Abstract
It is now clear that CD4+ T cells play a crucial role in the generation of CD8+ T effector and memory T-cell immune responses. In this study, we enhanced the CD4+ T-cell immune responses in mice by constructing a DNA vaccine encoding an invariant (Ii) chain in which the class II–associated Ii peptide (CLIP) region is replaced with a CD4+ T-helper epitope, PADRE (Ii-PADRE) (invariant Pan HLA-DR reactive epitope). C57BL/6 mice vaccinated with DNA encoding Ii-PADRE showed significantly greater PADRE-specific CD4+ T-cell immune responses than mice vaccinated with DNA encoding the Ii chain alone (Ii DNA). More important, administration of DNA encoding human papillomavirus (HPV) E6 or E7 antigen with DNA encoding Ii-PADRE led to significantly stronger E6- or E7-specific CD8+ T-cell immune responses and more potent protective and therapeutic anti-tumor effects against an E6/E7-expressing tumor model in mice than administration of E6 or E7 DNA with Ii DNA. Overall, our data indicate that administration of DNA vaccines with Ii-PADRE DNA represents an effective approach to enhancing the generation of CD4+ T cells and eliciting stronger antigen-specific CD8+ T-cell immune responses. Therefore, this strategy may be expected to have significant potential for clinical translation.
INTRODUCTION
DNA vaccines have emerged as a potentially important form of antigen-specific immunotherapy because of their safety, ease of production, and stability. Intradermal (i.d.) administration of DNA vaccines using a gene gun represents an efficient means of delivering DNA directly into dendritic cells, the most potent professional antigen-presenting cells (APCs). The DNA-expressing dendritic cells mature and migrate to the draining lymph nodes, where they prime helper and killer T cells in vivo.1,2 We have previously used this system to modify the properties of dendritic cells to enhance DNA vaccine potency (for a review, see ref. 3).
It is now clear that CD4+ T cells play a crucial role in the generation of CD8+ T effector and memory T-cell populations.4 CD4+ T cells at tumor sites can also interact with natural killer cells and macrophages to enhance tumor destruction.5,6 Thus, it is desirable to design an immunization regimen that is capable of generating antigen-specific CD4+ T cells, and for this task it is important to understand the mechanisms of antigen presentation to the CD4+ T cells through the major histocompatibility complex (MHC) class II pathway.
CD4+ T cells recognize antigens in the context of MHC class II molecules. In general, exogenous antigens are taken up by APCs through phagocytosis or endocytosis and are degraded into antigenic peptides by acid proteases in low-pH endosomal or lysosome-like compartments.7–10 The antigenic peptides later associate with the MHC class II molecules on the surface of the APCs for recognition by the CD4+ T cells. An essential component of this MHC class II–mediated antigen presentation is the invariant (Ii) chain molecule. In the endoplasmic reticulum, MHC class II molecules assemble and then bind with the Ii chain. The class II–associated Ii peptide (CLIP) region of the Ii chain occupies the MHC class II peptide-binding grove. The Ii chain is then degraded until only the CLIP region remains; this region prevents premature binding of the antigenic peptide into the MHC class II peptide-binding groove. In the lysosomes, CLIP is later replaced by one of the antigenic peptides.
Previous studies have demonstrated that transfection of MHC class II–positive cells with DNA encoding an Ii chain in which CLIP is replaced with a CD4+ T-helper epitope of an antigen of interest can lead to the presentation CD4+ T-cell epitope through the MHC class II pathway.11–18 We reasoned that the replacement of the CLIP region of the Ii chain with a high-affinity and “promiscuous” CD4+ T-cell epitope such as the Pan HLA-DR reactive epitope (PADRE)19 may lead to the stable presentation of PADRE through MHC class II molecules. We also hypothesized that immunization with DNA vaccines encoding an Ii chain in which the CLIP region is replaced with PADRE (Ii-PADRE DNA) may lead to the generation of PADRE-specific CD4+ T-cell immune responses in mice.
In the current study, we created a DNA vaccine encoding an Ii chain in which the CLIP region is replaced with PADRE (Ii-PADRE DNA). Vaccination of C57BL/6 mice with Ii-PADRE DNA increased the number of PADRE-specific CD4+ T cells in the immunized mice. In addition, administration of Ii-PADRE DNA with DNA vaccines containing either the E6 or E7 protein of human papillomavirus type 16 (HPV-16) led to enhanced HPV antigen-specific CD8+ T-cell immune responses and potent protective and therapeutic anti-tumor effects against an E6/E7-expressing tumor model, TC-1, in mice. These findings have clinical implications for enhancing the potency of DNA vaccines and for improving antigen-specific immune responses in many antigenic systems.
RESULTS
Vaccination with Ii-PADRE DNA
We constructed a DNA vaccine encoding an Ii chain in which the CLIP sequence (amino acids (aa) 81–102, KPVSQMRMATPLLMRPM) was replaced with the PADRE sequence (AKFVAAWTLKAAA) to form Ii-PADRE DNA. Figure 1a and b are schematic diagrams of the Ii chain protein and Ii-PADRE chimeric protein. Figure 1c is a schematic diagram of a typical MHC class II molecule associated with the Ii chain. The CLIP region of the Ii chain occupies the peptide-binding site and is eventually replaced by an antigenic peptide in the endosomal/lysosomal compartments. Figure 1d is a schematic diagram of an MHC class II molecule associated with the Ii-PADRE chimeric protein. The PADRE peptide remains attached to the peptide-binding site of the MHC class II molecule.
Figure 1. Schematic diagrams of the invariant (Ii) chain and the chimeric Ii–Pan HLA-DR reactive epitope (PADRE).
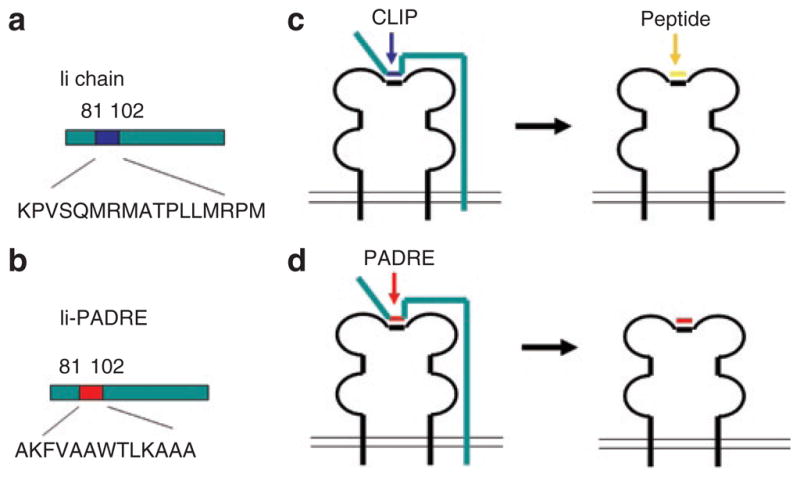
(a) Diagram of the Ii chain. The blue region indicates the location of the class II–associated Ii peptide (CLIP) (aa81–102). (b) Diagram of the Ii-PADRE chimeric protein. The red region indicates the location of PADRE, which replaces the CLIP region of the Ii chain. (c) Diagram of a typical major histocompatibility complex (MHC) class II molecule associated with the Ii chain. The CLIP region of the Ii chain occupies the peptide-binding site and is eventually replaced by an antigenic peptide in the endosomal/lysosomal compartments. (d) Diagram of an MHC class II molecule associated with the Ii-PADRE chimeric protein. The PADRE peptide remains attached to the peptide-binding site of the MHC class II molecule.
