Abstract
Objective:
To determine the relationship between proton magnetic resonance spectroscopy (1H MRS) metabolites and β-amyloid (Aβ) load and the effects of Aβ load on the association between 1H MRS metabolites and cognitive function in cognitively normal older adults.
Methods:
We studied 311 cognitively normal older adults who participated in the population-based Mayo Clinic Study of Aging from January 2009 through September 2010. Participants underwent 11C-Pittsburgh compound B (PiB) PET, 1H MRS from the posterior cingulate gyri, and neuropsychometric testing to assess memory, attention/executive, language, and visual-spatial domain functions within 6 months. Partial Spearman rank order correlations were adjusted for age, sex, and education.
Results:
Higher PiB retention was associated with abnormal elevations in myoinositol (mI)/creatine (Cr) (partial rs = 0.17; p = 0.003) and choline (Cho)/Cr (partial rs = 0.13; p = 0.022) ratios. Higher Cho/Cr was associated with worse performance on Auditory Verbal Learning Test Delayed Recall (partial rs = −0.12; p = 0.04), Trail Making Test Part B (partial rs = 0.12; p = 0.04), Wechsler Adult Intelligence Scale–Revised (WAIS-R) Digit Symbol (partial rs = −0.18; p < 0.01), and WAIS-R Block Design (partial rs = −0.12; p = 0.03). Associations between 1H MRS metabolites and cognitive function were not different among participants with high vs low PiB retention.
Conclusion:
In cognitively normal older adults, the 1H MRS metabolite ratios mI/Cr and Cho/Cr are associated with the preclinical pathologic processes in the Alzheimer disease cascade. Higher Cho/Cr is associated with worse performance on domain-specific cognitive tests independent of Aβ load, suggesting that Cho/Cr elevation may also be dependent on other preclinical dementia pathologies characterized by Cho/Cr elevation such as Lewy body or ischemic vascular disease in addition to Aβ load. Neurology® 2011;77:951–958
Biomarkers of preclinical Alzheimer disease (AD) pathology are critical for identifying at-risk individuals for preventive clinical trials.1 A potential imaging marker for early detection of AD pathology is proton magnetic resonance spectroscopy (1H MRS), which allows noninvasive assessment of brain biochemistry. 1H MRS metabolite abnormalities, characterized by increased levels of the glial metabolite myoinositol (mI) and decreased levels of the neuronal metabolite N-acetylaspartate (NAA), are associated with the severity of AD pathology.2 Both decreased NAA and increased mI levels have been detected in individuals who have a higher risk for developing AD, such as patients with amnestic mild cognitive impairment (MCI),3,4 and in presymptomatic carriers of familial AD mutations.5 Furthermore, an elevation in the choline (Cho)/creatine (Cr) ratio predicted future cognitive decline in cognitively normal older adults.6
Up to 30% of cognitively normal older adults have increased β-amyloid (Aβ) deposition on PET imaging with amyloid ligand 11C-Pittsburgh compound B (PiB).7 Understanding the relationship between Aβ load and 1H MRS abnormalities in cognitively normal older adults may give insights on the biologic basis of 1H MRS metabolite changes in early AD. We hypothesized that 1H MRS metabolite abnormalities are associated with cognitive function in cognitively normal older adults and that preclinical AD pathology is responsible for this association. In a population-based cohort of cognitively normal older adults, our primary objective was to determine the association between 1H MRS metabolite markers and Aβ load. Our secondary objective was to determine the association between 1H MRS markers and cognitive performance and whether Aβ load modifies this association.
METHODS
Participants.
We studied 311 cognitively normal older adults who participated in the population-based Mayo Clinic Study of Aging (MCSA) from January 2009 through September 2010. MCSA is a prospective population-based study of older adults without dementia.8 Individuals participating in the MCSA undergo clinical examinations, a battery of neuropsychological tests, and MRI/MRS examinations every 15 months. After completion of each evaluation, a consensus committee meeting is held involving the behavioral neurologists, neuropsychologists, and nurses who evaluated the subjects to assign a clinical diagnosis to the participant. PET studies have been offered to all MCSA participants since January 2009 and are performed within 6 months of the MRI/MRS and cognitive testing.
