Abstract
Objective:
To use a historical placebo control design to determine whether lithium carbonate slows progression of amyotrophic lateral sclerosis (ALS).
Methods:
A phase II trial was conducted at 10 sites in the Western ALS Study Group using similar dosages (300–450 mg/day), target blood levels (0.3–0.8 mEq/L), outcome measures, and trial duration (13 months) as the positive trial. However, taking riluzole was not a requirement for study entry. Placebo outcomes in patients matched for baseline features from a large database of recent clinical trials, showing stable rates of decline over the past 9 years, were used as historical controls.
Results:
The mean rate of decline of the ALS Functional Rating Scale–Revised was greater in 107 patients taking lithium carbonate (−1.20/month, 95% confidence interval [CI] −1.41 to −0.98) than that in 249 control patients (−1.01/month, 95% CI −1.11 to −0.92, p = 0.04). There were no differences in secondary outcome measures (forced vital capacity, time to failure, and quality of life), but there were more adverse events in the treated group.
Conclusions:
The lack of therapeutic benefit and safety concerns, taken together with similar results from 2 other recent trials, weighs against the use of lithium carbonate in patients with ALS. The absence of drift over time and the availability of a large database of patients for selecting a matched historical control group suggest that use of historical controls may result in more efficient phase II trials for screening putative ALS therapeutic agents.
Classification of evidence:
This study provided Class IV evidence that lithium carbonate does not slow the rate of decline of function in patients with ALS over 13 months. Neurology® 2011;77:973–979
Amyotrophic lateral sclerosis (ALS) is characterized by the progressive loss of motor neurons leading to death in 2–5 years. More than 30 clinical trials, enrolling nearly 10,000 patients, yielded one modestly effective treatment, riluzole.1 This record has prompted us to consider more efficient approaches to screen promising compounds in ALS.
A small pilot study reported that lithium carbonate combined with riluzole slowed disease progression by 60% in 16 treated patients with ALS compared with 28 control subjects and also slowed progression in a SOD1 mouse model.2 The presumed target was impaired autophagy.3–5 In response, we designed a clinical trial to test the reported benefit using a historical control design. This approach gave us the opportunity to test one method for improving the efficiency of screening promising agents in this disease.6–8 Sequential9 and adaptive10 designs have also improved efficiency of ALS trials. Our study took advantage of our database of 616 historical placebo controls from 6 earlier trials spanning 9 years. Two other trials, performed at the same time with the same question in mind, allowed us to compare these methods with other approaches.11,12
The primary research question was this: Does lithium carbonate slow the decline of function in patients with ALS over 13 months (Class IV evidence)?
METHODS
Patients.
This was an open-label, multicenter, 13-month, phase II screening trial of 107 patients taking lithium carbonate. Treated patients were matched against 249 placebo control subjects from the minocycline in ALS trial (MINO), completed from 2005 to 2007.13 That trial used similar inclusion criteria, recruitment and enrollment procedures, patient evaluations, and statistical measures.13
Selection criteria.
Patients were enrolled between May 2008 and February 2009. Inclusion criteria were a diagnosis of probable or definite ALS,14 age 21–85 years, forced vital capacity (FVC) ≥75% of predicted, and onset of weakness within 3 years. Patients taking riluzole were on a stable dose for at least 30 days.
Study drug.
Subjects started lithium carbonate at 150 mg twice daily. Trough levels, at 12 ± 2 hours after the previous dose, were measured after 2 weeks and repeated 2 weeks after any dose change. We aimed for levels of 0.3–0.8 mEq/L, and patients with levels lower than 0.3 mEq/L were asked to increase the dose to a maximum of 450 mg/day. If subjects did not tolerate a dose, investigators could taper the dose by 150-mg increments to the maximum tolerated dosage. Trough levels were repeated at 1, 6, and 12 months after therapy was begun. Lithium carbonate tablets (150 mg) were purchased from Roxane Laboratories, Inc. and dispensed at baseline and at months 3, 6, and 9 in sealed bottles of 100.
Procedures.
