Abstract
Objective:
To evaluate the effect of all-trans retinoic acid (ATRA) as treatment for chemotherapy-induced peripheral neuropathy in an experimental animal model and in a randomized, double-blinded, controlled trial in patients with non-small-cell lung cancer (NSCLC).
Methods:
Forty male Wistar rats were randomized in 5 groups: group A, control; groups B and C, treated with cisplatin; and groups D and E, treated with paclitaxel. ATRA (20 mg/kg PO) was administered for 15 days in groups C and E. We evaluated neuropathy and nerve regeneration–related morphologic changes in sciatic nerve, the concentration of nerve growth factor (NGF), and retinoic acid receptor (RAR)–α and RAR-β expression. In addition, 95 patients with NSCLC under chemotherapy treatment were randomized to either ATRA (20 mg/m2/d) or placebo. Serum NGF, neurophysiologic tests, and clinical neurotoxicity were assessed.
Results:
The experimental animals developed neuropathy and axonal degeneration, associated with decreased NGF levels in peripheral nerves. Treatment with ATRA reversed sensorial changes and nerve morphology; this was associated with increased NGF levels and RAR-β expression. Patients treated with chemotherapy had clinical neuropathy and axonal loss assessed by neurophysiology, which was related to decreased NGF levels. ATRA reduced axonal degeneration demonstrated by nerve conduction velocity and clinical manifestations of neuropathy grades ≥2.
Conclusions:
ATRA reduced chemotherapy-induced experimental neuropathy, increased NGF levels, and induced RAR-β expression in nerve. In patients, reduction of NGF in serum was associated with the severity of neuropathy; ATRA treatment reduced the electrophysiologic alterations.
Classification of evidence:
This study provides Class II evidence that ATRA improves nerve conduction in patients with chemotherapy-induced peripheral neuropathy. Neurology® 2011;77:987–995
Platinum-based chemotherapy is the first-line therapy for non-small-cell lung cancer (NSCLC) and improves both survival and disease-related symptomatology.1 However, chemotherapy-induced peripheral neuropathy is common and decreases quality of life (QOL) and may limit chemotherapy doses.2 Cisplatin promotes the formation of free radicals, while paclitaxel blocks axonal transport within peripheral nerves. As many as 38% of patients with NSCLC treated with these drugs develop a disabling sensory neuropathy.3,4
Retinoids play a critical role in a variety of biological functions, particularly in epithelial and neural differentiation.5 Different types of retinoids possess variable selectivity to retinoid-receptors that is the case of all-trans retinoic acid (ATRA), which binds and activates different subtypes of retinoic acid receptors (RARs).6 Moreover, ATRA stimulates nerve growth factor (NGF) and the expression of its receptor. In a feedback manner, ATRA and NGF may potentiate each other.7 RAR-β2 expression stimulates neurite outgrowth and promotes functional regeneration of adult rat nerve after peripheral nerve injury. We have previously demonstrated that ATRA administration prevents and reverses neuropathy-associated morphologic changes in diabetic animals.8,9 We conducted a study in an animal model and a clinical trial in patients using a randomized, controlled, double-blinded protocol, in order to evaluate the effect of ATRA as a treatment for peripheral neuropathy.
METHODS
Standard protocol approvals, registrations, and patient consent.
The animal study was approved by the Instituto Nacional de Cancerología (INCan) and the Instituto de Investigaciones Biomédicas (IIB) of the Universidad Nacional Autónoma de México (UNAM) (INCan/CC/138/05). The clinical trial was approved by the INCan (2005/047/OMI); written consent was obtained from each patient.
Animal care and experimental design.
Forty male Wistar rats (weight, 250 g) were randomized into 5 groups. Group A received saline solution daily for 4 weeks; groups B and C were treated with cisplatin (3 mg/kg, intraperitoneally, every 3 days for 4 weeks); groups D and E received paclitaxel (8 mg/kg, intraperitoneally, twice a week during 4 weeks).10 To prevent cisplatin-induced kidney toxicity, 2 mL of saline solution was administered subcutaneously.11 ATRA (20 mg/kg, PO, daily for 2 weeks) was administered only to groups C (cisplatin/ATRA) and E (paclitaxel/ATRA).8,9 At the end of week 4, all rats received a sodium pentobarbital overdose (100 mg/kg, intraperitoneally).
Measurement of nociceptive activity in rats.
Thermal hyperalgesia was assessed with the Hargraves method. Each rat was tested 3 times and an average of 3 responses was scored. Testing was carried out before treatment and at 2 and 4 weeks after cisplatin or paclitaxel injection. Tactile allodynia was tested as previously described. von Frey filaments (Stoelting, Wood Dale, IL) were employed to determine the 50% paw-withdrawal threshold utilizing the up-down method of Dixon.12 Allodynia was considered to be present when paw-withdrawal thresholds were <4 g. All evaluations were blinded to treatment.
NGF levels.
NGF were obtained by ELISA analysis of sciatic nerve samples13 in microtiter plates previously coated with antibodies against NGF (NGF antimouse β [2.5 S] 0.1 μg/mL). NGF antimouse was labeled with α-galactosidase (Boehringer, Germany). After 4 hours of incubation, the enzyme was developed with chlorophenol red galactopyranoside (Roche Molecular Biochemicals, Paris). Absorbance was read on a spectrophotometer 90 minutes later at 570 nm.
