Abstract
Normal murine metapodophalangeal sesamoid bones, closely associated with tendons, were examined in terms of their structure and mineralization with reference to their potential function following crystal deposition. This study utilized radiography, whole mount staining, histology and conventional electron microscopy to establish a maturation timeline of mineral formation in one- to six-week-old metapodophalangeal sesamoids from CD-1 mice. An intimate cellular and structural relationship was documented in more detail than previously described between the sesamoid bone, tendon, and fibrocartilage enthesis at the metapodophalangeal joint. Sesamoid calcification began in one-week lateral sesamoids of the murine metacarpophalangeal joint of the second digit. All sesamoids were completely calcified by four weeks. Transmission electron microscopy of two-week metacarpophalangeal sesamoids revealed extensive type I collagen in the associated tendon and fibrocartilage insertion sites and type II collagen and proteoglycan networks in the interior of the sesamoid. No extracellular matrix vesicles were documented. The results demonstrate that murine sesamoid bones consist of cartilage elaborated by chondrocytes that predominantly synthesize and secrete type II collagen and proteoglycan. Type II collagen and proteoglycans appear responsible for the onset and progression of mineral formation in this tissue. These data contribute to new understanding of the biochemistry, ultrastructure and mineralization of sesamoids in relation to other bones and calcifying cartilage and tendon of vertebrates. They also reflect on the potentially important but currently uncertain function of sesamoids as serving as a fulcrum point along a tendon, foreshortening its length and altering advantageously its biomechanical properties with respect to tendon-muscle interaction.
Keywords: Metapodophalangeal sesamoid bones, mineralization, type II collagen, proteoglycans, tendon
Introduction
Sesamoid bones of the vertebrate skeleton are typically small structures that form within a continuous band of regular dense connective tissue, either tendon or ligament, and develop from a cartilaginous anlage like many other vertebrate bones (Bizarro, 1921; Bland and Ashhurst, 1997; Clark and Stechschulte, 1998; Gray, 1918; Joseph, 1951; Le Minor, 1988). Few studies have investigated sesamoid development, with the exception of the patella (Bland and Ashhurst, 1997; Reese et al., 2001; Walmsley, 1940), but the position of the sesamoid in the deep surface of tendon/ligament at a joint is the defining trait of the majority of these bones (Bizarro, 1921). The formation of most sesamoids occurs first as cartilaginous anlagen that ultimately mineralize. It is unknown, however, if the heavy investment with the associated tendon contributes to the sesamoid bone mineralization (or equivalently, calcification) similar to calcifying avian tendon. Traditionally, sesamoids have been considered as accessory and insignificant bones, commonly lost in the preservation or handling of skeletal material, and therefore routinely marginalized and overlooked in research. On the other hand, there may be a fundamental importance in the mineralization and function of these small “accessory” bones since they are present in a great diversity of animals and are phylogenetically inherited. Given their close association with tendon, sesamoids may provide insight into the mechanism of mineralization of bone, an historically difficult process to document and one that is still open to speculation.
The functions of sesamoids are incompletely understood. They have been described as stabilizing the joint, reducing friction along a tendon or ligament, modifying pressure, or increasing the mechanical advantage of the muscle at a joint (Alexander and Dimery, 1985; Benjamin et al., 2006; Bland and Ashhurst, 1997; Currey, 2002; Goldberg and Nathan, 1987; Gray, 1918; Hildebrand, 1985; Vickaryous and Olson, 2007; Wood, 1984). While precise functions of sesamoids are still uncertain, the mineralization of these bones may be a consideration in understanding the nature of their possible biomechanical, biological, or other roles.
Regarding mineralization of sesamoids or of any normally calcifying vertebrate tissue, a basic tenet in skeletal biology is that mineral deposition is mediated by specific organic molecules synthesized intracellularly and then secreted into extracellular matrices of those tissues. Thus, certain organic components of calcifying tissues direct inorganic crystal nucleation, growth and development. A critical, yet unanswered question in this context concerns identification of the precise extracellular matrix component(s) that may be responsible for the initial deposition of mineral in these tissues. Several studies in biomineralization suggest that extracellular matrix vesicles are the first structures to initiate mineral formation in the organic matrix of calcifying cartilage (Ali et al., 1970; Anderson, 1969; Bonucci, 1969; Gerstenfeld et al., 1998; Hsu and Anderson, 1978; Kirsch et al., 1997; Plate et al., 1996), fibrocartilage (Yamada, 1976), primary dentin (Stratmann et al., 1997), fish scales (Lowenstam and Weiner, 1989), antler (Szuwart et al., 1998), and other vertebrate mineralizing tissues. Numerous additional reports provide support for collagen molecules as templates for mineral deposition in bone, dentin, cementum, and calcifying avian tendons (Arsenault, 1989; Bernard and Pease, 1969; Birkedal-Hansen et al., 1977; Glimcher, 1987; 1989; Landis et al., 1993; 1996; Landis and Silver, 2002; Landis and Song, 1991; Siperko and Landis, 2001).
The progression of mineralization in most vertebrate tissues, whether through matrix vesicles or collagen, has been difficult to document since developing minerals quickly overgrow and obscure the complex organic matrix components with which they associate (Lowenstam and Weiner, 1989). Normally mineralizing avian tendons, on the other hand, consist of a somewhat less complicated organic matrix compared to other calcifying vertebrate tissues and one in which both matrix vesicle- and collagen-mediated events promote mineral nucleation, growth, and development. Furthermore, such mineralization events appear spatially and temporally distinct in the avian tendon, a unique feature that then allows a degree of discrimination between the separate means by which vesicles and collagen affect mineral formation (Landis et al., 1993; 1996; Landis and Silver, 2002; Landis and Song, 1991; Siperko and Landis, 2001). Calcifying avian tendon has thus been considered a valuable model for investigating vesicles, collagen, and mineralization in defined temporal and spatial pathways, and it has served as a guide to vertebrate tissue mineralization in general (Bonucci, 2007).
Similar to the structural relationship between tendon and bone in the avian leg and wing, murine sesamoid bones, which form completely within tendon or ligament, may present features different from other endochondral mineralizing tissues and that might provide better understanding to their function. In this respect, the documented events of mineralization of avian tendon detailed above may be applicable to the manner of sesamoid mineral formation. The metapodophalangeal sesamoid bones, the sesamoids of principal interest in the present investigation, are very common in extant mammals, unlike calcifying tendons, and are embedded in the flexor digitorum tendons along the palmar/plantar surface of the autopod (Le Minor, 1988). Although sesamoids develop from within tendon, their placement is such that they do not interfere with the continuity of the tendon or its insertion onto bone, unlike calcifying avian tendons where the central core of the tissue becomes mineralized. As noted previously, sesamoid development has been little studied and, in addition, there have been only a few reports (Cooper and Misol, 1970; Rufai et al., 1996; Yamada, 1976) describing the ultrastructure and mineralization of entheses, structures which are often associated with sesamoids. Thus, the ultrastructural components possibly contributing to the mineralization of sesamoid bones and the process of mineral formation, whether by matrix vesicles, collagen or some alternate means, of these bones have not been determined.