ALS Functional Rating Scale–Revised (ALSFRS-R) score, quality of life (QOL), and FVC were measured at baseline. At months 1, 3, 6, 9, and 12, we obtained laboratory tests, interim history, ALSFRS-R, FVC, adverse events, QOL, body weight, and medication logs for compliance. At months 4.5, 7.5, 10.5, and 13, we reviewed adverse events and medications and measured ALSFRS-R telephonically.
Outcome measures.
The primary outcome measure was slope of the ALSFRS-R. Secondary measures consisted of changes in FVC slope, weight loss, the single-item ALS Quality of Life Scale,15 and time to failure (defined as death, endotracheal intubation, tracheostomy-assisted ventilation, or use of noninvasive ventilation 23 hours/day for 14 days). Safety was assessed by adverse events and abnormal laboratory studies.
Study monitoring and organization.
Monitoring and training procedures were identical to those reported previously.13 Ten sites from the Western ALS Study Group (WALS) participated; the Forbes Norris MDA/ALS Research Center served as the main coordinating site. A steering and safety committee met monthly. Three interim analyses of safety were conducted after 30 patients completed 6 months of treatment (April 2009), after 60 patients completed 6 months of treatment (October 2009), and after 60 patients completed 9 months of treatment (February 2010). At each point, an external safety monitor confirmed that the study should continue.
Standard protocol approvals, registrations, and patient consents.
Written informed consent was provided by all participants, and the study was approved by review boards of all sites. The study was registered with ClinicalTrials.gov and was assigned clinical trial identifier number NCT00790582.
Statistical analysis.
Primary outcome measure.
We compared ALSFRS-R slopes for patients taking lithium with those in the MINO placebo cohort. All patients with 2 or more visits were included. Our study had greater than 85% power (at a 2-sided 5% significance level) to detect a 30% or greater reduction in the mean slope of ALSFRS-R between MINO control subjects and individuals taking lithium. The sample size calculation was based on simulations of a linear mixed-effects model applied to data from MINO.13 In comparison, the 30% slope reduction is approximately half that observed in the Fornai et al.2 study that suggested lithium might be beneficial in ALS. ALSFRS-R scores were analyzed for all patients in the intention-to-treat population by use of a linear mixed-effects model with fixed-effects terms for intercept, placebo slope, and effect of lithium on slope. Random effects allowed for interpatient variability in intercept and slope as well as residual variation. Tests for lithium effect were based on a Wald statistic equal to the estimated effect divided by its SE.
Missing data were handled in a similar fashion for treated patients and control subjects. The missing data were assumed to be not random but to have a similar pattern in both groups. We did sensitivity analyses for the main model and additional explorative analyses, which extended the linear mixed-effects model by including fixed effects for covariates. Using different control groups allowed us to examine sensitivity due to this factor.
Tests of secondary outcomes.
The effect of lithium carbonate on FVC, time to failure, safety, weight loss, and QOL was tested by comparing mean slopes with those from the control group based on 2-sided z tests at the 5% level of significance. All patients taking lithium carbonate were included in our safety analysis. The number of patients who had adverse events and abnormal laboratory values were compared between groups by Fisher exact test. We collected compliance data for each visit and by group. The effects of dose, of riluzole use, and of the occurrence of adverse events on the primary outcome measure were examined post hoc by comparison of the rates of decline estimated by the linear mixed-effects model. The numbers of participants who completed the study in each group were compared by Fisher exact test.
Control groups.
Our database contains 6 completed clinical trials that specifically used the ALSFRS-R to measure functional decline. These are the trials of creatine,16 celecoxib (Celebrex),17 TCH346,18 coenzyme Q10,10 lithium,11 and minocycline.13 Our primary comparison was with placebo from MINO. We included 206 patients randomly assigned at 4 months (n = 206) plus 43 who dropped out during the 4-month lead-in. We used multiple control groups to determine whether results for our primary outcome measure were dependent on time or patient characteristics of different placebo groups, believing that lack of dependence would support use of historical placebo controls in future phase II ALS trials.
Role of the funding source.
This trial was initiated and coordinated by the WALS investigators. The funding source for this trial approved the design and protocol but had no involvement in the collection, analysis, and interpretation of data or in the writing of the report.
