Abstract
Background
Although C4d deposition in peritubular capillaries has been identified as a strong risk factor for subsequent renal allograft loss, the optimal cut-off for the fraction of peritubular capillaries needed to establish a positive stain in formalin-fixed paraffin-embedded material has not been systematically defined. The objective of this study was to establish the threshold for positive staining that best predicts renal outcome in renal biopsies in a multicenter study in which local and central pathology were compared.
Methods
Unstained renal biopsy slides were obtained from 296 patients. The percentage of peritubular capillaries staining positively for C4d was detected by immunoperoxidase staining.
Results
The percentage C4d deposition ranged from 0% to 90% with 44% (129/296) having a positive percentage of C4d staining. The median for positive cases was 25%. Local C4d+ results were reported qualitatively, with 28% recorded as positive for C4d. Using a centrally-determined cut-off of 10%, tests for agreement of local and central C4d staining were fair (Kappa 0.40, 95% CI 0.29-0.51). Raising the centrally-determined cut-off to 25% or 50% did not change the Kappa values (0.44 and 0.41, respectively). By Cox proportional hazards model, C4d positivity (centrally-determined assessment) using a cut-off of 10% was the strongest predictor of time to graft loss (HR 2.66, 95% CI [1.68, 4.21]). Centrally-determined C4d positivity correlated with Banff scores indicative of acute inflammation, but not with scores indicative of fibrosis/atrophy or transplant glomerulopathy.
Conclusions
Our findings indicate that C4d positivity, defined as ≥10% by immunoperoxidase, is a strong predictor of graft loss.
Keywords: C4d, graft survival, immunoperoxidase
INTRODUCTION
Peritubular capillary deposition of C4d has been identified as a risk factor for the development of progressive graft dysfunction and loss (1-3). Peritubular capillary staining for C4d and presence of donor specific antibodies have been incorporated into the revised Banff ‘97 classification scheme as criteria for the diagnosis of acute humoral rejection (4). Moreover, in patients with chronic graft dysfunction, C4d predicts adverse outcome and is associated with a variety of histopathologic alterations, including transplant glomerulopathy (1, 5). Based on these considerations, it has been recommended that staining for C4d be performed on all renal allograft biopsies (6).
Methods for detection of C4d deposition in peritubular capillaries include immunofluorescence performed on frozen tissue and immunoperoxidase performed on formalin-fixed paraffin-embedded tissue. There are advantages to both methods for detection of C4d – for example, it is recognized that detection of C4d by immunofluorescence provides the most sensitive and specific marker for C4d positivity (7-9). However, immunofluorescence studies require the acquisition of additional tissue, which may not be practical for protocol biopsies or for retrospective studies. Unfortunately, there is considerable variation between centers on thresholds for “focal” or “diffuse” positive staining, both by immunofluorescence and by immunoperoxidase, rendering it difficult to compare results for C4d positivity between centers. Furthermore, there are no data in the literature that systematically correlate various cut-off points for the detection of C4d with outcome. At the Banff 2007 Meeting on Allograft Pathology, the need to correlate C4d cut-offs with long-term outcome was emphasized (6).
The purpose of this study was to establish an optimal cut-off for C4d deposition, as assessed by central analysis of formalin-fixed paraffin-embedded tissues stained by the immunoperoxidase method. Study patients were enrolled in the Deterioration of Kidney Allograft Function (DeKAF) Study, a multicenter study to define clinical and histopathologic features that predict adverse outcome in renal biopsies performed for new onset late graft dysfunction (10). In this cohort of patients, recruited from seven centers in the US and Canada, we demonstrate that a cut-off of 10% of peritubular capillaries to establish a positive C4d stain provided the best fit for the association of C4d positivity with graft loss.
RESULTS
The study cohort consists of 296 patients who underwent a clinically-indicated renal biopsy for new-onset deterioration of function (defined as 25% increase in serum creatinine or new onset of proteinuria) for which both local and central C4d data are available. The percentage of peritubular capillaries staining positively for C4d was assessed in a blinded fashion. A representative photomicrograph showing diffuse positive and negative immunoperoxidase staining for C4d is shown in Figure 1. The percentage of C4d positivity ranged from 0% to 90% with 44% (129/296) having a positive percentage of C4d staining; among those with positive percentage C4d, the median percentage was 25%. Local C4d+ results were reported qualitatively, with 28% (83/296) recorded as positive for C4d. Using a centrally-determined cut-off of 10%, the Kappa score for agreement of local and centrally-determined C4d positivity was 0.40 [95% CI 0.29-0.51]. Raising the cut-off for centrally-determined C4d values did not appreciably change the Kappa values – using a centrally-determined cut-off of 25%, the Kappa value was 0.46 [95% CI 0.35-0.57], and using cut-off of 50% for centrally-determined positivity, the Kappa value was 0.42 [95% CI 0.30-0.54]. The overall proportion of observed agreement ranged between 69%-79% for all putative cut-offs examined. The proportion of specific agreement for C4d+ negativity (range 76%-87%) was higher than the proportion of specific agreement for positivity (54%-60%), indicating that observed agreement was superior on cases where either the local or central result was negative rather than on cases where at least one result was positive, for each putative cut-off value.