Matrix vesicles are small, globular, membrane-bound structures found within spaces of the extracellular matrix of many vertebrate mineralizing tissues. Matrix vesicles appear to facilitate mineralization by putatively sequestering nuclei and initiating crystal formation within the vesicle membranes that separate the vesicle volume from surrounding extracellular fluid (Ali et al., 1970; Anderson, 1969; Kirsch et al., 1997). The mineral crystals continue growing and are suggested ultimately to rupture the vesicular limiting membrane, thereby exposing the crystals to the extravesicular milieu (Kirsch et al., 1997). Calcification of the surrounding extracellular matrix then presumably proceeds as crystals released from vesicles coalesce into clusters to form multiple mineralization centers. The mineral centers and crystals are hypothesized to provide a source of Ca+2 and PO4−3 ions that resolubilize and diffuse to initiate calcification on nearby collagen fibers and at other sites (Hsu and Anderson, 1978). It is very difficult, however, to demonstrate the origin of such putative diffusible ions, and it remains unknown as to whether matrix vesicles are solely responsible for initiating mineralization of extracellular matrices in which they reside.
As noted earlier, evidence also suggests that type I collagen fibers in the calcifying extracellular matrix of avian tendons mediate mineralization distinct from that of matrix vesicles (Gupta et al., 2003; Landis et al., 1993; Landis et al., 1996; Landis and Song, 1991). Calcifying avian leg tendon, unlike calcifying cartilage, is unique in that it provides a model in which the progression of mineralization by both matrix vesicles and collagen may be detected and assessed in a relatively clear and direct spatial and temporal sequence (Landis and Song, 1991). In this tissue, mineral formation associated with linear arrays of numerous matrix vesicles, located between parallel bundles of type I collagen and the longitudinal columns of spindle-shaped tenocytes within the tendon, temporally precedes collagen-mediated nucleation events (Landis, 1986). Once mineral deposition occurs through vesicle action, collagen appears to initiate mineral formation at sites spatially independent from vesicles and by a mechanism different from that of vesicles (Landis, 1986; Landis et al., 1993; Landis and Song, 1991). Thus, vesicle- and collagen-mediated mineralization can persist simultaneously in normally calcified avian tendons.
Metapodophalangeal sesamoid bones have a large fibrocartilaginous insertion site, or enthesis, in the transition from the flexor digitorum tendons to bone. Unlike other bones, the extensive surface area investment of the sesamoid in the enthesis, in conjunction with its development within tendon, may be an important structural factor interrelating the tendon and/or fibrocartilage to mineralization of the bone. Four histological zones (tendon, fibrocartilage, calcified fibrocartilage, and bone) comprise an enthesis (Cooper and Misol, 1970), and fibrocartilage, itself, may promote sesamoid mineral formation (Benjamin and Evans, 1990). In this regard, fibrocartilage is compositionally similar to both tendon and cartilage (each consisting in part of type I and type II collagen), it mineralizes normally like tendon (Benjamin and Ralphs, 1998), and it maintains a structural association with the sesamoid.
The present study further details general morphology of murine metapodophalangeal sesamoid bones, defines a maturation timeline, identifies component cells and their organization in sesamoids and their associated tendons and entheses, and investigates the presence and potential role of extracellular organic constituents such as matrix vesicles and collagen in sesamoid bone mineralization. Radiography, whole mount staining, light microscopy, and transmission electron microscopy were utilized as principal analytical methods. This work with metapodophalangeal sesamoid bones examines the question of whether structural changes in the sesamoids and their fibrocartilaginous insertion sites leading to mineral formation may be important functionally for these small, seemingly ancillary bones.
Materials and Methods
Metapodophalangeal sesamoid bones were examined in twelve normal CD-1 mice (Charles River Laboratories, Wilmington, MA) throughout their first six weeks of postnatal development (n = 2 per week). Radiography, whole mount staining, and histology were applied to assess tissue morphology and mineral content. Animals were euthanized in a carbon dioxide chamber and their fore and hind paws were dissected. All procedures were approved by the NEOUCOM Institutional Animal Care and Use Committee. In preparation for radiography, whole fore and hind limbs (from one- to six-week-old mice) were fixed in 95% ethyl alcohol for 2 to 4 days and x-rayed with a mammography radiation unit (GE Lorad M-II, Bedford, MA) operated at 25 kvp for 0.2 sec at 2.4 mA. These same paws were then prepared for whole mount staining by removing skin from them. The paws were placed in vials containing 1% potassium hydroxide to clear the tissue until the bones were visible, and then they were stained with Alcian blue and/or Alizarin red S to identify cartilage and bone, respectively. Clearing and staining techniques followed protocols previously described by Clark (1981), Kaufman (1992), and Wassersug (1976). The intact, cleared and stained metapodophalangeal sesamoids were next placed in Petri dishes and covered with glycerine thinned with a few drops of 3% KOH. Specimens were viewed under a dissection microscope (Zeiss Stemi SV II, West Germany) and photographed at magnifications of 2 to 6×.
Based on the data from the radiography and whole mount staining, one- to three-week fore and hind paws were fixed in 10% neutral buffered formalin (NBF) for 24 hours and decalcified in 5.5% EDTA/10% NBF with continuous agitation at room temperature for 2 to 8 weeks in preparation for histology. The paws were then processed, infiltrated, and embedded in paraffin with the fifth digit of each intact paw positioned medial side down in embedding molds to obtain proper orientation of digits for subsequent sectioning on a microtome (American Optical 820 rotary microtome, Buffalo, NY). Sections (7 µm thick) cut from the decalcified paws were stained with toluidine blue, Picrosirius red, or Safranin-O red to determine the general development and morphology of the sesamoids, their association with tendon, and collagen and proteoglycan presence using a light microscope (Olympus Optical IX70-S1F2, Japan). In certain instances, with the use of Picrosirius red and toluidine blue staining, slides were examined under polarized light to examine details of collagen structure and orientation.
Following the identification of sesamoid mineralization from radiography, whole mount staining, and histology, the second digits from 2-week-old mice were selected for ultrastructural examination utilizing transmission electron microscopy (TEM). Matrix vesicles, typically ranging in diameter from 60–250 nm, are only identifiable using TEM. In preparation for electron microscopy, tissues were fixed and decalcified in a solution containing 2.5% glutaraldehyde/7.5% EDTA in 0.1M cacodylic buffer (pH 7.4), continuously agitated for a period of 7 days at 4°C. Selected samples were subsequently fixed in 0.01% osmium tetroxide (OsO4) in 0.1 M cacodylic buffer, processed in a series of ethanol and propylene oxide solutions, and infiltrated and embedded in EMBED 812 (Electron Microscopy Sciences, Hatfield, PA) at 70°C.