RESULTS
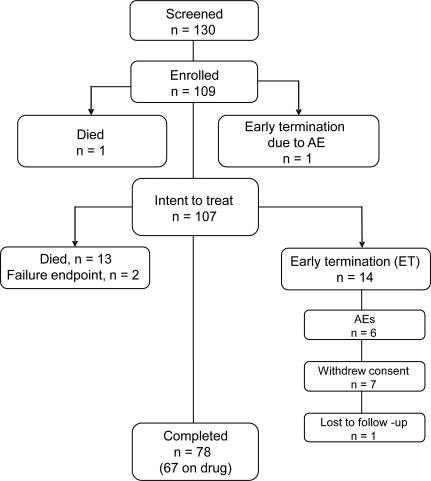
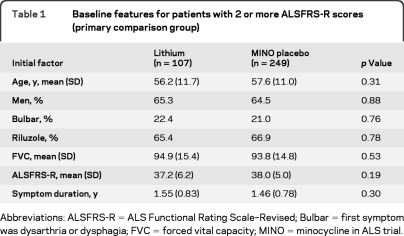
The patient flow and disposition in the trial are shown in figure 1. Patients in the treated group were well-matched for baseline features (table 1) to those in the historical control group from MINO. A total of 109 patients were enrolled, and 78 patients completed the full 13-month study (67 taking the drug). The intent-to-treat population included 107 patients. Of the 31 patients who did not complete the trial, 14 died (12 of ALS and 2 of cardiac arrest) and 2 others reached a failure endpoint. No deaths were considered to be related to the study drug. Fourteen patients withdrew consent (6 because of adverse events), and one was lost to follow-up. In total, 12 patients discontinued drug due to a variety of adverse events (anorexia, urinary urgency, confusion, joint swelling, EKG changes, depression, incoordination, nausea, lethargy, tremor, pulmonary embolism, and increased weakness). The completion rate of 72% for this 13-month study was greater than the 60% completion rate for the 13-month MINO.13
Figure 1. Flow chart.
The disposition of subjects in the study, including the number screened, enrolled, failed, early terminated (ET), and completed. AE = adverse event.
Table 1.
Baseline features for patients with 2 or more ALSFRS-R scores (primary comparison group)
Abbreviations: ALSFRS-R = ALS Functional Rating Scale–Revised; Bulbar = first symptom was dysarthria or dysphagia; FVC = forced vital capacity; MINO = minocycline in ALS trial.
Primary endpoint.
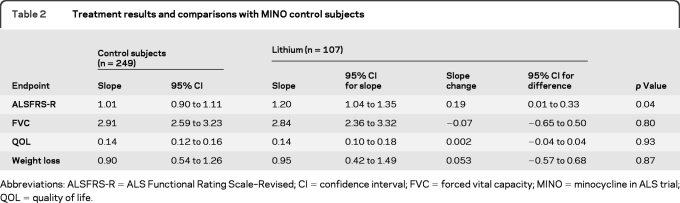
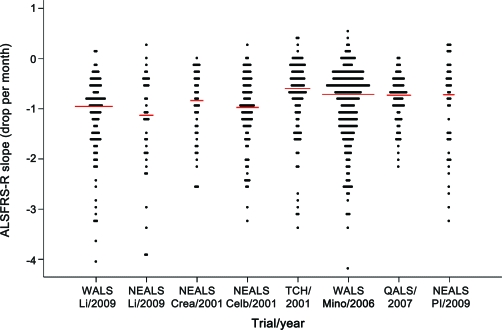
We found a significantly greater rate of decline of the ALSFRS-R slope in patients taking lithium (n = 107) compared with control subjects (n = 249). The estimated mean slope was 1.20/month for patients who took lithium carbonate vs 1.01/month for control subjects (table 2). The estimated difference in slope due to treatment was 0.19/month, which was significant (p = 0.04). Slopes for lithium in the other historical comparison groups are shown in figure 2. For additional analyses of control and lithium groups, see appendix e-1 on the Neurology® Web site at www.neurology.org.
Table 2.