Normal cognitive function was judged according to the published criteria,9 and the cognitively normal subjects received a Clinical Dementia Rating of 0. Subjects who had a contraindication for MRI, such as a pacemaker, or who were unable to participate in imaging studies because of severe illness were excluded. Subjects were not excluded due to neurologic, psychiatric, or systemic illnesses to preserve the representativeness of the study sample.
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Mayo Clinic Institutional Review Board, and informed consent for participation was obtained from every subject.
Neuropsychological testing.
Memory was evaluated by 30-minute delayed free recall scores for the Wechsler Memory Scale–Revised Logical Memory and Visual Reproduction subtests and also for the Rey Auditory Verbal Learning Test (AVLT). Language tests measured naming to confrontation with the Boston Naming Test and category fluency (i.e., naming animals, fruits, and vegetables). Attention/executive measures included the Trail Making Test Part B and the Wechsler Adult Intelligence Scale–Revised (WAIS-R) Digit Symbol subtest. Visual-spatial processing was examined by the WAIS-R Picture Completion and Block Design subtests. All tests were administered by experienced psychometrists and supervised by a clinical neuropsychologist.
MRI and 1H MRS.
MRI and single voxel (SV) 1H MRS examinations were performed at 3 T using an 8-channel phased array coil (GE Healthcare). A 3-dimensional high-resolution magnetization-prepared rapid gradient echo (MPRAGE) acquisition with repetition time/echo time/inversion time = 7/3/900 msec, flip angle 8 degrees, in-plane resolution of 1.0 mm, and a slice thickness of 1.2 mm was performed for anatomic segmentation and labeling of PiB PET scans and 1H MRS voxel localization. 1H MRS studies were performed using the automated MRS package Proton Brain Examination/SV. A point-resolved spectroscopy sequence with repetition time = 2,000, echo time = 30 msec, 2,048 data points, and 128 excitations was used for the examinations.10 An 8-cm3 (2 × 2 × 2 cm) 1H MRS voxel was prescribed on a midsagittal MPRAGE image, including right and left posterior cingulate gyri.3 Metabolite intensity ratios calculated at the end of each PROBE/SV acquisition were analyzed. Quantifying metabolite intensities by referencing to an internal standard is preferred in clinical 1H MRS, because internal referencing does not require correction for coil loading, atrophy, and relaxation times and can readily be used in clinical practice with standard equipment and vendor-provided processing software.
PiB PET.
PET images were acquired using a PET/CT scanner (DRX, GE Healthcare) operating in 3-dimensional mode. The subjects were injected with 292–729 MBq 11C-PiB. A CT image was obtained for attenuation correction. After a 40-minute uptake period, a 20-minute PiB scan was obtained. The PiB PET acquisition consisted of 4 5-minute dynamic frames, acquired from 40 to 60 minutes after injection. Standard corrections were applied. The pixel size for PET images was 1.0 mm, and the slice thickness was 3.3 mm. PiB PET quantitative image analysis was performed using the fully automated image processing pipeline as described in detail previously.11 In brief, the method includes gray matter (GM) sharpening of PET images using MRI and partial volume correction of CSF and tissue compartments using statistical parametric mapping 5. PiB PET cortical ratio images were calculated by dividing each PiB PET GM voxel value by the median value in the cerebellar GM region in the patient's MRI space. The global cortical PiB retention was calculated by taking the median value of the PiB PET GM ratio from the bilateral parietal, posterior cingulate, precuneus, temporal, prefrontal, orbitofrontal, and anterior cingulate GM regions in the in-house modified anatomic labeling atlas.11,12
Statistical analysis.
We summarize associations between numeric variables using Spearman partial rank order correlations, which we denote by partial rs.13 This statistic can be thought of as a nonparametric correlation between 2 variables (e.g., PiB and Cho/Cr) after partialling out, or controlling for, possible confounders. For testing associations involving MRS and PiB, we control for age and sex. In testing associations involving neuropsychological variables, we control for age, sex, and years of education. We used rank sum tests for between-group comparisons and partial rs to quantify associations because some neuropsychological variables were found to be highly non-normal with skewness and floor or ceiling effects, and the PiB distributions were highly skewed and possibly bimodal. In simulation studies, this statistic was found to perform well with highly non-normal data.13 As with other correlation measures, partial rs values are bounded between −1 and +1. A general interpretation of partial rs is that significant values indicate that as values in one variable increase, values in the other variable tend to increase (or decrease), after controlling for possible confounders. To estimate and test differences in partial rs between subjects found to have global PiB <1.5 vs those found to have global PiB ≥1.5, we used large-sample methods based on the estimated difference ± 1.96 SE, where SE is the estimated SE of the difference in partial rs. We used SAS software version 9.2.1 and the CORR procedure (SAS Institute Inc., Cary, NC; www.sas.com) to calculate estimates and confidence intervals for rs and differences in rs. Other analyses were performed using R version 2.11 (R Foundation for Statistical Computing, Vienna Austria, www.r-project.org).