Thin sectioning (~100 nm) of digits was conducted using a gem quality diamond knife. Sections were collected on naked or charged Formvar-coated grids (200 mesh) and positive-contrast-stained with 2% uranyl acetate and 2.6% lead citrate for improved viewing of the samples. Stained grids were examined in a JEM-100S TEM (JEOL Ltd., Peabody, MA) operated at 80 or 100 kV and using magnifications of 500 to 20,000×. Areas of interest were photographed and developed on Kodak 35 mm Panatomic-x Rapid Process Copy Film (Kodak Co., Rochester, NY). Images were scanned with an Epson Expression 1600 (Epson America, Inc., Long Beach, CA) at 1200 dpi for further investigation.
All collected mouse sesamoid data were analyzed to document cellular and extracellular ultrastructure and the presence of matrix vesicles and collagen in the tissues. Data were compared to previously reported structure, composition, and mineralization in calcifying cartilage (Ali et al., 1970; Gerstenfeld et al., 1998; Hsu and Anderson, 1978; Kirsch et al., 1997; Plate et al., 1996), avian tendons (Landis, 1986; Landis et al., 1993; Landis and Silver, 2002; Landis and Song, 1991; Siperko and Landis, 2001), and fibrocartilage (Benjamin et al., 1986; Cooper and Misol, 1970; Rufai et al., 1996; Yamada, 1976).
Results
Timing of mineralization
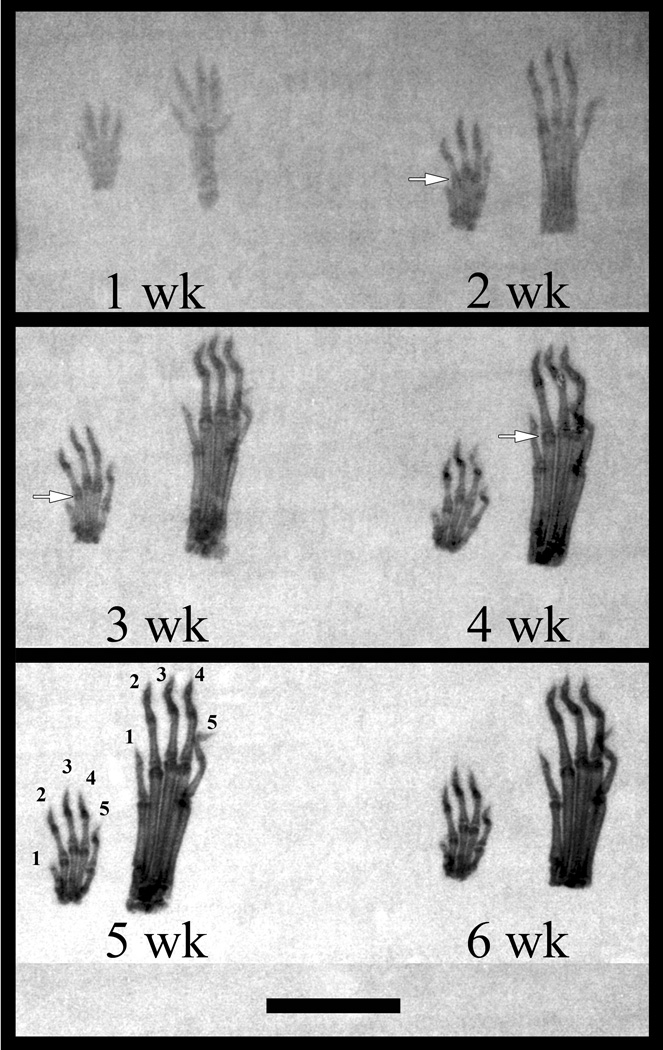
Skeletal indication of sesamoids in fore and hind paws of mice were not visible in the radiographs until three weeks of age (Fig. 1). The obvious presence of sesamoids at several metacarpophalangeal joints and their near absence in the hind paw suggested that the fore and hind paws of the CD-1 mouse developed at different ages. In addition, it appeared that the metacarpophalangeal sesamoids of the second, third, and fourth digit appeared first in the fore paw. Once completely mineralized in the five- and six-week-old samples, the metapodophalangeal sesamoids were clearly visible in the radiographs as dark condensations flanking either side of the distal end of the metapodia (Fig. 1). These results suggested that initial mineralization, the stage of mineralization which contains matrix vesicles and potential mineral crystals, would be observed in one- to three-week-old samples.
Figure 1.
Cleared and stained whole mount samples revealed that murine metapodophalangeal sesamoid bones began mineralization as early as one week of age in the metacarpophalangeal joint of the second digit (Fig. 2A). Evidence of mineral formation was the presence of a dense nodule within the cartilaginous core of the lateral-most sesamoid (Fig. 2A). The calcification of the epiphyseal plates of the metapodia and proximal phalanges began in advance of sesamoid mineral formation (Fig. 2B). Complete calcification was obtained between three and four weeks, resulting in a large, oblate sesamoid. Mineralization of these sesamoids occurred before the epiphyseal plates were fully fused, as observed in the five- and six-week-old samples (Fig. 1).
Figure 2.
Whole mount staining also revealed that sesamoids of the fore paw developed earlier than those of the hind when comparing the size of the sesamoids and the development of the epiphyseal plates of the metacarpals and proximal phalanges (Figs. 2A–B). It should be noted that the stages of overall mineralization represented in both fore and hind paws progressed in identical fashion at any time of animal age (data not shown). Individual digits of either fore or hind paws mineralized at different times in their maturation process. The second digit mineralized first (Fig. 2A), followed by the third, fourth, fifth, and finally the first digit.