Treatment results and comparisons with MINO control subjects
Abbreviations: ALSFRS-R = ALS Functional Rating Scale–Revised; CI = confidence interval; FVC = forced vital capacity; MINO = minocycline in ALS trial; QOL = quality of life.
Figure 2. ALS Functional Rating Scale–Revised (ALSFRS-R) slopes during the first 6 months of follow-up by study group.
Study groups are the following: Western ALS Study Group (WALS) LI = WALS lithium (n = 107), Northeast ALS Consortium (NEALS) LI = NEALS lithium (n = 39), NEALS Crea = NEALS creatine placebo (n = 45), NEALS Celb = NEALS Celebrex placebo (n = 95), TCH = Novartis TCH346 assigned placebo (n = 108), WALS Mino = minocycline control (n = 249), QALS = QALS coenzyme Q10 placebo (n = 75), and NEALS Pl = NEALS lithium placebo (n = 44). Each dot represents the slope of one patient. Only patients with symptom duration ≤3 years and initial forced vital capacity ≥75% are included. Small red bar indicates median slope for the study group. Slopes are limited to the first 6 months follow-up because that was the maximum for the NEALS creatine and lithium trials. Data are listed in chronologic order.
The detrimental lithium effect was greatest in patients taking lithium carbonate without riluzole. The mean slope for these 37 patients was −1.53/month (95% confidence interval [CI] −1.92 to −1.14) compared with a mean of −1.03/month (95% CI −1.14 to −0.91) for 70 patients taking lithium plus riluzole (p = 0.01 for the slope difference, tested in the linear mixed-effects model). For comparison, the lithium plus riluzole outcome was similar to the mean of −1.05 (95% CI −1.28 to −0.81, p = 0.84) for 166 control patients from MINO taking riluzole.
Secondary endpoints.
The mean slope of decline of the FVC was not significantly different between all patients taking lithium (2.84/month) and MINO control subjects (2.91/month) (p = 0.80) (table 2). The FVC decline from lithium alone did not reach significance (table 2), and patients taking lithium plus riluzole declined at a rate of 2.63/month (95% CI 2.12–3.15) vs 3.33/month for those taking lithium alone (95% CI 2.27–4.40) (p = 0.23). There was no difference in weight loss between patients taking lithium and control subjects (table 2).
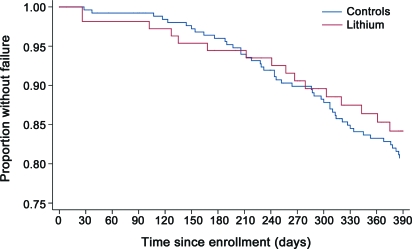
The Kaplan-Meier survival curve for patients taking lithium did not differ from that for control subjects (figure 3). A total of 16 patients either died14 or reached a failure endpoint compared with an expected total of 19, based on patients from MINO.2 Survival was not different when the subpopulations of patients taking riluzole were compared (p = 0.13 by log-rank test).
Figure 3. Survival comparison.
Kaplan-Meier survival curve of time to failure of lithium (red) vs placebo (blue).
QOL tended to decline over the course of the study, but there were no significant differences between the treated and control groups (table 2). Baseline QOL did not differ between patients taking lithium (7.5, 95% CI 7.2–7.8) and control subjects (7.4, 95% CI 7.2–7.7). The rate of decline for patients taking lithium was 0.139/month and for control subjects was 0.136/month (p = 0.93).
The 62 reported serious adverse events (SAEs) in patients taking lithium was greater than the 40 expected, based on rates from MINO (p < 0.001). The number of patients with SAE was also greater than expected (p = 0.02). Only one SAE was considered to be related to the study drug in a patient who experienced confusion, fever, and dehydration. Nonserious adverse events (NSAEs) were more frequent in patients treated with lithium. There were 146 falls (81 expected) and 123 neurologic NSAEs (88 expected). Other NSAEs did not differ between groups.
