Abstract
Wnt3a encodes a signal that is expressed in the primitive streak of the gastrulating mouse embryo and is required for paraxial mesoderm development. In its absence cells adopt ectopic neural fates. Embryos lacking the T-box-containing transcription factors, Brachyury or Tbx6, also lack paraxial mesoderm. Here we show that Brachyury is specifically down-regulated in Wnt3a mutants in cells fated to form paraxial mesoderm. Transgenic analysis of the T promoter identifies T (Brachyury) as a direct transcriptional target of the Wnt signaling pathway. Our results suggest that Wnt3a, signaling via Brachyury, modulates a balance between mesodermal and neural cell fates during gastrulation.
Keywords: Gastrulation, mesoderm, neural, cell fate, Wnt, Brachyury
The embryonic mesoderm of the mammalian embryo is formed by a series of inductive interactions first in the primitive streak, which gives rise to head and trunk mesoderm, and later in the tailbud, which generates the most posterior mesoderm of the tail. As development progresses, successively posterior structures are generated, leading to a posterior extension of the body axis. Previous studies have established that Wnt3a, which encodes a member of the Wnt family of secreted signaling molecules (for review, see Cadigan and Nusse 1997; Moon et al. 1997), is expressed in pluripotent ectoderm cells of the primitive streak during gastrulation (Takada et al. 1994). At early somite stages [8.0–8.5 days postcoitum (dpc)], the Wnt3a expression domain correlates with a domain of cells in the anterior primitive streak fated to give rise to paraxial mesoderm (for review, see Tam and Trainor 1994; Wilson and Beddington 1996). Moreover, the anterior and lateral limits of the Wnt3a expression domain lie between cells fated to give rise to paraxial mesoderm and cells that will give rise to neural ectoderm.
A requirement for Wnt3a in the specification of trunk and tail paraxial somitic mesoderm fates has been demonstrated by mutant analyses. Wnt3a homozygous null mutant embryos lack all but the anterior-most seven to nine somites (Takada et al. 1994; Greco et al. 1996; Yoshikawa et al. 1997). As a consequence, only the most rostral cervical vertebrae are formed. Histological and molecular analyses demonstrate that ectopic neural structures form in place of posterior paraxial mesoderm (Yoshikawa et al. 1997). Similar results have been reported recently for compound mutants in the high mobility group (HMG) domain containing transcriptional regulators Lef1 and Tcf1 (Galceran et al. 1999). Because members of this gene family function as transcriptional effectors of Wnt signaling (Nusse 1999), these results indicate that Lef1 and Tcf1 likely mediate Wnt3a's effects on paraxial mesoderm development.
Mesoderm specification is thought to be regulated, at least in part, by members of the T-box gene family of DNA-binding transcription factors (Smith 1999). Two of these, T and Tbx6, are coexpressed with Wnt3a in the primitive streak during gastrulation (Takada et al. 1994; Chapman et al. 1996). Mutations in either gene lead to a loss of trunk and tail mesoderm (Chesley 1935; Chapman and Papaioannou 1998). Ectopic neural tubes form in place of paraxial mesoderm in the Tbx6 mutants, but it is not clear how similar the neural tube abnormalities noted in the T homozygotes are to the Wnt3a phenotype. Given the similarities between the Wnt3a and T-box mutant phenotypes, we have investigated the possibility that Wnt3a signaling functions to regulate T-box activity.
Results and Discussion
To begin to unravel the potential relationships between Wnt3a, T, and Tbx6, we first examined the extent of the similarities between the Wnt3a and T mutant neural tube phenotypes. Histological analysis of serial cross sections through the posterior neural folds of an 8.0 dpc T homozygous mutant demonstrated abnormal folding and kinking of the neural folds that culminated in tubular epithelial structures lying ventral to the developing endogenous neural folds (data not shown). Whole-mount in situ hybridization analysis of 9.5 dpc T mutants for the expression of genes that mark the neural or mesoderm lineages reveals the neural character of the ectopic tubes. Although the expression of markers of paraxial mesoderm such as Mox1 (Fig. 1A) are significantly reduced in the posterior end of the T mutant embryo as expected (Fig. 1B), neural tube markers such as the dorsally expressed Wnt3a (Fig. 1C) and Hes5 (Fig. 1E) are expressed ectopically (Fig. 1D and F, respectively). Examination of sections of whole-mount stained mutant embryos demonstrates ectopic expression of Wnt3a (Fig. 1H), Hes5 (Fig. 1I), and Pax3 (Fig. 1J) in ectopic tubular epithelial structures that lie immediately ventral to the normal neural tube. Thus, as in Wnt3a null mutants (Takada et al. 1994; Yoshikawa et al. 1997), ectopic neural structures appear in embryos homozygous for the T mutation. Interestingly, small patches of Mox1 expression are observed occasionally in the ectopic tubes (Fig. 1B,G) suggesting that some cells may still maintain mesodermal character. Taken together, these results demonstrate the presence of ectopic neural tube-like structures in regions displaying reduced paraxial mesoderm, further emphasizing the similarities between the T, Tbx6, and Wnt3a mutant phenotypes.
