Highlights
► Recovery from viral encephalomyelitis requires a noncytolytic process for clearance of virus from neurons. ► Noncytolytic clearance of virus from neurons is dependent on antiviral antibody and IFN-γ produced by cells infiltrating the CNS. ► After clearance of infectious virus, viral RNA is slowly cleared, but remains at low levels. ► Long-term presence of residual viral RNA requires local antibody-secreting cells to prevent virus reactivation.
Abstract
Viruses that cause encephalomyelitis infect neurons and recovery from infection requires noncytolytic clearance of virus from the nervous system to avoid damaging these irreplaceable cells. Several murine model systems of virus infection have been used to identify clearance mechanisms. Quantitative analysis of Sindbis virus clearance over 6 months shows three phases: day 5–7, clearance of infectious virus, but continued presence of viral RNA; day 8–60, decreasing levels of viral RNA; day 60–180, maintenance of viral RNA at low levels. Antiviral antibody and interferon-γ have major roles in clearance with a likely role for IgM as well as IgG antibody. Long-term residence of virus-specific immune cells in the nervous system is necessary to prevent virus reactivation.
Introduction
RNA viruses that cause encephalomyelitis, including many arthropod-borne viruses, infect neurons, terminally differentiated, irreplaceable cells essential for function of the nervous system. Recovery from viral encephalomyelitis requires immune-mediated virus clearance from the brain and spinal cord, a process that is organ and cell type-dependent [1, 2]. For virus infections of many organs (e.g. lung and gut), cytotoxic processes that eliminate infected cells are an efficient mechanism for virus clearance. The infected cells targeted for elimination by T cells can be replaced quickly with new uninfected cells of the same type. However, recovery from neuronal infection is more challenging for the immune system because preservation of neuronal function requires survival of the infected cells and a noncytolytic clearance process.
In identifying the in vivo mechanisms involved in recovery from viral encephalomyelitis, it is useful to consider the multiple aspects of the clearance process, the time frames for the development of innate and adaptive responses in relationship to the phases of virus clearance and the potential role(s) of different components of the immune response in each of these phases. Clearance of infectious virus is the first step and the aspect of clearance most often measured by investigators. However, if infected cells that are no longer producing virus are allowed to survive, intracellular viral RNA must also be eliminated for clearance to be complete. If it is not, a mechanism for prevention of reactivation of virus replication must be established.
Models of RNA virus CNS infection
We have employed Sindbis virus (SINV) infection of mice as a model system for understanding recovery from encephalomyelitis in relationship to virus clearance from neurons. SINV is an enveloped plus-strand RNA virus that is geographically widespread and transmitted by mosquitoes. SINV causes rash and arthritis in humans and encephalomyelitis in mice [3]. After intracerebral or intranasal inoculation of mice, SINV quickly spreads throughout the central nervous system (CNS) with virus replication mostly in neurons of the olfactory tract and hippocampus, and motor neurons of the brainstem and spinal cord [4, 5]. Amounts of infectious virus in brain and spinal cord peak 2–3 days after infection. Clearance of infectious virus is initiated 4–5 days after infection and is complete by 7–8 days (Figure 1 ). Other encephalitic viruses that primarily infect neurons include the flavivirus West Nile virus [6] and rabies virus [7].
Figure 1.
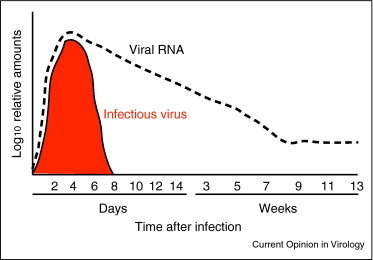
Schematic quantitative diagram of the phases of alphavirus clearance from the brain and spinal cord of mice. The period of detection of infectious virus by plaque assay is shaded red. The appearance, clearance and persistence of viral RNA as detected by quantitative RT-PCR is indicated by the dashed black line (Metcalf and Griffin, unpublished).
The coronavirus mouse hepatitis virus (MHV) and the picornavirus Theiler's murine encephalomyelitis virus (TMEV) provide other important mouse models for understanding the role of the immune response in viral encephalomyelitis. MHV and TMEV initially infect neurons to cause encephalomyelitis, but these viruses subsequently spread to glial cells and cause late demyelinating disease due to persistent virus infection [8, 9].