RESULTS
Study sample.
Characteristics of the study sample are listed in table 1. The demographic features of the MRI/MRS and PET study sample (participants) were on average similar to those of the MCSA subjects who were evaluated from January 2009 through September 2010 but did not participate in the MRI/MRS and PET studies (nonparticipants). The only exception was that the fraction of female participants was slightly less than the fraction of female nonparticipants. The cognitive performance of the participants was similar to that of the nonparticipants on neuropsychometric tests; however, the participants on average performed a few points better on tests that assessed memory, visual-spatial function, and attention/executive function.
Table 1.
Demographic characteristics and the raw scores on individual neuropsychometric tests in the participants (i.e., study sample), nonparticipants (i.e., MCSA subjects who did not participate in the current imaging study), and the entire cognitively normal MCSA cohort (combined group of PET/MRS study participants and nonparticipants) from January 2009 through September 2010
Abbreviations: AVLT = Rey Auditory Verbal Learning Test; CI = confidence interval; 1H MRS = proton magnetic resonance spectroscopy; MCSA = Mayo Clinic Study of Aging; WAIS-R = Wechsler Adult Intelligence Scale–Revised; WMS-R = Wechsler Memory Scale–Revised.
APOE genotype is missing in 17% of the participants, 24% of the nonparticipants, and 22% of the entire MCSA cognitively normal group.
Median (interquartile range), defined as the 25th and 75th percentiles (quartile 1, quartile 3), are given for neuropsychometric tests. The ranges of possible scores are indicated after the test names.
Correlations between Aβ load and 1H MRS metabolites.
Statistical summaries of 1H MRS metabolite ratios in the whole sample of PET/MRS study participants and in participants with low (<1.5) and high (≥1.5) PiB retention are listed in table 2. The 1H MRS metabolite ratios that correlated with the global cortical PiB uptake ratio were mI/Cr (Spearman partial rs = 0.17; p = 0.003) and Cho/Cr (Spearman partial rs = 0.13; p = 0.03) after adjusting for age and sex. Although NAA/Cr levels decreased with increasing PiB retention, there was no association between neuronal marker NAA/Cr and global cortical PiB retention (Spearman partial rs = −0.07; p = 0.24). Because 1H MRS metabolite ratios were acquired from the posterior cingulate gyrus, we also tested the associations between 1H MRS metabolite ratios and cortical PiB retention in the posterior cingulate gyrus. The strength of the associations with mI/Cr (Spearman partial rs = 0.15; p = 0.007) and Cho/Cr (Spearman partial rs = 0.15; p = 0.01) was similar to that of the associations we found with the global cortical PiB retention after adjustment for age and sex (figure 1).
Table 2.
Statistical summaries of 1H MRS metabolite ratios in the whole sample of PET/MRS study participants and in participants with low (<1.5) and high (≥1.5) PiB retention
Abbreviations: Cho = choline; CI = confidence interval; Cr = creatine; IQR = interquartile range, defined as the 25th and 75th percentiles; mI = myoinositol; MRS = magnetic resonance spectroscopy; NAA = N-acetylaspartate; PiB = 11C-Pittsburgh compound B.
p Value from rank sum tests comparing participants with high and low PiB retention.
Figure 1. Association between 1H magnetic resonance spectroscopy metabolite ratios and cortical 11C-Pittsburgh compound B (PiB) retention ratio.
Scatterplots demonstrate the association between log-transformed global cortical PiB retention ratio and choline (Cho)/creatine (Cr) and myoinositol (mI)/Cr (upper panels) and between log-transformed posterior cingulate cortical PiB retention ratio and Cho/Cr and mI/Cr (lower panels).