To determine whether Ii-PADRE DNA can generate PADRE-specific CD4+ T cells in vaccinated mice, we immunized C57BL/6 mice with Ii DNA or Ii-PADRE DNA i.d. using a gene gun. Splenocytes from vaccinated mice were harvested, stimulated with PADRE peptide, and characterized for the presence of PADRE-specific CD4+ T cells by intracellular cytokine staining for interferon-γ (IFN-γ) and staining for CD4+ followed by flow cytometry analysis (see Supplementary Figure S1). Mice vaccinated with Ii-PADRE DNA generated a significantly higher number of PADRE-specific CD4+ T cells than mice vaccinated with wild-type Ii DNA. These data suggest that the replacement of the CLIP region with the PADRE peptide sequence in the Ii chain DNA vaccine could lead to the presentation of PADRE through the MHC class II pathway to activate PADRE-specific CD4+ T cells in vaccinated mice.
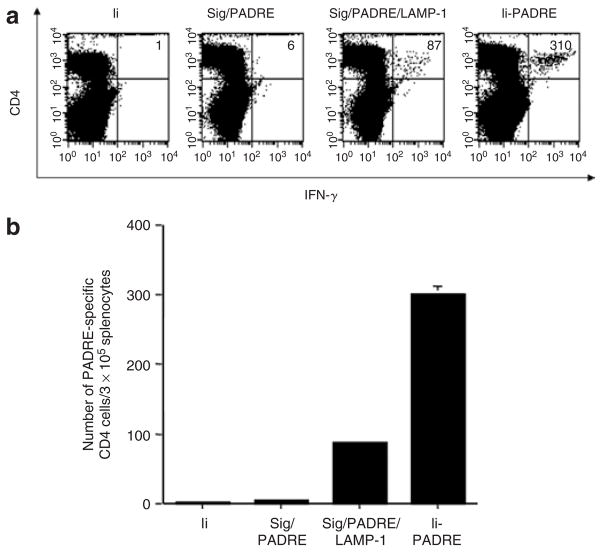
We next compared the various PADRE-containing DNA vaccines for their ability to generate PADRE-specific CD4+ T cells in vaccinated mice. These DNA vaccines included Sig/PADRE, Sig/PADRE/LAMP-1, and Ii-PADRE. We have previously shown that linkage of an antigen to the sorting signal of the lysosome-associated membrane protein type I (LAMP-1) can enhance presentation of the linked antigen to the MHC class II–restricted antigen-specific CD4+ T cells.20 In this study, we created a DNA vaccine encoding a chimeric protein linking the signal peptide, PADRE antigen, and LAMP-1 (Sig/PADRE/LAMP-1). In addition, we created a DNA vaccine encoding a signal peptide linked to PADRE (Sig/PADRE) for comparison. Furthermore, Ii DNA was used as a negative control. As shown in Figure 2, vaccination with Ii-PADRE DNA generated the most potent PADRE-specific CD4+ T-cell immune responses in mice among all the DNA vaccines tested. Our data indicate that vaccination with Ii-PADRE DNA represents an effective approach to enhancing PADRE-specific CD4+ T-cell immune responses in mice.
Figure 2. Flow cytometry analysis of interferon-γ (IFN-γ)-secreting CD4+ T cells in vaccinated mice.
C57BL/6 mice (five per group) were immunized twice with 2 μg per mouse of Ii DNA, Sig/PADRE DNA, Sig/PADRE/LAMP-1 DNA, or Ii-PADRE DNA, with a 1-week interval. Splenocytes from vaccinated mice were harvested 1 week after the second vaccination and stimulated overnight with the Pan HLA-DR reactive epitope (PADRE) peptide. (a) Representative figure of the flow cytometry data. The numbers in the upper right corner represent the number of IFN-γ-secreting CD4+ T cells per 3 × 105 splenocytes acquired. (b) Bar graph depicting the number of PADRE-specific CD4+ T cells per 3 × 105 splenocytes (means ± SE). The data presented in this figure are from one representative experiment of two performed.
Administration of Ii-PADRE DNA with SCT-E6 DNA i.d. using a gene gun generated both E6-specific CD8+ T cells and PADRE-specific CD4+ T cells in vaccinated mice
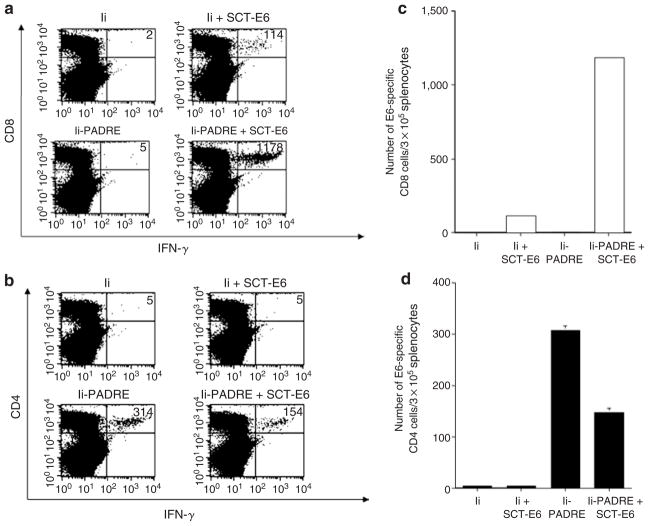
We have previously constructed a DNA vaccine encoding a single-chain trimer (SCT) of β2-microglobulin, the MHC class I heavy chain, and the immunodominant cytotoxic T-lymphocyte (CTL) epitope of HPV-16 E6 antigen (SCT-E6 DNA). C57BL/6 mice vaccinated with SCT-E6 DNA exhibited significantly increased E6 peptide–specific CD8+ T-cell immune responses and more potent anti-tumor effects against E6-expressing tumors than mice vaccinated with DNA encoding wild-type E6.21 Although vaccination with the SCT-E6 DNA could enhance E6-specific CD8+ T-cell immune responses, it could not generate antigen-specific CD4+ T-cell immune responses. It is now clear that CD4+ T cells are important for facilitating the activation of antigen-specific CD8+ T cells and the generation of long-term memory T cells. Having shown that vaccination with the Ii-PADRE DNA could generate a significantly increased number of PADRE-specific CD4+ T cells (see Supplementary Figure S1), we next explored whether administration of the Ii-PADRE DNA with the SCT-E6 DNA could enhance E6-specific CD8+ T-cell immune responses in vaccinated mice. We immunized mice with Ii-PADRE DNA plus SCT-E6 DNA or Ii DNA plus SCT-E6 DNA. In addition, we vaccinated mice with Ii-PADRE DNA or Ii DNA alone as controls. Splenocytes from vaccinated mice were harvested, stimulated with either E6 or PADRE peptide, and characterized for the presence of E6-specific CD8+ T cells or PADRE-specific CD4+ T cells by intracellular IFN-γ staining followed by flow cytometry analysis. As shown in Figure 3a and c, administration of Ii-PADRE DNA plus SCT-E6 DNA i.d. using a gene gun significantly enhanced E6-specific CD8+ T-cell immune responses compared with vaccination with Ii DNA plus SCT-E6. Vaccination with Ii DNA or Ii-PADRE DNA alone failed to generate E6-specific CD8+ T cells in immunized mice. In addition, vaccination with Ii-PADRE DNA alone or together with SCT-E6 DNA was able to generate PADRE-specific CD4+ T cells in immunized mice, whereas immunization with Ii DNA alone or in conjunction with SCT-E6 DNA failed to generate an appreciable number of PADRE-specific CD4+ T cells (Figure 3b and d). These data indicate that administration of the Ii-PADRE DNA with the SCT-E6 DNA can enhance the generation of E6-specific CD8+ T cells by SCT-E6 DNA vaccines.