After clearance of infectious virus, virus-infected cells must be eliminated to completely clear infection. Mature neurons are relatively resistant to both virus-induced and immune-mediated cytolysis. This is beneficial to the host, because if the immune clearance mechanism is damaging to the infected neuron, then the function of that neuron will be lost and the outcome will be the same as if the virus infection had caused neuronal death. If infected cells are allowed to survive, there must be mechanisms for inhibiting intracellular synthesis of virus nucleic acid and protein, for eliminating virus genomes from cells and preventing their replacement after degradation. After SINV infection, neurons survive the clearance of infectious virus and mice recover from infection uneventfully. However, it takes many weeks for the levels of viral RNA in the CNS to decrease (Figure 1).
Because the clearance process is not complete, mechanisms for preventing resumption of virus replication need to be in place to avoid chronic, progressive or relapsing disease [10, 11]. Thus, mouse models of CNS infection offer the opportunity to identify the multiple mechanisms required for recovery from viral encephalomyelitis and prevention of chronic disease.
Innate immune responses: Locally produced type I interferon (IFN) is important for initial control of virus replication [12, 13, 14]. IFN-β is a particularly important type I IFN in the CNS and can be produced by virus-infected neurons [15]. IFN-β-deficient mice have 10-fold higher virus titers during the first 3 days after infection than nondeficient mice (Figure 3) despite the presence of high levels of IFN-α [13]. Other factors produced in the CNS during the innate response include TNF, IL-1, IL6, CCL2, CCL3, CCL5, CXCL9 and CXCL10 that induce glial cell activation, expression of adhesion molecules on endothelial cells and cell migration [13, 16, 17].
Figure 3.
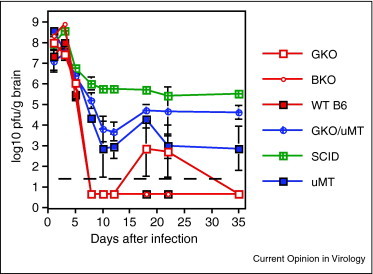
Effect of various immunodeficiencies on clearance of infectious virus from the brain after alphavirus infection of mice. SCID, severe combined immunodeficiency; BKO, IFN-β-deficient; μMT, antibody-deficient; GKO, interferon-γ-deficient; WT B6, wild type C57BL/6 [13].
Adaptive immune responses: The adaptive immune response is initiated in the draining cervical lymph nodes. Virus-specific CD4+ and CD8+ T cells rapidly expand, acquire effector functions and begin entering the circulation. The B cell response occurs in two phases: a rapid extrafollicular T cell-zone response and a slower follicular/germinal center B cell-zone response [18, 19]. Extrafollicular plasmablasts generated in the first phase proliferate extensively and rapidly produce low affinity antiviral antibody that is predominantly IgM [20, 21]. Germinal center B cells undergo class switch recombination, somatic hypermutation and selection resulting in plasmablasts and plasma cells that produce primarily high affinity IgG antibody. Both types of antibody-secreting cells enter the circulation and can home to sites of infection, including the CNS [22, 23].
Virus-specific T-lymphocytes and B-lymphocytes begin infiltrating the brain from the blood 3–4 days after infection. The earliest cells are CD8+ T cells followed by CD4+ T cells and then CD19+ B cells (Figure 2 ) and clearance of infectious virus begins (Figure 1). T cells are virus-specific and have effector phenotypes [8]. CD8+ T cells enter the parenchyma, have cytotoxic activity and produce IFN-γ while CD4+ T cells accumulate around vessels and produce several cytokines, including IFN-γ. The initial antibody-secreting B cells enter the CNS, produce IgM and are followed several days later by cells producing IgG and IgA [22, 24]. In the absence of an adaptive immune response (e.g. severe combined immunodeficiency [SCID] or Rag−/− mice), there is no clearance (Figure 3) and local production of IFN-α and IFN-β is sustained [4, 13, 25, 26]. Entry of mononuclear cells into the CNS is required for virus clearance and inhibition of CNS inflammation delays this process [27, 28].
Figure 2.
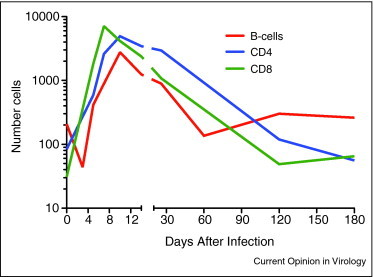
Quantitation of CD4+ T cells, CD8+ T cells and CD19+ B cells present at different times in the brain in response to alphavirus infection (Metcalf et al., unpublished).
