Abstract
Individuals with Parkinson disease (PD) who do not have dementia reliably demonstrate mild executive deficits on laboratory-based tests, but the impact of these deficits on occupational performance is unclear. The purpose of this study was to determine the relevance of executive dysfunction in PD without dementia to instrumental, leisure, and social activity participation. Twenty-four individuals with PD and 30 matched adult volunteers performed an experimental working memory test and rated their everyday executive function and activity participation. Participants with PD had worse working memory performance, tended to report more everyday executive problems, and reported lower activity participation compared to controls. Within PD, lower everyday executive function was associated with reduced activity participation after controlling for motor dysfunction and depressive symptoms. Executive function is an independent predictor of complex activity participation in early PD. These results suggest the need for occupational therapists to consider executive dysfunction during evaluation and treatment of individuals with PD.
Keywords: Parkinsonian disorders, cognition, activities of daily living
Approximately 1 million Americans are diagnosed as having Parkinson disease (PD), a progressive neurodegenerative disorder associated with considerable socioeconomic costs that are expected to rise as the population ages (Tanner & Aston, 2000). Despite the available medical and surgical treatments, PD results in progressive disability (Shulman et al., 2008). Individuals with PD are often referred to occupational therapy services for the restoration, maintenance, and promotion of participation in meaningful activities and roles with the goal of improving function and quality of life.
The motor manifestations of PD receive the most attention in clinical settings; however, non-motor manifestations of PD also contribute significantly to disability and reduced quality of life (Schrag, Jahanshahi, & Quinn, 2000; Weintraub, Moberg, Duda, Katz, & Stern, 2004). For example, cognitive dysfunction is common in PD. An estimated 40% of individuals with PD develop dementia (Emre, 2003). In addition, most (55% to 70%) individuals with PD who do not have dementia demonstrate cognitive deficits on neuropsychological tests (Green et al., 2002; Janvin, Aarsland, Larsen, & Hugdahl, 2003). Executive dysfunction, or impairment in the cognitive processes involved in planning, performing, and regulating complex, goal-directed behavior, is the hallmark feature of cognitive impairment in PD (Emre, 2003). Studies of individuals with PD but without dementia or depression reliably show impaired planning, sequencing, working memory, and set-shifting (Pillon, Boller, Levy, Dubois, & Cappa, 2001), all of which are important component processes of executive functioning.
Executive functions orchestrate many of our daily occupations, including instrumental activities of daily living (IADL), social interactions, and leisure pursuits. Research in other populations shows that executive dysfunction is related to limitations in these more complex activities, which, in turn, are related to reduced overall functional status and quality of life (Carlson et al., 1999; Eriksson, Kottorp, Borg, & Tham, 2009; Eriksson, Tham, & Borg, 2006; Royall, Palmer, Chiodo, & Polk, 2004). The impact of executive dysfunction on occupational performance in PD is unclear.
Most research on daily function in PD has focused on basic activities of daily living (ADL), which rely on physical ability and procedural memory and remain relatively unaffected by executive dysfunction. One study that looked at basic ADL and IADL in PD identified selective relationships with motor and executive functioning, respectively (Cahn et al., 1998). However, this study’s inclusion of participants with possible dementia and use of the Trail Making Test Part B as an executive function measure make it difficult to separate the potential effects of global cognitive impairment and motor slowness from those of executive dysfunction. In a study that included only participants with PD without dementia, executive function was an independent predictor of health-related quality of life (Klepac, Trkulja, Relja, & Babic, 2008). The authors hypothesized that this relationship was mediated by IADL function, but they did not assess it directly. The effect of executive dysfunction in the absence of dementia on IADL or other complex activities has not been explicitly investigated in PD.
The purpose of this study was to determine whether individuals with PD and no dementia have executive function deficits that are relevant for their participation in instrumental, social, and leisure activities. Because PD is a complex disorder with many disease-related factors that could potentially influence this relationship, we limited our sample to individuals with relatively mild disease, no dementia, and no current psychiatric diagnoses. We used a laboratory-based measure of working memory to assess executive function objectively and self-report measures to assess perceived everyday executive function and activity participation. Establishing an association between executive function and participation is a necessary step in determining the value of considering executive dysfunction in OT practice with this population.