Figure 1.
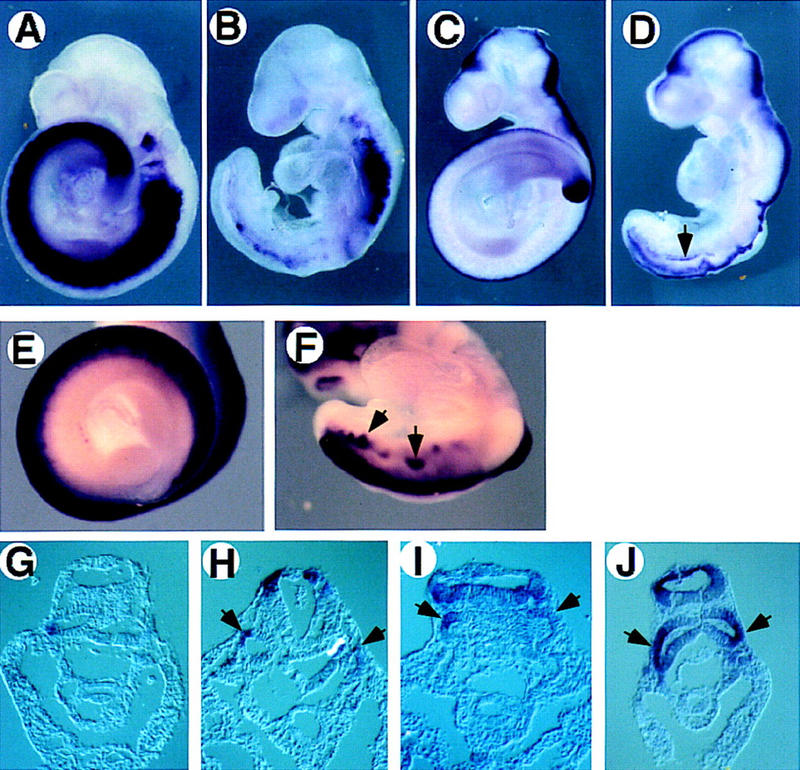
Expression of mesodermal and neural markers indicates ectopic neural tube formation in T/T mutant embryos. Wild-type (A,C,E) and T/T mutant (B,D,F,G,H,I,J) embryos were hybridized with Mox1 (A,B,G), Wnt3a (C,D,H), Hes5 (E,F,I), and Pax3 (J) probes. Whole-mount embryos (A–F) and transverse sections posterior to the forelimb level (G–J) are depicted. In a T/T mutant embryo, several neural markers including Wnt3a, Hes5, and Pax3 are expressed in an ectopic tube lying ventral to the endogenous neural tube (arrows in D,F,H,I,J) demonstrating the neural character of the ectopic tube.
To determine whether T or Tbx6 could function in the Wnt3a signaling pathway, we examined their expression in embryos lacking Wnt3a function. Activation of T and Tbx6 transcription is independent of Wnt3a, as both genes are expressed prior to the onset of Wnt3a expression at 7.5 dpc (Wilkinson et al. 1990; Takada et al. 1994; Chapman et al. 1996). However, Wnt3a could be required to maintain their transcription. Because the first 7 somites form in T, Tbx6, and Wnt3a homozygous mutants, and the precursors of the more posterior somites 8–12 leave the streak at the 3- to 7-somite stage (Wilson and Beddington 1996; Tam and Beddington 1987), we reasoned that examination of T and Tbx6 gene expression in Wnt3a mutant embryos should focus on these early somite stages. At this time, T mRNA is normally expressed throughout the entire anterior–posterior (A-P) length of the primitive streak, as well as the node and notochord (Fig. 2A,B). However, T expression was completely absent from the anterior half of the primitive streak of 2- to 4-somite Wnt3a−/− embryos (arrows, Fig. 2C,D). The down-regulation of T in the anterior streak was observed as early as the 0-somite stage (data not shown), several hours before any morphological abnormalities were evident in the mutant. The remainder of the T expression domain in the posterior streak, node, and notochord was unaffected at these stages except for an abnormal domain of T expression in the posterior region of the node (data not shown). Thus, the specific down-regulation of T in the region of the streak fated to give rise to somites is consistent with the view that T is a target of Wnt3a signaling during the regulation of paraxial mesoderm fates.