Current understanding of virus clearance from neurons
In studies to determine the components of the adaptive immune response that effect infectious virus clearance from the CNS, antiviral antibody, CD8+ T cells and IFN-γ have been identified as important contributors to clearance from neurons [4, 8, 25].
Antibody: Studies using passive transfer of antibody to persistently infected SCID mice have shown that IgG antibody to the SINV E2 glycoprotein is effective in clearing infectious virus by a noncytolytic process from all types of neurons in the brain and spinal cord [25]. Antibody is also critical for clearance of rabies virus from neurons [29]. In vitro studies of the mechanism(s) of IgG-mediated clearance have shown that antibody binds to the surface of infected cells and blocks virus budding. Antibody interaction with infected cells produces a dose-dependent restoration of host protein synthesis, Na+K+ATPase pump function, membrane potential and response to IFN-α/β [26, 30, 31]. During the first hours after antibody treatment of infected cells, synthesis of the nonstructural proteins is prolonged and synthesis of viral RNA is increased before a subsequent decrease in viral RNA synthesis [30]. There is a requirement for bivalent antibody, but neither complement nor leukocytes are necessary for antibody-mediated clearance [25].
CD8 + T cells: CD8+ T cells that infiltrate the parenchyma are important for perforin-dependent clearance of WNV from neurons [32] and clearance of MHV from microglial cells, astrocytes and macrophages [8]. Although neurons express very little surface MHC class I protein, in vivo imaging has shown that CD8+ T cells can interact directly with TMEV-infected neurons, a process that may mediate clearance or damage [33•].
IFN-γ: IFN-γ is implicated in the clearance of measles virus, lymphocytic choriomeningitis virus and vesicular stomatitis virus from neurons [34, 35•]. Studies of B cell-deficient (μMT) mice infected with SINV showed that antibody is required for clearance of infectious virus from the brain, but not spinal cord neurons. Therefore, a mechanism for antibody-independent clearance exists for motor neurons, but not for hippocampal or cortical neurons [4, 13]. Depletion of either CD4+ or CD8+ T cells from μMT mice impairs virus clearance to a similar extent, suggesting overlapping functions, most likely through cytokine secretion. Using cytokine-expressing recombinant viruses, we showed that infectious SINV could be cleared by local production of IFN-γ, but not TNF-α [4].
In vitro studies showed that IFN-γ treatment of persistently infected differentiated neuronal cells has rapid and profound effects on infected cells [36]. Neuronal survival is improved and host protein synthesis is restored. Viral RNA synthesis shows an initial increase (3–6 h after treatment) and a decrease in the ratio of genomic to subgenomic RNA followed a few hours later by the termination of viral RNA synthesis. The suppression of virus replication is mediated by signaling through the Jak/STAT pathway [35•, 37•]. The specific intracellular effectors are not known, but generation of nitric oxide has been suggested [34].
Outstanding research questions
How is infectious virus cleared during primary infection in vivo?
Clearance of infectious virus from the CNS begins within 5 days after infection and is complete by day 7–8 (Figure 1). The first cells of the adaptive immune response to appear in the CNS are IFN-γ-producing CD8+ T cells followed by IFN-γ-producing CD4+ T cells and IgM-producing B cells (Figure 2). These three types of effector cells are present in the CNS during the clearance process, but the relative roles of these cells and subsets of these cells in in vivo clearance of infectious virus have not been defined.
Although IgG antibody to the E2 glycoprotein is effective in clearing infectious SINV from the CNS, production of virus-specific IgG does not begin until approximately 10 days after infection. The initiation of clearance 4–5 days after infection suggests an important in vivo role for SINV-specific IgM, rather than IgG. Studies of antibody-mediated protection from fatal encephalitis suggest that IgM MAbs are less protective than IgG MAbs with the same or similar specificity, but IgM from plasma crosses poorly into the brain due to blood–brain barrier size restrictions on protein entry. Therefore, lack of IgM efficacy compared to IgG when passively transferred could be due to the inefficiency of IgM entry into the CNS, rather than a lack of biologic activity. Studies with rabies virus infection have shown that infiltrating antibody-secreting cells are much more effective for virus clearance that serum antibody, so local antibody production by infiltrating IgM-producing extrafollicular B cell plasmablasts may be very efficacious [29].
