The Diels-Alder reaction of 2-pyrones provides a direct and versatile access to synthetically valuable multifunctional bicyclic chiral building blocks.1,2 However, as electron-deficient dienes of aromatic character, 2-pyrones are known to be reluctant diene partners for Diels-Alder reactions.1,3 This presents a distinct challenge for the development of a catalytic asymmetric variant for this synthetically useful class of Diels-Alder reactions. Thus, it comes as no surprise that, in spite of the great strides made on the development of asymmetric Diels-Alder reactions, a highly diastereoselective and enantioselective catalytic Diels-Alder reaction with 2-pyrones has not yet been realized.1,4 In fact, to our knowledge, even for chiral auxiliary-directed asymmetric Diels-Alder reactions with 2-pyrones, only a single example was reported.4b Herein, we wish to describe the development of an efficient asymmetric Diels-Alder reaction of 2-pyrones using cinchona alkaloid-derived bifunctional organic catalysts.
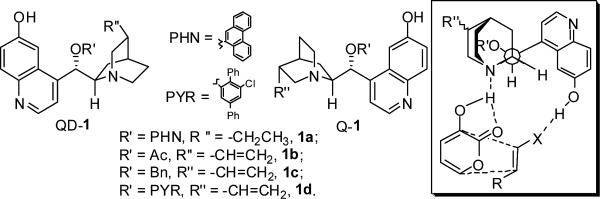
Nakatani and coworkers explored the use of natural cinchona alkaloids to promote a Diels-Alder reaction of 3-hydroxy-2-pyrone (3a) with N-methylmaleimide.4a However, the enantioselectivity afforded by natural cinchona alkaloids were modest. Nakatani proposed that the mode of action by natural cinchona alkaloids was to activate and orient 3a only in the Diels-Alder reaction. We recently demonstrated that 6’-OH cinchona alkaloids 1 (Figure 1) are effective organic catalysts for asymmetric conjugate additions,5 aldol reactions6 and Friedel-Crafts reactions.7 Mechanistic studies from our laboratories indicate that the hydrogen bond donor and acceptor motifs in 1 activate and orient the nucleophiles and electrophiles, respectively, through multiple hydrogen bonding interactions.5a-b This mechanistic insight in turn implies that 1 might function as efficient bifunctional catalysts for asymmetric Diels-Alder of pyrones 3 with electron-deficient dienophiles by simultaneously raising the energy of the HOMO of the former and lowering the energy of the LUMO of the latter while orienting the two reactants to exert stereochemistry control (Figure 1).8-10
Figure 1.
Bifunctional Catalysis for D-A Reactions of 2-Pyrone 3
Guided by this hypothesis we investigated the reaction of 3-hydroxy-2-pyrone 3a and trans-3-benzoylacrylic ester 4A with various cinchona alkaloids as catalysts. As summarized in table 1, the reaction readily proceeded to completion with the expected sense of chemoselectivity to afford cycloadducts endo/exo-5aA as a mixture of diastereomers (Table 1). Importantly, the 6’-OH cinchona alkaloids QD-1 afforded significantly better catalytic efficiency than that by natural cinchona alkaloids, the monofunctional cinchona alkaloid DHQD-PHN and the conformationally rigid 6’-OH cinchona alkaloid β-ICD (Table 1, entries 5-8 vs. 1-4). These results illustrated that both the structure of the tunable 9-substitutent and the bifunctional nature of catalysts 1 are critically important to their catalytic efficiency for the asymmetric Diels-Alder reaction. After further optimizations we achieved a highly diastereoselective and enantioselective reaction with 5 mol% of QD-1a, to afford exo-5aA in 93:7 dr and 91% ee.
Table 1.
D-A Reaction with Cinchona Alkaloidsa
 | |||||||
|---|---|---|---|---|---|---|---|
| entry | catalyst | drb exo:endo | ee (%)b of exo-5aA | entry | catalyst | drb exo:endo | ee (%)b of exo-5aA |
| 1 | Quinidine | 66:34 | 5 | 6 | QD-1c | 87:13 | 80 |
| 2 | Cinchonine | 62:38 | -5 | 7 | QD-1d | 85:15 | 82 |
| 3 | DHQD-PHN | 66:34 | 33 | 8 | QD-1a | 88:12 | 88 |
| 4 | β–ICD | 94:6 | 22 | 9c | QD-1a | 93:7 | 89 |
| 5 | QD-1b | 90:10 | 57 | 10d | QD-1a | 93:7 | 91 |
See Supporting Information (SI) for details.