Expression of RAR-α and RAR-β.
The sciatic nerve sample was obtained and RNA was extracted with TRIzol®. Quantitative reverse transcriptase and real-time PCR (qRT-PCR) were made by one-step kit using reactive RT-PCR master mix reagents. Probes (Applied Biosystems, Carlsbad, CA) to detect the relative expression of RAR-α and RAR-β were FAM-coupled. RNAase-P was used as endogenous control. RT-PCR conditions were followed by the Applied Biosystems equipment protocol in real time 7,500 (Applied Biosystems, Carlsbad, CA).
Ultrastructural evaluation of sciatic nerve.
The left sciatic nerve was obtained by dissection. Classification of peripheral nerve elements was conducted as previously described.14 The myelinated and unmyelinated axons, number of Schwann cell nuclei, regeneration bands, remyelinating axons characterized as a thin myelin band, and the percentage of myelinated axons with ultrastructural variations in myelin were quantified by square millimeter under a transmission electron microscopy EM-10A (Carl-Zeiss, Mexico City, Mexico). Morphologic evaluation was performed by an independent observer in a blinded fashion.
Clinical study.
Baseline characteristics of the patients.
Patients from the INCan with histologically confirmed stage IIIB/IV NSCLC were enrolled in the study. A double-blind, randomized study was carried out between August 2005 and December 2008. Complete medical history and a physical examination were performed. Inclusion criteria for patients comprised Eastern Cooperative Oncology Group performance status 0–2, no prior chemotherapy, adequate laboratory parameters, and life expectancy >12 weeks. Patients with known CNS metastatic disease were eligible provided that at least 2 months had elapsed since completion of radiation therapy (RT). Patients with clinical neuropathy prior to study enrollment were excluded.
Trial design.
All patients received standard treatment based on cisplatin 80 mg/m2 and paclitaxel 175 mg/m2 (CP) every 21 days for 6 courses.15 In a double-blinded design, patients were randomized to receive ATRA 20 mg/m2/d (ATRA/CP) or placebo (placebo/CP) 1 week prior to treatment until completion of 2 courses.16 The randomization plan used a table of random numbers blinded to the investigators. Physical examination, laboratory tests, and neurotoxicity events using National Cancer Institute Common Toxicity Criteria and Adverse Events version 3.0 were evaluated for each patient.17
NGF serum levels.
Whole blood was obtained by peripheral vein puncture to measure NGF. ELISA was performed at initiation and after completion of the second chemotherapy course.
Electrophysiologic peripheral nerve functional study.
Prior to initiation of the first and after completion of the second chemotherapy course, each participant underwent a complete standard bilateral sensory-motor nerve conduction study that consisted of recording motor conduction velocities from median, ulnar, peroneal, and tibial nerves, as well as sensitive conduction velocities from median, ulnar, and sural nerves at the Instituto Nacional de Neurologia y Neurocirugía. Distal latencies and peak amplitudes were evaluated. Room temperature was maintained at the same temperature (26°C). Grade of damage was ranked from 0 to 3 for latency and amplitude of each nerve in all patients.18 Measurements and data analysis were performed with VikingSelect master software version 8.1 (Nicolet Viasys, San Diego, CA) and was performed by an independent observer without previous knowledge of the specimen source group.
Statistical analysis.
Continuous variables were summarized as arithmetic means, medians, and SD; categorical variables as proportions. For the human study, the primary outcome for the clinical trial was the reduction of chemotherapy-induced peripheral neuropathy damage determined by electrophysiologic peripheral nerve functional study. Inferential comparisons among animal groups were conducted with one-way analysis of variance and post hoc (Tukey) test or with Kruskal-Wallis test; for comparison between control or ATRA patient groups, the Student t test and Pearson χ2 or Fisher exact test were used. For multivariate analysis (logistic regression), the variables included were those considered to have clinical significance and those shown as significant or nearly statistically significant (p < 0.1) in the univariate analysis. Within-group sensitivity changes, NGF levels, and latency time before and after treatment were compared with paired Student t test, McNemar, or Wilcoxon test, according to the distribution type determined by the Kolmogorov-Smirnov test. Statistical significance was determined when p < 0.05 in a 2-sided test. SPSS software package (version 17.0, SPSS, Inc., Chicago, IL) was employed for data analysis.
RESULTS
Experimental study in rats.
Sensitivity test.