Figure 2.
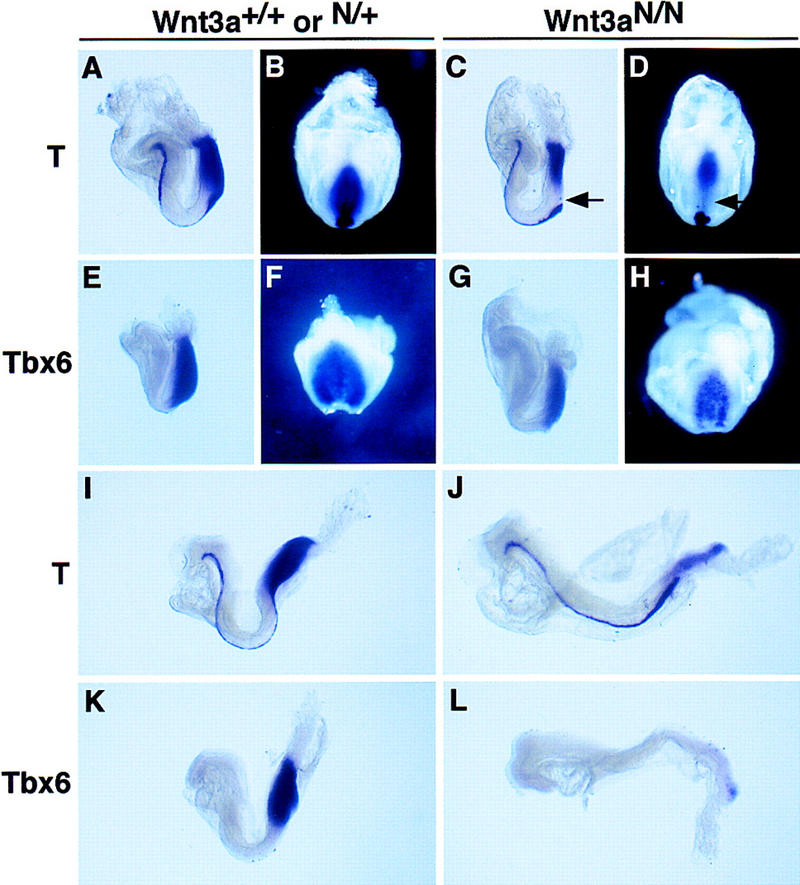
In situ hybridization analysis of T-box gene expression in early somite-stage embryos lacking Wnt3a activity. Embryos are viewed laterally and oriented such that anterior is to the left and dorsal is up, with the exception of B, D, F, and H, which are ventral–posterior views. (A,B) T is expressed throughout the entire A-P extent of the primitive streak ectoderm and mesoderm, and in the node and notochord of wild-type 2-somite-stage embryos. (C,D) T mRNA is absent in the anterior primitive streak of equivalent stage Wnt3a−/− embryos (arrows), but expression is unaffected in the posterior primitive streak, node, and notochord. (E,F) Tbx6 is expressed throughout the entire A-P extent of the primitive streak and paraxial presomitic mesoderm of wild-type embryos. (G,H) Unlike T, Tbx6 continues to be expressed in the anterior primitive streak of early somite stage embryos lacking Wnt3a. (I,J) After the 6-somite stage, T is significantly down-regulated throughout the entire primitive streak in Wnt3a mutants. In contrast, normal expression levels are observed in the notochord. (K,L) Only a small domain of Tbx6 expression is detected in the posterior-most end of the primitive streak of Wnt3a−/− embryos around the 6 somite stage, whereas strong expression is found throughout the primitive streak and paraxial mesoderm of the wild-type control.