Method
Participants
Participants were 24 individuals with PD and 30 age-matched control volunteers. Participants with PD were recruited from the Movement Disorders Center at Washington University School of Medicine. Spouses of the participants with PD were preferentially recruited as controls and additional control participants were recruited from the community. Exclusionary criteria included current psychiatric disorders or history of psychosis; suspected dementia or global cognitive impairment (score of < 27 on the Mini-Mental State Examination); history of head injury, neurosurgery, or neurological condition other than PD; treatment with medications known to interfere with cognitive functioning; and biological family history of PD (control participants). Participants with PD were diagnosed as having idiopathic PD by a movement disorders specialist, were Hoehn and Yahr stage I or II (indicating relatively mild disease; Hoehn & Yahr, 1967), and had a mean disease duration of 4.5 years (standard deviation [SD] = 2.6).
Procedure
This study was approved by the Human Research Protection Office at Washington University School of Medicine and was completed in accordance with the tenets of the Declaration of Helsinki. All participants gave written informed consent before data were collected. A movement disorders research nurse performed motor dysfunction ratings (Unified Parkinson’s Disease Rating Scale Motor subscale, UPDRS; Fahn et al., 1987) on the participants with PD while they were receiving and not receiving antiparkinsonian medications and brief neurological evaluations on the control participants. Participants completed the Wechsler Test of Adult Reading (Wechsler, 2001) as an estimate of premorbid intelligence and the 15-item Geriatric Depression Scale (GDS; Yesavage et al., 1982) to rate depressive symptoms. Details for the key measures reported in this article are provided below.
Working Memory Performance: Serial Set Task
This laboratory-based task is a modified Sternberg short-term memory tasks (Oberauer, 2001) measure that varies the demands on maintenance and manipulation aspects of working memory. Participants viewed a series of four or five letters presented sequentially on a computer screen and were then cued to remember the letters in the presented order (maintain) or to rearrange them alphabetically (reorder). After a 10-second delay, a single letter and position-number probe was presented, and participants were to decide whether the letter resided in the position indicated by the number. There were four conditions in this test, representing the four combinations of set size (4, 5) and task (maintenance, manipulation), with 30 trials per condition. Accuracy (proportion correct) and verbal response times were recorded.
Reported Everyday Executive Function
The Dys-executive Questionnaire (DEX; Burgess, Alderman, Evans, Emslie, & Wilson, 1998) is a 20-item questionnaire that measures self-reported frequency of dys-executive behaviors in everyday life. Participants rate each item according to how often they experience the behavior on a 5-point scale (0 = Never and 4 = Very Often). This yields a total score of 0 to 80, with higher scores indicating worse everyday executive dysfunction. All participants completed the self-report version of this questionnaire (self-DEX), and participants with PD were rated with an equivalent informant-report version (proxy-DEX).
Complex Activity Participation
The Activity Card Sort (ACS; Baum & Edwards, 2001) measures the activity patterns of older adults. It consists of 80 photographic cards depicting common complex activities that fall into four domains: instrumental activities, low physical-demand leisure activities, high physical-demand leisure activities, and social activities. In this study, participants sorted the activity cards into piles according to their perceived level of current participation. The piles were then assigned numerical values on a weighted scale: 0 = Do not do, 1 = Do now at the level I want, and 0.5 = Do less than I want. Current participation scores for each activity domain were calculated by summing the item values within that domain.
Statistical Analyses
Descriptive statistics were calculated for all variables. Parametric analyses (repeated measures general linear models, t tests) were used for group comparisons except where noted. Correlational analyses (Pearson r, hierarchical linear regression) were performed to assess within-groups relationships between variables. All statistical tests were two-tailed, and an alpha level of p < .05 was considered significant.
Results
Participant Characteristics
Characteristics of the sample are presented in the table. There were no significant group effects for gender, ethnicity, age, education, or estimated pre-morbid intelligence (ps > 0.43; chi-square tests were used for ethnicity and gender). The PD group reported significantly more depressive symptoms than the control group as measured by the GDS, t = −3.30; p = .002. However, no participants in either group reported depressive symptoms above the GDS screening cutoff for depressive disorder. The mean UPDRS motor score of the PD group was significantly lower (indicating better motor functioning) while receiving compared to not receiving their antiparkinsonian medications (t = −2.70, p = .01).
