Abstract
In the field of molecular oncology, the Myc basic helix-loop-helix family of transcription factors has been extensively studied. The Myc proto-oncogene c-Myc binds DNA, activates or represses gene transcription, and consequently affects cellular proliferation. However, emerging evidence presents the existence of c-Myc variants that lack transcriptional activity. A cytoplasmic variant of c-Myc called “Myc-nick,” which arises from calpain-mediated cleavage of c-Myc, assists in stable microtubule assembly. Furthermore, Myc-nick promotes MyoD-mediated myogenic differentiation, thus antagonizing its precursor. These results provide exciting new opportunities in formulating molecular approaches for treatment of cancer and in our understanding of cell differentiation.
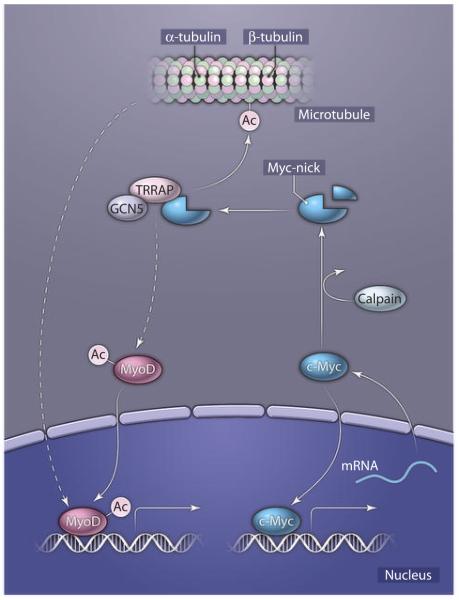
The Myc family of transcription factors participates in key tumor growth processes (1, 2). Although most studies have focused mainly on their gene regulatory activity, rare reports have indicated the existence of several cytoplasmic c-Myc variants with unexplored functions (3, 4). Interest in c-Myc degradation led Conacci-Sorrell et al. to identify a previously unknown member of Myc family, which they termed “Myc-nick” (5). Myc-nick was generated in the cytoplasm of dense populations of murine and human cell lines and primary cultures, as well as that of various normal and cancer cell lines, indicating that the generation of Myc-nick is a widespread phenomenon. Myc-nick was generated by proteolytic cleavage of full-length c-Myc in the cytoplasm. The use of various protease inhibitors (such as MG132 or calpeptin) and RNA interference revealed the proteases responsible for c-Myc cleavage to be the regulatory subunit of calpains, which preferentially cleaved at Lys298 of c-Myc. When expressed in Myc-null fibroblasts, Myc-nick induced additional morphological changes and cytoskeletal reorganization compared with full-length c-Myc. c-Myc associated with tubulins (6, 7), and stable microtubules were more prominent in Myc-nick–expressing cells, as evidenced by the presence of acetylated α-tubulin and resistance to destabilization by nocodazole. Deletion of the Myc-box motif II (ΔMBII) of c-Myc, which is the binding site for TRRAP (transformation/transcription domain-associated protein) and the acetyltransferase GCN5 (8), reverted the acetylation of α-tubulin to control levels, suggesting that Myc-nick promotes acetylation of microtubules by recruiting GCN5. Addition of c-Myc to either cytoplasmic extract or purified microtubules potentiated acetylation of α-tubulin by GCN5. Therefore, these data suggest a role for a Myc-nick–GCN5 complex in tubulin acetylation and, hence, reorganization of the cytoplasmic compartment (Fig. 1).
Fig. 1.
Myc-nick generation and regulation of cellular processes in the cytoplasm. Calpain-mediated cleavage of c-Myc results in the generation of Myc-nick. Myc-nick interacts with TRRAP and GCN5 and assists in the acetylation of tubulins, which results in more stable microtubular structures. The link between stable microtubule formation and MyoD-mediated gene transcription is currently unknown (indicated by the dashed lines). Ac, acetyl group.
Myc-nick was abundant in mature skeletal muscle and the brain. However, because tubulin acetylation increased during tissue formation (9, 10), the authors sought to delineate the role of Myc-nick in this process, using myogenic differentiation in culture as a model system. Increased abundance or activity of calpains, the emergence of Myc-nick, and elevated α-tubulin acetylation were observed in differentiated muscle cells (myotubes). In differentiated myotubes, c-Myc was predominantly cytoplasmic and colocalized with acetylated α-tubulin. Furthermore, introduction of Myc-nick with a myogenic transcription factor (MyoD) enhanced the conversion and differentiation of fibroblasts, as measured by expression of myogenic genes. Distinct from acetylation of α-tubulin, induction of myogenic genes was only partially dependent on the MBII domain of Myc-nick. This observation suggests that, in addition to acetylation, Myc-nick may regulate myogenesis through unidentified mechanisms. Alternatively, other acetyltransferases beside GCN5 might interact with the Myc-nick mutant lacking the MBII domain. Cells expressing a c-Myc mutant that lacked the calpain-recognition domain were less efficient in undergoing myogenic differentiation. Collectively, these results show that Myc-nick cooperates with MyoD in cellular programming and myogenic differentiation.