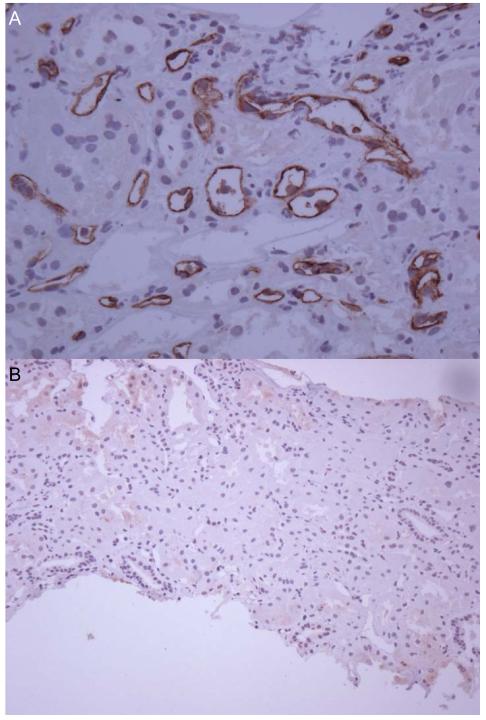
Figure 1.
Representative histopathologic section of renal biopsy stained by immunoperoxidase for C4d showing diffuse positive staining within peritubular capillaries (panel A). Representative section of a renal biopsy showing no significant staining for C4d (panel B).
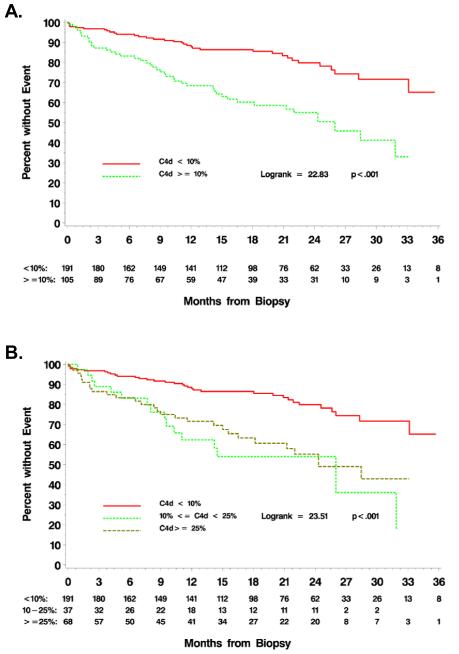
Cox proportional hazards models were used to estimate the unadjusted risk of graft loss by local and central C4d results. Selection of an optimal cut-off for central C4d positivity was performed by a maximum Chi square search (Table 1), or, since all models have the same number of degrees of freedom, equivalently by AIC (Akaike Information Criterion). The model with a C4d cut-off of 10% provided the best-fitting model by these measures. A Kaplan-Meier graph of centrally-determined C4d positivity using a cut-off threshold of 10% is given in Figure 2A.
Table 1.
Hazard ratios from univariate models for time-to-graft failure (including death) by central C4d positivity cut-off or local determination.
| C4d Positivity | HR (95% CI)a | Chi-Square | P-value | AICb | C-indexc |
|---|---|---|---|---|---|
| >0% | 2.75 ([1.73,4.38]) | 19.719 | <0.001 | 775.214 | 0.63 |
| ≥5% | 2.85 ([1.79,4.54]) | 21.239 | <0.001 | 773.848 | 0.63 |
| ≥10% | 2.88 ([1.83-4.52])d | 22.849 | <0.001 | 773.479 | 0.63 |
| ≥15% | 2.40 ([1.53,3.78]) | 15.235 | <0.001 | 780.939 | 0.60 |
| ≥20% | 2.24 ([1.42,3.55]) | 12.537 | <0.001 | 783.368 | 0.59 |
| ≥25% | 2.08 ([1.30-3.32]) | 9.763 | 0.002 | 785.781 | 0.58 |
| ≥30% | 1.81 ([1.11,2.94]) | 5.789 | 0.016 | 789.179 | 0.56 |
| ≥40% | 1.42 ([0.84,2.42]) | 1.743 | 0.187 | 792.713 | 0.52 |
| ≥50% | 1.33 ([0.76-2.35]) | 1.007 | 0.317 | 793.385 | 0.51 |
| Local C4d positive (cut-off unknown) |
1.47 ([0.90-2.38]) | 2.430 | 0.119 | 792.108 | 0.53 |
Hazard Ratio (HR) and 95% Confidence Interval (CI)
Akaike Information Criterion, a penalized likelihood, and measure of general. Smaller values are better.