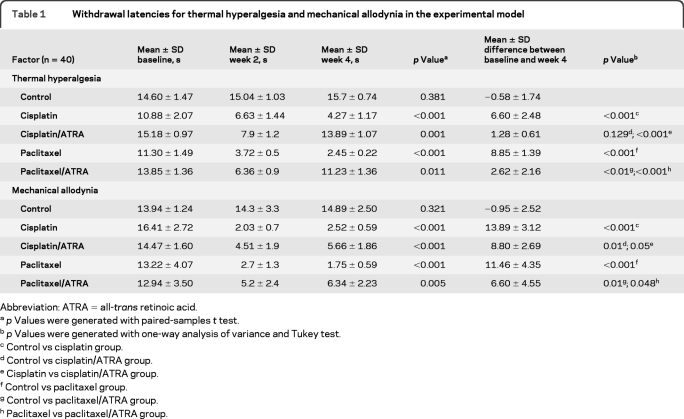
All rats survived the 4 weeks of treatment. Latency time (temperature sensitivity) was not different at baseline among the 5 groups (p = 0.889). In groups receiving cisplatin and paclitaxel, there was a reduction of withdrawal latency before and after chemotherapy (week 2). Means of differences between baseline and week 4 were less in animals treated with ATRA than in those only receiving chemotherapy (table 1). Threshold response to mechanical allodynia was not different at baseline among the 5 groups (p = 0.143). There was a reduction of withdrawal pressure (week 2) in all groups that received cisplatin or paclitaxel. Moreover, means of differences between baseline and week 4 were less in ATRA-treated groups than in those receiving only chemotherapy (table 1).
Table 1.
Withdrawal latencies for thermal hyperalgesia and mechanical allodynia in the experimental model
ATRA =all-trans retinoic acid
p Values were generated with paired-samples t test.
p Values were generated with one-way analysis of variance and Tukey test.
Control vs cisplatin group.
Control vs cisplatin/ATRA group.
Cisplatin vs cisplatin/ATRA group.
Control vs paclitaxel group.
Control vs paclitaxel/ATRA group.
Paclitaxel vs paclitaxel/ATRA group.
NGF content.
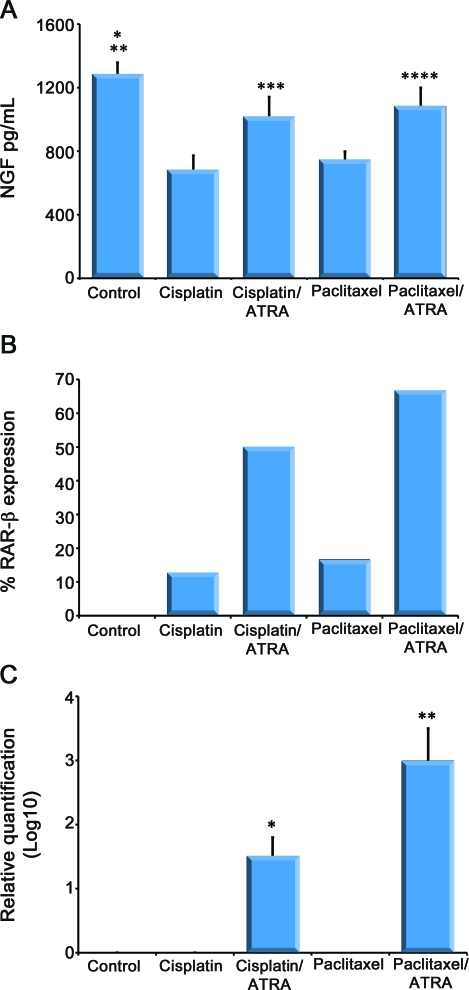
At the end of the experiment, the groups receiving either cisplatin (679.8 [95% confidence interval (CI) 560–798]; p < 0.001) or paclitaxel (745.4 [687–805]; p < 0.001) had reduced NGF levels when compared to controls (1282.4 [1,224–1,340]). Moreover, ATRA treatment reversed the changes induced in NGF by cisplatin (1,016.3 [1,046–985]; p < 0.001) or paclitaxel (1,083.9 [1,052–1,113]; p = 0.05) (figure 1A).
Figure 1. Effect of all-trans retinoic acid (ATRA) on nerve growth factor (NGF) contents and retinoic acid receptor (RAR)-β expression in sciatic nerve.
(A) Content of NGF in sciatic nerve. ATRA increased NGF content in sciatic nerve of rat treated with chemotherapy. *p < 0.001 when compared with cisplatin, cisplatin/ATRA, and paclitaxel; **p = 0.004 when compared with paclitaxel/ATRA; ***p < 0.0001 when compared with cisplatin; ****p = 0.052 when compared with paclitaxel. (B) Percentage of RAR-β expression in sciatic nerve. (C) Relative quantification of RAR-β expressed by logarithm base 10. RAR-β is not expressed in peripheral nerve control group but ATRA increased its expression in rats treated with chemotherapy. *p = 0.22 when compared with cisplatin; **p < 0.001 when compared with paclitaxel.
Expression of RAR-α and RAR-β in sciatic nerve.
Analysis of RT-PCR for RAR-α showed no expression. RAR-β expression was found in 3%, 12.5%, 66.6%, 16.6%, and 50% in control, cisplatin, cisplatin/ATRA, paclitaxel, and paclitaxel/ATRA groups, respectively (figure 1B). RAR-β expression was 1.5- and 3-fold higher in both cisplatin/ATRA and paclitaxel/ATRA groups, respectively, than in control and in their respective non-ATRA-treated group (figure 1C).
Ultrastructural evaluation.