The study by Conacci-Sorrell et al. highlights the pleiotropic functions of the Myc family of transcription factors. By losing its nuclear localization and DNA binding domains, c-Myc becomes mainly cytoplasmic and promotes acetylation of α-tubulin. These observations raise interesting questions regarding the processivity of c-Myc by calpains. For example, can calpain activity be modulated in tumor cells such that it results in cleavage of c-Myc and regression of tumor growth? If so, can pharmacological agents be devised to selectively activate the regulatory subunit of calpains? Other questions relate to how calcium homeostasis influences calpain activity in cancer cells or how c-Myc may escape calpain cleavage in rhabdomyosarcoma cells.
The data of Conacci-Sorrell et al. suggest a role for Myc-nick in promoting myogenic differentiation. Whereas previous studies indicated that c-Myc interferes with MyoD activity (11), Myc-nick assists in the MyoD-dependent conversion of fibroblasts. In this regard, we speculate that Myc-nick may modulate the activity of MyoD indirectly through crosstalk with assembled microtubules. This proposed mechanism may be analogous to the release of the myocardin-related coactivator of Serum Response Factor (SRF) from polymerized actin in the cytoplasm (12). Alternatively, recruitment of Myc-nick by GCN5 may acetylate and activate MyoD en route from cytoplasm to nucleus (13). Purification of Myc-nick–associated proteins should help in identifying additional binding partners (Fig. 1). It will be interesting to see as the field advances and unravels details in these Myc-nick–assisted processes.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Pelengaris S, Khan M, Evan G. c-MYC: More than just a matter of life and death. Nat. Rev. Cancer. 2002;2:764–776. doi: 10.1038/nrc904. [DOI] [PubMed] [Google Scholar]
- 3.Wakamatsu Y, Watanabe Y, Shimono A, Kondoh H. Transition of localization of the N-Myc protein from nucleus to cytoplasm in differentiating neurons. Neuron. 1993;10:1–9. doi: 10.1016/0896-6273(93)90236-k. [DOI] [PubMed] [Google Scholar]
- 4.Bai MK, Costopoulos JS, Christoforidou BP, Papadimitriou CS. Immunohistochemical detection of the c-myc oncogene product in normal, hyperplastic and carcinomatous endometrium. Oncology. 1994;51:314–319. doi: 10.1159/000227356. [DOI] [PubMed] [Google Scholar]
- 5.Conacci-Sorrell M, Ngouenet C, Eisenman RN. Myc-nick: a cytoplasmic cleavage product of Myc that promotes α-tubulin acetylation and cell differentiation. Cell. 2010;142:480–493. doi: 10.1016/j.cell.2010.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexandrova N, Niklinski J, Bliskovsky V, Otterson GA, Blake M, Kaye FJ, Zajac-Kaye M. The N-terminal domain of c-Myc associates with α-tubulin and microtubules in vivo and in vitro. Mol. Cell. Biol. 1995;15:5188–5195. doi: 10.1128/mcb.15.9.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niklinski J, Claassen G, Meyers C, Gregory MA, Allegra CJ, Kaye FJ, Hann SR, Zajac-Kaye M. Disruption of Myc-tubulin interaction by hyperphosphorylation of c-Myc during mitosis or by constitutive hyperphosphorylation of mutant c-Myc in Burkitt’s lymphoma. Mol. Cell. Biol. 2000;20:5276–5284. doi: 10.1128/mcb.20.14.5276-5284.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMahon SB, Wood MA, Cole MD. The essential cofactor TRRAP recruits the histone acetyltransferase hGCN5 to c-Myc. Mol. Cell. Biol. 2000;20:556–562. doi: 10.1128/mcb.20.2.556-562.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Creppe C, Malinouskaya L, Volvert ML, Gillard M, Close P, Malaise O, Laguesse S, Cornez I, Rahmouni S, Ormenese S, Belachew S, Malgrange B, Chapelle JP, Siebenlist U, Moonen G, Chariot A, Nguyen L. Elongator controls the migration and differentiation of cortical neurons through acetylation of alpha-tubulin. Cell. 2009;136:551–564. doi: 10.1016/j.cell.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 10.Gundersen GG, Khawaja S, Bulinski JC. Generation of a stable, posttranslationally modified microtubule array is an early event in myogenic differentiation. J. Cell Biol. 1989;109:2275–2288. doi: 10.1083/jcb.109.5.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miner JH, Wold BJ. c-myc inhibition of MyoD and myogenin-initiated myogenic differentiation. Mol. Cell. Biol. 1991;11:2842–2851. doi: 10.1128/mcb.11.5.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–342. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- 13.Sartorelli V, Puri PL, Hamamori Y, Ogryzko V, Chung G, Nakatani Y, Wang JY, Kedes L. Acetylation of MyoD directed by PCAF is necessary for the execution of the muscle program. Mol. Cell. 1999;4:725–734. doi: 10.1016/s1097-2765(00)80383-4. [DOI] [PubMed] [Google Scholar]