Harrell’s C-index, an analogue of area under the Receiver Operator Characteristic Curve for censored data, bootstrap-corrected for optimism using 200 bootstrap samples. Values range from 0 to 1, with the uninformative null model having a value of 0.5. Larger values indicate better discrimination between high and low risk cases. The values are rounded to two decimal places, with the maximal value occurring at the 10% cut-off.
The table contains the hazard ratio estimated directly from the data. The cross-validated estimate of the hazard ratio for central C4d positivity defined by a 10% cut-off is 2.66 (95% CI [1.68,4.21]), with likelihood ratio test χ2=19.9, 1 df, p<0.001.
Figure 2.
Time to graft failure by C4d status. Immunoperoxidase staining for C4d was centrally performed and read in a blinded fashion. A) Time to graft failure as a function of C4d status, using a centrally-defined cut-off threshold of 10%. B) Time to graft failure as a function of C4d status, using centrally-defined cut-off values of <10% (negative), 10-24%, or ≥25%.
To correct for over-fitting (“optimism”) in the fitting of the model as a result of multiple comparisons employed in the maximum Chi-square search, 2-fold cross-validation was employed. Cross-validation analysis estimated a statistically significant association of C4d+ positivity (likelihood ratio χ2=19.9, p<0.0001) with a hazard ratio of 2.66 (95% CI [1.68, 4.21]), compared with a 2.88 hazard ratio for the direct univariate analysis.
Harrell’s c-index corrected for over-fitting via bootstrapping shows the discrimination of C4d positivity is also greatest at a 10% cut-off (c=0.63), whereas the discrimination of local C4d positivity (c=0.53) is comparable to the null model (c=0.50). Calibration of the model with positivity cut-off of 10% was assessed through bootstrapping the difference between predicted model-based and observed Kaplan-Meier estimates of year 1 and year 2 survival to estimate the degree of over-fitting in the model (11). The direct estimate of survival and its standard error at year 1 post-biopsy was 81.0%±3.0%, with an estimated survival rate corrected for optimism of 81.2%. Likewise, at year 2, the direct estimate of survival was 71.0%±4.4% while the estimate corrected for optimism is 71.1%. Thus, the model appears to be well-calibrated in prediction of time to graft loss.
Increasing the centrally-determined C4d-positive threshold beyond 10% weakened the association between C4d positivity and outcome – using a centrally-determined cut-off of 40%, there was no significant association between C4d positivity and outcome. Of note, there was no significant association between locally-determined C4d positivity (cut-off not specified) and outcome (HR 1.47, 95% CI 0.9-2.38, p=0.121) (Table 1).
To further demonstrate the risk associated with relatively low levels of C4d positivity, the time to graft failure analyses was compared between the three subgroups defined by central C4d+ results of <10%, 10-24%, and ≥25%. A Kaplan-Meier graph showing these three risk groups is given in Figure 2B. In proportional hazards regression comparing to the group with <10% central C4d, the hazard ratio for graft failure for the group with central C4d from 10-24% was 3.3 (95% CI [1.8,6.0], p<0.001), while the estimated hazard ratio for those with ≥25% central C4d was 2.7 (95% CI [1.6,4.5], p<0.001). The two higher risk groups did not differ significantly in risk of graft failure (p=0.534).
Central histopathologic analysis of hematoxylin & eosin (H&E), periodic acid-Schiff (PAS), trichrome, and silver-stained sections was performed according to the Banff ‘07 Classification Scheme modified to include assessment of inflammation and tubulitis in areas of atrophy as well as in non-atrophic regions (6). Distribution of Banff scores according to C4d status is summarized in Table 2. By the Wilcoxon two-sample test, positive C4d staining correlated with the presence of peritubular capillary infiltrates (p<0.001), acute inflammation (Banff i score, p=0.004), acute tubulitis (t, p=0.026) and a marginal but not significant association with transplant glomerulopathy (Banff cg-score, p=0.051). Positive C4d staining was significantly associated with inflammation in areas of atrophy (p=0.001) and tubulitis in areas of atrophy (p=0.030). Positive C4d staining did not significantly correlate with chronic Banff scores, including interstitial fibrosis (ci), tubular atrophy (ct), vascular sclerosis (cv), arteriolar hyalinosis (ah), or mesangial matrix expansion (mm).
Table 2.
Selected centrally-determined Banff scores as a function of centrally-determined C4d positivity (≥10%).