Basic histomorphology
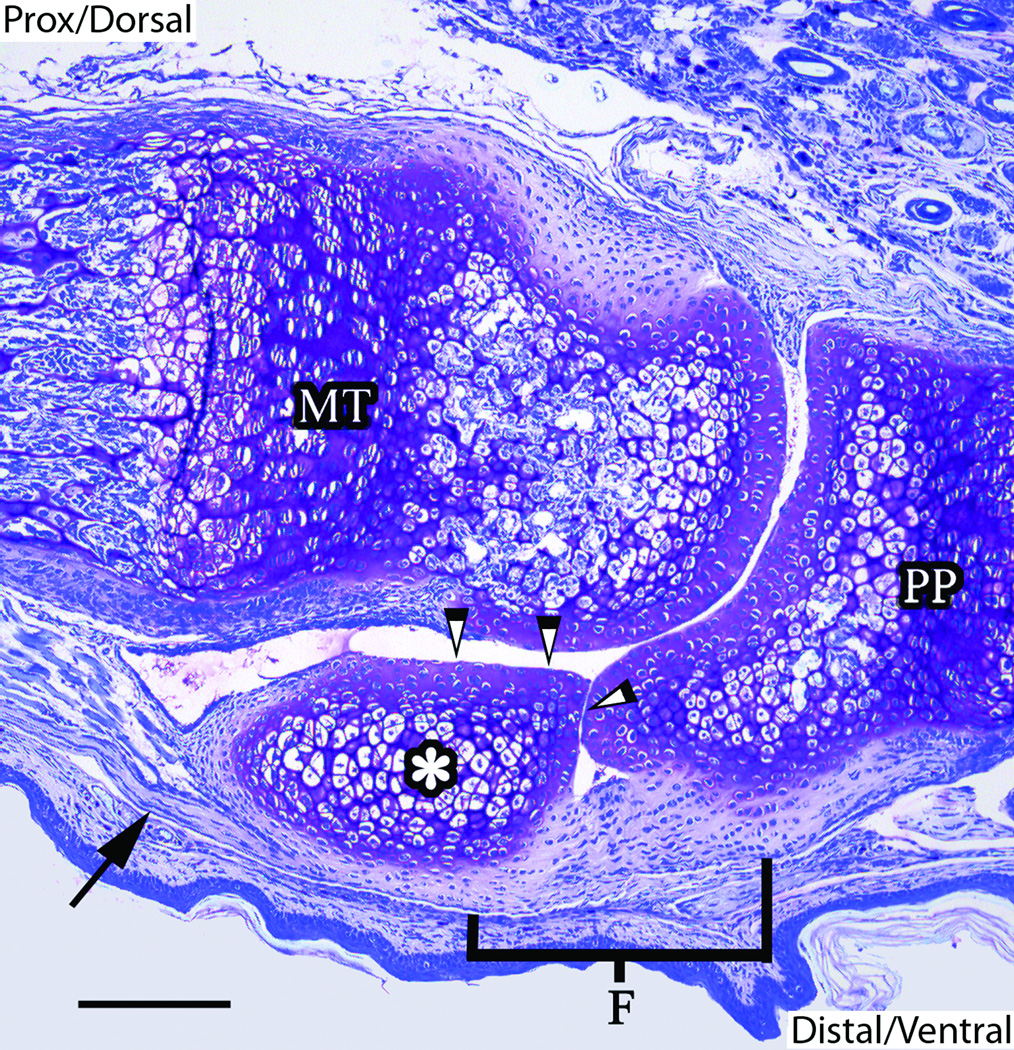
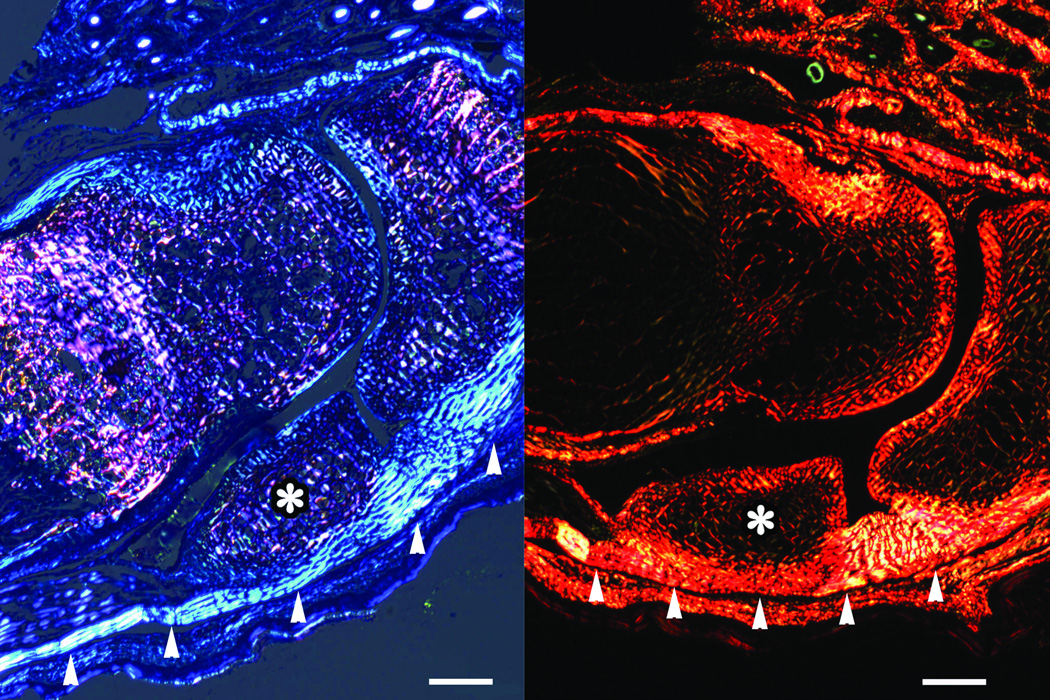
Evidence of sesamoid calcification was not observed in histological slides in the fore paws of mice until three weeks of age. Although samples were necessarily decalcified for histological preparation, the appearance of irregular trabeculae and marrow cavities (that is, the signatures of spongy bone) was indicative of mineralization in these sesamoids (data not shown). The sesamoids of each metapodophalangeal joint were found on either side of the distal end of the metapodia (Fig. 3). These small bones articulated with the metapodia and proximal phalanx on the ventral surface of the joint. Articular cartilage was apparent along the distal and dorsal surfaces of the sesamoids. The flexor digitorum tendons attached to, and ran the length of, the ventral surface of the sesamoids (Fig. 4). These tendons terminated along the ventral surface of the proximal phalanx at a fibrocartilaginous insertion and were especially visible under polarized light microscopy of both toluidine blue- and Picrosirius red-stained sections (Fig. 5). The distinct polarization at the sesamoid insertion site in these slides was indicative of abundant parallel collagen fibers, similar to the general pattern found in tendon (Bancroft and Gamble, 2002; Leeson et al., 1985).
Figure 3.
Figure 4.
Figure 5.
Tissue morphology
Two major cell types, chondrocytes and fibroblasts, were associated with the sesamoids in the transition from tendon, fibrocartilage, cartilage, and bone (Fig. 4). The dorsal and distal surfaces of the sesamoids contained chondrocytes that maintained the articular cartilage in contact with the metapodia and proximal phalanges. In unmineralized sesamoids, round, metachromatic, hypertrophic chondrocytes, residing in distinct lacunae, dominated the interior region of the sesamoid resembling those same cells found in the epiphyseal plates of adjacent metatarsals and proximal phalanges (Fig. 4). The degree of hypertrophy appeared to increase within the more central region of the sesamoids. Proteoglycans, extracellular matrix molecules commonly documented in calcifying tissues, were abundant in the developing sesamoids, metapodia, and proximal phalanges of both the fore and hind paws of one-week-old mice (Fig. 6).
Figure 6.
Fibroblasts were also abundant in the samples examined. Spindle-shaped fibroblasts comprising the tendon (also called tenocytes) were documented in longitudinal columns parallel to the collagen fibers within the tendon anterior to attachment with the sesamoid (Fig. 4). Fibroblasts associated with fibrocartilage transitioned from tenocyte-like cells of the tendon into ovoid-shaped cells approaching the more metachromatic chondrocytes and cartilage of the sesamoid. These transitional cells were arranged in linear isogenous groups at fibrocartilaginous insertions (entheses) and they surrounded the proximal, ventral, and distal borders of the sesamoids. Closer to the interior region of the sesamoid, the fibrocartilage cells became rounder and resided in distinct lacunae as they changed toward a hypertrophic state (Fig. 4). Other chondrocytes appeared distinct from these fibrocartilage and hypertrophic cells and comprised articular surfaces of the sesamoids at the dorsal and portions of their distal borders.