The mean dose of lithium carbonate throughout the trial was 375 mg/day, and the mean blood level was 0.36 mEq/L (range 0.2–0.8) at the end of the initial titration period (n = 107). The mean level was 0.35 mEq/L for 87 patients still taking medication at 6 months and 0.37 mEq/L for 60 patients taking medication at 12 months. Early in the trial, after just 11 patients had completed the baseline, 2 patients taking the original maximal dosage of 600 mg/day experienced dizziness and confusion, with 1 patient withdrawing from the study. This experience, combined with the report that low doses were more effective in the mouse model,2 led us to decrease the maximum to 450 mg/day to address concerns that too many patients would develop toxicity.
Comparisons with additional control groups.
A primary concern in the use of historical controls is whether results would differ depending on the time period or the particular control group used. We conducted several additional analyses to determine the sensitivity of our primary result to these factors. We found some variability, but no significant differences in ALSFRS-R slopes among the different placebo groups (p = 0.53, when comparisons were restricted to patients with similar initial FVC and symptom duration). We found no trend with time for ALSFRS-R slopes (p = 0.72 for a linear trend in slopes over time). If we used placebo from the 5 other trials in place of the MINO placebo, the results were similar to those for the primary comparison of ALSFRS-R (estimated lithium slope difference = 0.29, p = 0.001). In addition, the slopes for 2 lithium-treated groups (Northeast ALS Consortium and WALS) were similar (p = 0.66 for testing slope difference). These results are shown in figure 2 and are explained in detail in appendix e-1.
DISCUSSION
Our findings refute a beneficial effect of lithium in ALS and broaden the conclusions reached in 2 earlier trials.11,12 One enrolled 84 patients taking riluzole and was powered to detect the large positive effect reported in the initial trial.2 It was stopped at the first interim analysis for futility against this endpoint. The other enrolled 171 patients who were randomly assigned to either a subtherapeutic or a higher therapeutic dose of lithium.12 That study was stopped prematurely because of excessive dropouts and found no differences in efficacy measures between the groups or compared with control subjects from a disease registry.12 Our findings provide additional information about longer treatment duration, safety concerns, and a trial design with historical controls.
A variety of factors may explain the optimistic results of the initial study.2 Among them, the small size of that trial may have contributed to a chance outcome. In addition, the trial enrolled an unusual patient population for which the mean disease duration was more than 3 years at enrollment, combined with relatively high mean baseline ALSFRS-R scores of 39.9 in treated subjects and 40.1 in control subjects. These numbers compute to a very slow pretrial estimated rate of progression of 0.2 ALSFRS-R points/month, compared with 0.58 in this trial and 0.57 in MINO control subjects. Thus, the apparent lithium beneficial effect in the initial study could have been due to randomizing many patients with slowly progressive disease to the treated group.
Although our trial was designed to examine the large reported benefit of lithium, we also view it as an intermediate step in developing more efficient screening trials.19 By showing that the large reported beneficial effect is unlikely and that the outcome is no better than that for placebo control subjects from our database, we conclude that lithium is a poor candidate for further testing, and we predict that ultimately it would not prove beneficial in a larger trial. That our results appear similar to those of the other 2 placebo-controlled trials of lithium described above suggests that this approach warrants further examination.
The most critical issue in assessing the validity of historical controls in ALS is to ensure that slope outcomes are remaining constant over time in different trials. In Parkinson disease, in which the use of historical controls has been questioned, a systematic drift in slopes is presumably due to factors related to symptomatic treatments for that disease.20 In contrast, in ALS there are no treatments that clearly modify the course of the disease, and our analysis suggests that there has been no systematic drift. Although there has been some variation in the reported slope outcomes from placebo patients in past control groups, much of this can be explained by enrollment criteria. In particular, varied cutoffs for disease duration and baseline FVC allow for patients with faster or slower progression to enroll in trials. These variables account for most differences in slope. By using a matched control population and standardized analytic methods, we find that mean slopes have been remarkably constant over the past 9 years.