Correlations between 1H MRS and cognition and the effect of Aβ load on these correlations.
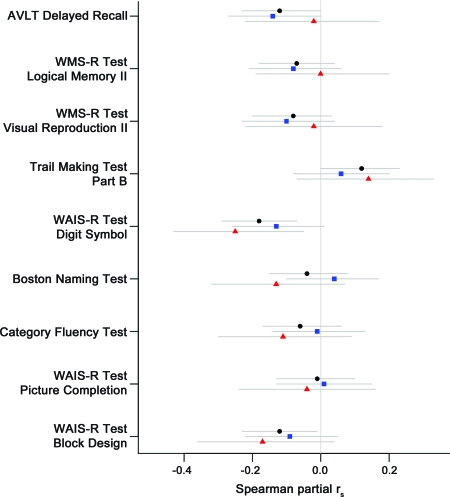
Among the 3 metabolite ratios, only Cho/Cr correlated with cognitive function after adjustment for age, sex, and education. A higher Cho/Cr ratio was associated with worse performance on the AVLT Delayed Recall (Spearman partial rs = −0.12; p = 0.04), Trail Making Test Part B (Spearman partial rs = 0.12; p = 0.04), WAIS-R Digit Symbol (Spearman partial rs = −0.18; p = 0.001), and WAIS-R Block Design Tests (Spearman partial rs = −0.12; p = 0.03) after adjustment for age, sex, and education. To determine the effects of Aβ load, we tested for the association between Cho/Cr ratio and cognitive function in the high and low PiB retention groups separately as demonstrated in figure 2. Higher Cho/Cr levels were associated with lower WAIS-R Digit Symbol scores in the high PiB retention group (Spearman partial rs = −0.25; p = 0.014), but this was only a trend in the low PiB retention group (Spearman partial rs = −0.13; p = 0.071). Nonetheless, we did not find any evidence that the Spearman partial correlations between 1H MRS variables and neuropsychological scores were significantly different between participants with high vs low PiB retention (table e-1 on the Neurology® Web site at www.neurology.org).
Figure 2. Spearman partial correlations between choline (Cho)/creatine (Cr) and cognitive performance.
Black circles indicate partial rs in the whole sample of PET/magnetic resonance spectroscopy study participants, blue squares indicate the partial rs in participants with low 11C-Pittsburgh compound B (PiB) retention, and red triangles indicate the partial rs in participants with high PiB retention. The gray lines are the confidence intervals. For the Trail Making Test Part B, a higher score indicates worse performance. For all other neuropsychometric tests, a lower score indicates worse performance. A higher Cho/Cr ratio was associated with worse performance on Rey Auditory Verbal Learning Test (AVLT) Delayed Recall (p = 0.04), Trail Making Test Part B (p = 0.04), Wechsler Adult Intelligence Scale–Revised (WAIS-R) Digit Symbol (p = 0.001), and WAIS-R Block Design (p = 0.03) after adjustment for age, sex, and education. However, we did not find any evidence that the Spearman partial rs values between Cho/Cr and neuropsychological scores were significantly different between participants with high vs low PiB retention (table e-1).
DISCUSSION
In a population-based sample of cognitively normal older adults, we showed that 1) elevated Cho/Cr and mI/Cr ratios on 1H MRS correlate with increased PiB retention, 2) the Cho/Cr ratio correlates with memory, attention/executive, and visual-spatial performance, and 3) the correlation between Cho/Cr ratio and cognitive function is not modified by PiB retention.
We observed high levels of PiB retention (≥1.5 global cortical retention ratio) in 33% of the cognitively normal individuals at or older than 70 years. This is on a par with the 20%–30% rate of high cortical PiB retention ratio that we and others have reported in the literature7,11,14,15 but lower than the 47% rate reported in the AD Neuroimaging Initiative cognitively normal cohort.16 The differences are most likely due to the methods of subject ascertainment and recruitment. In the current study, we recruited subjects who had been randomly selected from the Olmsted County population. This is in contrast to the previously studied cohorts of cognitively normal volunteers recruited through advertisements or memory clinics,7,11,14,15 which increases the potential for selection or volunteer bias. Although it is possible that people with poor general health would be less likely to participate in imaging studies, we did not exclude subjects due to neurologic, psychiatric, or systemic illnesses to study a representative sample of the population. The cognitive function in participants and nonparticipants in the randomly sampled population was on average similar, although participants performed a few points better on tests that assessed memory, visual-spatial function, and attention/executive function, suggesting minimal nonparticipation bias, if any.