Figure 3. Intracellular cytokine staining followed by flow cytometry analysis to determine the number of E6-specific CD8+ T cells and Pan HLA-DR reactive epitope (PADRE)–specific CD4+ T cells in vaccinated mice.
C57BL/6 mice (five per group) were immunized twice intradermally using a gene gun with 2 μg per mouse of Ii DNA, Ii DNA plus SCT-E6 DNA, Ii-PADRE DNA, or Ii-PADRE DNA plus SCT-E6 DNA, with a 1-week interval. Splenocytes from vaccinated mice were harvested 1 week after the second vaccination and stimulated with E6 or PADRE peptide. Splenocytes without peptide stimulation were used as a negative control. The splenocytes were stained for CD8 or CD4 and intracellular interferon-γ (IFN-γ). (a, b) Representative figures of the flow cytometry data. The numbers in the upper right corner represent the number of (a) E6-specific CD8+ T cells or (b) PADRE-specific CD4+ T cells per 3 × 105 splenocytes acquired. (c, d) Bar graph depicting the numbers of (a) E6-specific CD8+ T-cells or (d) PADRE-specific CD4+ T cells per 3 × 105 splenocytes (means ± SE). The data presented in this figure are from one representative experiment of two performed.
Vaccination with Ii-PADRE DNA plus SCT-E6 DNA
To determine whether the observed increase in the number of E6-specific CD8+ T cells generated by vaccination with Ii-PADRE DNA plus SCT-E6 DNA can be translated into better anti-tumor effects, we performed an in vivo protection experiment using a previously characterized E6-expressing tumor model, TC-1.7 As shown in Figure 4, 100% of mice vaccinated with Ii-PADRE DNA plus SCT-E6 DNA remained tumor-free 63 days after subcutaneous (s.c.) challenge with TC-1 tumor cells. In contrast, only 60% of mice vaccinated with Ii DNA plus SCT-E6 DNA remained tumor-free 63 days after TC-1 tumor challenge, and all of the mice immunized with Ii DNA or Ii-PADRE DNA developed tumors within 14 days of TC-1 tumor challenge.
Figure 4. In vivo tumor protection experiments.
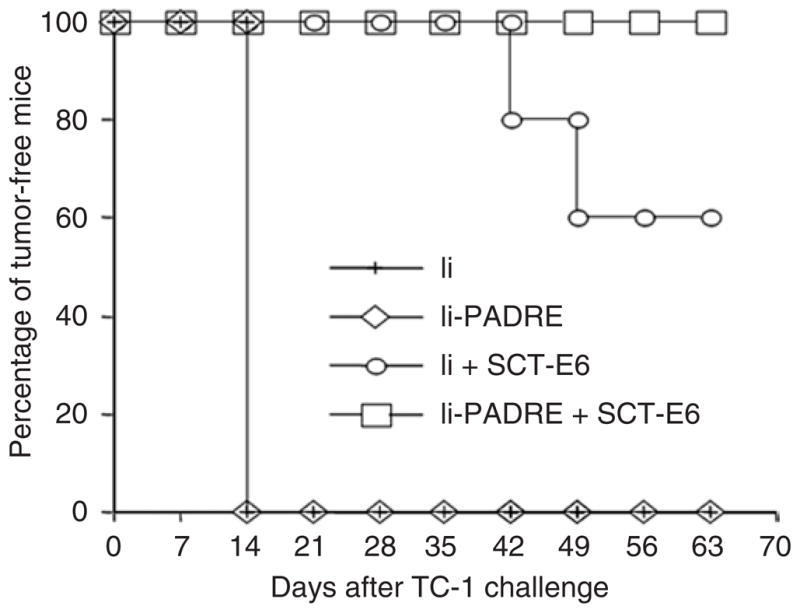
C57BL/6 mice (five per group) were immunized twice using a gene gun with 2 μg per mouse of Ii DNA, Ii–Pan HLA-DR reactive epitope (PADRE) DNA, Ii DNA plus SCT-E6 DNA, or Ii-PADRE DNA plus SCT-E6 DNA, with a 1-week interval. One week after the second vaccination, the vaccinated mice were challenged subcutaneously with 5 × 104 TC-1 cells per mouse. The mice were monitored for evidence of tumor growth by inspection and palpation twice a week. The data shown here are from one representative experiment of two performed.
We further assessed the therapeutic potential of each vaccine by performing an in vivo tumor treatment experiment using a s.c. TC-1 tumor challenge model. Mice were challenged with TC-1 tumor cells and then treated with the various DNA vaccines 3 days later. Whereas 60% of the mice treated with Ii-PADRE DNA plus SCT-E6 DNA remained tumor-free 42 days after TC-1 tumor challenge, all of the mice treated with Ii DNA, Ii-PADRE DNA, or Ii DNA plus SCT-E6 DNA exhibited tumor growth 14 days after the tumor challenge (see Supplementary Figure S2). These data indicate that administration of Ii-PADRE DNA with SCT-E6 DNA can elicit potent anti-tumor effects against challenge with an E6-expressing tumor cell line.