Electron microscopy
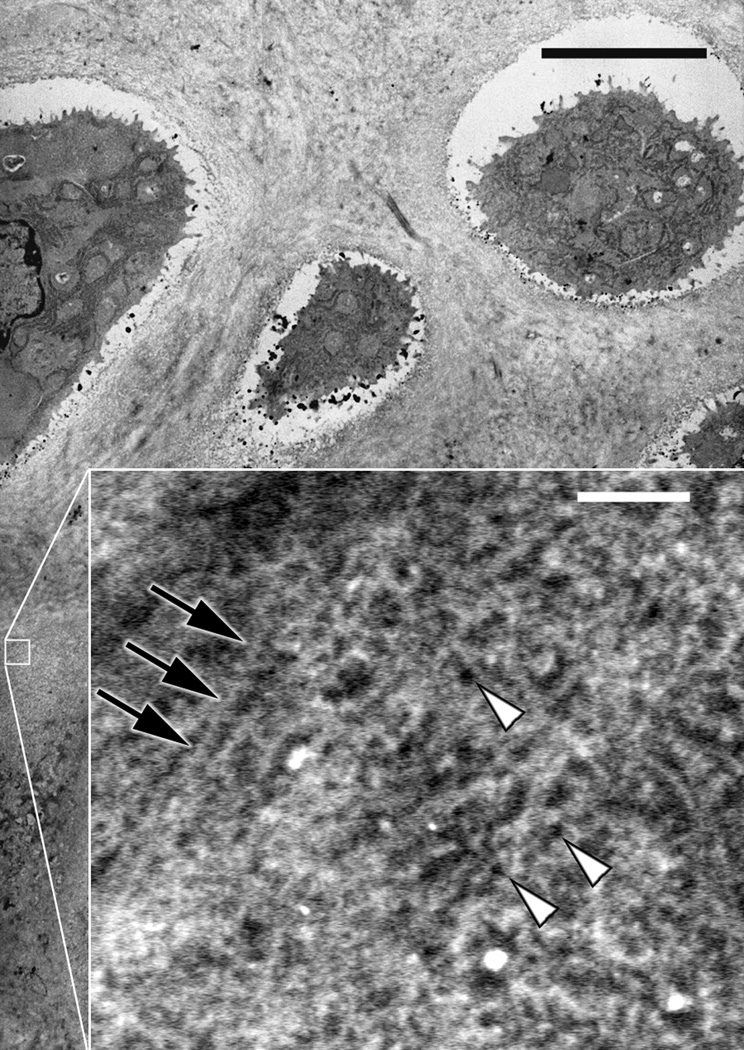
Evidence from radiography, whole mount staining, and histology suggested that the onset and early progression of mineralization events had been established in two-week-old metacarpophalangeal lateral sesamoids. Thus, these tissues were considered most likely to contain matrix vesicles and they were examined by TEM. Analysis showed extracellular vesicle-like structures in a few small clusters in the fibrocartilaginous areas of the sesamoid, except in a single case where they were associated with chondrocytes near the periphery of the sesamoid calcifying core (Fig. 7). None of these vesicles, however, could be definitively identified as matrix vesicles that contributed to the mineralization process in the metapodophalangeal sesamoid bones. In addition, type I collagen fibers were present in large abundance in the associated tendon and fibrocartilage of the sesamoid bones (Fig. 8). The mineralizing cores of the two-week-old sesamoids were devoid of matrix vesicle-like structures and any obvious indications of the large, type I collagen fibers, but extensive proteoglycan networks were evident as well as type II collagen fibers (Fig. 9).
Figure 7.
Figure 8.
Figure 9.
Discussion
The intimate association between the sesamoid bone and tendon at the metapodophalangeal joint has been previously described for numerous vertebrate species (Bizarro, 1921; Hall, 2005; Vickaryous and Olson, 2007). In the present study, this relationship has been investigated in the mouse by light microscopy and polarized light to examine more carefully cell phenotype and cellular organization. The study also included electron microscopy to define the previously undescribed ultrastructural extracellular matrix composition and the means of mineralization in these tissues. Few research reports have considered sesamoid development with the exception of the patella (Bland and Ashhurst, 1997; Reese et al., 2001; Vickaryous and Olson, 2007; Walmsley, 1940). In early patellar development, a cell cluster develops within the patellar tendon shortly after the epiphyses of the femur and tibia are established. The cell cluster grows from the deep surface of the tendon to form a cartilaginous model from which bone develops (Bland and Ashhurst, 1997). Mineralization centers quickly replace the cartilage, and the tendon inserts into the bone through a fibrocartilage interface (Clark and Stechschulte, 1998).
The cellular arrangement and phenotype of the sesamoid-associated entheses investigated here were comparable to that reported in the literature (Benjamin and Evans, 1990; Benjamin and Ralphs, 1998; Cooper and Misol, 1970). Fibroblasts were typical within both the sesamoid-associated tendon and fibrocartilage insertion sites. The flat, spindle-shaped tenocytes of the tendon were arranged in extensive columns parallel to the orientation of collagen fibers. Fibrocartilage cells transitioned from tenocytes into chondrocytes in columnar groups, characteristic of that documented in the four transition zones of entheses (Cooper and Misol, 1970). Chondrocytes comprised the articular surfaces and interior of the sesamoids. Hypertrophic chondrocytes were found within the calcifying core of the sesamoid and these were similar to those within epiphyseal growth plates of the calcifying metapodophalangeal bones and phalanges.
Radiography and whole mount staining provided information regarding sesamoid mineral development in the mouse paw samples, identification and location of mineral at the metapodophalangeal joints, and apparent differences in bone growth between front and hind paws of the animals. Clear evidence of mineral formation in CD-1 murine metapodophalangeal sesamoid bones was not apparent in radiography and histology until three weeks of age. Whole mount-stained results of this study suggest, on the other hand, that mineral is first detectable by one week of age. The mineral is localized to the central core of a cartilaginous model, which expands gradually in size as calcification progresses within the tissue. In addition, the differential onset of calcification of the fore and hind paws reported here has been documented in both the mouse and rat (Johnson, 1933; Spark and Dawson, 1928; Strong, 1925). In these animals, fore paws are reported to begin mineralization in advance of hind paws shortly before and after birth. Seven days after birth, hind paws give the appearance of delayed development because of their greater size compared to that of the fore paws (Spark and Dawson, 1928). The mineralization stages reflected in digits appear to progress in like manner in both paws regardless of animal age.