In contrast to T, Tbx6 continued to be expressed strongly in the anterior streak of Wnt3a mutant embryos at the 0- to 2-somite stage (data not shown) and only moderately down-regulated in Wnt3a mutant embryos at the 2- to 4-somite stage (Fig. 2G,H). These results suggest that T, and not Tbx6, may be a direct target of Wnt3a signaling. By the 6-somite stage, low-level T expression was observed in the streak ectoderm of Wnt3a−/− mutants but not in migrating mesodermal precursors (Fig. 2I,J). Consistent with this observation, Tbx6, which at this time is expressed almost exclusively in migrating mesodermal cells, was virtually absent from the primitive streak (Fig. 2K,L). T expression in the notochord remained unaffected. Thus, in the absence of Wnt3a, early T expression was lost specifically in the anterior primitive streak in the cells normally fated to give rise to paraxial mesoderm. This was followed by a more general reduction of Tbx6 and T expression in the mesoderm of the primitive streak, presumably reflecting the widespread loss of mesoderm progenitors at these later stages.
To address the possibility that T is a direct target of Wnt3a signaling, we examined the transcriptional regulation of T. It is now well established that a subclass of Wnt family members regulate target gene expression through the nuclear translocation of a transcriptional complex containing β-catenin and a member of the Tcf family (Nusse 1999). Although Lef1 and Tcf1 single mutants form normal somites, double mutants display a paraxial mesoderm phenotype identical to that observed in Wnt3a mutants, indicating that these factors have redundant roles in transducing Wnt3a signals (Galceran et al. 1999). Both Lef1 and Tcf1 were expressed in the primitive streak of Wnt3a mutants in a normal fashion at 7.5 dpc and early somite stages, indicating that their expression is independent of Wnt3a (data not shown).
To establish whether T is a direct target of Lef1 and Tcf1, we examined the T promoter for Lef1/Tcf1 (hereafter referred to as Tcf) binding sites. Examination of a proximal 500-bp region of the mouse T promoter that contains elements that are sufficient to drive expression of a reporter gene in the primitive streak domain, but not in the node and notochord (Fig. 3C; Clements et al. 1996), identified two canonical Tcf1 binding sites (Fig. 3A; van de Wetering et al. 1991). A distal site was located at position −358 to −352, relative to the start of transcription, and a proximal site, in reverse orientation, was located at position −191 to −185 (Fig. 3A). The two Tcf binding sites found in the T promoter are identical to sites found in several vertebrate and invertebrate gene regulatory elements that are known to be Wnt-responsive and capable of binding Lef1 or Tcf1 with high affinity, and in a sequence-specific fashion (Tetsu and McCormick 1999 and references therein). The presence of putative Tcf binding sites is consistent with direct regulation of T by Wnt3a signaling. Interestingly, consensus E boxes presumably capable of binding bHLH transcription factors were also found in the T promoter adjacent to each of these two Tcf binding sites, approximately one turn of the DNA helix away from the adjacent Tcf binding site. The same relative orientation of Tcf binding sites and E boxes was found in the proximal 225 bp of the Xbra (the Xenopus T homolog), and Xbra2 (a pseudogene of Xbra) promoters (Fig. 3A,B; Artinger et al. 1997; Latinkic et al. 1997), which demonstrates a conservation within the regulatory region between these vertebrates, suggesting that these sequences may participate in the assembly of multiprotein enhancer complexes.
Figure 3.
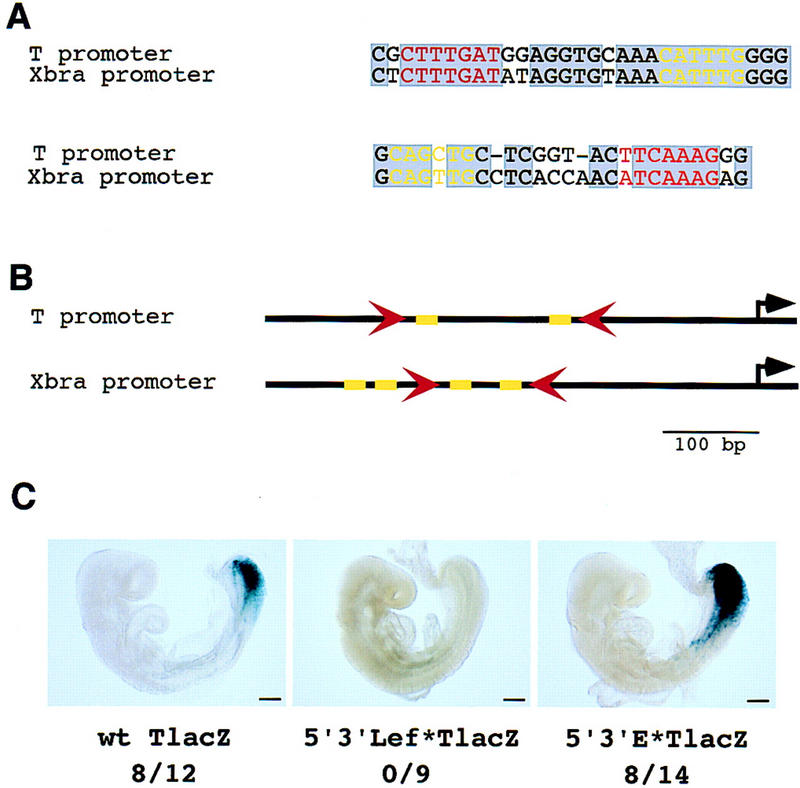