Vaccination with Ii-PADRE DNA plus SCT-TRP2 DNA
We explored whether the Ii-PADRE DNA vaccine could enhance the generation of antigen-specific CD8+ T-cell immune responses in mice vaccinated with DNA vaccine employing an SCT technology targeting other tumor antigenic peptides. Tyrosine-related protein 2 (TRP2) has been shown to be a tumor-associated antigen that is highly expressed in murine melanomas such as B-16. Furthermore, the immunodominant epitope has been shown to be located at aa181–188, VYDFFVWL. We therefore created a DNA vaccine encoding an SCT linking β2-microglobulin, the MHC class I heavy chain, and the immunodominant CTL epitope of TRP2 antigen (SCT-TRP2). To determine whether the administation of Ii-PADRE DNA with SCT-TRP2 DNA i.d. using a gene gun could enhance TRP2-specific CD8+ T cell–mediated immune responses, C57BL/6 mice were vaccinated with either Ii DNA plus SCT-TRP2 DNA or Ii-PADRE DNA plus SCT-TRP2 DNA. We then characterized the presence of TRP2-specific CD8+ T-cell precursors using splenocytes from vaccinated mice by flow cytometry analysis. Administration of Ii-PADRE DNA plus SCTTRP2 DNA generated a significantly higher frequency of TRP2-specific CD8+ T-cell precursors than the administration of Ii DNA plus SCT-TRP2 DNA (see Supplementary Figure S3, P < 0.01). These data indicate that administration of the Ii-PADRE DNA with the SCT DNA can also enhance antigen-specific CD8+ T-cell immune responses in other antigenic systems.
Administration of Ii-PADRE DNA with CRT-E7 DNA significantly enhanced E7-specific CD8+ T cell–mediated immune responses
We have previously shown that vaccination with DNA encoding Calreticulin (CRT) linked to E7 antigen (CRT-E7) i.d. using a gene gun could significantly enhance E7-specific CD8+ T cells in mice compared with vaccination with wild-type E7 DNA.22 To determine whether administration of Ii-PADRE DNA with CRT-E7 DNA could enhance E7-specific CD8+ T cells, we vaccinated mice with Ii-PADRE DNA plus CRT-E7 DNA or Ii DNA plus CRT-E7 DNA. As shown in Figure 5a and b, gene gun administration of Ii-PADRE DNA plus CRT-E7 DNA generated a higher number of E7-specific CD8+ T cells in mice than vaccination with Ii DNA plus CRT-E7 DNA (P < 0.01). Vaccination with Ii-PADRE DNA plus CRT-E7 DNA also generated a significantly increased number of PADRE-specific CD4+ T cells in mice (Figure 5c and d). Our data indicate that antigen-specific CD8+ T cells generated by DNA vaccines employing an intracellular targeting strategy can be enhanced by administration with the Ii-PADRE DNA.
Figure 5. Characterization of E7-specific interferon-γ (IFN-γ)-secreting CD8+ T cells and Pan HLA-DR reactive epitope (PADRE)-specific CD4+ T cells by flow cytometry analysis in vaccinated mice.
C57BL/6 mice (five per group) were immunized twice intradermally using a gene gun with 2 μg per mouse of Ii DNA plus CRT-E7 DNA or Ii-PADRE DNA plus CRT-E7 DNA, with a 1-week interval. Splenocytes from vaccinated mice were harvested 1 week after the second vaccination and stimulated with E7 peptide or PADRE peptide. Splenocytes without peptide stimulation were used as a negative control. The splenocytes were stained for both CD8 and intracellular IFN-γ. (a, b) Representative figures of the flow cytometry data. The numbers in the upper right corner represent the number of (a) E7-specific IFN-γ-secreting CD8+ T cells or (b) PADRE-specific CD4+ T cells per 3 × 105 splenocytes acquired. (c, d) Bar graphs depicting the number of (c) E7-specific T-cells or (d) PADRE-specific CD4+ T cells per 3 × 105 splenocytes (means ± SE). The data presented in this figure are from one representative experiment of two performed.
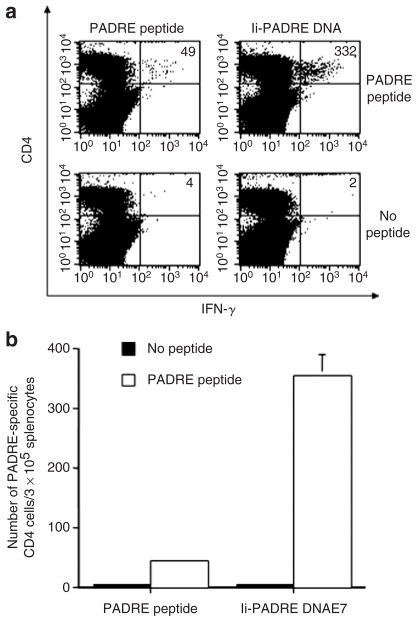
Vaccination with Ii-PADRE DNA generates more PADRE-specific CD4+ T cells than vaccination with PADRE peptide
To determine whether vaccination with Ii-PADRE DNA could elicit better PADRE-specific CD4+ T-cell immune responses in vaccinated mice than vaccination with PADRE peptide, we vaccinated C57BL/6 mice with Ii-PADRE DNA i.d. using a gene gun or with PADRE peptide mixed with incomplete Freund’s adjuvant s.c. Splenocytes from vaccinated mice were isolated and characterized for the presence of PADRE-specific CD4+ T-cell precursors by intracellular cytokine staining with flow cytometry analysis. As shown in Figure 6, vaccination with Ii-PADRE DNA generated a significantly higher number of PADRE-specific CD4+ T cells than immunization with a PADRE peptide.
Figure 6. Flow cytometry analysis to characterize Pan HLA-DR reactive epitope (PADRE)-specific CD4+ T cells in mice vaccinated with PADRE peptide or Ii-PADRE DNA.
C57BL/6 mice (five per group) were immunized twice with 100 μg per mouse of PADRE peptide subcutaneously or 2 μg per mouse of Ii-PADRE DNA intradermally, with a 1-week interval. Splenocytes from vaccinated mice were harvested 1 week after the second vaccination and stimulated with PADRE peptide. Splenocytes without peptide stimulation were used as a negative control. The splenocytes were stained for both CD4 and intracellular interferon-γ (IFN-γ). (a) Representative figure of the flow cytometry data. The numbers in the upper right corner represent the number of PADRE-specific CD4+ T cells per 3 × 105 splenocytes acquired. (b) Bar graph depicting the number of PADRE-specific CD4+ T cells per 3 × 105 splenocytes (means ± SE). The data presented in this figure are from one representative experiment of two performed.