| Central C4d Status | Central Banff i-score p=0.004 |
Total | Central Banff t-score p=0.026 |
Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Frequency/Row
Percent |
0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | ||
| Negative | 117 69% |
21 12% |
18 10% |
13 8% |
169 | 127 75% |
11 7% |
18 11% |
13 8% |
169 |
| Positive | 48 49% |
21 22% |
22 23% |
6 6% |
97 | 59 61% |
11 11% |
20 21% |
7 7% |
97 |
| Total | 165 | 42 | 40 | 19 | 266 | 186 | 22 | 38 | 20 | 266 |
| Central C4d Status | Central Banff ptc-score p<0.001 |
Total | Central Banff cg-score p=0.051 |
Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Frequency/Row
Percent |
0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | ||
| Negative | 90 53% |
55 33% |
11 7% |
13 8% |
169 | 121 72% |
14 8% |
12 7% |
22 13.02 |
169 |
| Positive | 28 29% |
44 45% |
18 19% |
7 7% |
97 | 58 60% |
12 12% |
8 8% |
19 20% |
97 |
| Total | 118 | 99 | 29 | 20 | 266 | 179 | 26 | 20 | 41 | 266 |
| Central C4d Status | Inflammation in Atrophic Regions P<0.001 |
Total | |||
|---|---|---|---|---|---|
|
Frequency/Row Percent |
0 | 1 | 2 | 3 | |
| Negative | 67 40% |
51 30% |
37 22% |
14 8% |
169 |
| Positive | 17 18% |
35 36% |
34 35% |
11 11% |
97 |
| Total | 84 | 86 | 71 | 25 | 266 |
P-values computed via the Wilcoxon Two-Sample test.
The number of centrally-defined C4d positive biopsies, using a cut-off threshold of 10%, as a function of locally-determined primary or secondary clinicopathologic diagnosis is shown in Table 3. By Fisher’s Exact Test, C4d positivity was associated with a local diagnosis of acute cellular rejection (p<0.001), antibody-mediated rejection (p=0.024), and chronic allograft nephropathy (CAN) (p=0.039). Acute tubular necrosis was significantly associated with a negative C4d status (p=0.016).
Table 3.
Local primary or secondary diagnoses by centrally-determined C4d status
| Central C4d Status | |||||
|---|---|---|---|---|---|
| Negative | Positive | ||||
| Local Primary or Secondary Diagnoses |
N | Pct* | N | Pct* | P-value** |
| Rejection: Cellular | 26 | 13.7 | 32 | 30.5 | <0.001 |
| Rejection: Antibody-Mediated | 10 | 5.3 | 14 | 13.3 | 0.024 |
| Borderline Changes | 14 | 7.4 | 6 | 5.7 | 0.639 |
| ATN | 10 | 5.3 | 0 | 0.0 | 0.016 |
| CAN | 79 | 41.6 | 57 | 54.3 | 0.039 |
| CNI Toxicity | 56 | 29.5 | 29 | 27.6 | 0.789 |
| Glomerulopathy (de novo) | 12 | 6.3 | 5 | 4.8 | 0.795 |
| Arterial Nephrosclerosis | 21 | 11.1 | 5 | 4.8 | 0.086 |
| Polyoma Virus | 7 | 3.7 | 1 | 1.0 | 0.267 |
| Recurrent Disease | 20 | 10.5 | 20 | 19.0 | 0.050 |
| Transplant Glomerulopathy | 37 | 19.5 | 25 | 23.8 | 0.456 |
| No Abnormalities | 6 | 3.2 | 1 | 1.0 | 0.428 |
| Other | 52 | 27.4 | 23 | 21.9 | 0.331 |
| No Diagnosis | 1 | 0.5 | 0 | 0.0 | >0.999 |
| Total Cases | 169 | 100 | 97 | 100 | |
Percents are percents of C4d negative (resp. positive) cases. Percentages in a given column may add to more than 100% due to individual participants having one or several secondary diagnoses.
P-values are computed via Fisher’s Exact Test.
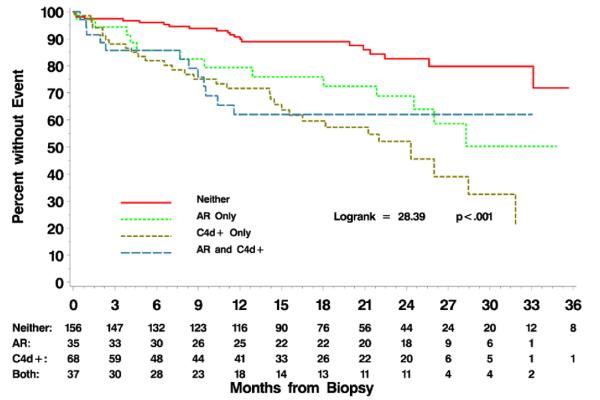
Twenty-three percent of the 296 biopsies studied were C4d-positive (centrally-determined), 12% had acute rejection (Banff i2 t2 or greater), and 12.5% had both acute rejection and positive C4d staining. The presence of positive C4d staining was at least as strong a predictor of adverse outcome as the presence of acute rejection (Figure 3). Compared to patients with biopsies that were C4d-negative and had no evidence of rejection (neither), hazard ratios for patients with biopsies that were C4d-positive, acute rejection-positive, or both ranged from 2.4 to 4.0. However, these differences in hazard ratios for the latter three groups were not significantly different.
Figure 3.
Time to graft failure in patients (n=296) whose biopsies show positive C4d staining (n=68), acute rejection (n=35), both (n=37), or neither (n=156).