There are still many factors that need to be examined. Bias relating to the use of historical controls may stem from the types of patients who enroll in these studies, other studies enrolling concurrently that shunt subsets of patients to one trial or another, or altered evaluations of patients taking open-label agents. The validity of these trials can be improved by examining whether patients who enroll in open-label trials are the same as those who enroll in controlled trials. Our results suggest that further investigation of historical control data from ALS trials may be valuable to ensure the absence of drift and to examine factors that contribute to variability between trials. This approach could lead to a substantial increase in statistical power (for a discussion of methods to estimate power with historical controls, see Zhang et al.21), may increase the number of patients enrolling in ALS trials (now less than 20% of patients), and would yield information to supplement animal models and laboratory data in making better informed decisions about whether to proceed to phase III. In this study, the clear lack of benefit suggests that larger trials of lithium are not likely to be fruitful. Finally, the stability of our primary outcome measure over time and the large database of historical placebo controls from which to select a matched historical group for comparison suggest that historically controlled trials in a disease such as ALS deserve further consideration.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the patients and caregivers who participated and the external safety monitor, Professor H. Mitsumoto, for his guidance, the NEALS group for sharing their data, and the following contributors for their substantial help: California Pacific Medical Center: Giovanna Kushner and Thais Zayas-Bazan (Study Coordinators), Mark Spitalny (Data Manager), and Chow Saephanh (word processing the manuscript); Kansas University Medical Center: Laura Herbelin (Clinic Evaluator) and Maureen Walsh (Study Coordinator); Mayo Clinic: Joseph Verheijde (Clinic Evaluator) and Teri Radam (Study Coordinator); Methodist Neurological Institute: Luis Lay, Jr. (Study Coordinator); Providence ALS Center: Nancy Hoke (Study Coordinator); University of California at Irvine: Veronica Martin (Study Coordinator), Sana Thara, and Patricia Tully (Study Coordinators); University of California at Los Angeles: Rebecca Alvarez (Study Coordinator) and Juliana Albu (Clinic Evaluator); University of Pennsylvania: Katie Hoskins (Study Coordinator); University of Utah: Narneice Craven and Summer Davis (Study Coordinators); Washington University: Charlie Wulf and Pamela Townsend (Study Coordinators).
- ALS
- amyotrophic lateral sclerosis
- ALSFRS-R
- ALS Functional Rating Scale–Revised
- CI
- confidence interval
- FVC
- forced vital capacity
- MINO
- minocycline in ALS trial
- NSAE
- nonserious adverse event
- QOL
- quality of life
- SAE
- serious adverse event
- WALS
- Western ALS Study Group.
Editorial, page 936
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Miller: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, study supervision, and obtaining funding. Dr. Moore: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, and statistical analysis. D.A. Forshew: drafting/revising the manuscript, study concept or design, acquisition of data, and study supervision. Dr. Katz: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, contribution of vital reagents/tools/patients, acquisition of data, statistical analysis, study supervision, and obtaining funding. Dr. Barohn: drafting/revising the manuscript. Dr. Valan: study concept or design, analysis or interpretation of data, and study supervision. Dr. Bromberg: study concept or design, acquisition of data, and study supervision. Dr. Goslin: study concept or design, analysis or interpretation of data, acquisition of data, and study supervision. Dr. Graves: study concept or design, analysis or interpretation of data, and acquisition of data. Dr. McCluskey: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, study supervision, and obtaining funding. Dr. McVey: analysis or interpretation of data, acquisition of data, and study supervision. Dr. Mozaffar: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, and study supervision. Dr. Florence: drafting/revising the manuscript, acquisition of data, and assistance in the training of the clinical evaluators in the outcome measures and assessments. Dr. Pestronk: drafting/revising the manuscript, study concept or design, and analysis or interpretation of data. Dr. Ross: drafting/revising the manuscript, study concept or design, acquisition of data, and study supervision. Dr. Simpson: drafting/revising the manuscript and acquisition of data. Dr. Appel: study concept or design and obtaining funding.
COINVESTIGATORS
WALS Study Group Sites: California Pacific Medical Center: Susan Woolley-Levine, PhD (neuropsychologic data analysis); Kansas University Medical Center: Mazen Dimachkie, MD (site investigator), Yunxia Wang, MD (site investigator); Mayo Clinic, Scottsdale: E.P. Bosch, MD, site investigator; University of Pennsylvania, Philadelphia; Lauren Elman (site investigator).