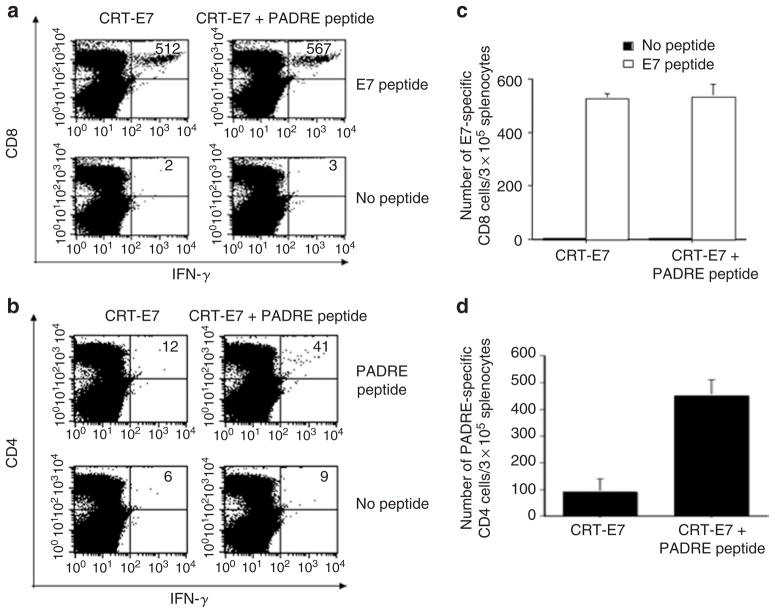
Administration of PADRE peptide with CRT-E7 DNA failed to enhance E7-specific CD8+ T-cell immune responses
We also assessed whether the E7-specific CD8+ T cells generated by vaccination with CRT-E7 DNA i.d. can be enhanced by PADRE peptide mixed with incomplete Freund’s adjuvant administered s.c. As shown in Figure 7a and b, mice vaccinated with CRT-E7 DNA in conjunction with PADRE peptide (mixed with incomplete Freund’s adjuvant) failed to generate a significantly higher number of E7-specific CD8+ T cells than mice vaccinated with CRT-E7 DNA in conjunction with incomplete Freund’s adjuvant. However, the combination of CRT-E7 DNA and PADRE peptide led to an increase of PADRE-specific CD4+ T cells compared with vaccination with CRT-E7 DNA with incomplete Freund’s adjuvant (Figure 7c and d).
Figure 7. Characterization of E7-specific interferon-γ (IFN-γ)-secreting CD8+ T cells and Pan HLA-DR reactive epitope (PADRE)-specific CD4+ T cells in mice vaccinated with PADRE peptide and CRT-E7.
C57BL/6 mice (five per group) were immunized twice with 2 μg per mouse of CRT-E7 DNA intradermally via gene gun and 100 μg per mouse of PADRE in 200 μl incomplete Freund’s adjuvant by subcutaneous tail-base injection, with a 1-week interval. Mice vaccinated with 2 μg CRT-E7 DNA using a gene gun and 200 μl incomplete Freund’s adjuvant by subcutaneous tail-base injection were used as a negative control. Splenocytes were harvested 1 week after the second vaccination and stimulated with E7 peptide or PADRE peptide. Splenocytes without peptide stimulation were used as a negative control. The splenocytes were stained for both CD8 and intracellular IFN-γ. (a, b) Representative figures of the flow cytometry data. The numbers in the upper right corner represent the numbers of (a) E7-specific IFN-γ-secreting CD8+ T cells or (b) PADRE-specific CD4+ T cells per 3 × 105 splenocytes acquired. (c, d) Bar graphs depicting the numbers of (c) E7-specific CD8+ T cells or (d) PADRE-specific CD4+ T cells per 3 × 105 splenocytes (means ± SE).
Administration of DNA vaccines with Ii-PADRE DNA is more effective than administration with PADRE peptide in enhancing antigen-specific CD8+ T cells
We next determined whether C57BL/6 mice vaccinated with CRT-E7 DNA plus Ii-PADRE DNA could generate better E7-specific CD8+ T-cell immune responses than mice immunized with CRT-E7 DNA plus PADRE peptide. The mice were immunized twice with CRT-E7 DNA (i.d.) plus Ii DNA (i.d.), CRT-E7 DNA (i.d.) plus Ii-PADRE DNA (i.d.), CRT-E7 DNA (i.d.) plus adjuvant (s.c.), or CRT-E7 DNA (i.d.) plus PADRE peptide (s.c.), with a 1-week interval. Splenocytes from immunized mice were harvested 1 week after the second vaccination and stimulated with E7 peptide. The splenocytes were characterized for the presence of E7-specific CD8+ T cells using intracellular staining for IFN-γ and staining for CD8 followed by flow cytometry analysis. As shown in Figure 8, mice vaccinated with CRT-E7 DNA plus Ii-PADRE DNA generated a significantly higher number of E7-specific CD8+ T cells than mice immunized with CRT-E7 DNA plus PADRE peptide. CRT-E7 DNA administered with PADRE peptide generated similar levels of E7-specific CD8+ T cells compared to administration with adjuvant alone or DNA encoding the Ii chain. These data indicate that DNA vaccines in conjunction with Ii-PADRE DNA are more effective in enhancing antigen-specific CD8+ T cells than the combination of DNA vaccines with PADRE peptide.
Figure 8. Flow cytometry analysis to characterize E7-specific CD8+ T cells in vaccinated mice.
C57BL/6 mice (five per group) were immunized twice with CRT-E7 DNA (intradermally, i.d.) plus Ii DNA (i.d.), CRTE7 DNA (i.d.) plus Ii-PADRE DNA (i.d.), CRT-E7 DNA (i.d.) plus adjuvant (subcutaneously, s.c.), or CRT-E7 DNA (i.d.) plus PADRE peptide (s.c.), with a 1-week interval. Splenocytes were harvested 1 week after the second vaccination and stimulated with E7 peptide. The splenocytes were stained for both CD8 and intracellular IFN-γ. The bar graph depicts the number of E7-specific CD8+ T cells per 3 × 105 splenocytes (means ± SE).
DISCUSSION
Our study demonstrated that vaccination with the Ii-PADRE DNA could increase the number of PADRE-specific CD4+ T cells in immunized mice. In addition, administration of DNA vaccines with Ii-PADRE DNA led to an increase in the number of antigen-specific CD8+ T cells, resulting in potent protective and therapeutic anti-tumor effects. Furthermore, we showed not only that this strategy to enhance CD4+ T-cell immune responses by Ii-PADRE DNA applied to other antigenic systems, but also that it could be used to enhance DNA vaccine potency when employed in conjunction with other DNA vaccination strategies such as an intracellular targeting strategy.