DISCUSSION
Although C4d has been touted as a marker of humoral immunity and adverse outcome (12, 13), interpretation of results is highly method-dependent (immunofluorescence versus immunoperoxidase, and cut-offs for positivity ranging from “focal” to “diffuse”). Studies have suggested that immunofluorescence detection of C4d is more sensitive than peroxidase-based methods, but it may be impractical to obtain an additional biopsy core for immunofluorescence studies at some centers. Furthermore, the clinical utility of various cut-off points for positive C4d staining, particularly by immunoperoxidase, has not been previously established.
In this study, Kappa values for agreement between central and local determination of C4d were fair (0.40 for a cut-off of 10%) and did not appreciably change when higher cut-off values for centrally-determined C4d positivity were employed. These Kappa values are similar to those obtained in a study comparing immunofluorescence and immunoperoxidase detection of C4d which were performed on the same biopsy cases (Kappa 0.3) (9). Based upon a maximum Chi-square search, a cut-off of 10% for central C4d positivity best predicted graft failure/death. The cross-validated estimate of the hazard ratio of central C4d positivity using a 10% cut-off was 2.66 (95% CI [1.68, 4.21], Likelihood ratio χ2=19.9, 1 df, p<0.001). The association of C4d positivity with graft failure/death became less significant as higher cut-off thresholds for centrally-determined C4d were employed; there was no significant association between C4d positivity and graft loss when a threshold of 50% was employed. Those with central C4d between 10% and 24% (inclusive) had a significantly higher risk of failure than those with <10% C4d, and furthermore had similar risk of graft-failure compared to those with ≥25% C4d, strengthening the assertion that relatively low levels of C4d are associated with a higher risk of graft failure. There was no significant association between local C4d determinations and outcome, as defined by time to graft failure/death (p=0.121). This may be due to the center-to-center variation in method to detect C4d and the lack of a validated threshold for determination of C4d positivity.
One limitation of our study is an inability to investigate whether a lower cut-off than 10% would have significantly more or less predictive power than the currently selected cut-off of 10% due to insufficient numbers of events among individuals with central C4d >0% and <10%. This question may become open to analysis as additional events accrue with continued follow-up.
By central histopathologic analysis, positive C4d scores correlated with the presence of peritubular capillary infiltrates, acute inflammation, acute tubulitis, and inflammation in areas of atrophy. Other investigators have found C4d positivity in approximately 30-40% of biopsies with acute cellular rejection, and an association with steroid-resistant acute rejection (12-15). Our data confirm this finding, as the presence of C4d in biopsies demonstrating acute cellular rejection is associated with adverse outcome (14-18). Widespread peritubular capillary staining for C4d in association with peritubular capillary inflammatory infiltrates and/or glomerulitis is thought representative of acute humoral rejection (13, 19). Positive C4d staining has also been identified in 25-40% of patients with chronic antibody-mediated rejection, as defined by presence of donor-specific antibody, double-contouring of glomerular capillary loop basement membranes (transplant glomerulopathy), mononuclear inflammatory cell infiltrates within peritubular capillaries, and arterial intimal thickening with mononuclear cell infiltrates (5, 18, 20-22). In patients with transplant glomerulopathy, the presence of peritubular capillary staining for C4d has been associated with adverse outcome (23). However, the prevalence of C4d staining in the presence of transplant glomerulopathy is only 25-40% (23, 24).
Histopathologic correlates of positive C4d staining did not correlate with chronic Banff scores, including interstitial fibrosis, tubular atrophy, vascular sclerosis, arteriolar hyalinosis, or mesangial matrix expansion. One possible explanation for the lack of correlation of C4d staining with chronic lesions is that there is considerable variability of C4d staining in sequential biopsies, with both late appearance and disappearance of C4d staining observed in serial biopsies obtained from the same patient (20). Nevertheless, a positive C4d stain in patients with chronic allograft dysfunction, when present, is a strong marker of adverse outcome. In a retrospective analysis of 80 patients with CAN but no evidence of transplant glomerulopathy, peritubular capillary staining for C4d was associated with a worse outcome than C4d-negative biopsies (25).
Based on these considerations, we conclude that immunoperoxidase detection of C4d, with a cut-off threshold of 10%, predicts adverse graft outcome in this cohort of patients with graft dysfunction. In patients with acute cellular rejection, positive C4d staining may predict adverse outcome and may provide the basis for alternative therapeutic strategies to target a humoral immune response. Although a positive C4d predicted adverse outcome in patients with chronic histopathologic alterations, C4d was not a sensitive marker for the presence of transplant glomerulopathy. Future studies are needed to develop an optimal model that predicts graft failure, using a cut-off value of 10% for C4d positivity in association with other biomarkers of chronic graft dysfunction. Nevertheless, validation of C4d as a definitive marker using paraffin-embedded material is an important first step in the development of therapeutic protocols to improve long-term graft function.
