra PS. Food intake elicited by central administration of orexins/hypocretins: identification of hypothalamic sites of action. Brain Res. 1999;842:473–477. doi: 10.1016/s0006-8993(99)01824-7. [DOI] [PubMed] [Google Scholar]
- Edwards CM, Abusnana S, Sunter D, Murphy KG, Ghatei MA, Bloom SR. The effect of the orexins on food intake: comparison with neuropeptide Y, melanin-concentrating hormone and galanin. J Neuroendocrinol. 1999;160:R7–12. doi: 10.1677/joe.0.160r007. [DOI] [PubMed] [Google Scholar]
- Erhuma A, Bellinger L, Langley-Evans SC, Bennett AJ. Prenatal exposure to undernutrition and programming of responses to high-fat feeding in the rat. Br J Nutr. 2007;98:517–524. doi: 10.1017/S0007114507721505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbatyuk O, Hokfelt T. Effect of inhibition of glucose and fat metabolism on galanin-R1 receptor mRNA levels in the rat hypothalamic paraventricular and supraoptic nuclei. Neuroreport. 1998;9:3565–3569. doi: 10.1097/00001756-199811160-00005. [DOI] [PubMed] [Google Scholar]
- Gustafson EL, Smith KE, Durkin MM, Gerald C, Branchek TA. Distribution of a rat galanin receptor mRNA in rat brain. Neuroreport. 1996;7:953–957. doi: 10.1097/00001756-199603220-00025. [DOI] [PubMed] [Google Scholar]
- Hallbeck M, Blomqvist A. Spinal cord-projecting vasopressinergic neurons in the rat paraventricular hypothalamus. J Comp Neurol. 1999;411:201–211. [PubMed] [Google Scholar]
- Han SK, Chong W, Li LH, Lee IS, Murase K, Ryu PD. Noradrenaline excites and inhibits GABAergic transmission in parvocellular neurons of rat hypothalamic paraventricular nucleus. J Neurophysiol. 2002;87:2287–2296. doi: 10.1152/jn.2002.87.5.2287. [DOI] [PubMed] [Google Scholar]
- Hawes JJ, Brunzell DH, Wynick D, Zachariou V, Picciotto MR. GalR1, but not GalR2 or GalR3, levels are regulated by galanin signaling in the locus coeruleus through a cyclic AMP-dependent mechanism. J Neurochem. 2005;93:1168–1176. doi: 10.1111/j.1471-4159.2005.03105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 2001;103:777–797. doi: 10.1016/s0306-4522(01)00033-1. [DOI] [PubMed] [Google Scholar]
- Hobson SA, Bacon A, Elliot-Hunt CR, Holmes FE, Kerr NC, Pope R, Vanderplank P, Wynick D. Galanin acts as a trophic factor to the central and peripheral nervous systems. EXS. 2010;102:25–38. doi: 10.1007/978-3-0346-0228-0_3. [DOI] [PubMed] [Google Scholar]
- Hook V, Toneff T, Baylon S, Sei C. Differential activation of enkephalin, galanin, somatostatin, NPY, and VIP neuropeptide production by stimulators of protein kinases A and C in neuroendocrine chromaffin cells. Neuropeptides. 2008;42:503–511. doi: 10.1016/j.npep.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YQ, Kawashima H, Iwasaki Y, Uchida K, Sugimoto K, Itoi K. Differential effects of forced swim-stress on the corticotropin-releasing hormone and vasopressin gene transcription in the parvocellular division of the paraventricular nucleus of rat hypothalamus. Neurosci Lett. 2004;358:201–204. doi: 10.1016/j.neulet.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Karatayev O, Barson JR, Chang GQ, Leibowitz SF. Hypothalamic injection of non-opioid peptides increases gene expression of the opioid enkephalin in hypothalamic and mesolimbic nuclei: Possible mechanism underlying their behavioral effects. Peptides. 2009;30:2423–2431. doi: 10.1016/j.peptides.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchgessner AL, Sclafani A, Nilaver G. Histochemical identification of a PVN-hindbrain feeding pathway. Physiol Behav. 1988;42:529–543. doi: 10.1016/0031-9384(88)90154-0. [DOI] [PubMed] [Google Scholar]
- Kodadek T, Cai D. Chemistry and biology of orexin signaling. Mol Biosyst. 2010;6:1366–1375. doi: 10.1039/c003468a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrkouli SE, Stanley BG, Leibowitz SF. Galanin: stimulation of feeding induced by medial hypothalamic injection of this novel peptide. Eur J Pharmacol. 1986;122:159–160. doi: 10.1016/0014-2999(86)90175-5. [DOI] [PubMed] [Google Scholar]
- Landry M, Hokfelt T. Subcellular localization of preprogalanin messenger RNA in perikarya and axons of hypothalamo-posthypophyseal magnocellular neurons: an in situ hybridization study. Neuroscience. 1998;84:897–912. doi: 10.1016/s0306-4522(97)00567-8. [DOI] [PubMed] [Google Scholar]