Furthermore, several lines of evidence suggest that locally produced IgM may be sufficient for clearance of infectious SINV from the CNS. T cell-deficient athymic nu/nu mice produce virus-specific IgM, but not T cells, and little IgG, but are able to clear CNS virus with normal kinetics [24, 38]. Because bivalent antibody is required for suppression of SINV replication [26], it is possible that multivalent IgM antibody may be even more effective than IgG.
After infectious virus is cleared how is viral RNA decreased?
In immunologically normal mice, viral RNA levels gradually decrease over several weeks after infectious virus has been cleared (Figure 1). In SCID mice that have cleared virus in response to passively transferred antibody, virus replication is renewed in the brains of most mice once antibody levels have decayed [39]. Thus, viral RNA persists and the RNA that remains is capable of renewing virus production if there is a secondary failure of immune control. Therefore, immune-mediated decrease of viral RNA during the second phase of clearance is likely to be particularly important for prevention of renewed virus replication.
During this phase of infection there is a rapid contraction in the numbers of CD8+ T cells in the CNS while CD4+ T cell numbers are maintained and B cell numbers increase (Figure 2). Mice deficient in IFN-γ or in the response to IFN-γ often display transient reactivation of virus production 12–22 days after infection [13] (Figure 3 ), suggesting that IFN-γ is needed to continue to decrease levels of viral RNA. It is possible that CD8+ T cells provide early local IFN-γ production and that CD4+ T cells are essential for continued local production of IFN-γ and potentially for providing local help to maintain both CD8+ T cell function [40, 41] and antibody production by B cells [42]. Antibody produced in the CNS during this phase of clearance is high avidity IgG and IgA which may also regulate intracellular production of viral RNA.
After recovery, why are immune cells still maintained in the CNS?
Approximately 2 months after infection, viral RNA in the CNS has been decreased to a stable low level (Figure 1). CD8+ T cells expressing the integrin CD103 are maintained with IL-15 independent homeostatic proliferation in the CNS for prolonged periods of time after CNS infection [43, 44•]. B cells secreting virus-specific antibody are also present at stable low levels and local antibody synthesis continues within the infected CNS for many months [8, 26]. Evidence suggests that this antibody is essential for preventing recrudescence of virus replication [45, 46].
Long-term intrathecal production of virus-specific antibody characterizes the recovery phase of viral encephalitis in humans, as well as mice [47, 48, 49]. Further evidence of the importance of sustained suppression of virus replication in the CNS comes from clinical experience with the use of rituximab (anti-CD20) for elimination of B cells as a treatment for B cell lymphoma and autoimmune disease and of natalizumab (anti-VLA-4) for prevention of entry of inflammatory cells into the CNS. A major complication of these treatments has been reactivation of virus infection in the CNS [9, 50•, 51].
Conclusions
B cell production of antiviral antibody and T cell-production of IFN-γ within the infected nervous system are important for noncytolytic clearance of infectious virus and viral RNA and also for prevention of virus reactivation. However, many aspects of in vivo clearance are not understood. These include the role of IgM in early clearance of infectious virus, the mechanism by which production of viral RNA is suppressed in neurons no longer producing infectious virus and the environment in the brain that supports long-term residence of immune cells in regions of infection.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
Acknowledgements
Work from the authors’ laboratory was supported by research grant R01 NS038932 and training grant T32 AI007417 from the National Institutes of Health.