In crude reaction mixtures.
Reaction was run in Et2O.
Reaction was run in Et2O, the concentration of 3a was 0.1 M.
Catalyst QD-1a was found to tolerate a significant degree of alterations in both pyrones 3 and dienophiles 4 (Table 2). The reactions between pyrone 3a and dienophiles of different substitution patterns (4A-D) proceeded in 76:24 to 93:7 dr, and the major diastereoisomers were generated mostly in greater than 90% ee. It is noteworthy that even the relatively unreactive dienophile 4D could be employed in this reaction, thereby generating optically active chiral building blocks containing two adjacent tetrasubstituted stereocenters. Moreover, catalyst QD-1a was able to furnish useful levels of enantioselectivity and diastereoselectivity for reactions of dienophile 4A with pyrones 3b-e bearing various substituents (entries 5-8, Table 2).
Table 2.
D-A Reaction with QD-1a and Q-1a (in parentheses)a
 | |||||||
|---|---|---|---|---|---|---|---|
| entry | pyrone | dienophile | solvent | temp (°C) | exo:endo | yieldb (%) | eeb (%) |
| 1 | 3a |
|
Et2O | rt | 93:7 (94:6) | 87 (90) | 94 (87) |
| 2 | 3a |

|
Et2O | rt | 91:9 (94:6) | 91 (93) | 91 (83) |
| 3 | 3a |

|
Et2O | rt | 93:7 | 100c | 90 |
| 4d | 3a |

|
Et2O | rt | 24:76 (26:74) | 65 (63) | 91 (90) |
| 5 | 3b | 4A | Et2O | 0 | 95:5 | 84 | 85 |
| 6 | 3c | 4A | Et2O | 0 | 88:12 | 87 | 82 |
| 7 | 3d | 4A | EtOAc | rt | 86:14 | 77 | 84 |
| 8 | 3e | 4A | EtOAc | rt | 85:15 | 75 | 83 |
However, QD-1a was found to be ineffective for reactions of 3a with fumaronitrile 4E (Table 3, entry 1). Although the 9-thiourea cinchona alkaloids 2 were found to afford low diastereoselectivity and enantioselectivity for the reaction of 3a with 4A,11 their high efficiency for the activation of acrylonitriles toward conjugate additions12 led us to evaluate 2 as catalysts for reactions of 3a with dienophiles 4E-G. Gratifyingly, QD-2 and Q-2 afforded drastically improved enantioselectivity and diastereoselectivity, generating the corresponding exo-adduct in 85 to 98% ee and 89:11 to >97:3 dr (Table 3, entries, 2-4). The results obtained with reactions involving fumaronitrile (4E) and maleonitrile 4F illustrate the ability of 2 to tolerate dienophiles with either an E- or a Z-double bond. It is also noteworthy that these reactions are stereospecific with respect to the geometry of the double bond. These results are consistent with a concerted cycloaddition mechanism.13
Table 3.
D-A Reactions with 4E-F Catalyzed by QD-2 and Q-2 (in parentheses)a
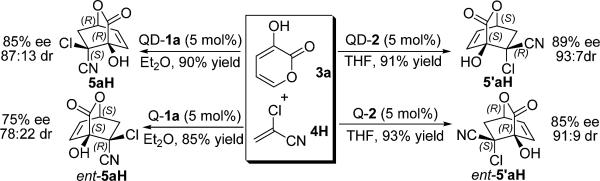
We recently demonstrated that bifunctional organic catalysts containing the hydrogen bond donor and acceptor in different spatial relationships, such as 1a and 2, could afford complementary diastereoselectivities for asymmetric reactions creating two stereocenters.5f,12 Prompted by this finding, we investigated the reaction of 3a and α-chloroacrylonitrile 4H with catalysts QD-1a and QD-2, respectively (Scheme 1). Indeed the former was found to be endo-selective and while the latter exo-selective. Consequently, cinchona alkaloids 1a and 2 derived from quinine and quinidine, respectively, afforded selective pathways to each of the four possible stereoisomers that could be generated from 3a and 4H (Scheme 1).14
Scheme 1.