- Landry M, Liu HX, Shi TJ, Brumovsky P, Nagy F, Hokfelt T. Galaninergic mechanisms at the spinal level: focus on histochemical phenotyping. Neuropeptides. 2005;39:223–231. doi: 10.1016/j.npep.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Lang R, Gundlach AL, Kofler B. The galanin peptide family: receptor pharmacology, pleiotropic biological actions, and implications in health and disease. Pharmacol Ther. 2007;115:177–207. doi: 10.1016/j.pharmthera.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Akabayashi A, Wang J. Obesity on a high-fat diet: role of hypothalamic galanin in neurons of the anterior paraventricular nucleus projecting to the median eminence. J Neurosci. 1998;18:2709–2719. doi: 10.1523/JNEUROSCI.18-07-02709.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz SF, Avena NM, Chang GQ, Karatayev O, Chau DT, Hoebel BG. Ethanol intake increases galanin mRNA in the hypothalamus and withdrawal decreases it. Physiol Behav. 2003;79:103–111. doi: 10.1016/s0031-9384(03)00110-0. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Chang GQ, Dourmashkin JT, Yun R, Julien C, Pamy PP. Leptin secretion after a high-fat meal in normal-weight rats: strong predictor of long-term body fat accrual on a high-fat diet. Am J Physiol Endocrinol Metab. 2006;290:E258–267. doi: 10.1152/ajpendo.00609.2004. [DOI] [PubMed] [Google Scholar]
- Lindberg J, Saetre P, Nishino S, Mignot E, Jazin E. Reduced expression of TAC1, PENK and SOCS2 in Hcrtr-2 mutated narcoleptic dog brain. BMC Neurosci. 2007;8:34. doi: 10.1186/1471-2202-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Bartfai T. Analyzing the validity of GalR1 and GalR2 antibodies using knockout mice. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:417–420. doi: 10.1007/s00210-009-0394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- McLean S, Hoebel BG. Feeding induced by opiates injected into the paraventricular hypothalamus. Peptides. 1983;4:287–292. doi: 10.1016/0196-9781(83)90134-1. [DOI] [PubMed] [Google Scholar]
- Meister B. Gene expression and chemical diversity in hypothalamic neurosecretory neurons. Mol Neurobiol. 1993;7:87–110. doi: 10.1007/BF02935638. [DOI] [PubMed] [Google Scholar]
- Meister B, Cortes R, Villar MJ, Schalling M, Hokfelt T. Peptides and transmitter enzymes in hypothalamic magnocellular neurons after administration of hyperosmotic stimuli: comparison between messenger RNA and peptide/protein levels. Cell Tissue Res. 1990a;260:279–297. doi: 10.1007/BF00318631. [DOI] [PubMed] [Google Scholar]
- Meister B, Hokfelt T, Geffard M, Oertel W. Glutamic acid decarboxylase- and gamma-aminobutyric acid-like immunoreactivities in corticotropin-releasing factor-containing parvocellular neurons of the hypothalamic paraventricular nucleus. Neuroendocrinology. 1988;48:516–526. doi: 10.1159/000125058. [DOI] [PubMed] [Google Scholar]
- Meister B, Villar MJ, Ceccatelli S, Hokfelt T. Localization of chemical messengers in magnocellular neurons of the hypothalamic supraoptic and paraventricular nuclei: an immunohistochemical study using experimental manipulations. Neuroscience. 1990b;37:603–633. doi: 10.1016/0306-4522(90)90094-k. [DOI] [PubMed] [Google Scholar]
- Melander T, Hokfelt T, Rokaeus A. Distribution of galaninlike immunoreactivity in the rat central nervous system. J Comp Neurol. 1986;248:475–517. doi: 10.1002/cne.902480404. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Lopez FJ, Negro-Vilar A. Anatomy and physiology of central galanin-containing pathways. Prog Neurobiol. 1993;40:711–769. doi: 10.1016/0301-0082(93)90012-h. [DOI] [PubMed] [Google Scholar]
- Mitchell V, Habert-Ortoli E, Epelbaum J, Aubert JP, Beauvillain JC. Semiquantitative distribution of galanin-receptor (GAL-R1) mRNA-containing cells in the male rat hypothalamus. Neuroendocrinology. 1997;66:160–172. doi: 10.1159/000127234. [DOI] [PubMed] [Google Scholar]
- Nagase H, Nakajima A, Sekihara H, York DA, Bray GA. Regulation of feeding behavior, gastric emptying, and sympathetic nerve activity to interscapular brown adipose tissue by galanin and enterostatin: the involvement of vagal-central nervous system interactions. J Gastroenterol. 2002;37(Suppl 14):118–127. doi: 10.1007/BF03326430. [DOI] [PubMed] [Google Scholar]
- Naleid AM, Grace MK, Chimukangara M, Billington CJ, Levine AS. Paraventricular opioids alter intake of high-fat but not high-sucrose diet depending on diet preference in a binge model of feeding. Am J Physiol Regul Integr Comp Physiol. 2007;293:R99–105. doi: 10.1152/ajpregu.00675.2006. [DOI] [PubMed] [Google Scholar]