MATERIALS AND METHODS
Patients and Enrollment
Patients were enrolled in the Cross-Sectional Cohort of the DeKAF Study, a multicenter study to define clinical and histopathologic features that predict adverse outcome in renal biopsies performed for new onset late graft dysfunction (10). Recipients were eligible for enrollment if transplanted prior to October 1, 2005, having a baseline serum creatinine <2.0 mg/dL as of January 1, 2006, and subsequently developing new-onset dysfunction, defined as a ≥25% increase in serum creatinine, or new-onset proteinuria (albumin/Cr ratio >0.2 or protein/Cr ratio >0.5) leading to an allograft biopsy. Enrollment occurred at the time of the biopsy. The study is registered at www.clinicaltrials.gov (NCT00270712). Institutional Review Board approval was obtained at all participating sites.
The current study included patients with late-onset graft dysfunction, as defined above, for which both local and central C4d data were available. Local C4d determinations were performed in conjunction with standard histopathologic analysis of the clinically-indicated renal biopsy, and were reported as negative or positive using locally-established cut-off points. Unstained slides were submitted for central C4d staining. Serum creatinine, urine protein and creatinine, demographic information, current immunosuppression therapy, intervening illnesses, date and cause of graft loss were collected from all enrolled patients every 6 months.
Immunoperoxidase stain for C4d
Central C4d staining of formalin-fixed paraffin-embedded tissues from the clinically-indicated renal biopsies was performed by immunoperoxidase using a polyclonal anti-C4d antibody (Alpco Diagnostics, Salem, NH) following antigen retrieval (26). To facilitate consistency, slides were batched and stained on a Dako autostainer (Dako, Carpinteria, CA). C4d stains were read blindly, without clinical or pathologic information. The percentage of peritubular capillaries staining positively for C4d was recorded.
Histopathologic analysis
Allograft biopsies were read by the local pathologist, and the local pathologic diagnosis was used to guide clinical care and immunosuppressive management. Representative sections were submitted to a central laboratory for analysis. All biopsies were interpreted by the central pathologist in a blinded fashion, without knowledge of C4d status. In addition to the “standard” Banff classification scheme (6), a simplified semiquantitative estimate of inflammation in areas of atrophy was developed and scored as follows: 0 = inflammation in <10% of atrophic regions; 1 = inflammation in 10-25% of atrophic regions; 2 = inflammation in 26-50% of atrophic regions; 3 = inflammation in >50% of atrophic regions.
Statistical analysis
Agreement between local and central C4d positivity was assessed by Kappa analysis, proportion of observed agreement, and proportions of specific agreement for C4d+ positivity and for C4d+ negativity (27). Methods of survival analysis were used to analyze time-to-event outcomes. Non-parametric assessment (Kaplan-Meier) was employed to analyze time to graft failure – as defined by return to dialysis, retransplantation, or death – for biopsies staining positively or negatively for C4d. Cox proportional hazard models were used to estimate unadjusted hazard ratios for graft loss by local or central C4d results.
For both the survival methods and the agreement analyses, all observed values of percentage C4d up to 50% were assessed as putative cut-offs for determination of central C4d positivity. Values greater than 50% were not considered due to the paucity of observations with C4d above this value (41/296).
Selection of the optimal cut-off for determination of central C4d positivity was made using the maximum Chi-square search, following Mazumdar et al. (28). Briefly, the optimal cut-off for C4d positivity is determined by the largest Chi Square value (smallest p-value) for the Likelihood Ratio test in a univariate model of C4d positivity determined by the putative cut-off. Two-fold cross-validation was employed to obtain a hazard ratio estimate and p-value for the optimal cut-off to correct for the multiple testing employed in the maximum Chi Square search. The predictive performance of the final model was assessed in terms of discrimination via Harrell’s c-index (11). The calibration of the final model was assessed by bootstrap estimation of the bias due to over-fitting at 1-year and 2-years post-biopsy. Both the c-index and the calibration were corrected for over-fitting (“optimism”) through bootstrap estimation with 200 replications.
Associations between C4d staining and histologic features were assessed by the two-sample Wilcoxon test. Associations between C4d staining and local diagnoses were assessed by Fisher’s exact test. Two-sample cross-validation, computation of the c-index and calibration estimates were performed using the R (29), using the rms package of Harrell (30). All other analyses were performed using SAS/STAT software, Version 9.2 of the SAS System for Unix.
ACKNOWLEDGEMENTS
The DeKAF Team consists of Arthur Matas, MD, University of Minnesota; Fernando Cosio, MD, Mayo Clinic; Joseph Grande, MD, Mayo Clinic; Sita Gourishankar, MD, University of Alberta; Philip Halloran, MD, University of Alberta; J. Michael Cecka, PhD, University of California (UCLA); Lawrence Hunsicker, University of Iowa; David Rush, MD, University of Manitoba; Robert Gaston, MD, University of Alabama; Roslyn Mannon, MD, University of Alabama; Bertram Kasiske, MD, Hennepin County Medical Center; John Connett, PhD, University of Minnesota; Robert Leduc, PhD, University of Minnesota; Behzad Najafian, MD, University of Minnesota; Gretchen Crary, MD, Hennepin County Medical Center; Kim Solez, MD, University of Alberta; Ramesh Nair, MD, University of Iowa; Ian Gibson, MD, University of Manitoba; and William Cook, MD, University of Alabama.