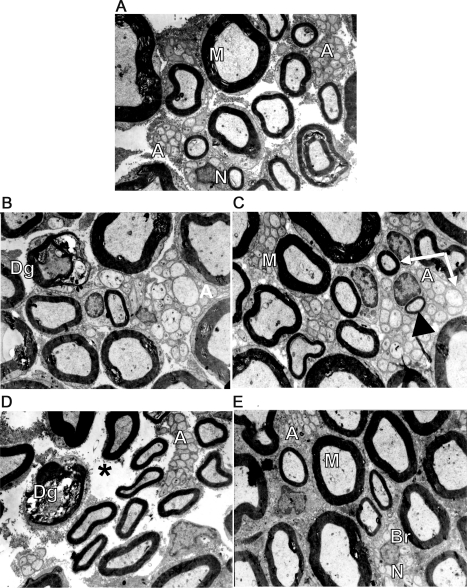
The number of the axonal degeneration bands in mm2 was higher in both the cisplatin (6.1 ± 1.2; p = 0.022) and paclitaxel (9 ± 1.8; p < 0.001) groups when compared to controls (0.27 ± 0.27). However, there were no differences in the number of degenerated axons between controls and cisplatin/ATRA (4.2 ± 1.4; p = 0.21) or paclitaxel/ATRA (2.6 ± 0.5; p = 0.6). Furthermore, the paclitaxel group had more degenerated axons (figure 2D) when compared to the paclitaxel/ATRA group (p = 0.003) (figure 2E). Nerve regeneration evaluated by regeneration bands was higher in cisplatin/ATRA (653.7 ± 63; p = 0.01) and paclitaxel/ATRA (766 ± 140; p = 0.01; figure 2, C and E) than in controls (1,910 ± 54; p < 0.001) (figure 2A), as well as in cisplatin (263 ± 70; p < 0.015) and paclitaxel (329 ± 78; p = 0.012) groups (figure 2, B and D). Number of Schwann cell nuclei was higher in cisplatin (1,658 ± 176), cisplatin/ATRA (1,686 ± 180; p = 0.001); paclitaxel (1,131 ± 180), and paclitaxel/ATRA (1,834 ± 193; p = 0.001) than in controls (718 ± 138; p < 0.001). The number of long amyelinic axons was higher in cisplatin/ATRA (2.1 ± 0.4; p = 0.021) and paclitaxel/ATRA (1.6 ± 0.3; p = 0.05) groups when compared to controls; no differences were found among groups according to number of myelinic (control 18,687 ± 1,033; cisplatin 20,709 ± 1,175; cisplatin/ATRA 23,556 ± 744; paclitaxel 22,360 ± 416; paclitaxel/ATRA 24,244 ± 639) and amyelinic axons (control 38,465 ± 4,869; cisplatin 46,988 ± 4,784; cisplatin/ATRA 63,297 ± 9,373; paclitaxel 53,402 ± 2,765; paclitaxel/ATRA 59,321 ± 4,255).
Figure 2. Electronic micrographics of sciatic nerve of rats who received chemotherapy with or without all-trans retinoic acid (ATRA) and controls.
(A) Myelinated axons with a myelin band according to their axonal diameter (M) in control group; preserved unmyelinated axons (AA) are shown. (B) Cisplatin group without ATRA treatment; degenerated myelinated axons (Dg) with altered myelin band and altered unmyelinated axons (A) were observed. (C) Cisplatin plus ATRA group showed normal appearance, myelinated axons with a myelin band according to their axonal diameter (M), conserved unmyelinated axons (A), and some had a diameter similar to those of small myelinated axons (arrows), probably indicating a remyelinated process, denoted by the thin myelin band (arrowhead). (D) In the paclitaxel-only treated group, degenerated myelinated axons (Dg) with altered myelin band and altered unmyelinated axons (A) are shown. There is endoneural damage denoted by spaces between myelinated axons and unmyelinated axon groups (asterisk). (E) Paclitaxel- and ATRA-treated group showed normal appearance, myelinated axons with a myelin band according to their axonal diameter (M), and conserved unmyelinated axons (A). There is a Schwann cell nucleus (N) surrounded by unmyelinated axon sprouts forming a regeneration band (Br). All electronic micrographics are at 6,860× and the quantifications are per square millimeter. Eight to 10 photographs were taken for each rat.
Clinical trial.
Patients.
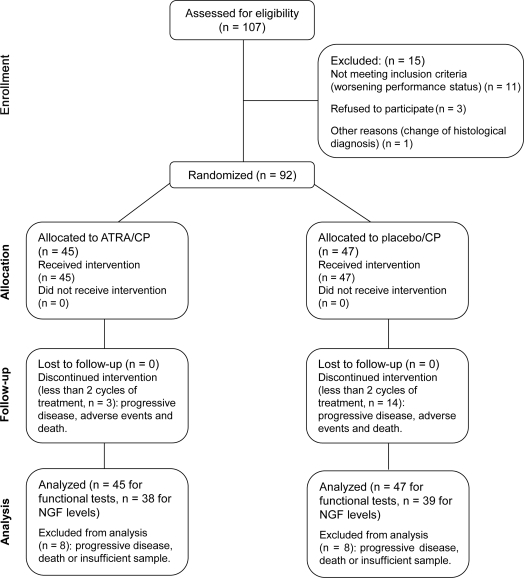
A total of 107 patients with NSCLC were assessed for eligibility. Ninety-two of them fulfilled the selection criteria; 47 and 45 patients were randomized to chemotherapy/placebo and chemotherapy/ATRA groups, respectively (figure 3). We did not find differences regarding age. Diabetes mellitus (DM) was present in 14% (13) of all patients; of these, 8 belonged to the control group and 5 to the experimental group. There were no differences in the baseline characteristics among groups (table e-1 on the Neurology® Web site at www.neurology.org).