Table.
Background Characteristics for Each Group (N = 54)
| Variable | PD | Control |
|---|---|---|
| No. | 24 | 30 |
| Male/female ratio | 14/10 | 14/16 |
| Ethnicity | ||
| White | 24 | 28 |
| African American | 0 | 2 |
| Age in years (SD) | 59.0 (7.8) | 60.0 (7.8) |
| Education in years (SD) | 14.9 (2.3) | 15.3 (3.2) |
| WTAR (SD) | 105.7 (14.2) | 107.1 (9.2) |
| GDSa (SD) | 2.3 (1.3) | 0.9 (1.1) |
| UPDRS on medication (SD) | 14.2 (6.2) | – |
| UPDRS off medication (SD) | 17.7 (6.7) | – |
PD = Parkinson disease; SD = standard deviation; WTAR = Wechsler Test of Adult Reading standard score; GDS = 15-item Geriatric Depression Scale score; UPDRS = Unified Parkinson Disease Rating Scale motor score.
p = .002.
Group Comparisons of Executive Function and Activity Participation
We included serial set accuracy as the dependent variable in a repeated measures general linear model with set size (4, 5) and task (maintenance, manipulation) as within-subjects factors and participant group as the between-subjects factor. Overall, performance was better with four-letter lists compared with five-letter lists, F(1, 52) = 125.60, p < .001, and there was a trend toward better performance on the maintenance compared to the manipulation task, F(1, 52) = 3.36, p = 0.07. Participants with PD performed worse than control participants across all test conditions, F(1, 52) = 5.45, p = .02. There were no interaction effects among any of the model factors (ps > 0.33), so for parsimony we averaged accuracy scores across all test conditions to calculate an overall working memory score for each participant. The resulting value (PD: M = 0.82, SD = 0.10; control: M = 0.87, SD = 0.06) was used for the correlational analyses reported below.
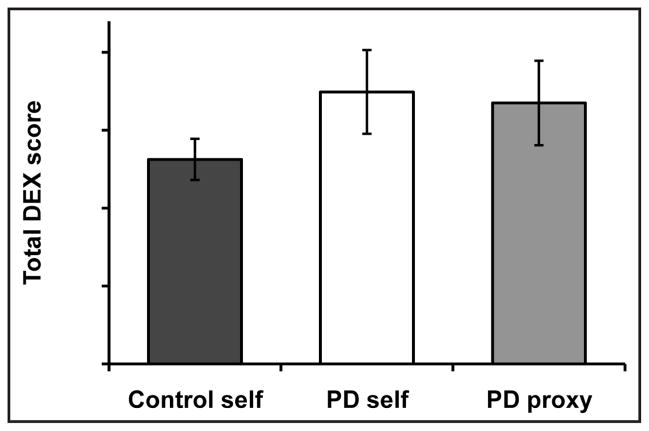
Although the PD group’s self- and proxy-DEX scores tended to be higher (worse) than the control group’s self-DEX scores, these differences were not statistically significant, most likely due to the large variance in the PD group’s scores (ps > 0.13; Fig. 1). The self- and proxy-DEX scores of the participants with PD were not significantly different (t = 0.21, p = .84), and they were moderately correlated with each other, r = 0.37, p = .08.
Figure 1.
Mean (± standard error of the mean) total Dysexecutive Questionnaire (DEX) scores for the control and Parkinson disease (PD) groups’ self-ratings (control self, PD self) and the PD group’s proxy-ratings (PD proxy).