This work is supported by National Institutes of Health Grant U01 AI58013 ClinicalTrials.gov Identifier: NCT00270712
ABBREVIATIONS
- AIC
Akaike Information Criterion
- CAN
Chronic allograft nephropathy
- DeKAF
Deterioration of Kidney Allograft Function
Contributor Information
Gretchen S. Crary, Department of Pathology and Laboratory Medicine, Hennepin County Medical Center.
Yassaman Raissian, Department of Laboratory Medicine & Pathology, Mayo Clinic.
Robert C. Gaston, Division of Nephrology, University of Alabama.
Sita M. Gourishankar, Division of Nephrology and Immunology, University of Alberta.
Robert E. Leduc, Division of Biostatistics, University of Minnesota.
Roslyn B. Mannon, Department of Nephrology, University of Alabama at Birmingham.
Arthur J. Matas, Department of Surgery, University of Minnesota.
Joseph P. Grande, Department of Laboratory Medicine & Pathology, Mayo Clinic.
REFERENCES
- 1.Feucht HE, Mihatsch MJ. Diagnostic value of C4d in renal biopsies. Current Opinion in Nephrology & Hypertension. 2005;14(6):592. doi: 10.1097/01.mnh.0000168943.54115.ac. [DOI] [PubMed] [Google Scholar]
- 2.Wang R, Wang H, Chen J, et al. C4d deposition in allograft renal biopsies is an independent risk factor for graft failure. Nephrology. 2009;14(5):527. doi: 10.1111/j.1440-1797.2009.01095.x. [DOI] [PubMed] [Google Scholar]
- 3.Herzenberg AM, Gill JS, Djurdjev O, Magil AB. C4d deposition in acute rejection: an independent long-term prognostic factor. Journal of the American Society of Nephrology. 2002;13(1):234. doi: 10.1681/ASN.V131234. [DOI] [PubMed] [Google Scholar]
- 4.Racusen LC, Colvin RB, Solez K, et al. Antibody-mediated rejection criteria - an addition to the Banff 97 classification of renal allograft rejection. American Journal of Transplantation. 2003;3(6):708. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 5.Mauiyyedi S, Pelle PD, Saidman S, et al. Chronic humoral rejection: identification of antibody-mediated chronic renal allograft rejection by C4d deposits in peritubular capillaries. J Am Soc Nephrol. 2001;12(3):574. doi: 10.1681/ASN.V123574. [DOI] [PubMed] [Google Scholar]
- 6.Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. American Journal of Transplantation. 2008;8(4):753. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 7.Ludovico-Martins H, Silva C, Teodoro WR, Martini Filho D, Noronha IL. Analysis of different staining techniques for c4d detection in renal allograft biopsies. Transplant Proc. 2009;41(3):862. doi: 10.1016/j.transproceed.2009.01.073. [DOI] [PubMed] [Google Scholar]
- 8.Troxell ML, Weintraub LA, Higgins JP, Kambham N. Comparison of C4d immunostaining methods in renal allograft biopsies. Clinical Journal of The American Society of Nephrology: CJASN. 2006;1(3):583. doi: 10.2215/CJN.00900805. [DOI] [PubMed] [Google Scholar]
- 9.Seemayer CA, Gaspert A, Nickeleit V, Mihatsch MJ. C4d staining of renal allograft biopsies: a comparative analysis of different staining techniques. Nephrology Dialysis Transplantation. 2007;22(2):568. doi: 10.1093/ndt/gfl594. [DOI] [PubMed] [Google Scholar]
- 10.Gourishankar S, Leduc R, Connett J, et al. Pathological and Clinical Characterization of the ‘Troubled Transplant’: Data from the DeKAF Study. Am J Transplant. 2010;10(2):324. doi: 10.1111/j.1600-6143.2009.02954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrell FE, Jr., Lee KL, Califf RM, Pryor DB, Rosati RA. Regression modelling strategies for improved prognostic prediction. Stat Med. 1984;3(2):143. doi: 10.1002/sim.4780030207. [DOI] [PubMed] [Google Scholar]
- 12.Crespo M, Pascual M, Tolkoff-Rubin N, et al. Acute humoral rejection in renal allograft recipients: I. Incidence, serology and clinical characteristics. Transplantation. 2001;71(5):652. doi: 10.1097/00007890-200103150-00013. [DOI] [PubMed] [Google Scholar]
- 13.Mauiyyedi S, Crespo M, Collins AB, et al. Acute humoral rejection in kidney transplantation: II. Morphology, immunopathology, and pathologic classification. J Am Soc Nephrol. 2002;13(3):779. doi: 10.1681/ASN.V133779. [DOI] [PubMed] [Google Scholar]