In this study, we observed that administration of DNA vaccines with Ii-PADRE DNA led to the generation of PADRE-specific CD4+ T cells, resulting in the enhancement of antigen-specific CD8+ T-cell immune responses. Several models have been proposed to illustrate the roles of CD4+ T cells in facilitating the generation of antigen-specific CD8+ T-cell immune responses. In the “three cell interaction” model, APCs deliver co-stimulatory signals to the CD4+ Th cells, which in turn generate interleukin-2. This interleukin-2 production is necessary for CTL activation. 23–25 The “sequential two-cell interactions” model proposes that the engagement of CD4+ T cells with APCs leads to the maturation of APCs, which subsequently activate CD8+ T cells.25–27 A recently proposed model suggests that APCs can directly transfer MHC class I antigen complexes and co-stimulatory molecules to expanding populations of interleukin-2-producing T helper cells, which then function as APCs to stimulate CTL activation directly.25 More recently, dendritic cell/CD4 T-cell interaction has been shown to lead to the production of CCL3 and CCL4 chemokines (also known as MIP-1alpha and MIP-1beta). These chemokines may be important for attracting antigen-specific CD8+ T cells to the antigen-expressing dendritic cells.28 All these models suggest that CD4+ T helper cells are important for the activation and proliferation of CD8+ T cells.
The concern was raised that PADRE might activate CD4+CD25+ T regulatory cells. In a study by Phan et al. it was found that in patients with metastatic melanoma who were immunized with an MHC class II–restricted peptide in addition to MHC class I–restricted peptides, the immunologic response of circulating peripheral blood mononuclear cells to a class I–restricted peptide was diminished.29 Their results raised the possibility that class II–restricted peptides may activate CD4+CD25+ T regulatory cells. To rule out that possibility, we examined the PADRE-specific CD4+ T cells generated by i.d. vaccination with DNA encoding Ii-PADRE for their expression of FoxP3, a marker for CD4+CD25+ T regulatory cells. We found that the PADRE-specific CD4+ T cells did not express FoxP3 (data not shown). Furthermore, we have observed that administration of Ii-PADRE DNA with the DNA vaccine led to significant enhancement rather than suppression of the antigen-specific CD8+ T cells. The discrepancy between the observed results and previous reports may be due to the form of the PADRE-related vaccine or the antigen used in the study. Thus, these results indicate that PADRE in the context of the Ii-PADRE DNA vaccine does not activate CD4+CD25+ T regulatory cells.
The success of Ii-PADRE DNA in enhancing antigen-specific CD8+ T-cell immune responses warrants further exploration of innovative strategies that are capable of generating CD4+ T-cell immune responses to enhance CD8+ T-cell immune responses. One potential strategy is to generate a single-chain MHC class II molecule linking a CD4+ T-helper epitope. For instance, Thayer et al. described the design of a single-chain I-Ab:antigenic peptide complex, with a linker that connects the α-chain, β-chain, and peptide. The chimeric molecule was used to stabilize an antigenic peptide in the peptide-binding groove of an MHC class II molecule.18 Cells transfected with DNA encoding such a chimeric molecule have been shown to stimulate an interleukin-2 response from an antigen-specific T-cell hybridoma.18 Thus, it will be interesting to generate a DNA construct encoding a similar chimeric molecule that targets PADRE and to explore whether such a DNA vaccine can lead to the activation of PADRE-specific CD4+ T cells in immunized mice.
MATERIALS AND METHODS
Mice
C57BL/6 mice (6–8 weeks old) were purchased from the National Cancer Institute (Frederick, MD). All animals were maintained under specific pathogen-free conditions at the Johns Hopkins Hospital (Baltimore, MD). All procedures were performed according to the Johns Hopkins Institutional Care and Use Committee approved protocols and in accordance with recommendations for the proper care of laboratory animals.
Cells
In brief, TC-1 cells were obtained by co-transformation of primary C57BL/6 mouse lung epithelial cells with HPV-16 E6 and E7 and an activated ras oncogene as described previously.7 The expression of E6 in TC-1 cells has also been characterized previously by He et al.30
DNA constructs
A DNA vaccine encoding an SCT composed of an immunodominant CTL epitope of HPV-16 E6, β2-microglobulin, and the H-2Kb heavy chain (SCT-E6) has been previously described.21 A DNA vaccine encoding an SCT composed of a CTL epitope (aa181–188, VYDFFVWL) of TRP2, β2-microglobulin, and the H-2Kb heavy chain (SCT-TRP2)31 was constructed. In brief, an insert containing the immunodominant TRP2 aa181–188 epitope and flanking AgeI/NheI restriction enzyme sites was made by annealing two single-stranded oligonucleotides (5′-CCGGTTTGTATGCTGTGTATGACTTTTTTGTGTGGCTCGGAGGAGGTG-3′ and 5′-CTAGCACCTCCTCCGAGCCACACAAAAAAGTCATACACAGCATACAA-3′). It was then cloned into pIRES-OVA-Kb (ref. 21) using AgeI/NheI sites to replace the OVA epitope, generating pIRES-E6-β2m-Kb.
A DNA vaccine encoding an Ii chain was constructed by real-time polymerase chain reaction (PCR) amplification using RNA isolated from dendritic cells and primers (5′-aaagaattcatggatgaccaacgcgacctc-3′ and 5′-aaaggatcctcacagggtgacttgacccag-3′). The real-time PCR product was cloned into the EcoRI/BamHI sites of pcDNA3.1(−) to generate pcDNA3-Ii. For the generation of pcDNA3-li-PADRE (Ii-PADRE DNA), we first generated an Ii-PADRE DNA fragment in which the CLIP epitope in the Ii chain was replaced by PADRE. In brief, the DNA fragments encoding Ii chain aa1–80 and PADRE were amplified by PCR with a set of primers (5′-aaagaattcatggatgaccaacgcgacctc-3′ and 5′-tccaggcagccacgaacttggcaagcttcatgcgaaggctct-3′). The DNA fragment encoding the PADRE and Ii chain aa103–279 was amplified by PCR with a set of primers (5′-aaaggatcctcacagggtgacttgacccag-3′, 5′-ctggaccctgaaggctgccgctatggataacatgctccttgg-3′, and 5′-gccaagttcgtggctgcctggaccctgaaggctgccgct-3′). The overlapping PCR fragments were then used as a template to create Ii-PADRE using PCR with a set of primers (5′-aaagaattcatggatgaccaacgcgacctc-3′ and 5′-aaaggatcctcacagggtgacttgacccag-3′). The DNA fragment encoding Ii-PADRE was further cloned into the EcoRI/BamHI sites of the pcDNA3.1(−) vector to generate pcDNS3-Ii-PADRE. To generate pcDNA3-sig, a signal peptide of LAMP-1 was PCR-amplified using template pcDNA3-sigE7L1 and a set of primers (5′-AAATCTAGAATGGCGGCCCCCGGCGCCCG-3′ and 5′-GGGGAATTCTAGATCCTCAAAGAGTGCTG-3′) and cloned into the XbaI/EcoRI sites of pcDNA3.1(−). To generate pcDNA3-sigPADRE, a set of oligos encoding PADRE (5′-AATTCGCCAAGTTCGTGGCTGCCTGGACCCTGAAGGCTGCCGCTTGAA-3′ and 5′-AGCTTTCAAGCGGCAGCCTTCAGGGTCCAGGCAGCCACGAACTTGGCG-3′) was cloned into the EcoRI/HindIII sites of pcDNA3-sig. To generate pcDNA3/sig/PADRE/LAMP-1, the PADRE-LAMP-1 fragment was amplified using pcDNA3/sigE7L1 as a template and a set of primers (5′-AAAGAATTCGCCAAGTTCGTGGCTGCCTGGACCCTGAAGGCTGCCGCTCTTAACAACATGTTGATCCCC-3′ and 5′-TTTGGATCCCTAGATGGTCTGATAGCCGG-3′) and cloned into EcoRI/BamHI sites of pcDNA3/sig.