Catalyst-controlled exo/endo Selectivity
In summary, by exploring cinchona alkaloid-based bifunctional organic catalysts, we have developed an unprecedented highly enantioselective and diastereoselective catalytic Diels-Alder reaction with pyrones. To our knowledge, the current study also provides the first example of an organic molecule as an efficient acid-base bifunctional catalyst for a Diels-Alder reaction. Furthermore, we demonstrated the possibility of using such catalysts to control the endo/exo selectivity in a Diels-Alder reaction. Studies are underway to expand the scope of this reaction and to explore its application in asymmetric synthesis.
Supplementary Material
Acknowledgement
We thank National Institute of Health (GM-61591) for financial support. We thank the National Science Foundation for the partial support of this work through grant (CHE-0521047) for the purchase of a new X-ray diffractometer.
Footnotes
Supporting Information Available: Experimental procedures and characterization of the products. X-ray analysis data (CIF) for 5aH and 5’aH. This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.For reviews, see: Afarinkia K, Vinader V, Nelson TD, Posner GH. Tetrahedron. 1992;48:9111.Woodard BT, Posner GH. Advances in Cycloaddition. 1999;5:47.
- 2.For synthetic applications of Diels-Alder reactions of 2-pyrones, see: Corey EJ, Kozikowski AP. Tetrahedron Lett. 1975:2389.Nicolaou KC, Liu JJ, Hwang C-K, Dai W-M, Guy RK. J. Chem. Soc., Chem. Commun. 1992:1118.Nicolaou KC, Yang Z, Liu JJ, Ueno H, Nantermet PG, Guy RK, Clalborne CF, Renaud J, Couladouros EA, Paulvannan K, Sorensen EJ. Nature. 1994;367:630. doi: 10.1038/367630a0.Okamura H, Shimizu H, Nakamura Y, Iwagawa T, Nakatani M. Tetrahedron Lett. 2000;41:4147.Shimizu H, Okamura H, Iwagawa T, Nakatani M. Tetrahedron. 2001;57:1903.Baran PS, Burns NZ. J. Am. Chem. Soc. 2006;128:3908. doi: 10.1021/ja0602997.
- 3.Gladysz JA, Lee SJ, Tomasello JAV, Yu YS. J. Org. Chem. 1977;42:4170. [Google Scholar]
- 4.Okamura H, Nakamura Y, Iwagawa T, Nakatani M. Chem. Lett. 1996:193.Okamura H, Morishige K, Iwagawa T, Nakatani M. Tetrahedron Lett. 1998;39:1211. For asymmetric inverse electron-demand Diels-Alder (IEDDA) reaction of 2-pyrones, see: Posner GH, Carry J-C, Lee JK, Bull DS, Dai H. Tetrahedron Lett. 1994;35:1321.Posner GH, Eydoux F, Lee JK, Bull DS. Tetrahedron Lett. 1994;41:7541.Posner GH, Dai H, Bull DS, Lee JK, Eydoux F, Ishihara Y, Welsh W, Pryor N, Petr. S., Jr. J. Org. Chem. 1996;61:671. doi: 10.1021/jo9515900. For catalytic asymmetric IEDDA reactions, see: Markó IE, Evans GR, Seres P, Chellé I, Janousek Z. Pure & Appl. Chem. 1996;68:113.Markó IE, Chellé-Regnaut I, Leroy B, Warriner SL. Tetrahedron Lett. 1997;38:4269.