DISCLOSURE
Dr. Miller serves on scientific advisory boards for Cytokinetics, Inc. and Neuraltus Pharmaceuticals, Inc.; has received funding for travel and speaker honoraria from Avanir Pharmaceuticals; serves on the editorial board of Lancet Neurology; serves as a consultant for Gilead Sciences, Inc.; and receives research support from the Muscular Dystrophy Association. Dr. Moore serves as a consultant for Neuraltus Pharmaceuticals, Inc., Knopp Neurosciences Inc., Sangamo BioSciences, Celgene, and IntelliGeneScan, Inc. and receives research support from Neuraltus Pharmaceuticals, Inc., IntelliGeneScan, Inc., the Phoenix Neurological Association, the Palo Alto Medical Foundation, Knopp Neurosciences Inc., Sangamo BioSciences, the NIH/NCI, and the Muscular Dystrophy Association. D.A. Forshew receives research support from the Muscular Dystrophy Association. Dr. Katz has received research support from Pfizer Inc/Eisai Inc., and the ALS Association, and has received honoraria from Crescent Healthcare, Inc., Blue Cross, Talecris Biotherapeutics, and CSL Behring. Dr. Barohn has served on the speakers' bureau for Talecris Biotherapeutics and receives research support from the NIH. Dr. Valan reports no disclosures. Dr. Bromberg serves on a scientific advisory board for Accordant Health Services, Inc.; has received funding for travel and speaker honoraria from Rx Solutions and Talecris Biotherapeutics; serves as an Assistant Editor for Muscle & Nerve and the Journal of Neuromuscular Diseases and as Editor of Clinical Neurophysiology; receives publishing royalties for Handbook of Peripheral Neuropathy (Taylor & Francis, 2005) and Quality of Life Measurement in Neurodegenerative and Related Conditions (Cambridge, 2010); serves on the speakers' bureau for Talecris Biotherapeutics; and receives research support from Knopp Neurosciences Inc., and the NIH. Dr. Goslin reports no disclosures. Dr. Graves has served on the speakers' bureau for Talecris Biotherapeutics; has served as a consultant for Avanir Pharmaceuticals, the American Red Cross, Cephalon, Inc., the Guillain-Barré Support Group International, and Sanofi-Aventis; receives research support from Regeneron Pharmaceuticals Inc., Avanir Pharmaceuticals, Amgen, Novartis, Genentech, Inc., Sanofi-Aventis, Knopp Neurosciences Inc., the NIH, and the Muscular Dystrophy Association; and is a stakeholder in GE Healthcare. Dr. McCluskey and Dr. McVey report no disclosures. Dr. Mozaffar serves on the Medical and Scientific Advisory Board for the Myositis Association; has served as a consultant and/or on the speakers' bureau for and received funding for travel from Genzyme Corporation, Talecris Biotherapeutics, Crescent Healthcare, Inc., Allergan, Inc., and Avanir Pharmaceuticals, Inc. Dr. Florence serves on a scientific advisory board for Prosensa; serves on the editorial board of Neuromuscular Disorders; and serves as a consultant for Prosensa, GlaxoSmithKline, and Acceleron Pharma. Dr. Pestronk has served on a speakers' bureau for and received speaker honoraria from Athena Diagnostics, Inc.; owns stock in Johnson & Johnson; is director of the Washington University Neuromuscular Clinical Laboratory which performs antibody testing and muscle and nerve pathology analysis, procedures for which the Washington University Neurology Department bills; may accrue revenue on patents re: TS-HDS antibody, GALOP antibody, GM1 ganglioside antibody, and Sulfatide antibody; has received license fee payments from Athena Diagnostics, Inc. for patents re: antibody testing; and receives/has received research support from Genzyme Corporation, Insmed Inc., Knopp Neurosciences Inc., Prosensa, Isis Pharmaceuticals, Inc., Sanofi-Aventis, the NIH (5R01-NS04326407 [site PI]), CINRG Children's Hospital, Washington, DC, and the Muscular Dystrophy Association. Dr. Ross and Dr. Simpson report no disclosures. Dr. Appel serves on a scientific advisory board for Neuraltus Pharmaceuticals, Inc.; has received a speaker honorarium from Avanir Pharmaceuticals; receives research support from the NIH and the Muscular Dystrophy Association; and has served as an expert consultant in a medico-legal case.