Figure 3. Consort diagram of clinical setting.
ATRA = all-trans retinoic acid; CP = cisplatin and paclitaxel; NGF = nerve growth factor.
Clinical neuropathy.
Development of neuropathy grades 2 and 3 was present in 53% (95% CI 45.6–60.4; 49/92) and 12% (9.7–14.3; 11/92) of all patients, respectively. No patient had neuropathy grade 4. Neuropathy grades ≥2 were present in 75% (64.3–85.7; 35/47) and in 56% (38.7–67.2; 25/45) of patients from chemotherapy/placebo and chemotherapy/ATRA groups (p = 0.056). When we excluded diabetic patients, neuropathy grades ≥2 were present in 78.9 (95% CI 67–93; 30/38) and 51.2% (66–36; 21/41) of patients from chemotherapy/placebo and chemotherapy/ATRA groups (p = 0.01). Other factors associated with near statistical significance were male gender (73 vs 55%; p = 0.07) and stage IV (68 vs 46%; p = 0.091). In the multivariate analysis, only female gender (odds ratio [OR] 0.4 [0.16–1.0]; p = 0.05) and ATRA treatment (OR 0.4 [0.16–1.0]; p = 0.05) were associated with reduction in clinical neuropathy.
Serum NGF contents.
Serum NGF levels before and after treatments were evaluated in only 77 (83.7%) patients due to death or insufficient tissue sample. There was a reduction of both baseline and post-treatment NGF level for all patients. No differences were found on content in NGF in both groups (table e-2). However, patients with a high difference in NGF levels between baseline and post-treatment (median, ≥436 pg/mL) developed clinical neuropathy grade 2 (61% [95% CI 51–71] vs 18% [10–26]; p = 0.009) and 3 (27% [17–37] vs 9% [1–19]; p = 0.082) more frequently.
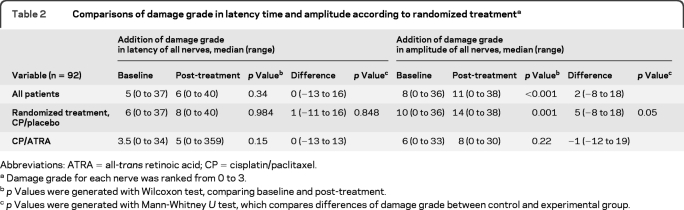
Electrophysiologic peripheral nerve functional study.
Ninety-two patients were evaluated with electrophysiologic peripheral nerve tests before and after ATRA/placebo therapy during 6 weeks (2 chemotherapy cycles). No difference was found among groups in latency time between baseline and post-treatment measurements (table 2). When patients with and without DM were compared, no differences were found with regard to latency time (p = 0.581) and amplitude damage (p = 0.521). However, patients ≥60 years of age had alterations in basal latency time (mean grade rank, 37.7 vs 56; p = 0.001) and amplitude damage (37 vs 57; p < 0.001). Neuronal damage evaluated by amplitude of evoked potentials was higher after treatment than at baseline in the chemotherapy/placebo group (32.1 Vm vs 218 Vm; p = 0.001 for each comparison). The chemotherapy/ATRA group showed no such difference (p = 0.22). Moreover, comparisons between both groups showed that patients in the chemotherapy/ATRA group had lower amplitude damage differences at baseline and post-treatment than those in the chemotherapy/placebo group (28.3s vs 38.4s; p = 0.05). In the multivariate analysis, no differences were found in DM (p = 0.84), brain metastases (p = 0.47), and age (p = 0.48); only ATRA treatment was associated with a nearly statistical significance with reduction of amplitudes (OR 0.42 [95% CI 0.17–1.03]; p = 0.06).
Table 2.
Comparisons of damage grade in latency time and amplitude according to randomized treatmenta
Abbreviations: ATRA = all-trans retinoic acid; CP = cisplatin/paclitaxel
Damage grade for each nerve was ranked from 0 to 3.
p Values were generated with Wilcoxon test, comparing baseline and post-treatment.
p Values were generated with Mann-Whitney U test, which compares differences of damage grade between control and experimental group.
Toxicity.
The adverse effect found between placebo and ATRA treatment groups was hypertriglyceridemia grades 3 and 4 and was present in 5 of the 45 patients. These patients were treated with diet and gemfibrozil (table e-3).