References
- 1.Matthews A.E., Weiss S.R., Shlomchik M.J., Hannum L.G., Gombold J.L., Paterson Y. Antibody is required for clearance of infectious murine hepatitis virus A59 from the central nervous system, but not the liver. J Immunol. 2001;167:5254–5263. doi: 10.4049/jimmunol.167.9.5254. [DOI] [PubMed] [Google Scholar]
- 2.Kundig T.M., Hengartner H., Zinkernagel R.M. T cell-dependent IFN-gamma exerts an antiviral effect in the central nervous system but not in peripheral solid organs. J Immunol. 1993;150:2316–2321. [PubMed] [Google Scholar]
- 3.Griffin D.E., Alphaviruses . In: Field's Virology. 5th ed. Knipe D.L., Howley P.M., Griffin D.E., Lamb R.A., Martin M.A., Roizman B., Straus S., editors. Lippincott Williams & Wilkins; 2007. pp. 1023–1067. [Google Scholar]
- 4.Binder G., Griffin D. Interferon-γ mediated site-specific clearance of alphavirus from CNS neurons. Science. 2001;293:303–306. doi: 10.1126/science.1059742. [DOI] [PubMed] [Google Scholar]
- 5.Tucker P.C., Strauss E.G., Kuhn R.J., Strauss J.H., Griffin D.E. Viral determinants of age-dependent virulence of Sindbis virus in mice. J Virol. 1993;67:4605–4610. doi: 10.1128/jvi.67.8.4605-4610.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diamond M.S., Mehlhop E., Oliphant T., Samuel M.A. The host immunologic response to West Nile encephalitis virus. Front Biosci. 2009;14:3024–3034. doi: 10.2741/3432. [DOI] [PubMed] [Google Scholar]
- 7.Lafon M. Immune evasion, a critical strategy for rabies virus. Dev Biol (Basel) 2008;131:413–419. [PubMed] [Google Scholar]
- 8.Bergmann C.C., Lane T.E., Stohlman S.A. Coronavirus infection of the central nervous system: host-virus stand-off. Nat Rev Microbiol. 2006;4:121–132. doi: 10.1038/nrmicro1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsunoda I., Fujinami R.S. Neuropathogenesis of Theiler's murine encephalomyelitis virus infection, an animal model for multiple sclerosis. J Neuroimmune Pharmacol. 2010;5:355–369. doi: 10.1007/s11481-009-9179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorries R. The role of T-cell-mediated mechanisms in virus infections of the nervous system. Curr Top Microbiol Immunol. 2001;253:219–245. doi: 10.1007/978-3-662-10356-2_11. [DOI] [PubMed] [Google Scholar]
- 11.Marten N.W., Stohlman S.A., Bergmann C.C. Role of viral persistence in retaining CD8(+) T cells within the central nervous system. J Virol. 2000;74:7903–7910. doi: 10.1128/jvi.74.17.7903-7910.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ireland D.D., Stohlman S.A., Hinton D.R., Atkinson R., Bergmann C.C. Type I interferons are essential in controlling neurotropic coronavirus infection irrespective of functional CD8 T cells. J Virol. 2008;82:300–310. doi: 10.1128/JVI.01794-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burdeinick-Kerr R., Wind J., Griffin D. The synergistic roles of antibody and interferon in noncytolytic clearance of Sindbis virus from different regions of the central nervous system. J Virol. 2007;81:5628–5636. doi: 10.1128/JVI.01152-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byrnes A.P., Durbin J.E., Griffin D.E. Control of Sindbis virus infection by antibody in interferon-deficient mice. J Virol. 2000;74:3905–3908. doi: 10.1128/jvi.74.8.3905-3908.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prehaud C., Megret F., Lafage M., Lafon M. Virus infection switches TLR-3-positive human neurons to become strong producers of beta interferon. J Virol. 2005;79:12893–12904. doi: 10.1128/JVI.79.20.12893-12904.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savarin C., Bergmann C.C. Neuroimmunology of central nervous system viral infections: the cells, molecules and mechanisms involved. Curr Opin Pharmacol. 2008;8:472–479. doi: 10.1016/j.coph.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein R.S., Diamond M.S. Immunological headgear: antiviral immune responses protect against neuroinvasive West Nile virus. Trends Mol Med. 2008;14:286–294. doi: 10.1016/j.molmed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 18.MacLennan I.C., Toellner K.M., Cunningham A.F., Serre K., Sze D.M., Zuniga E., Cook M.C., Vinuesa C.G. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 19.Acosta-Rodriguez E.V., Merino M.C., Montes C.L., Motran C.C., Gruppi A. Cytokines and chemokines shaping the B-cell compartment. Cytokine Growth Factor Rev. 2007;18:73–83. doi: 10.1016/j.cytogfr.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Chang W.L., Coro E.S., Rau F.C., Xiao Y., Erle D.J., Baumgarth N. Influenza virus infection causes global respiratory tract B cell response modulation via innate immune signals. J Immunol. 2007;178:1457–1467. doi: 10.4049/jimmunol.178.3.1457. [DOI] [PubMed] [Google Scholar]