Although 1H MRS metabolite ratios were measured from the posterior cingulate gyri, both global cortical PiB retention and posterior cingulate gyri PiB retention similarly correlated with the 1H MRS metabolite levels. This result is expected given that the regional variability of PiB retention in cognitively normal individuals is typically low among regions that are included in the global cortical PiB retention ratio (i.e., posterior cingulate gyri and temporal, parietal, and frontal lobe association cortices).11,14 In our sample, we found a modest correlation between PiB retention and both mI/Cr and Cho/Cr. However, in an earlier imaging–autopsy correlation study, only antemortem mI/Cr levels correlated with the density of neuritic plaques in subjects at autopsy, who spanned the cognitive spectrum.2 Therefore, mechanisms underlying the association between PiB retention and mI/Cr are likely to be different from the mechanisms underlying the association between PiB retention and Cho/Cr.
1H MRS findings in patients with MCI3,17,18 and mild AD19 and in presymptomatic carriers of the familial AD mutations5 suggest that mI/Cr elevation is an early event in the progression of AD pathology. The mI peak consists of glial metabolites that are responsible for osmoregulation.20–23 mI/Cr levels increase with age in the APP-PS1 mouse model of AD, coinciding with the timing of microglial activation in these mice.24 It is thought that the elevation of the mI peak is related to glial proliferation and astrocytic and microglial activation in AD.25 In the human brain, PiB labels both dense core and diffuse plaques, although labeling of diffuse plaques is less prominent than that of compact/cored plaques.26–28 Although most of the amyloid deposits in cognitively normal individuals are noncompact or diffuse and display only minimal glial reaction, the dense cored amyloid deposits in AD are surrounded by clusters of microglia and astrocytes.29 If mI/Cr is a marker of astrocytic and microglial activation associated with the amyloid pathology of AD, then the association we observed between mI/Cr levels and PiB retention may be directly related to the presence of compact/cored Aβ plaques. Based on this, we might expect to find an association between higher mI/Cr and worse cognitive performance. The absence of this expected correlation requires further investigation.
The only 1H MRS marker that significantly correlated with cognitive function in cognitively normal older adults specifically in the attention/executive, memory, and visual-spatial function domains was Cho/Cr ratio. This is in agreement with a previous population-based study showing that Cho/Cr levels predict future cognitive decline in cognitively normal adults.6 The biologic significance of Cho/Cr elevation in patients with AD and in patients with mild cognitive impairment is not fully understood.30 The Cho peak is composed of cytosolic glycerophosphocholine and phosphocholine, which are the products of membrane phosphatidylcholine breakdown and precursors of choline and acetylcholine synthesis.31 One possible explanation for the elevation of Cho in AD is increased membrane turnover due to neurodegeneration. It has also been hypothesized that the elevation of the Cho peak is the consequence of membrane phosphatidylcholine catabolism to provide free choline for the chronically deficient acetylcholine production in AD.32,33 Elevated Cho/Cr levels in patients with dementia with Lewy bodies characterized by a profound cholinergic deficit further support the hypothesis that elevation in Cho/Cr may be associated with cholinergic dysfunction.34 Cho/Cr levels decrease with cholinergic agonist treatment in AD, suggesting that downregulation of choline acetyltransferase activity may be responsible for the elevation of Cho.35 If indeed there is a relationship between Cho/Cr and cholinergic function in cognitively normal older adults, then Cho/Cr would be a potential biomarker for the cholinergic response in preclinical AD.
We did not find a correlation between NAA/Cr and cognitive function, nor did we find a significant correlation between NAA/Cr and Aβ load in cognitively normal older adults. Although the neuronal integrity marker NAA/Cr level is associated with cognitive function in patients with AD,36,37 both cross-sectional and longitudinal studies in patients with amnestic MCI and AD suggest that the elevation in mI/Cr and Cho/Cr precedes the decline in NAA/Cr in the temporal sequence of 1H MRS changes in AD.30,38 Although NAA/Cr gradually declines in the course of AD starting from the amnestic MCI stage, NAA/Cr levels have been relatively stable in cognitively normal older adults during longitudinal studies in different cohorts.39,40 The relative stability of NAA/Cr in cognitively normal older adults suggests that the decline in NAA/Cr is associated with a diagnosis of amnestic MCI or AD but is not a significant feature of the preclinical disease process. The lack of an association between NAA/Cr and cognitive function in the cognitively normal subjects of this study agrees with this hypothesis.