Further, the present study shows that the murine metacarpophalangeal sesamoids of the second digits (as well as this digit itself) mineralize before other metapodophalangeal sesamoids and their associated digits. The second digit (and associated sesamoids) is followed in a matter of days by the development of the third and fourth digit, then the fifth, and lastly the first digit in these animals. The results reported here are consistent with published literature concerning the maturation of the fore and hind paws of mice and rats (Patton and Kaufman, 1995; Spark and Dawson, 1928; Strong, 1925). A similar calcification pattern is documented in a variety of animals, including humans (Davies and Parsons, 1927). The related progression of the mineralization of the autopod in different species signifies genetically controlled and regulated development of the paws, digits, and sesamoids, much like the other elements of the skeleton (Atchley and Hall, 1991; Karaplis, 2002).
In the current investigation, ultrastructural results failed to demonstrate the involvement of either matrix vesicles or type I collagen in initiating mineralization of metacarpophalangeal sesamoid bones. The apparent absence of extracellular matrix vesicles in these tissues was somewhat unanticipated since vesicles are abundant in extracellular matrices of calcifying tendon, calcifying cartilage, and early bone (Anderson, 1969; Bernard and Pease, 1969; Landis, 1986; Yamada, 1970), tissues resembling the sesamoid and related structures. Previous analyses of matrix vesicles in calcifying cartilage provide evidence that their structure and enzyme composition (particularly alkaline phosphatase) support accumulation, supersaturation, nucleation, and growth of mineral ions (Ali et al., 1970; Anderson, 1969). Thus, the absence of vesicles excludes their effects on mineralization in the cartilaginous anlage of murine sesamoids and implies that other extracellular matrix molecules are responsible for initiating sesamoid mineral formation.
Like vesicles, type I collagen in the tendon and entheses of the metapodophalangeal sesamoids could not be identified as contributing to the mineralization process of these bones. However, because these samples were decalcified for electron microscopy and planes of sections of embedded specimen blocks examined here may not have been optimally aligned, identification of mineral associated with collagen was not possible. In contrast, calcifying avian leg and wing tendons support mineral nucleation events associated with collagen fibrils and fibers (Landis et al., 1993; 1996; Landis and Song, 1991). In these tissues, nucleation may occur on the surface of collagen fibrils and fibers and/or within their periodic hole and overlap zones (Hodge and Petruska, 1963) as the extracellular matrix of the tendon calcifies. Surface mineral crystals and those growing from inside the fibers presumably fuse or coalesce to form a continuous mineralized structure with the associated collagen (Landis, 1986; Landis et al., 1993; 1996; Landis and Song, 1991).
In a related context, no definitive electron microscopic evidence of remnants of mineralization, such as crystal ghosts described by Bonucci (1969), was noted in, or associated with, any extracellular structure of the specimens. In these circumstances, it does not appear that the extensive enthesis or associated tendon contributes to the mineralization of metapodophalangeal sesamoid bones even though the sesamoid develops entirely within the tendon. Since fibrocartilage is composed of type I and type II collagen and often normally calcifies at the enthesis, it was expected that this tissue would participate in the mineralization of murine metacarpophalangeal sesamoids because of its close involvement with the developing sesamoid.
On the other hand, the presence of extensive proteoglycan networks and type II collagen fibrils suggests these two molecules may be principal mineralization mediators of the calcifying cartilage of sesamoid bones in the absence of matrix vesicles and type I collagen. The precise role of proteoglycans in the mineralization process has been historically difficult to determine (Bonucci, 2007; Lowenstam and Weiner, 1989). Some evidence has suggested, however, that molecules such as proteoglycans comprised in part of acid groups are intimately associated with the initial crystal nodules of calcifying vertebrate tissues (Bonucci, 2007). Proteoglycans consist of numerous sulfated and carboxylated polysaccharides and are considered to be both inhibitors and facilitators of mineralization events in calcifying tissues in which they are found (Byers et al., 1997; Mwale et al., 2000). Type II collagen has not been as extensively examined as type I collagen in relation to the mineralization process of vertebrates, regardless of the fact that it is the most abundant collagen in calcifying cartilage. There are currently no definitive data suggesting that mineralization is mediated by type II collagen, although its importance as a structural framework for mineral deposition has been acknowledged (Bonucci, 2007).
In summary, the composite cell types and their organization, sesamoid morphological interrelations, and a developmental timeline leading to mineralization have been reexamined in new and more complete detail for murine metapodophalangeal sesamoid bones and their associated tendons and fibrocartilaginous entheses. In addition, ultrastructural extracellular matrix constituents involved in specific mineralization events have been investigated and described for the first time for these tissues. No evidence of vesicle- or type I collagen-mediated mineralization events was found in either the sesamoids or their tendon/fibrocartilage, although mineral formation had occurred in the sesamoids themselves by two weeks of age and it increased progressively with time and maturation of these bones. Such mineral deposition was clearly associated with the extracellular matrix at the cartilage core of the sesamoids, composed principally of type II collagen and proteoglycans.
The genetic maintenance of sesamoids in numerous vertebrate species, in addition to their unique spatial association with tendon/fibrocartilage and their development and mineralization localized within tendon, reflect on the yet unclear potentially important function(s) for these highly conserved skeletal components. Among the most often cited hypotheses of direct sesamoid function are that they serve to stabilize the joint (Hildebrand, 1985), reduce friction and wear on the tendon (Goldberg and Nathan, 1987; Gray, 1918; Le Minor, 1988), and resist compressive forces (Bland and Ashhurst, 1997; Gray, 1918; Kaplan, 1984). Based on the features of metapodophalangeal sesamoids elaborated in the present study, including mineralization of sesamoids at particular sites in tendon and attachment of sesamoids through their associated tendon to adjacent tissues, it is possible that these bones also function as fulcrum points that effectively foreshorten tendon length. A result of this circumstance would be to alter biomechanical properties of the tendon and increase them advantageously as the muscle executes its effects on the associated tendon invested in its sesamoid. This concept is consistent with previous suggestions that sesamoids act to increase the mechanical advantage of the muscle at a joint (Alexander and Dimery, 1985; Benjamin et al., 2006; Currey, 2002).
Besides the question of a defined sesamoid function(s), equally intriguing and unknown is the basis for the specific location within a tendon at which a sesamoid bone develops, whether through genetic determinants or predisposition of the involved cells, local force concentration or perturbation, or some other means. These and other aspects of function for the sesamoid, and for vertebrate mineralization in general, are undergoing further investigation in this laboratory in the context of comparative anatomy and tissue adaptation among different species.
Acknowledgements
The authors are especially grateful to Ms. Jeanette Killius and Dr. Hans Thewissen (Department of Anatomy and Neurobiology, Northeastern Ohio Universities Colleges of Medicine and Pharmacy) for training in electron microscopy and support of this study, respectively. The authors also thank Dr. Chris Vinyard, Mr. Nick Robl, and Ms. Brooke Armfield (Department of Anatomy and Neurobiology, Northeastern Ohio Universities Colleges of Medicine and Pharmacy) for their helpful comments during the editing process of this manuscript. This work was funded by research grants from the Skeletal Biology Research Focus Area of the Northeastern Ohio Universities Colleges of Medicine and Pharmacy, the Graduate Student Senate of Kent State University, and the National Institutes of Health, Washington, DC (Grant AR 41452 to WJL).