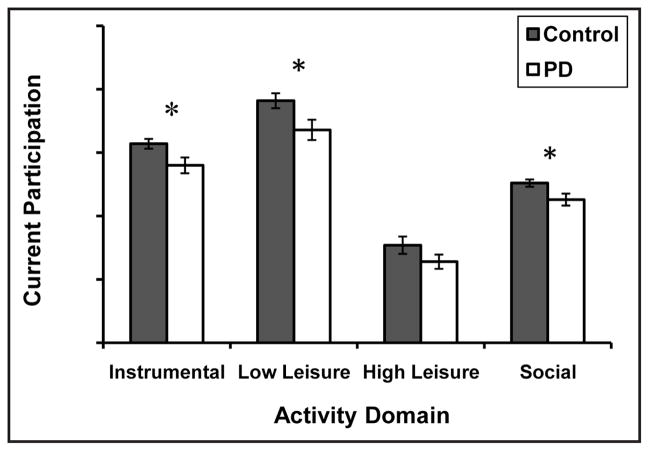
The PD group reported less participation overall than the control group, F(1, 52) = 7.5, p = .008 (Fig. 2). Comparisons of each activity domain revealed that participants with PD participated in fewer instrumental, low physical-demand leisure, and social activities compared to control participants (ps < 0.03). High physical-demand leisure participation did not differ between the groups (t = 1.58, p = .15), likely due to the low levels of participation reported in this domain overall. We summed the values from all 80 items on the ACS to obtain a total participation score, which was used for the remaining analyses. Participants with PD reported less total participation (M = 46.8, SD = 8.5) than control participants (M = 55.1, SD = 8.0; t = 2.74, p = .008).
Figure 2.
Mean (± standard error of the mean) Activity Card Sort scores for each activity domain for the control and Parkinson disease (PD) groups (*p < .05)
Within-Groups Associations Between Laboratory and Everyday Executive Function and Activity Participation
To determine the relationship between objectively measured working memory and reported everyday executive function, we correlated overall working memory scores with DEX scores. Working memory performance was not significantly correlated to self-DEX ratings in either group (ps > 0.34). However, the proxy-DEX ratings of the participants with PD were significantly correlated with their working memory performance (i.e., lower working memory performance in the laboratory was associated with more proxy-observed dysexecutive behaviors in everyday life [r = −0.51; p = .01]).
To determine the relationships between executive function and activity participation, we correlated working memory and DEX scores with total ACS scores. In the PD group, working memory performance was not significantly correlated with total ACS scores (r = 0.22, p = .31). However, the PD group’s self- and proxy-DEX ratings were correlated with total ACS scores (i.e., more reported everyday executive dysfunction was associated with less complex activity participation [self-DEX: r = −0.63, p = .001; proxy-DEX: r = −0.50, p = .02]). These measures did not show significant correlations in the control group (ps > 0.56).
Independent Association Between Everyday Executive Function and Activity Participation in PD
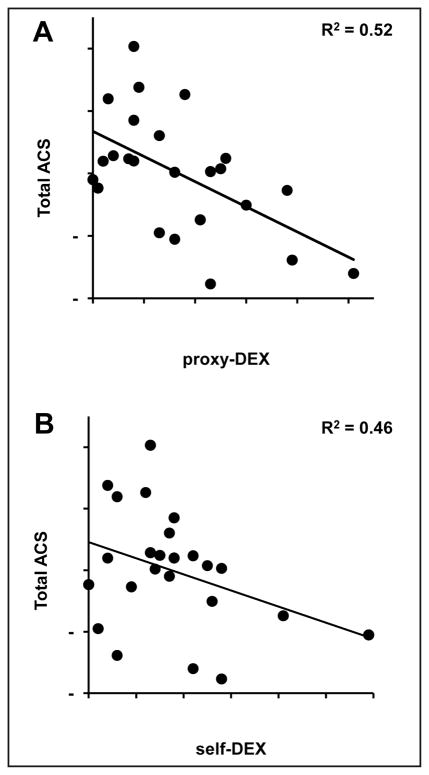
Finally, we wanted to determine the independent contribution of everyday executive dysfunction to complex activity participation in our PD group after accounting for the effects of other potential influential variables. We performed hierarchical linear regression analyses with total ACS score as the dependent variable and motor dysfunction (UPDRS motor score while not receiving medications), depressive symptoms (GDS), and DEX scores force-entered as predictors. Two separate models were tested: one with proxy-DEX scores and one with self-DEX scores entered in the final step. Motor dysfunction accounted for an initial 14% (p = .07) of the variance in total activity participation and depressive symptoms for an additional 16% (p = .04). Proxy-DEX and self-DEX scores each accounted for unique portions of the variance in total activity participation after controlling for motor dysfunction and depressive symptoms (proxy-DEX: ΔR2 = 0.22, p = .007; self-DEX: ΔR2 = 0.16, p = .03). Both of the overall models were significant. The model with proxy-DEX accounted for 52% of the variance in total activity participation, F(3, 20) = 7.22, p = .002 (Fig. 3A), whereas the model with self-DEX accounted for 46% of the variance in total activity participation, F(3, 20) = 5.64, p = .006 (Fig. 3B).