- 21.Baumgarth N. A two-phase model of B-cell activation. Immunol Rev. 2000;176:171–180. doi: 10.1034/j.1600-065x.2000.00606.x. [DOI] [PubMed] [Google Scholar]
- 22.Tschen S.I., Stohlman S.A., Ramakrishna C., Hinton D.R., Atkinson R.D., Bergmann C.C. CNS viral infection diverts homing of antibody-secreting cells from lymphoid organs to the CNS. Eur J Immunol. 2006;36:603–612. doi: 10.1002/eji.200535123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blink E.J., Light A., Kallies A., Nutt S.L., Hodgkin P.D., Tarlinton D.M. Early appearance of germinal center-derived memory B cells and plasma cells in blood after primary immunization. J Exp Med. 2005;201:545–554. doi: 10.1084/jem.20042060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tyor W.R., Moench T.R., Griffin D.E. Characterization of the local and systemic B cell response of normal and athymic nude mice with Sindbis virus encephalitis. J Neuroimmunol. 1989;24:207–215. doi: 10.1016/0165-5728(89)90118-5. [DOI] [PubMed] [Google Scholar]
- 25.Levine B., Hardwick J.M., Trapp B.D., Crawford T.O., Bollinger R.C., Griffin D.E. Antibody-mediated clearance of alphavirus infection from neurons. Science. 1991;254:856–860. doi: 10.1126/science.1658936. [DOI] [PubMed] [Google Scholar]
- 26.Griffin D.E. Recovery from viral encephalomyelitis: immune-mediated noncytolytic virus clearance from neurons. Immunol Res. 2010;47:123–133. doi: 10.1007/s12026-009-8143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savarin C., Stohlman S.A., Atkinson R., Ransohoff R.M., Bergmann C.C. Monocytes regulate T cell migration through the glia limitans during acute viral encephalitis. J Virol. 2010;84:4878–4888. doi: 10.1128/JVI.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greene I.P., Lee E.-Y., Prow N.A., Ngwang B., Griffin D.E. Protection from fatal viral encephalomyelitis: AMPA receptor antagonists have a direct effect on the inflammatory response to infection. PNAS. 2008;105:3575–3580. doi: 10.1073/pnas.0712390105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hooper D.C., Phares T.W., Fabis M.J., Roy A. The production of antibody by invading B cells is required for the clearance of rabies virus from the central nervous system. PLoS Negl Trop Dis. 2009;3:e535. doi: 10.1371/journal.pntd.0000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Despres P., Griffin J.W., Griffin D.E. Effects of anti-E2 monoclonal antibody on Sindbis virus replication in AT3 cells expressing bcl-2. J Virol. 1995;69:7006–7014. doi: 10.1128/jvi.69.11.7006-7014.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Despres P., Griffin J.W., Griffin D.E. Antiviral activity of alpha interferon in Sindbis virus-infected cells is restored by anti-E2 monoclonal antibody treatment. J Virol. 1995;69:7345–7348. doi: 10.1128/jvi.69.11.7345-7348.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shrestha B., Samuel M.A., Diamond M.S. CD8+ T cells require perforin to clear West Nile virus from infected neurons. J Virol. 2006;80:119–129. doi: 10.1128/JVI.80.1.119-129.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33•.McDole J.R., Danzer S.C., Pun R.Y., Chen Y., Johnson H.L., Pirko I., Johnson A.J. Rapid formation of extended processes and engagement of Theiler's virus-infected neurons by CNS-infiltrating CD8 T cells. Am J Pathol. 2010;177:1823–1833. doi: 10.2353/ajpath.2010.100231. [DOI] [PMC free article] [PubMed] [Google Scholar]; Direct demonstration of the interaction of CD8+ T cells with virus-infected neurons in vivo.
- 34.Rottenberg M., Kristensson K. Effects of interferon-gamma on neuronal infections. Viral Immunol. 2002;15:247–260. doi: 10.1089/08828240260066206. [DOI] [PubMed] [Google Scholar]
- 35•.Stubblefield P., Sr., Widness M., Levine A.D., Patterson C.E. T cell-, interleukin-12-, and gamma interferon-driven viral clearance in measles virus-infected brain tissue. J Virol. 2011;85:3664–3676. doi: 10.1128/JVI.01496-10. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using brain slice cultures, the authors demonstrate IFN-γ-mediated virus clearance from neurons.