References
- Alexander RM, Dimery NJ. The significance of sesamoids and retro-articular processes for the mechanics of joints. J Zool. 1985;205:357–371. [Google Scholar]
- Ali SY, Sajdera SW, Anderson HC. Isolation and characterization of calcifying matrix vesicles from epiphyseal cartilage. Proc Natl Acad Sci USA. 1970;67:1513–1520. doi: 10.1073/pnas.67.3.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson HC. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol. 1969;41:59–72. doi: 10.1083/jcb.41.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenault AL. A comparative electron microscopic study of apatite crystals in collagen fibrils of rat bone, dentin and calcified turkey leg tendons. Bone Miner. 1989;6:165–177. doi: 10.1016/0169-6009(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Atchley WR, Hall BK. A model for development and evolution of complex morphological structures. Biol Rev. 1991;66:101–157. doi: 10.1111/j.1469-185x.1991.tb01138.x. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Evans EJ. Fibrocartilage. J Anat. 1990;171:1–15. [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Evans EJ, Copp L. The histology of tendon attachments to bone in man. J Anat. 1986;149:89–100. [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Ralphs JR. Fibrocartilage in tendons and ligaments: An adaptation to compressive load. J Anat. 1998;193:481–494. doi: 10.1046/j.1469-7580.1998.19340481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Toumi H, Ralphs JR, Bydder G, Best TM, Milz S. Where tendons and ligaments meet bone: Attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J Anat. 2006;208:471–490. doi: 10.1111/j.1469-7580.2006.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard GW, Pease DC. An electron microscopic study of initial intramembranous osteogenesis. Am J Anat. 1969;125:271–290. doi: 10.1002/aja.1001250303. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H, Butler WT, Taylor RE. Proteins of the periodontium: Characterization of the insoluble collagens of bovine dental cementum. Calcif Tissue Res. 1977;23:39–44. doi: 10.1007/BF02012764. [DOI] [PubMed] [Google Scholar]
- Bizarro AH. On sesamoid and supernumerary bones of the limbs. J Anat. 1921;55:256–268. [PMC free article] [PubMed] [Google Scholar]
- Bland YS, Ashhurst DE. Fetal and postnatal development of the patella, patellar tendon and suprapatella in the rabbit; changes in the distribution of the fibrillar collagens. J Anat. 1997;190:327–342. doi: 10.1046/j.1469-7580.1997.19030327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonucci E. Further investigation on the organic-inorganic relationship in calcifying cartilage. Calcif Tissue Res. 1969;3:38–54. doi: 10.1007/BF02058644. [DOI] [PubMed] [Google Scholar]
- Bonucci E. Biological Calcification: Normal and Pathological Processes in the Early Stages. New York: Springer-Verlag; 2007. [Google Scholar]
- Byers S, van Rooden JC, Foster BK. Structural changes in the large proteoglycan, aggrecan, in different zones of the ovine growth plate. Calcif Tissue Int. 1997;60:71–78. doi: 10.1007/s002239900188. [DOI] [PubMed] [Google Scholar]
- Callis GM. Bone. In: Bancroft JD, Gamble M, editors. Theory and Practice of Histological Techniques. New York: Churchill Livingstone; 2002. pp. 274–279. [Google Scholar]
- Clark G. Staining Procedures used by the Biological Stain Commission. Baltimore: Williams and Wilkins Co.; 1981. pp. 183–184. [Google Scholar]
- Clark J, Stechschulte DJ. The interface between bone and tendon at an insertion site: A study of the quadriceps tendon insertion. J Anat. 1998;193:605–616. doi: 10.1046/j.1469-7580.1998.19240605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper RR, Misol S. Tendon and ligament insertion: A light and electron microscopic study. J Bone Joint Surg Am. 1970;52:1–20. [PubMed] [Google Scholar]
- Currey JD. Bones: Structure and Mechanics. Princeton: Princeton University Press; 2002. Bones, tendons, and muscles; pp. 277–280. [Google Scholar]
- Davies DA, Parsons FG. The age order of the appearance and union of the normal epiphyses as seen by x-rays. J Anat. 1927;62:58–71. [PMC free article] [PubMed] [Google Scholar]
- Gerstenfeld LC, Toma CD, Schaffer JL, Landis WJ. Chondrogenic potential of skeletal cell populations: Selective growth of chondrocytes and their morphogenesis and development in vitro. Microsc Res Tech. 1998;43:156–173. doi: 10.1002/(SICI)1097-0029(19981015)43:2<156::AID-JEMT8>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Glimcher MJ. The nature of the mineral component of bone and the mechanism of calcification. Instr Course Lect. 1987;36:49–69. [PubMed] [Google Scholar]
- Glimcher MJ. Mechanism of calcification: Role of collagen fibrils and collagen-phosphoprotein complexes in vitro and in vivo. Anat Rec. 1989;224:139–153. doi: 10.1002/ar.1092240205. [DOI] [PubMed] [Google Scholar]
- Goldberg I, Nathan H. Anatomy and pathology of the sesamoid bones: The hand compared to the foot. Int Orthop. 1987;11:141–147. doi: 10.1007/BF00266700. [DOI] [PubMed] [Google Scholar]
- Gray H. The sesamoid bones. In: Lewis WH, editor. Anatomy of the Human Body. Philadelphia: Lea and Febiger; 1918. p. 1396. [Google Scholar]
- Gupta HS, Roschger P, Zizak I, Fratzl-Zelman N, Nader A, Klaushofer K, Fratzl P. Mineralized microstructure of calcified avian tendons: A scanning small angle X-ray scattering study. Calcif Tissue Int. 2003;72:567–576. doi: 10.1007/s00223-002-1031-8. [DOI] [PubMed] [Google Scholar]
- Hall BK. Bones and Cartilage: Developmental and Evolutionary Skeletal Biology. San Diego: Elsevier Academic Press; 2005. Tendons and sesamoids; pp. 120–122. [Google Scholar]
- Hildebrand M. Digging of quadrupeds. In: Hildebrand M, Bramble DM, Liem KF, Wake DB, editors. Functional Vertebrate Morphology. Cambridge: The Belknap Press of Harvard University Press; 1985. pp. 102–104. [Google Scholar]
- Hodge AJ, Petruska JA. Recent studies with the electron microscope on ordered aggregates of the tropocollagen macromolecule. In: Ramachandran GN, editor. Aspects of Protein Structure. New York: Academic Press; 1963. pp. 289–300. [Google Scholar]