DISCUSSION
Chemotherapy-induced peripheral neuropathy (CIPN) is the most important nonhematologic toxicity of cisplatin and paclitaxel therapy, as seen in both experimental animals and patients. Symptoms of CIPN began soon after therapy in 65% of patients treated with a cumulative dose of 200–300 mg/m.19 Overall incidence of symptomatic neuropathy in paclitaxel-treated patients ranges from 5% to75%, developing severe symptoms (grade 3 or higher) in 10%–20% of them.20
Several mechanisms for CIPN development have been associated with decrease of NGF in serum, demyelination, and axonal loss.21 Paclitaxel and cisplatin alters axonal transport of growth factors.22 Similar to other studies,23 we have observed a decrease in NGF contents both in sciatic nerves of rats and serum of patients treated with cisplatin and paclitaxel, suggesting a role in CIPN physiopathology. In mice, NGF plays a key role in the recovery from numerous peripheral neuronal and non-neuronal insults, reducing or preventing neuropathy.21,24,25
Ultrastructural evaluation of rat sciatic nerves revealed axonal damage without demyelination; similarly, we found amplitude alterations in the patients suggesting axonal demyelination.26 ATRA is capable of reversing morphologic and functional changes due to peripheral neuropathy, by increasing NGF concentrations in nerve and serum.9,27 One mechanism through which ATRA might influence neurite outgrowth is by regulating the neurotrophin transcription of receptor genes, which regulate actin polymerization and advancement of the growth cone.28 Moreover, by positive feedback, NGF induces production of retinaldehyde dehydrogenase type 2, which synthesizes retinoic acid, stimulating intracellular RAR pathways.7 In our study, alterations in the sensitivity tests were reduced in ATRA-treated rats, achieving latency times similar to controls, with minor neural damage in functional tests apparently related to prevention of NGF depletion, inducing morphologic changes suggestive of regeneration. Neural content of NGF increases with ATRA treatment in CIPN rats, although these changes were not as notorious as in rats with diabetic neuropathy.8,9 ATRA-treated patients had a lower prevalence of neuropathy grades 2 and 3 than those of the chemotherapy/placebo group. Treatment with ATRA did not prevent NGF depletion in serum; therefore, the increase of NGF could be related to regeneration from neural damage, rather than neural protection from CIPN.
ATRA binds and activates different RAR subtypes (RAR-α, RAR-β, and RAR-γ), which regulate the expression of the different genes and activate their own promoter regions.29 It also stimulates neurite proliferation and length in a variety of embryonic neural cells.27,30 RAR-α and RAR-β differ in their cell localization; the former is expressed in the dendritic region, and the latter in the cell soma; neural damage increases the concentrations of both receptors.31 In degenerating nerves, an RAR-α increase is detected between 7 and 14 days, after the complete nerve transection, and RAR-β is detected between 14 and 21 days. After day 21, RAR-α is no longer detected. Our results showed that RAR-β expression was higher in chemotherapy/ATRA groups than in the non-ATRA-treated group. RAR-β expression stimulates neurite outgrowth and promotes functional regeneration of sensory axons in spinal cord,7 however, RAR-α expression was not detected in our study groups, perhaps due to the fact that RAR-α expression reaches its nadir between 4 and 7 days after neural injury.31
Several studies have tried diverse neuroprotective substances to prevent CIPN; in most of them the results have been negative.32 The challenge is to find an agent capable of both effective reduction of neurotoxicity and increase of tolerance to chemotherapy agents. Recently, a neuroprotective effect of vitamin E against CIPN has been reported.33
Pharmacologic studies are necessary to determine dose and duration of ATRA therapy in CIPN. We selected the doses used in this study based on previous experimental reports on tolerance.8,9 In humans, optimal retinoid doses and drug schedules are known, but some studies suggest that ATRA at low doses could also be effective. The doses of ATRA evaluated in our study presented a fair toxicity profile16 and were lower than those employed in APL treatment.34 In the treatment of solid tumors, higher ATRA doses (150 mg/m2/d) as single agent have been poorly tolerated.35
Our study has the limitation of short follow-up (6 weeks) after 2 cycles of chemotherapy, which induce a low frequency of severe neuropathy (grades 3 and 4). The time course of peripheral nerve injury and recovery from chemotherapy depends on the dose intensity and chemotherapy type; usually, 10%–28% of patients treated with paclitaxel and cisplatin or carboplatin have persistent symptoms 6 weeks after the end of treatment34,36; therefore, it would be interesting to conduct long-term studies to assess ATRA as a potential neuroprotector agent. However, the rapid disease progression and short global survival of our patients with lung cancer did not allow a long follow-up.
Supplementary Material
- ATRA
- all-trans retinoic acid
- CI
- confidence interval
- CIPN
- chemotherapy-induced peripheral neuropathy
- CP
- cisplatin/paclitaxel
- DM
- diabetes mellitus
- NGF
- nerve growth factor
- NSCLC
- non-small-cell lung cancer
- OR
- odds ratio
- QOL
- quality of life
- qRT-PCR
- quantitative reverse transcriptase and real-time PCR
- RAR
- retinoic acid receptor
- RT
- radiation therapy
Presented in part at the 99th annual meeting of the American Association for Cancer Research, April 12–16, 2008, San Diego, California.