Although the associations between 1H MRS metabolites and Aβ pathology are modest, our data give insights on the significance of 1H MRS metabolite markers in cognitively normal older adults. Our data showed that Cho/Cr level was associated with cognitive function independent of PiB retention. Cho/Cr correlated with WAIS-R Digit Symbol scores even in participants with low PiB retention, suggesting that the elevation of Cho/Cr is dependent on other pathophysiologic mechanisms characterized by elevated Cho/Cr levels in addition to Aβ pathology. These mechanisms may be increased membrane turnover due to preclinical vascular disease6 or cholinergic dysfunction due to preclinical Lewy body disease.34 Longitudinal follow-up and postmortem pathologic confirmation may further clarify the underpinnings of MRS metabolite changes in cognitively normal older adults.
Supplementary Material
Glossary
GLOSSARY
- Aβ
β-amyloid
- AD
Alzheimer disease
- AVLT
Auditory Verbal Learning Test
- Cho
choline
- Cr
creatine
- GM
gray matter
- 1H MRS
proton magnetic resonance spectroscopy
- MCI
mild cognitive impairment
- MCSA
Mayo Clinic Study of Aging
- mI
myoinositol
- MPRAGE
magnetization-prepared rapid gradient echo
- NAA
N-acetylaspartate
- PiB
11C-Pittsburgh compound B
- SV
single voxel
- WAIS-R
Wechsler Adult Intelligence Scale–Revised
Footnotes
Editorial, page 932
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Kantarci: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, and obtaining funding. Dr. Lowe: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, contribution of vital reagents/tools/patients, acquisition of data, study supervision, and obtaining funding. S.A. Przybelski: analysis or interpretation of data and statistical analysis. M.L. Senjem: analysis or interpretation of data, and contribution of vital reagents/tools/patients. S.D. Weigand: analysis or interpretation of data and statistical analysis. Dr. Ivnik: study concept or design and acquisition of data. Dr. Roberts: drafting/revising the manuscript, study concept or design, acquisition of data, study supervision, and obtaining funding. Dr. Geda: drafting/revising the manuscript and acquisition of data. Dr. Boeve: drafting/revising the manuscript and acquisition of data. Dr. Knopman: drafting/revising the manuscript and acquisition of data. Dr. Petersen: drafting/revising the manuscript and obtaining funding. Dr. Jack: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, study supervision, and obtaining funding.
DISCLOSURE
Dr. Kantarci receives research support from the NIH. Dr. Lowe serves on scientific advisory boards for Bayer Schering Pharma and GE Healthcare and receives research support from GE Healthcare, Siemens Molecular Imaging, the NIH (NIA, NCI), the MN Partnership for Biotechnology and Medical Genomics, and the Leukemia & Lymphoma Society. S.A. Przybelski reports no disclosures. M.L. Senjem has received research support from Pfizer Inc. S.D. Weigand reports no disclosures. Dr. Ivnik serves on the editorial boards of The Clinical Neuropsychologist and Aging, Neuropsychology, and Cognition; receives publishing royalties for Clinical Interpretation of the WAIS-III and WMS-III (Academic Press, 2003); and receives research support from the NIH/NIA. Dr. Roberts receives research support from the NIH. Dr. Geda receives research support from the NIH. Dr. Boeve has served as a consultant to GE Healthcare; receives publishing royalties for The Behavioral Neurology of Dementia (Cambridge University Press, 2009); and receives research support from Cephalon, Inc., Allon Therapeutics, Inc., the NIH/NIA, the Alzheimer's Association, and the Mangurian Foundation. Dr. Knopman serves as Deputy Editor for Neurology®; has served on a data safety monitoring board for Eli Lilly and Company; has served as a consultant for Elan/Janssen AI; is an investigator in clinical trials sponsored by Elan/Janssen AI, Baxter International Inc., and Forest Laboratories, Inc.; and receives research support from the NIH. Dr. Petersen serves on scientific advisory boards for the Alzheimer's Association, the National Advisory Council on Aging (NIA), Elan/Janssen AI, Pfizer Inc (Wyeth), and GE Healthcare; receives publishing royalties from Mild Cognitive Impairment (Oxford University Press, 2003); serves as a consultant for Elan/Janssen AI and GE Healthcare; and receives research support from the NIH/NIA. Dr. Jack serves on scientific advisory boards for Elan/Janssen AI, Eli Lilly & Company, GE Healthcare, and Eisai Inc.; receives research support from Baxter International Inc., Allon Therapeutics, Inc., Pfizer Inc, the NIH/NIA, and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Foundation; and holds stock/stock options in Johnson & Johnson.