REFERENCES
- 1. Miller RG, Jackson CE, Kasarskis EJ, et al. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2009;73:1218–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fornai F, Longone P, Cafaro L, et al. Lithium delays progression of amyotrophic lateral sclerosis. Proc Natl Acad Sci USA 2008;105:2052–2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fornai F, Longone P, Ferrucci M, et al. Autophagy and amyotrophic lateral sclerosis: the multiple roles of lithium. Autophagy 2008;4:527–530 [DOI] [PubMed] [Google Scholar]
- 4. Sarkar S, Floto RA, Berger Z, et al. Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol 2005;170:1101–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sarkar S, Rubinsztein DC. Inositol and IP3 levels regulate autophagy: biology and therapeutic speculations. Autophagy 2006;2:132–134 [DOI] [PubMed] [Google Scholar]
- 6. Czaplinski A, Haverkamp LJ, Yen AA, Simpson EP, Lai EC, Appel SH. The value of database controls in pilot or futility studies in ALS. Neurology 2006;67:1827–1832 [DOI] [PubMed] [Google Scholar]
- 7. Gordon PH. A placebo arm is not always necessary in clinical trials of amyotrophic lateral sclerosis. Muscle Nerve 2009;39:858–860 [DOI] [PubMed] [Google Scholar]
- 8. Simmons Z. Can we eliminate placebo in ALS clinical trials? Muscle Nerve 2009;39:861–865 [DOI] [PubMed] [Google Scholar]
- 9. Groeneveld GJ, Veldink JH, van der Tweel I, et al. A randomized sequential trial of creatine in amyotrophic lateral sclerosis. Ann Neurol 2003;53:437–445 [DOI] [PubMed] [Google Scholar]
- 10. Kaufmann P, Thompson JL, Levy G, et al. Phase II trial of CoQ10 for ALS finds insufficient evidence to justify phase III. Ann Neurol 2009;66:235–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aggarwal SP, Zinman L, Simpson E, et al. Safety and efficacy of lithium in combination with riluzole for treatment of amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010;9:481–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chio A, Borghero G, Calvo A, et al. Lithium carbonate in amyotrophic lateral sclerosis: lack of efficacy in a dose-finding trial. Neurology 2010;75:619–625 [DOI] [PubMed] [Google Scholar]
- 13. Gordon PH, Moore DH, Miller RG, et al. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol 2007;6:1045–1053 [DOI] [PubMed] [Google Scholar]
- 14. Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1:293–299 [DOI] [PubMed] [Google Scholar]
- 15. Robbins RA, Simmons Z, Bremer BA, Walsh SM, Fischer S. Quality of life in ALS is maintained as physical function declines. Neurology 2001;56:442–444 [DOI] [PubMed] [Google Scholar]
- 16. Shefner JM, Cudkowicz ME, Schoenfeld D, et al. A clinical trial of creatine in ALS. Neurology 2004;63:1656–1661 [DOI] [PubMed] [Google Scholar]
- 17. Cudkowicz ME, Shefner JM, Schoenfeld DA, et al. Trial of celecoxib in amyotrophic lateral sclerosis. Ann Neurol 2006;60:22–31 [DOI] [PubMed] [Google Scholar]
- 18. Miller R, Bradley W, Cudkowicz M, et al. Phase II/III randomized trial of TCH346 in patients with ALS. Neurology 2007;69:776–784 [DOI] [PubMed] [Google Scholar]
- 19. Cudkowicz ME, Katz J, Moore DH, et al. Toward more efficient clinical trials for amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2010;11:259–265 [DOI] [PubMed] [Google Scholar]
- 20. NINDS NET-PD Investigators A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology 2006;66:664–671 [DOI] [PubMed] [Google Scholar]
- 21. Zhang S, Cao J, Ahn C. Calculating sample size in trials using historical controls. Clin Trials 2010;7:343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.