DNA vaccination using a gene gun
DNA-coated gold particles were prepared, and gene gun particle-mediated DNA vaccination was performed, according to a protocol described previously. 32 Gold particles coated with DNA vaccines were delivered to the shaved abdominal regions of mice using a helium-driven gene gun (Bio-Rad, Hercules, CA) with a discharge pressure of 400 lb/in2. Mice were immunized with 2 μg of the DNA vaccine and received one boost with the same dose after a 1-week interval. Splenocytes were harvested 1 week after the second vaccination.
Peptide vaccination
Mice were immunized twice with 100 μg of the peptide emulsified in 200 μl incomplete Freund’s adjuvant by s.c. injection at the base of their tails, with a 1-week interval between immunizations.
Intracellular cytokine staining and flow cytometry analysis
Pooled splenocytes from the vaccinated mice were harvested 1 week after the second vaccination and incubated overnight with 1 μg/ml E6 peptide (aa50–57) or PADRE peptide (AKFVAAWTLKAAA) in the presence of GolgiPlug (BD Pharmingen, San Diego, CA) (1 μl/ml). The stimulated splenocytes were then washed once with FACScan buffer and stained with phycoerythrin-conjugated monoclonal rat anti-mouse CD8a (clone 53.6.7). Cells were subjected to intracellular cytokine staining using the Cytofix/Cytoperm kit according to the manufacturer’s instruction (BD Pharmingen, San Diego, CA). Intracellular IFN-γ was stained with fluorescein-conjugated rat anti-mouse IFN-γ. All antibodies were purchased from BD Pharmingen. Flow cytometry analysis was performed using FACSCalibur with CELLQuest software (BD Biosciences, Mountain View, CA).
In vivo tumor protection experiment
For the in vivo tumor protection experiment, C57BL/6 mice (five per group) were immunized using a gene gun with 1 μg pcDNA3 plus 1 μg pcDNA3-Ii (Ii DNA), 1 μg of pcDNA3 plus 1 μg of pcDNA3-Ii-PADRE (Ii-PADRE DNA), 1 μg pcDNA3-Ii plus SCT-E6 (Ii DNA plus SCT-E6 DNA), or 1 μg pcDNA3-Ii-PADRE plus SCT-E6 (Ii-PADRE DNA plus SCT-E6 DNA). Mice were given a booster once with the same dose and vaccination regimen. One week after the second vaccination, mice were challenged with 5 × 104 TC-1 tumor cells per mouse s.c. in the right leg and monitored twice a week by inspection and palpation.
In vivo tumor treatment experiment
For the in vivo tumor treatment experiment, 1 × 104 TC-1 tumor cells were injected into 5–8-week-old C57BL/6 mice (five per group) s.c. in the right leg. After 3 days, the mice were immunized with the DNA vaccines as described above. After 1 week, these mice were given one booster with the same immunization regimen. Mice were monitored once a week by inspection and palpation.
Statistical analysis
All data expressed as means ± SE are representative of at least two different experiments. Data for intracellular cytokine staining with flow cytometry analysis were evaluated using analysis of variance. Comparisons between individual data points were made using a Student’s t-test. For statistical analysis of the tumor protection experiment, we used Kaplan–Meier analysis.
Supplementary Material
Figure S1. Intracellular cytokine staining with flow cytometry analysis to determine the number of PADRE-specific CD4+ T cells in mice vaccinated with various DNA constructs.
Figure S2. In vivo tumor treatment experiments to compare the anti-tumor effects of various DNA vaccines in mice.
Figure S3. Flow cytometry analysis to characterize TRP2-specific CD8+ T-cell responses in vaccinated mice.
Acknowledgments
We gratefully acknowledge Richard Roden (Johns Hopkins Medical Institutions) for helpful discussions. We also acknowledge Flight Attendant Medical Research Institute. We would also like to thank Roanne Calizo and Shaw-Wei D. Tsen of the Johns Hopkins Medical Institutions for helping with the preparation of the manuscript. This work was supported by grant 1 RO1 CA114425-01 and the Cervical Cancer SPORE Program (P50 CA098252) of the National Cancer Institute.