REFERENCES
- 1. Thal LJ, Kantarci K, Reiman EM, et al. The role of biomarkers in clinical trials for Alzheimer disease. Alzheimer Dis Assoc Disord 2006;20:6–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kantarci K, Knopman DS, Dickson DW, et al. Alzheimer disease: postmortem neuropathologic correlates of antemortem 1H MR spectroscopy metabolite measurements. Radiology 2008;248:210–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kantarci K, Jack CR, Jr, Xu YC, et al. Regional metabolic patterns in mild cognitive impairment and Alzheimer's disease: a 1H MRS study. Neurology 2000;55:210–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chao LL, Schuff N, Kramer JH, et al. Reduced medial temporal lobe N-acetylaspartate in cognitively impaired but nondemented patients. Neurology 2005;64:282–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Godbolt AK, Waldman AD, MacManus DG, et al. MRS shows abnormalities before symptoms in familial Alzheimer disease. Neurology 2006;66:718–722 [DOI] [PubMed] [Google Scholar]
- 6. den Heijer T, Sijens PE, Prins ND, et al. MR spectroscopy of brain white matter in the prediction of dementia. Neurology 2006;66:540–544 [DOI] [PubMed] [Google Scholar]
- 7. Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol 2010;67:122–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008;30:58–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men: the Mayo Clinic Study of Aging. Neurology 2010;75:889–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Webb PG, Sailasuta N, Kohler SJ, Raidy T, Moats RA, Hurd RE. Automated single-voxel proton MRS: technical development and multisite verification. Magn Reson Med 1994;31:365–373 [DOI] [PubMed] [Google Scholar]
- 11. Jack CR, Jr, Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain 2008;131:665–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lowe VJ, Kemp BJ, Jack CR, Jr, et al. Comparison of 18F-FDG and PiB PET in cognitive impairment. J Nucl Med 2009;50:878–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schemper M. Non-parametric partial association revisited. J R Stat Soc Ser D 1991;40:73–76 [Google Scholar]
- 14. Aizenstein HJ, Nebes RD, Saxton JA, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol 2008;65:1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pike KE, Savage G, Villemagne VL, et al. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer's disease. Brain 2007;130:2837–2844 [DOI] [PubMed] [Google Scholar]
- 16. Jagust WJ, Bandy D, Chen K, et al. The Alzheimer's Disease Neuroimaging Initiative positron emission tomography core. Alzheimers Dement 2010;6:221–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chantal S, Braun CM, Bouchard RW, Labelle M, Boulanger Y. Similar 1H magnetic resonance spectroscopic metabolic pattern in the medial temporal lobes of patients with mild cognitive impairment and Alzheimer disease. Brain Res 2004;1003:26–35 [DOI] [PubMed] [Google Scholar]
- 18. Catani M, Cherubini A, Howard R, et al. 1H-MR spectroscopy differentiates mild cognitive impairment from normal brain aging. Neuroreport 2001;12:2315–2317 [DOI] [PubMed] [Google Scholar]
- 19. Huang W, Alexander GE, Chang L, et al. Brain metabolite concentration and dementia severity in Alzheimer's disease: a 1H MRS study. Neurology 2001;57:626–632 [DOI] [PubMed] [Google Scholar]
- 20. Urenjak J, Williams SR, Gadian DG, Noble M. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci 1993;13:981–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci 1993;15:289–298 [DOI] [PubMed] [Google Scholar]
- 22. Fisher SK, Novak JE, Agranoff BW. Inositol and higher inositol phosphates in neural tissues: homeostasis, metabolism and functional significance. J Neurochem 2002;82:736–754 [DOI] [PubMed] [Google Scholar]