Figure 3.
Relationship between (A) proxy-DEX or (B) self-DEX and total standardized Activity Card Sort (ACS) scores after accounting for motor dysfunction and depressive symptoms within the Parkinson disease group (overall model adjusted R2’s shown; N = 24). DEX = Dysexecutive Questionnaire.
Discussion
We found that individuals with early PD and without dementia have mild executive function deficits that are associated with reduced participation in IADL and leisure and social activities. This effect exists over and above the effects of motor and psychiatric problems. Thus, executive dysfunction is relevant to occupational performance in PD.
The subtle difference in working memory performance across groups emphasizes the mild nature of disease in our participants with PD. Nonetheless, they have executive function deficits that likely interfere with their ability to participate in activities essential for independence, health, and well-being. Interestingly, motor dysfunction was the weakest predictor in our models, suggesting it does not contribute to participation restrictions as much as cognitive dysfunction and other non-motor impairments early in the course of PD.
Our findings are consistent with research in other populations (Eriksson et al., 2006; Royall et al., 2004), but they challenge the prevailing views of PD that motor impairments interfere with daily life more than other, less obvious disease manifestations (Shulman et al., 2008) and that cognitive deficits in the absence of dementia do not significantly affect daily activities (York & Alvarez, 2008). These views have likely resulted from an over-focus on basic ADL; however, the importance of considering the impact of PD on broader occupational and social functioning is gaining recognition in clinical research (Rahman, Griffin, Quinn, & Jahanshahi, 2008). Our results suggest that the relationship between executive dysfunction and quality of life in early PD may not only be mediated by IADL function (Klepac et al., 2008), but also by engagement in leisure and social activities. The consequences of mild cognitive dysfunction in PD may become more apparent as we gain a better understanding of complex activity participation in this population.
The findings reveal high variability in the everyday executive function ratings of the participants with PD. Although some individuals experienced noticeable executive problems in daily life, others—who presumably had the same neural dysfunction and likely demonstrated some degree of executive dysfunction in the laboratory—did not seem to have as many real-world difficulties. Whether this is an effect of individual differences in cognitive compensation, level of daily cognitive challenge, or reliability of subjective reports warrants further investigation. In addition, informant ratings of everyday executive function may more accurately reflect inherent cognitive ability than self-ratings, as evidenced by their stronger association with working memory performance. This could be an effect of mild depressive tendencies in some individuals with PD causing exaggerated sensitivity to problems. Our data support this interpretation, because self-reports of executive dysfunction shared more variance with depressive symptoms than did informant reports. Alternatively, impaired insight into cognitive deficits could be a direct manifestation of higher order cognitive dysfunction in PD, although empirical evidence for this is limited and inconclusive (Seltzer, Vasterling, Mathias, & Brennan, 2001). In either case, these findings highlight the importance of incorporating information from multiple sources to obtain the most comprehensive and accurate assessment of a person’s cognitive functioning.
Executive dysfunction is a potentially overlooked factor in the management of PD. Most therapies for PD target its physical effects and do not address cognition unless more severe impairment is present. Our results suggest that individuals with subtle changes in cognition may benefit from cognitive rehabilitation strategies to support continued participation in valued activities and roles. In addition to interfering with daily occupational performance, executive dysfunction could influence the ability of clients with PD to derive the full benefit from occupational therapy interventions, particularly self-management strategies (Lowenstein & Tickle-Degnen, 2008), which can place considerable demand on executive processes such as planning, initiation, and self-monitoring. Therefore, consideration of executive function may enhance both the outcome and process of rehabilitation for individuals with PD.
This is the first study to look specifically at the potential effects of executive dysfunction in PD on participation in the broad range of complex activities that give people’s lives meaning. Strengths of this study include sensitive measurement and quantification of working memory and activity participation and our conservative approach to study inclusion. Distinguishing between individuals with and without dementia is important for rehabilitation research because these individuals likely require different intervention approaches. Limitations of this study include use of a small, non-representative sample of individuals with PD and the cross-sectional design. Longitudinal investigation and path analyses with a larger and more representative cohort are necessary to confirm the current results and determine the causal nature of the relationship between executive dysfunction and participation in PD. In addition, measurement of activity performance and a broader range of executive processes (e.g., the Executive Function Performance Test; Baum et al., 2008) is an important next step in guiding the development of more comprehensive and targeted interventions for this population.