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Conception and design: Oscar Arrieta. Financial support: Oscar Arrieta. Administrative support: Alma Delia Campos-Parra. Provision of study material or patients: Oscar Arrieta, Norma Hernández-Pedro, María del Carmen Fernández González-Aragón, David Saavedra-Pérez, Miguel Ángel Ríos-Trejo, Tania Cerón-Lizárraga, and Luis Martínez-Barrera. Collection and assembly of data: Norma Hernández-Pedro, María del Carmen Fernández González-Aragón, Miguel Ángel Ríos-Trejo, Tania Cerón-Lizárraga, Benjamín Pineda, Graciela Ordóñez, and Alma Ortiz-Plata. Data analysis and interpretation: Oscar Arrieta, Norma Hernández-Pedro, María del Carmen Fernández González-Aragón, David Saavedra-Pérez, Luis Martínez-Barrera, Vinicio Granados-Soto, Alma Delia Campos-Parra, and Julio Sotelo. Manuscript writing: Oscar Arrieta, Norma Hernández-Pedro, David Saavedra-Pérez, Alma Delia Campos-Parra, Vinicio Granados-Soto, and Julio Sotelo. Final approval of manuscript: Oscar Arrieta, Norma Hernández-Pedro, María del Carmen Fernández González-Aragón, David Saavedra-Pérez, Alma Delia Campos-Parra, Miguel Ángel Ríos-Trejo, Tania Cerón-Lizárraga, Luis Martínez-Barrera, Benjamín Pineda, Graciela Ordóñez, Alma Ortiz-Plata, Vinicio Granados-Soto, and Julio Sotelo.
DISCLOSURE
Dr. Arrieta, Dr. Hernández-Pedro, Dr. Fernández González-Aragón, Dr. Saavedra-Pérez, Dr. Campos-Parra, Dr. Ríos-Trejo, Dr. Cerón-Lizárraga, Dr. Martínez-Barrera, Dr. Pineda, Dr. Ordóñez, and Dr. Ortiz-Plata report no disclosures. Dr. Granados-Soto serves on the editorial board of Anesthesia and Analgesia. Dr. Sotelo reports no disclosures.
REFERENCES
- 1. Higgins MJ, Ettinger DS. Chemotherapy for lung cancer: the state of the art in 2009. Expert Rev Anticancer Ther 2009;9:1365–1378 [DOI] [PubMed] [Google Scholar]
- 2. Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol 2002;249:9–17 [DOI] [PubMed] [Google Scholar]
- 3. Arrieta O, Michel Ortega RM, Villanueva-Rodriguez G, et al. Association of nutritional status and serum albumin levels with development of toxicity in patients with advanced non-small cell lung cancer treated with paclitaxel-cisplatin chemotherapy: a prospective study. BMC Cancer 2010;10:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Strumberg D, Brugge S, Korn MW, et al. Evaluation of long-term toxicity in patients after cisplatin-based chemotherapy for non-seminomatous testicular cancer. Ann Oncol 2002;13:229–236 [DOI] [PubMed] [Google Scholar]
- 5. Zhong D, Zhang D, Song Y. The role of all-trans-retinoic acid on the proliferation and differentiation of rat embryonic neural stem cells. Chin J Reparative Reconstruct Surg 2008;22:206–211 [PubMed] [Google Scholar]
- 6. Clarke N, Germain P, Altucci L, Gronemeyer H. Retinoids: potential in cancer prevention and therapy. Expert Rev Mol Med 2004;6:1–23 [DOI] [PubMed] [Google Scholar]
- 7. Corcoran J, Maden M. Nerve growth factor acts via retinoic acid synthesis to stimulate neurite outgrowth. Nat Neurosci 1999;2:307–308 [DOI] [PubMed] [Google Scholar]
- 8. Hernandez-Pedro N, Ordonez G, Ortiz-Plata A, et al. All-trans retinoic acid induces nerve regeneration and increases serum and nerve contents of neural growth factor in experimental diabetic neuropathy. Translational Res 2008;152:31–37 [DOI] [PubMed] [Google Scholar]
- 9. Arrieta O, Garcia-Navarrete R, Zuniga S, et al. Retinoic acid increases tissue and plasma contents of nerve growth factor and prevents neuropathy in diabetic mice. Eur J Clin Invest 2005;35:201–207 [DOI] [PubMed] [Google Scholar]
- 10. Cavaletti G, Tredici G, Braga M, Tazzari S. Experimental peripheral neuropathy induced in adult rats by repeated intraperitoneal administration of Taxol. Exp Neurol 1995;133:64–72 [DOI] [PubMed] [Google Scholar]
- 11. Verdu E, Vilches JJ, Rodriguez FJ, Ceballos D, Valero A, Navarro X. Physiological and immunohistochemical characterization of cisplatin-induced neuropathy in mice. Muscle Nerve 1999;22:329–340 [DOI] [PubMed] [Google Scholar]
- 12. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53:55–63 [DOI] [PubMed] [Google Scholar]
- 13. Ordonez G, Fernandez A, Perez R, Sotelo J. Low contents of nerve growth factor in serum and submaxillary gland of diabetic mice: a possible etiological element of diabetic neuropathy. J Neurol Sci 1994;121:163–166 [DOI] [PubMed] [Google Scholar]
- 14. Jacobs JM, Love S. Qualitative and quantitative morphology of human sural nerve at different ages. Brain 1985;108:897–924 [DOI] [PubMed] [Google Scholar]