- Hsu HT, Anderson HC. Calcification of isolated matrix vesicles and reconstituted vesicles from fetal bovine cartilage. J Cell Biol. 1978;75:3805–3808. doi: 10.1073/pnas.75.8.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ML. The time and order of appearance of ossification centers in the albino mouse. Am J Anat. 1933;52:241–271. [Google Scholar]
- Joseph J. The sesamoid bones of the hand and the time of fusion of the epiphyses of the thumb. J Anat. 1951;85:230–241. [PMC free article] [PubMed] [Google Scholar]
- Kaplan EB. The thumb. In: Spinner M, editor. Kaplan’s Functional and Surgical Anatomy of the Hand. Philadelphia: Lippincott; 1984. pp. 119–121. [Google Scholar]
- Karaplis AC. Embryonic development of bone and the molecular regulation of intramembranous and endochondral bone formation. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of Bone Biology. San Diego: Academic Press; 2002. pp. 33–58. [Google Scholar]
- Kaufman MH. The Atlas of Mouse Development. San Diego: Academic Press; 1992. [Google Scholar]
- Kirsch T, Nah HD, Shapiro IM, Pacifici M. Regulated production of mineralization-competent matrix vesicles in hypertrophic chondrocytes. J Cell Biol. 1997;137:1149–1160. doi: 10.1083/jcb.137.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis WJ. A study of calcification in the leg tendons from the domestic turkey. J Ultrastruct Mol Struct Res. 1986;94:217–238. doi: 10.1016/0889-1605(86)90069-8. [DOI] [PubMed] [Google Scholar]
- Landis WJ, Hodgens KJ, Song MJ, Arena J, Kiyonaga S, Marko M, Owen C, McEwen BF. Mineralization of collagen may occur on fibril surfaces: Evidence from conventional and high-voltage electron microscopy and three-dimensional imaging. J Struct Biol. 1996;117:24–35. doi: 10.1006/jsbi.1996.0066. [DOI] [PubMed] [Google Scholar]
- Landis WJ, Silver FH. The structure and function of normally mineralizing avian tendons. Comp Biochem Physiol A. 2002;133:1135–1157. doi: 10.1016/s1095-6433(02)00248-9. [DOI] [PubMed] [Google Scholar]
- Landis WJ, Song MJ. Early mineral deposition in calcifying tendon characterized by high voltage electron microscopy and three-dimensional graphic imaging. J Struct Biol. 1991;107:116–127. doi: 10.1016/1047-8477(91)90015-o. [DOI] [PubMed] [Google Scholar]
- Landis WJ, Song MJ, Leith A, McEwen L, McEwen BF. Mineral and organic matrix interaction in normally calcifying tendon visualized in three dimensions by high-voltage electron microscopic tomography and graphic image reconstruction. J Struct Biol. 1993;110:39–54. doi: 10.1006/jsbi.1993.1003. [DOI] [PubMed] [Google Scholar]
- Le Minor JM. The ventral metacarpo- and metatarso-phalangeal sesamoid bones: Comparative anatomy and evolutionary aspects. Gegenbaurs Morphol Jahrb. 1988;134:693–731. [PubMed] [Google Scholar]
- Leeson CR, Leeson TS, Paparo AA. Textbook of Histology. Philadelphia: W. B. Saunders Company; 1985. [Google Scholar]
- Lowenstam HA, Weiner S. On Biomineralization. New York: Oxford University Press; 1989. [Google Scholar]
- Mwale F, Billinghurst C, Wu W, Alini M, Webber C, Reiner A, Ionescu M, Poole J, Poole AR. Selective assembly and remodeling of collagens II and IX associated with expression of the chondrocyte hypertrophic phenotype. Dev Dyn. 2000;218:648–662. doi: 10.1002/1097-0177(200008)218:4<648::AID-DVDY1022>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Patton JT, Kaufman MH. The timing of ossification of the limb bones, and growth rates of various long bones of the fore and hind limbs of the prenatal and early postnatal laboratory mouse. J Anat. 1995;186:175–185. [PMC free article] [PubMed] [Google Scholar]
- Plate U, Tkotz T, Wiesmann HP, Stratmann U, Joos U, Höhling HJ. Early mineralization of matrix vesicles in the epiphyseal growth plate. J Microsc. 1996;183:102–107. doi: 10.1046/j.1365-2818.1996.67430.x. [DOI] [PubMed] [Google Scholar]
- Reese S, Pfuderer UR, Bragulla H, Loeffler K, Budras KD. Topography, structure and function of the patella and the patelloid in marsupials. Anat Histol Embryol. 2001;30:189–294. doi: 10.1046/j.1439-0264.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- Rufai A, Ralphs JR, Benjamin M. Ultrastructure of fibrocartilage at the insertion of the rat achilles tendon. J Anat. 1996;189:185–191. [PMC free article] [PubMed] [Google Scholar]
- Siperko LM, Landis WJ. Aspects of mineral structure in normally calcifying avian tendon. J Struct Biol. 2001;135:313–320. doi: 10.1006/jsbi.2001.4414. [DOI] [PubMed] [Google Scholar]
- Spark C, Dawson AB. The order and time of appearance of centers of ossification in the fore and hind limbs of the albino rat, with special reference to the possible influence of the sex factor. Am J Anat. 1928;41:411–445. [Google Scholar]
- Stratmann U, Schaarschmidt K, Wiesmann HP, Plate U, Höhling HJ, Szuwart T. The mineralization of mantle dentine and of circumpulpal dentine in the rat: An ultrastructural and element-analytical study. Anat Embryol. 1997;195:289–297. doi: 10.1007/s004290050048. [DOI] [PubMed] [Google Scholar]
- Strong RM. The order, time, and rate of ossification of the albino rat (Mus Norvegicus albinus) skeleton. Am J Anat. 1925;36:313–355. [Google Scholar]
- Szuwart T, Kierdorf H, Kierdorf U, Clemen G. Ultrastructural aspects of cartilage formation, mineralization, and degeneration during primary antler growth in fallow deer (Dama dama) Ann Anat. 1998;180:501–510. doi: 10.1016/S0940-9602(98)80055-1. [DOI] [PubMed] [Google Scholar]
- Vickaryous MK, Olson WM. Sesamoids and ossicles in the appendicular skeleton. In: Hall BK, editor. Fins into Limbs: Evolution, Development, and Transformation. Chicago: University of Chicago Press; 2007. pp. 323–341. [Google Scholar]
- Walmsley R. The development of the patella. J Anat. 1940;74:360–370. [PMC free article] [PubMed] [Google Scholar]
- Wassersug RJ. A procedure for differential staining of cartilage and bone in whole formalin-fixed vertebrates. Stain Technol. 1976;51:131–134. doi: 10.3109/10520297609116684. [DOI] [PubMed] [Google Scholar]
- Wood VE. The sesamoid bones of the hand and their pathology. J Hand Surg. 1984;9:261–264. doi: 10.1016/0266-7681(84)90038-x. [DOI] [PubMed] [Google Scholar]
- Yamada M. Ultrastructural and cytochemical studies on the calcification of the tendon-bone joint. Arch Histol Jpn. 1976;39:347. doi: 10.1679/aohc1950.39.347. [DOI] [PubMed] [Google Scholar]