References
- 1.Condon C, Watkins SC, Celluzzi CM, Thompson K, Falo LD., Jr DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122–1128. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 2.Porgador A, Irvine KR, Iwasaki A, Barber BH, Restifo NP, Germain RN. Predominant role for directly transfected dendritic cells in antigen presentation to CD8+ T cells after gene gun immunization. J Exp Med. 1998;188:1075–1082. doi: 10.1084/jem.188.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hung CF, Wu TC. Improving DNA vaccine potency via modification of professional antigen presenting cells. Curr Opin Mol Ther. 2003;5:20–24. [PubMed] [Google Scholar]
- 4.Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu Rev Immunol. 2006;24:519–540. doi: 10.1146/annurev.immunol.23.021704.115825. [DOI] [PubMed] [Google Scholar]
- 5.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marzo AL, Kinnear BF, Lake RA, Frelinger JJ, Collins EJ, Robinson BW, et al. Tumor-specific CD4+ T cells have a major “post-licensing” role in CTL mediated anti-tumor immunity. J Immunol. 2000;165:6047–6055. doi: 10.4049/jimmunol.165.11.6047. [DOI] [PubMed] [Google Scholar]
- 7.Lin K-Y, Guarnieri FG, Staveley-O’Carroll KF, Levitsky HI, August T, Pardoll DM, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–26. [PubMed] [Google Scholar]
- 8.Yewdell JW, Bennink JR. The binary logic of antigen processing and presentation to T cells. Cell. 1990;62:203–206. doi: 10.1016/0092-8674(90)90356-j. [DOI] [PubMed] [Google Scholar]
- 9.Neefjes JJ, Stollorz V, Peters PJ, Geuze HJ, Ploegh HL. The biosynthetic pathway of MHC class II but not class I molecules intersects the endocytic route. Cell. 1990;61:171–183. doi: 10.1016/0092-8674(90)90224-3. [DOI] [PubMed] [Google Scholar]
- 10.Blum JS, Cresswell P. Role for intracellular proteases in the processing and transport of class II HLA antigens. Proc Natl Acad Sci USA. 1988;85:3975–3979. doi: 10.1073/pnas.85.11.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujii S, Senju S, Chen YZ, Ando M, Matsushita S, Nishimura Y. The CLIP-substituted invariant chain efficiently targets an antigenic peptide to HLA class II pathway in L cells. Hum Immunol. 1998;59:607–614. doi: 10.1016/s0198-8859(98)00058-5. [DOI] [PubMed] [Google Scholar]
- 12.Malcherek G, Wirblich C, Willcox N, Rammensee HG, Trowsdale J, Melms A. MHC class II-associated invariant chain peptide replacement by T cell epitopes: engineered invariant chain as a vehicle for directed and enhanced MHC class II antigen processing and presentation. Eur J Immunol. 1998;28:1524–1533. doi: 10.1002/(SICI)1521-4141(199805)28:05<1524::AID-IMMU1524>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 13.van Bergen J, Schoenberger SP, Verreck F, Amons R, Offringa R, Koning F. Efficient loading of HLA-DR with a T helper epitope by genetic exchange of CLIP. Proc Natl Acad Sci USA. 1997;94:7499–7502. doi: 10.1073/pnas.94.14.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Tienhoven EA, ten Brink CT, van Bergen J, Koning F, van Eden W, Broeren CP. Induction of antigen specific CD4+ T cell responses by invariant chain based DNA vaccines. Vaccine. 2001;19:1515–1519. doi: 10.1016/s0264-410x(00)00330-3. [DOI] [PubMed] [Google Scholar]
- 15.van Bergen J, Camps M, Offringa R, Melief CJ, Ossendorp F, Koning F. Superior tumor protection induced by a cellular vaccine carrying a tumor-specific T helper epitope by genetic exchange of the class II-associated invariant chain peptide. Cancer Res. 2000;60:6427–6433. [PubMed] [Google Scholar]
- 16.Nagata T, Higashi T, Aoshi T, Suzuki M, Uchijima M, Koide Y. Immunization with plasmid DNA encoding MHC class II binding peptide/CLIP-replaced invariant chain (Ii) induces specific helper T cells in vivo: the assessment of Ii p31 and p41 isoforms as vehicles for immunization. Vaccine. 2001;20:105–114. doi: 10.1016/s0264-410x(01)00310-3. [DOI] [PubMed] [Google Scholar]
- 17.Nagata T, Aoshi T, Suzuki M, Uchijima M, Kim Y-H, Yang Z, et al. Induction of protective immunity to Listeria monocytogenes by immunization with plasmid DNA expressing a helper T-cell epitope that replaces the class II-associated invariant chain peptide of the invariant chain. Infect Immun. 2002;70:2676–2680. doi: 10.1128/IAI.70.5.2676-2680.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thayer WP, Dao CT, Ignatowicz L, Jensen PE. A novel single chain I-A(b) molecule can stimulate and stain antigen-specific T cells. Mol Immunol. 2003;39:861–870. doi: 10.1016/s0161-5890(03)00010-5. [DOI] [PubMed] [Google Scholar]
- 19.Alexander J, Sidney J, Southwood S, Ruppert J, Oseroff C, Maewal A, et al. Development of high potency universal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity. 1994;1:751–761. doi: 10.1016/s1074-7613(94)80017-0. [DOI] [PubMed] [Google Scholar]
- 20.Wu T-C, Guarnieri FG, Staveley-O’Carroll KF, Viscidi RP, Levitsky HI, Hedrick L, et al. Engineering an intracellular pathway for major histocompatibility complex class II presentation of antigens. Proc Natl Acad Sci USA. 1995;92:11671–11675. doi: 10.1073/pnas.92.25.11671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang CH, Peng S, He L, Tsai YC, Boyd DA, Hansen TH, et al. Cancer immunotherapy using a DNA vaccine encoding a single-chain trimer of MHC class I linked to an HPV-16 E6 immunodominant CTL epitope. Gene Ther. 2005;12:1180–1186. doi: 10.1038/sj.gt.3302519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng WF, Hung CF, Chai CY, Hsu KF, He L, Ling M, et al. Tumor-specific immunity and antiangiogenesis generated by a DNA vaccine encoding calreticulin linked to a tumor antigen. J Clin Invest. 2001;108:669–678. doi: 10.1172/JCI12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchison NA, O’Malley C. Three-cell-type clusters of T cells with antigen-presenting cells best explain the epitope linkage and noncognate requirements of the in vivo cytolytic response. Eur J Immunol. 1987;17:1579–1583. doi: 10.1002/eji.1830171109. [DOI] [PubMed] [Google Scholar]
- 24.Bennett SR, Carbone FR, Karamalis F, Miller JF, Heath WR. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J Exp Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiang J, Huang H, Liu Y. A new dynamic model of CD8+ T effector cell responses via CD4+ T helper-antigen-presenting cells. J Immunol. 2005;174:7497–7505. doi: 10.4049/jimmunol.174.12.7497. [DOI] [PubMed] [Google Scholar]
- 26.Bousso P, Robey E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat Immunol. 2003;4:579–585. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- 27.Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 28.Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 29.Phan GQ, Touloukian CE, Yang JC, Restifo NP, Sherry RM, Hwu P, et al. Immunization of patients with metastatic melanoma using both class I- and class II-restricted peptides from melanoma-associated antigens. J Immunother. 2003;26:349–356. doi: 10.1097/00002371-200307000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He Z, Wlazlo AP, Kowalczyk DW, Cheng J, Xiang ZQ, Giles-Davis W, et al. Viral recombinant vaccines to the E6 and E7 antigens of HPV-16. Virology. 2000;270:146–161. doi: 10.1006/viro.2000.0271. [DOI] [PubMed] [Google Scholar]
- 31.Bloom MB, Perry-Lalley D, Robbins PF, Li Y, el-Gamil M, Rosenberg SA, et al. Identification of tyrosinase-related protein 2 as a tumor rejection antigen for the B16 melanoma. J Exp Med. 1997;185:453–459. doi: 10.1084/jem.185.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen C-H, Wang T-L, Hung C-F, Yang Y, Young RA, Pardoll DM, et al. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res. 2000;60:1035–1042. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Intracellular cytokine staining with flow cytometry analysis to determine the number of PADRE-specific CD4+ T cells in mice vaccinated with various DNA constructs.
Figure S2. In vivo tumor treatment experiments to compare the anti-tumor effects of various DNA vaccines in mice.
Figure S3. Flow cytometry analysis to characterize TRP2-specific CD8+ T-cell responses in vaccinated mice.