- Nichol KA, Depczynski BB, Cunningham AM. Characterization of hypothalamic neurons expressing a neuropeptide receptor, GALR2, using combined in situ hybridization-immunohistochemistry. Methods (San Diego, Calif) 1999;18:481–486. doi: 10.1006/meth.1999.0816. [DOI] [PubMed] [Google Scholar]
- O’Donnell D, Ahmad S, Wahlestedt C, Walker P. Expression of the novel galanin receptor subtype GALR2 in the adult rat CNS: distinct distribution from GALR1. J Comp Neurol. 1999;409:469–481. [PubMed] [Google Scholar]
- Odorizzi M, Max JP, Tankosic P, Burlet C, Burlet A. Dietary preferences of Brattleboro rats correlated with an overexpression of galanin in the hypothalamus. Eur J Neurosci. 1999;11:3005–3014. doi: 10.1046/j.1460-9568.1999.00722.x. [DOI] [PubMed] [Google Scholar]
- Okere CO, Waterhouse BD. Inter- and intra-nuclear differences in galanin expression between the hypothalamic paraventricular and supraoptic nuclei in colchicine-untreated rats. Brain Res. 2003;972:222–228. doi: 10.1016/s0006-8993(03)02524-1. [DOI] [PubMed] [Google Scholar]
- Palkovits M. Peptidergic Transmitter Systems. In: D’haenen HAH, den Boer JA, Willner P, editors. Biological Psychiatry. Vol. 1. John Wiley & Sons, Ltd; West Sussex, England: 2002. pp. 85–97. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Elsevier Academic Press; New York: 2005. [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham T, Guerrini S, Wong H, Reeve J, Jr., Sternini C. Distribution of galanin receptor 1 immunoreactivity in the rat stomach and small intestine. J Comp Neurol. 2002;450:292–302. doi: 10.1002/cne.10311. [DOI] [PubMed] [Google Scholar]
- Qi Y, Namavar MR, Iqbal J, Oldfield BJ, Clarke IJ. Characterization of the projections to the hypothalamic paraventricular and periventricular nuclei in the female sheep brain, using retrograde tracing and immunohistochemistry. Neuroendocrinology. 2009;90:31–53. doi: 10.1159/000221304. [DOI] [PubMed] [Google Scholar]
- Rada P, Avena NM, Leibowitz SF, Hoebel BG. Ethanol intake is increased by injection of galanin in the paraventricular nucleus and reduced by a galanin antagonist. Alcohol (Fayetteville, NY) 2004;33:91–97. doi: 10.1016/j.alcohol.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Sakaue M, Saito N, Taniguchi H, Baba S, Tanaka C. Immunohistochemical localization of gamma-aminobutyric acid in the rat pituitary gland and related hypothalamic regions. Brain Res. 1988;446:343–353. doi: 10.1016/0006-8993(88)90893-1. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. The organization of forebrain afferents to the paraventricular and supraoptic nuclei of the rat. J Comp Neurol. 1983;218:121–144. doi: 10.1002/cne.902180202. [DOI] [PubMed] [Google Scholar]
- Sweet DC, Levine AS, Kotz CM. Functional opioid pathways are necessary for hypocretin-1 (orexin-A)-induced feeding. Peptides. 2004;25:307–314. doi: 10.1016/j.peptides.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Tasker JG, Dudek FE. Local inhibitory synaptic inputs to neurones of the paraventricular nucleus in slices of rat hypothalamus. J Physiol. 1993;469:179–192. doi: 10.1113/jphysiol.1993.sp019810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taslimi Z, Haghparast A, Hassanpour-Ezatti M, Safari MS. Chemical stimulation of the lateral hypothalamus induces conditioned place preference in rats: Involvement of OX1 and CB1 receptors in the ventral tegmental area. Behav Brain Res. 2011;217:41–46. doi: 10.1016/j.bbr.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Tempel DL, Leibowitz KJ, Leibowitz SF. Effects of PVN galanin on macronutrient selection. Peptides. 1988;9:309–314. doi: 10.1016/0196-9781(88)90265-3. [DOI] [PubMed] [Google Scholar]
- Wortley KE, Chang GQ, Davydova Z, Leibowitz SF. Peptides that regulate food intake: orexin gene expression is increased during states of hypertriglyceridemia. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1454–1465. doi: 10.1152/ajpregu.00286.2002. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Yamanaka A, Kunii K, Tsujino N, Goto K, Sakurai T. Orexin-mediated feeding behavior involves both leptin-sensitive and -insensitive pathways. Physiol Behav. 2002;77:251–257. doi: 10.1016/s0031-9384(02)00843-0. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Brennan M, Sabino V, Lu X, Bartfai T. Galanin type 1 receptor knockout mice show altered responses to high-fat diet and glucose challenge. Physiol Behav. 2007;91:479–485. doi: 10.1016/j.physbeh.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]