We found that executive function problems contribute significantly to decreased activity participation in individuals with mild and early PD. Although the mechanism for this relationship warrants further investigation, our results suggest the need to expand the focus of rehabilitative management for this population. Empirical investigations into the effects of occupational therapy for individuals with PD reveal only small to moderate improvements (Dixon et al., 2007; Murphy & Tickle-Degnen, 2001). It is possible that the beneficial effects of occupational therapy for individuals with PD could be enhanced if evaluating and addressing executive dysfunction was common practice.
Acknowledgments
This research was supported by the Greater St. Louis Chapter of the American Parkinson’s Disease Association, the McDonnell Center for Higher Brain Function, NS41248, KL2RR024994, and UL1RR024992. Some of these data were presented at the American Occupational Therapy Association annual conference, April 10–13, 2008, Long Beach, California.
Footnotes
The authors have no financial or proprietary interest in the materials presented herein.
Contributor Information
Erin R. Foster, Program in Occupational Therapy and Departments of Psychiatry and Neurology, Washington University in St. Louis School of Medicine, St. Louis, Missouri.
Tamara Hershey, Departments of Psychiatry, Neurology, and Radiology, Washington University in St. Louis School of Medicine, St. Louis, Missouri.
References
- Baum CM, Connor LT, Morrison T, Hahn M, Dromerick AW, Edwards DF. Reliability, validity, and clinical utility of the Executive Function Performance Test: A measure of executive function in a sample of people with stroke. American Journal of Occupational Therapy. 2008;62:446–455. doi: 10.5014/ajot.62.4.446. [DOI] [PubMed] [Google Scholar]
- Baum CM, Edwards DF. Activity Card Sort (ACS) St. Louis, MO: Program in Occupational Therapy, Washington University School of Medicine; 2001. [Google Scholar]
- Burgess PW, Alderman N, Evans J, Emslie H, Wilson BA. The ecological validity of tests of executive function. Journal of the International Neuropsychological Society. 1998;4:547–558. doi: 10.1017/s1355617798466037. [DOI] [PubMed] [Google Scholar]
- Cahn DA, Sullivan EV, Shear PK, Pfefferbaum A, Heit G, Silverberg G. Differential contributions of cognitive and motor component processes to physical and instrumental activities of daily living in Parkinson’s disease. Archives of Clinical Neuropsychology. 1998;13:575–583. [PubMed] [Google Scholar]
- Carlson MC, Fried LP, Xue QL, Bandeen-Roche K, Zeger SL, Brandt J. Association between executive attention and physical functional performance in community-dwelling older women. The Journals of Gerontology Series B, Psychological Sciences and Social Sciences. 1999;54:S262–S270. doi: 10.1093/geronb/54b.5.s262. [DOI] [PubMed] [Google Scholar]
- Dixon L, Duncan D, Johnson P, Kirkby L, O’Connell H, Taylor H, et al. Occupational therapy for patients with Parkinson’s disease. Cochrane Database of Systematic Reviews. 2007;(3):CD002813. doi: 10.1002/14651858.CD002813.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emre M. What causes mental dysfunction in Parkinson’s disease? Movement Disorders. 2003;18(Suppl 6):S63–S71. doi: 10.1002/mds.10565. [DOI] [PubMed] [Google Scholar]
- Eriksson G, Kottorp A, Borg J, Tham K. Relationship between occupational gaps in everyday life, depressive mood and life satisfaction after acquired brain injury. Journal of Rehabilitation Medicine. 2009;41:187–194. doi: 10.2340/16501977-0307. [DOI] [PubMed] [Google Scholar]
- Eriksson G, Tham K, Borg J. Occupational gaps in everyday life 1–4 years after acquired brain injury. Journal of Rehabilitation Medicine. 2006;38:159–165. doi: 10.1080/16501970500415322. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL, Marsden CD, Goldstein M, Calne DB Members of the UDC. Recent developments in Parkinson’s disease. New York: Macmillan; 1987. Unified Parkinson’s disease rating scale; pp. 153–163. [Google Scholar]