- 5.a Li H, Wang Y, Tang L, Deng L. J. Am. Chem. Soc. 2004;126:9906. doi: 10.1021/ja047281l. [DOI] [PubMed] [Google Scholar]; b Li H, Wang Y, Tang L, Wu F, Liu X, Guo C, Foxman BM, Deng L. Angew. Chem., Int. Ed. 2005;44:105. doi: 10.1002/anie.200461923. [DOI] [PubMed] [Google Scholar]; c Li H, Song J, Liu X, Deng L. J. Am. Chem. Soc. 2005;127:8948. doi: 10.1021/ja0511063. [DOI] [PubMed] [Google Scholar]; d Wu F, Li H, Hong R, Deng L. Angew. Chem,. Int. Ed. 2006;45:947. doi: 10.1002/anie.200502658. [DOI] [PubMed] [Google Scholar]; e Wu F, Hong R, Khan J, Liu X, Deng L. Angew. Chem., Int. Ed. 2006;45:4301. doi: 10.1002/anie.200600867. [DOI] [PubMed] [Google Scholar]; f Wang Y, Liu X, Deng L. J. Am. Chem. Soc. 2006;128:3928. doi: 10.1021/ja060312n. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Wang B, Deng L. J. Am. Chem. Soc. 2006;128:732. doi: 10.1021/ja057237l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H, Wang Y-Q, Deng L. Org. Lett. 2006;8:4063. doi: 10.1021/ol061552a. [DOI] [PubMed] [Google Scholar]
- 8.For studies on hydrogen bond-promoted asymmetric Diels-Alder reaction, see: Huang Y, Unni AK, Thadani AN, Rawal VH. Nature. 2003;424:146. doi: 10.1038/424146a.Unni AK, Takenaka N, Yamamoto H, Rawal VH. J. Am. Chem. Soc. 2005;127:1336. doi: 10.1021/ja044076x. For a review of hydrogen bond-based asymmetric catalysis, see: Taylor MS, Jacobsen EN. Angew. Chem., Int. Ed. 2006;45:1520. doi: 10.1002/anie.200503132.
- 9.For an asymmetric inverse electron-demand Hetero-Diels-Alder reaction with dual activation see: Abraham CJ, Paull DH, Scerba MT, Grebinski JW, Lectka T. J. Am. Chem. Soc. 2006;128:13370. doi: 10.1021/ja065754d.
- 10.For other asymmetric Diels-Alder reactions by orgnaocatalysts, see: Ahrendt KA, Borths CJ, MacMillan DWC. J. Am. Chem. Soc. 2000;122:4243.Juhl K, Jørgensen KA. Angew. Chem., Int. Ed. 2003;42:1498. doi: 10.1002/anie.200250652.Ramachary DB, Chowdari NS, Barbas CF., III Angew. Chem., Int. Ed. 2003;42:4233. doi: 10.1002/anie.200351916.Sundén H, Ibrahem I, Eriksson L, Córdova A. Angew. Chem., Int. Ed. 2005;44:4877. doi: 10.1002/anie.200500811.Ishihara K, Nakano K. J. Am. Chem. Soc. 2005;127:10504. doi: 10.1021/ja053368a.Kano T, Tanaka Y, Maruoka K. Org. Lett. 2006;8:2687. doi: 10.1021/ol060621i.Itoh J, Fuchibe K, Akiyama T. Angew. Chem., Int. Ed. 2006;45:4796. doi: 10.1002/anie.200601345.He M, Struble JR, Bode JW. J. Am. Chem. Soc. 2006;128:8418. doi: 10.1021/ja062707c.He M, Uc GJ, Bode JW. J. Am. Chem. Soc. 2006;128:15088. doi: 10.1021/ja066380r.Riant O, Kagan HB. Tetrahedron. 1994;50:4543.Shen J, Nguyen TT, Goh Y-P, Ye W, Fu X, Xu J, Tan C-H. J. Am. Chem. Soc. 2006;128:13692. doi: 10.1021/ja064636n.Wolfer J, Bekele T, Abraham CJ, Dogo-Isonagie C, Lectka T. Angew. Chem., Int. Ed. 2006;45:7398. doi: 10.1002/anie.200602801.
- 11.The exo-5aA was obtained in 78:22 dr and 37% ee.
- 12.Wang B, Wu F, Wang Y, Liu X, Deng L. J. Am. Chem. Soc. 2007;129:768. doi: 10.1021/ja0670409. [DOI] [PubMed] [Google Scholar]
- 13.Okamura H, Iwagawa T, Nakatani M. Tetrahedron Lett. 1995;36:5939. [Google Scholar]
- 14.See SI for the determination of absolute configurations of 5aH and 5’aH.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.