- 14.Poduval RD, Kadambi PV, Josephson MA, et al. Implications of immunohistochemical detection of C4d along peritubular capillaries in late acute renal allograft rejection. Transplantation. 2005;79(2):228. doi: 10.1097/01.tp.0000148987.13199.10. [DOI] [PubMed] [Google Scholar]
- 15.Banasik M, Boratynska M, Nowakowska B, et al. C4D deposition and positive posttransplant crossmatch are not necessarily markers of antibody-mediated rejection in renal allograft recipients. Transplantation Proceedings. 2007;39(9):2718. doi: 10.1016/j.transproceed.2007.08.064. [DOI] [PubMed] [Google Scholar]
- 16.Herzenberg AM, Gill JS, Djurdjev O, Magil AB. C4d deposition in acute rejection: an independent long-term prognostic factor. J Am Soc Nephrol. 2002;13(1):234. doi: 10.1681/ASN.V131234. [DOI] [PubMed] [Google Scholar]
- 17.Kawamura N, Tomita M, Hasegawa M, et al. Complement C4d deposition in transplanted kidneys: preliminary report on long-term graft survival. Clinical Transplantation. 2005;19(Suppl 14):27. doi: 10.1111/j.1399-0012.2005.00401.x. [DOI] [PubMed] [Google Scholar]
- 18.Ranjan P, Nada R, Jha V, Sakhuja V, Joshi K. The role of C4d immunostaining in the evaluation of the causes of renal allograft dysfunction. Nephrology Dialysis Transplantation. 2008;23(5):1735. doi: 10.1093/ndt/gfm843. [DOI] [PubMed] [Google Scholar]
- 19.Gibson IW, Gwinner W, Brocker V, et al. Peritubular capillaritis in renal allografts: prevalence, scoring system, reproducibility and clinicopathological correlates. American Journal of Transplantation. 2008;8(4):819. doi: 10.1111/j.1600-6143.2007.02137.x. [DOI] [PubMed] [Google Scholar]
- 20.Regele H, Bohmig GA, Habicht A, et al. Capillary deposition of complement split product C4d in renal allografts is associated with basement membrane injury in peritubular and glomerular capillaries: a contribution of humoral immunity to chronic allograft rejection. J Am Soc Nephrol. 2002;13(9):2371. doi: 10.1097/01.asn.0000025780.03790.0f. [DOI] [PubMed] [Google Scholar]
- 21.Vongwiwatana A, Gourishankar S, Campbell PM, Solez K, Halloran PF. Peritubular Capillary Changes and C4d Deposits Are Associated with Transplant Glomerulopathy But Not IgA Nephropathy. Am J Transplant. 2004;4(1):124. doi: 10.1046/j.1600-6143.2003.00294.x. [DOI] [PubMed] [Google Scholar]
- 22.Sis B, Campbell PM, Mueller T, et al. Transplant glomerulopathy, late antibody-mediated rejection and the ABCD tetrad in kidney allograft biopsies for cause. American Journal of Transplantation. 2007;7(7):1743. doi: 10.1111/j.1600-6143.2007.01836.x. [DOI] [PubMed] [Google Scholar]
- 23.Kieran N, Wang X, Perkins J, et al. Combination of peritubular c4d and transplant glomerulopathy predicts late renal allograft failure. Journal of the American Society of Nephrology. 2009;20(10):2260. doi: 10.1681/ASN.2009020199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimizu T, Ishida H, Shirakawa H, et al. Clinicopathological analysis of transplant glomerulopathy cases. Clinical Transplantation. 2009;23(Suppl 20):39. doi: 10.1111/j.1399-0012.2009.01008.x. [DOI] [PubMed] [Google Scholar]
- 25.David-Neto E, Prado E, Beutel A, et al. C4d-positive chronic rejection: a frequent entity with a poor outcome. Transplantation. 2007;84(11):1391. doi: 10.1097/01.tp.0000288807.52520.5e. [DOI] [PubMed] [Google Scholar]
- 26.Matas A, Leduc R, Rush D, et al. Histopathologic clusters differentiate subgroups within the nonspecific diagnoses of CAN or CR: Preliminary data from the DeKAF Study. Am J Transplant. 2010;10(2):315. doi: 10.1111/j.1600-6143.2009.02943.x. [DOI] [PubMed] [Google Scholar]
- 27.Fleiss J, Levin B, Cho Paik M. Statistical Methods for Rates and Proportions. 3 ed. John Wiley & Sons; Hoboken, NJ: 2003. [Google Scholar]
- 28.Mazumdar M, Smith A, Bacik J. Methods for categorizing a prognostic variable in a multivariable setting. Stat Med. 2003;22(4):559. doi: 10.1002/sim.1333. [DOI] [PubMed] [Google Scholar]
- 29.The R Development Core Team . R: A Language and Environment for Statistical Computing version 2.11.0. R Foundation for Statistical Computing; Vienna, Austria: 2010. http://cran.r-project.org/doc/manuals/refman.pdf. [Google Scholar]
- 30.Harrell FE., Jr. rms: Regression Modeling Strategies, R package verson 2.1-0. 2009 http://CRAN.R-project.org[mgn]package=rms.