- Green J, McDonald WM, Vitek JL, Evatt M, Freeman A, Haber M, et al. Cognitive impairments in advanced PD without dementia. Neurology. 2002;59:1320–1324. doi: 10.1212/01.wnl.0000031426.21683.e2. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: Onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Janvin C, Aarsland D, Larsen JP, Hugdahl K. Neuropsychological profile of patients with Parkinson’s disease without dementia. Dementia and Geriatric Cognitive Disorders. 2003;15:126–131. doi: 10.1159/000068483. [DOI] [PubMed] [Google Scholar]
- Klepac N, Trkulja V, Relja M, Babic T. Is quality of life in non-demented Parkinson’s disease patients related to cognitive performance? A clinic-based cross-sectional study. European Journal of Neurology. 2008;15:128–133. doi: 10.1111/j.1468-1331.2007.02011.x. [DOI] [PubMed] [Google Scholar]
- Lowenstein N, Tickle-Degnen L. Developing an occupational therapy home program for patients with Parkinson’s disease. In: Trail M, Protas EJ, Lai EC, editors. Neurorehabilitation in Parkinson’s Disease: An Evidence-Based Treatment Model. Thorofare, NJ: SLACK Incorporated; 2008. pp. 231–243. [Google Scholar]
- Murphy S, Tickle-Degnen L. The effectiveness of occupational therapy-related treatments for persons with Parkinson’s disease: A meta-analytic review. American Journal of Occupational Therapy. 2001;55:385–392. doi: 10.5014/ajot.55.4.385. [DOI] [PubMed] [Google Scholar]
- Oberauer K. Removing irrelevant information from working memory: A cognitive aging study with the modified Sternberg task. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27:948–957. [PubMed] [Google Scholar]
- Pillon B, Boller F, Levy R, Dubois B. Cognitive deficits and dementia in Parkinson’s disease. In: Boller F, Cappa SF, editors. Handbook of Neuropsychology. 2. Vol. 6. Philadelphia: Elsevier Science; 2001. pp. 311–371. [Google Scholar]
- Rahman S, Griffin HJ, Quinn NP, Jahanshahi M. Quality of life in Parkinson’s disease: The relative importance of the symptoms. Movement Disorders. 2008;23:1428–1434. doi: 10.1002/mds.21667. [DOI] [PubMed] [Google Scholar]
- Royall DR, Palmer R, Chiodo LK, Polk MJ. Declining executive control in normal aging predicts change in functional status: The Freedom House Study. Journal of the American Geriatrics Society. 2004;52:346–352. doi: 10.1111/j.1532-5415.2004.52104.x. [DOI] [PubMed] [Google Scholar]
- Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson’s disease? Journal of Neurology, Neurosurgery & Psychiatry. 2000;69:308–312. doi: 10.1136/jnnp.69.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer B, Vasterling JJ, Mathias CW, Brennan A. Clinical and neuropsychological correlates of impaired awareness of deficits in Alzheimer disease and Parkinson disease: A comparative study. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 2001;14:122–129. [PubMed] [Google Scholar]
- Shulman LM, Gruber-Baldini AL, Anderson KE, Vaughan CG, Reich SG, Fishman PS, et al. The evolution of disability in Parkinson disease. Movement Disorders. 2008;23:790–796. doi: 10.1002/mds.21879. [DOI] [PubMed] [Google Scholar]
- Tanner CM, Aston DA. Epidemiology of Parkinson’s disease and akinetic syndromes. Current Opinion in Neurology. 2000;13:427–430. doi: 10.1097/00019052-200008000-00010. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading Manual. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Weintraub D, Moberg PJ, Duda JE, Katz IR, Stern MB. Effect of psychiatric and other nonmotor symptoms on disability in Parkinson’s disease. Journal of the American Geriatrics Society. 2004;52:784–788. doi: 10.1111/j.1532-5415.2004.52219.x. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- York MK, Alvarez JA. Cognitive impairments associated with Parkinson’s disease. In: Trail M, Protas EJ, Lai EC, editors. Neurorehabilitation in Parkinson’s disease: An evidence-based treatment model. Thorofare, NJ: SLACK Incorporated; 2008. pp. 71–100. [Google Scholar]