- 36.Burdeinick-Kerr R., Griffin D.E. Gamma interferon-dependent, noncytolytic clearance of Sindbis virus infection from neurons in vitro. J Virol. 2005;79:5374–5385. doi: 10.1128/JVI.79.9.5374-5385.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37•.Burdeinick-Kerr R., Govindarajan D., Griffin D.E. Noncytolytic clearance of Sindbis virus infection from neurons by gamma interferon is dependent on Jak/STAT signaling. J Virol. 2009;83:3429–3435. doi: 10.1128/JVI.02381-08. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstration that the antiviral response of neurons to IFN-γ is dependent on the Jak/STAT signaling pathway.
- 38.Hirsch R.L., Griffin D.E. The pathogenesis of Sindbis virus infection in athymic nude mice. J Immunol. 1979;123:1215–1218. [PubMed] [Google Scholar]
- 39.Levine B., Griffin D.E. Persistence of viral RNA in mouse brains after recovery from acute alphavirus encephalitis. J Virol. 1992;66:6429–6435. doi: 10.1128/jvi.66.11.6429-6435.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bevan M.J. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 41.Zhou J., Hinton D.R., Stohlman S.A., Liu C.P., Zhong L., Marten N.W. Maintenance of CD8+ T cells during acute viral infection of the central nervous system requires CD4+ T cells but not interleukin-2. Viral Immunol. 2005;18:162–169. doi: 10.1089/vim.2005.18.162. [DOI] [PubMed] [Google Scholar]
- 42.Ozaki K., Spolski R., Feng C.G., Oi C.F., Cheng J., Sher A., Morse H.C., Liu C., Schwartzberg P.L., Leonard W.J. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–1634. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 43.Zuo J., Stohlman S.A., Parra G.I., Bergmann C.C. IL-15 independent maintenance of virus-specific CD8(+) T cells in the CNS during chronic infection. J Neuroimmunol. 2009;207:32–38. doi: 10.1016/j.jneuroim.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Wakim L.M., Woodward-Davis A., Bevan M.J. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci USA. 2010;107:17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]; Identification of homing receptors on CD8+ T cells that are required for maintenance in the brain after clearance of infectious virus.
- 45.Ramakrishna C., Stohlman S.A., Atkinson R.D., Shlomchik M.J., Bergmann C.C. Mechanisms of central nervous system viral persistence: the critical role of antibody and B cells. J Immunol. 2002;168:1204–1211. doi: 10.4049/jimmunol.168.3.1204. [DOI] [PubMed] [Google Scholar]
- 46.Ramakrishna C., Bergmann C.C., Atkinson R., Stohlman S.A. Control of central nervous system viral persistence by neutralizing antibody. J Virol. 2003;77:4670–4678. doi: 10.1128/JVI.77.8.4670-4678.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gunther G., Haglund M., Lindquist L., Skoldenberg B., Forsgren M. Intrathecal IgM, IgA and IgG antibody response in tick-borne encephalitis. Long-term follow-up related to clinical course and outcome. Clin Diagn Virol. 1997;8:17–29. doi: 10.1016/s0928-0197(97)00273-0. [DOI] [PubMed] [Google Scholar]
- 48.Fryden A., Link H., Norrby E. Cerebrospinal fluid and serum immunoglobulins and antibody titers in mumps meningitis and aseptic meningitis of other etiology. Infect Immun. 1978;21:852–861. doi: 10.1128/iai.21.3.852-861.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burke D.S., Nisalak A., Ussery M.A., Laorakpongse T., Clantavibul S. Kinetics of Japanese encephalitis virus immunoglobulin M and G antibodies in human serum and cerebrospinal fluid. J Infect Dis. 1985;151:1093–1099. doi: 10.1093/infdis/151.6.1093. [DOI] [PubMed] [Google Scholar]
- 50•.Carson K.R., Evens A.M., Richey E.A., Haberman T.M., Focosi D., Seymour J.F., Lauback J., Bawn S.C., Gordon L.I., Winter J.N. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009;113:4834–4840. doi: 10.1182/blood-2008-10-186999. [DOI] [PMC free article] [PubMed] [Google Scholar]; The complications of treatment with anti-CD20 antibody provide evidence that B cells are important to prevent reactivation of virus in the central nervous system.
- 51.Kiani-Alikhan S., Skoulidis F., Barroso A., Nuovo G., Ushiro-Lumb I., Breuer J., Lister A., Mattes F. Enterovirus infection of neuronal cells post-Rituximab. Br J Haematol. 2009;146:333–335. doi: 10.1111/j.1365-2141.2009.07748.x. [DOI] [PubMed] [Google Scholar]