- 23. Glanville NT, Byers DM, Cook HW, Spence MW, Palmer FB. Differences in the metabolism of inositol and phosphoinositides by cultured cells of neuronal and glial origin. Biochim Biophys Acta 1989;1004:169–179 [DOI] [PubMed] [Google Scholar]
- 24. Marjanska M, Curran GL, Wengenack TM, et al. Monitoring disease progression in transgenic mouse models of Alzheimer's disease with proton magnetic resonance spectroscopy. Proc Natl Acad Sci USA 2005;102:11906–11910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ross BD, Bluml S, Cowan R, Danielsen E, Farrow N, Tan J. In vivo MR spectroscopy of human dementia. Neuroimaging Clin N Am 1998;8:809–822 [PubMed] [Google Scholar]
- 26. Lockhart A, Lamb JR, Osredkar T, et al. PIB is a non-specific imaging marker of amyloid-beta (Abeta) peptide-related cerebral amyloidosis. Brain 2007;130:2607–2615 [DOI] [PubMed] [Google Scholar]
- 27. Kantarci K, Yang C, Schneider JA, et al. Ante mortem amyloid imaging and beta-amyloid pathology in a case with dementia with Lewy bodies. Neurobiol Aging Epub 2010 Oct 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ikonomovic MD, Klunk WE, Abrahamson EE, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer's disease. Brain 2008;131:1630–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dickson DW. The pathogenesis of senile plaques. J Neuropathol Exp Neurol 1997;56:321–339 [DOI] [PubMed] [Google Scholar]
- 30. Kantarci K. 1H magnetic resonance spectroscopy in dementia. Br J Radiol 2007;80:S146–S152 [DOI] [PubMed] [Google Scholar]
- 31. Klein J. Membrane breakdown in acute and chronic neurodegeneration: focus on choline-containing phospholipids. J Neural Transm 2000;107:1027–1063 [DOI] [PubMed] [Google Scholar]
- 32. MacKay S, Meyerhoff DJ, Constans JM, Norman D, Fein G, Weiner MW. Regional gray and white matter metabolite differences in subjects with AD, with subcortical ischemic vascular dementia, and elderly controls with 1H magnetic resonance spectroscopic imaging. Arch Neurol 1996;53:167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wurtman RJ, Blusztajn JK, Maire JC. Autocannibalism of choline-containing membrane phospholipids in the pathogenesis of Alzheimer's disease: a hypothesis. Neurochem Int 1985;7:369–372 [DOI] [PubMed] [Google Scholar]
- 34. Kantarci K, Petersen RC, Boeve BF, et al. 1H MR spectroscopy in common dementias. Neurology 2004;63:1393–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Satlin A, Bodick N, Offen WW, Renshaw PF. Brain proton magnetic resonance spectroscopy (1H-MRS) in Alzheimer's disease: changes after treatment with xanomeline, an M1 selective cholinergic agonist. Am J Psychiatry 1997;154:1459–1461 [DOI] [PubMed] [Google Scholar]
- 36. Jessen F, Block W, Träber F, et al. Decrease of N-acetylaspartate in the MTL correlates with cognitive decline of AD patients. Neurology 2001;57:930–932 [DOI] [PubMed] [Google Scholar]
- 37. Kantarci K, Smith GE, Ivnik RJ, et al. 1H magnetic resonance spectroscopy, cognitive function, and apolipoprotein E genotype in normal aging, mild cognitive impairment and Alzheimer's disease. J Int Neuropsychol Soc 2002;8:934–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Valenzuela MJ, Sachdev P. Magnetic resonance spectroscopy in AD. Neurology 2001;56:592–598 [DOI] [PubMed] [Google Scholar]
- 39. Kantarci K, Weigand SD, Petersen RC, et al. Longitudinal 1H MRS changes in mild cognitive impairment and Alzheimer's disease. Neurobiol Aging 2007;28:1330–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schott JM, Frost C, MacManus DG, Ibrahim F, Waldman AD, Fox NC. Short echo time proton magnetic resonance spectroscopy in Alzheimer's disease: a longitudinal multiple time point study. Brain 2010;133:3315–3322 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.