- 15. Smit EF, van Meerbeeck JP, Lianes P, et al. Three-arm randomized study of two cisplatin-based regimens and paclitaxel plus gemcitabine in advanced non-small-cell lung cancer: a phase III trial of the European Organization for Research and Treatment of Cancer Lung Cancer Group–EORTC 08975. J Clin Oncol 2003;21:3909–3917 [DOI] [PubMed] [Google Scholar]
- 16. Arrieta O, Gonzalez-De la Rosa CH, Arechaga-Ocampo E, et al. Randomized phase II trial of All-trans-retinoic acid with chemotherapy based on paclitaxel and cisplatin as first-line treatment in patients with advanced non-small-cell lung cancer. J Clin Oncol 2010;28:3463–3471 [DOI] [PubMed] [Google Scholar]
- 17. Postma TJ, Heimans JJ. Grading of chemotherapy-induced peripheral neuropathy. Ann Oncol 2000;11:509–513 [DOI] [PubMed] [Google Scholar]
- 18. Wampler MA, Topp KS, Miaskowski C, Byl NN, Rugo HS, Hamel K. Quantitative and clinical description of postural instability in women with breast cancer treated with taxane chemotherapy. Arch Phys Med Rehabil 2007;88:1002–1008 [DOI] [PubMed] [Google Scholar]
- 19. von Schlippe M, Fowler CJ, Harland SJ. Cisplatin neurotoxicity in the treatment of metastatic germ cell tumour: time course and prognosis. Br J Cancer 2001;85:823–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bhagra A, Rao RD. Chemotherapy-induced neuropathy. Curr Oncol Rep 2007;9:290–299 [DOI] [PubMed] [Google Scholar]
- 21. Aloe L, Manni L, Properzi F, De Santis S, Fiore M. Evidence that nerve growth factor promotes the recovery of peripheral neuropathy induced in mice by cisplatin: behavioral, structural and biochemical analysis. Auton Neurosci 2000;86:84–93 [DOI] [PubMed] [Google Scholar]
- 22. Cata JP, Weng HR, Lee BN, Reuben JM, Dougherty PM. Clinical and experimental findings in humans and animals with chemotherapy-induced peripheral neuropathy. Minerva Anestesiol 2006;72:151–169 [PubMed] [Google Scholar]
- 23. Cavaletti G, Bogliun G, Marzorati L, et al. Early predictors of peripheral neurotoxicity in cisplatin and paclitaxel combination chemotherapy. Ann Oncol 2004;15:1439–1442 [DOI] [PubMed] [Google Scholar]
- 24. Apfel SC, Arezzo JC, Lipson L, Kessler JA. Nerve growth factor prevents experimental cisplatin neuropathy. Ann Neurol 1992;31:76–80 [DOI] [PubMed] [Google Scholar]
- 25. Hayakawa K, Itoh T, Niwa H, Mutoh T, Sobue G. NGF prevention of neurotoxicity induced by cisplatin, vincristine and Taxol depends on toxicity of each drug and NGF treatment schedule: in vitro study of adult rat sympathetic ganglion explants. Brain Res 1998;794:313–319 [DOI] [PubMed] [Google Scholar]
- 26. Sahenk Z, Barohn R, New P, Mendell JR. Taxol neuropathy: electrodiagnostic and sural nerve biopsy findings. Arch Neurol 1994;51:726–729 [DOI] [PubMed] [Google Scholar]
- 27. Corcoran J, Shroot B, Pizzey J, Maden M. The role of retinoic acid receptors in neurite outgrowth from different populations of embryonic mouse dorsal root ganglia. J Cell Sci 2000;113:2567–2574 [DOI] [PubMed] [Google Scholar]
- 28. Davies AM. Neurotrophins: more to NGF than just survival. Curr Biol 2000;10:R374–R376 [DOI] [PubMed] [Google Scholar]
- 29. Manshouri T, Yang Y, Lin H, et al. Downregulation of RAR alpha in mice by antisense transgene leads to a compensatory increase in RAR beta and RAR gamma and development of lymphoma. Blood 1997;89:2507–2515 [PubMed] [Google Scholar]
- 30. Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res 2002;43:1773–1808 [DOI] [PubMed] [Google Scholar]
- 31. Zhelyaznik N, Mey J. Regulation of retinoic acid receptors alpha, beta and retinoid X receptor alpha after sciatic nerve injury. Neuroscience 2006;141:1761–1774 [DOI] [PubMed] [Google Scholar]
- 32. Rao RD, Flynn PJ, Sloan JA, et al. Efficacy of lamotrigine in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled trial, N01C3. Cancer 2008;112:2802–2808 [DOI] [PubMed] [Google Scholar]
- 33. Pace A, Giannarelli D, Galie E, et al. Vitamin E neuroprotection for cisplatin neuropathy: a randomized, placebo-controlled trial. Neurology 2010;74:762–766 [DOI] [PubMed] [Google Scholar]
- 34. van der Hoop RG, van der Burg ME, ten Bokkel Huinink WW, van Houwelingen C, Neijt JP. Incidence of neuropathy in 395 patients with ovarian cancer treated with or without cisplatin. Cancer 1990;66:1697–1702 [DOI] [PubMed] [Google Scholar]
- 35. Treat J, Friedland D, Luginbuhl W, et al. Phase II trial of all-trans retinoic acid in metastatic non-small cell lung cancer. Cancer Invest 1996;14:415–420 [DOI] [PubMed] [Google Scholar]
- 36. Pignata S, De Placido S, Biamonte R, et al. Residual neurotoxicity in ovarian cancer patients in clinical remission after first-line chemotherapy with carboplatin and paclitaxel: the Multicenter Italian Trial in Ovarian cancer (MITO-4) retrospective study. BMC Cancer 2006;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.