Canonical Tcf binding sites in the T promoter are required for expression in the primitive streak and paraxial mesoderm of transgenic embryos. (A) Two canonical Tcf binding sites (red) are present in the mouse T promoter and conserved in the Xenopus Xbra promoter. A canonical E box motif (yellow) flanks each of the Tcf sites in both the Xenopus and mouse T promoters. (B) A schematic diagram illustrating the relative orientation of the Tcf binding sites (red arrowheads) and the E boxes (yellow rectangles) in the mouse and frog T promoters. (C) (Left) The expression of a lacZ reporter driven by the wild-type T promoter in the primitive streak and paraxial presomitic mesoderm of a transgenic 8.75 dpc embryo; (middle) a representative example of an embryo carrying a T promoter transgene mutated in each of the two Tcf binding sites; (right) an embryo carrying a T promoter transgene mutated in each of the two putative E boxes. The fraction of embryos expressing the lacZ transgene in the primitive streak is indicated below. Bar, 200 μm.
To test the model, we first generated mutations in both of the Tcf sites and examined the activity of a reporter gene in transgenic embryos. Eight of 12 (66.6%) embryos transgenic for the wild-type T promoter expressed the lacZ reporter in the 8.75 dpc primitive streak and hindgut (Fig. 3C, left). The remaining four transgenic embryos had no β-galactosidase activity at all. Interestingly, no β-galactosidase activity was detected in the primitive streaks of any of nine independent transgenic embryos carrying the mutated transgene (Fig. 3C, middle). These results demonstrate that the Tcf sites are essential for expression of T in the primitive streak. The distal Tcf site alone, or both sites together, is required for activity, as a 95-bp deletion that removes the distal site abolishes promoter activity (Clements et al. 1996). In contrast, a transgene containing mutations generated in both E boxes of the T promoter was expressed in 8/14 (57%) transgenic embryos (Fig. 3C, right), a similar percentage to the unmodified construct. Thus, despite their position and conservation, the E boxes are not necessary for expression of T in the primitive streak. From these results we conclude that regional expression of T in the primitive streak is most likely directly regulated by signaling through the Wnt3a pathway. Furthermore, this regulatory interaction is essential for the development of most paraxial mesoderm.
Although we have established that T is a target of the Wnt3a pathway, we were interested in determining whether Wnt3a itself could be a target of T, thereby establishing an autoregulatory positive feedback loop essential for paraxial mesoderm development. Previous reports have documented that activation of Wnt3a transcription does not appear to require T activity, as Wnt3a expression is initially normal in the streak of T mutants (Rashbass et al. 1994). Furthermore, Wnt3a transcripts continue to be expressed in Wnt3a mutants in the anterior primitive streak of early somite stage embryos when T is no longer expressed (data not shown). Together, these results indicate that Wnt3a is not a target of T; however, the overlapping expression of other T-box-encoding genes with T during gastrulation, such as Tbx6 (Chapman et al. 1996), complicates this analysis. TWis mutants, which arose through the insertion of a transposable element into the T locus (Shedlovsky et al. 1988; Herrmann et al. 1990), have a more severe phenotype than T mutants even though these mutants carry a deletion of the T gene that completely removes activity (Herrmann et al. 1990). TWis homozygotes lack somites completely, whereas T homozygotes form 7 somites. These data suggest that the TWis allele may dominantly inhibit the activity of other T-box members (Herrmann 1991). Interestingly, we observed significantly reduced Wnt3a expression in the primitive streak of TWis/TWis embryos at the 0- to 2-somite stage (Fig. 4D,F; data not shown). In contrast, the loss of Wnt3a transcripts observed in T/T homozygotes was not observed until the 4- to 5-somite stage (Rashbass et al. 1994). The correlation between the stage at which Wnt3a transcription is down-regulated and the severity of the T allele suggests that Wnt3a may be regulated by T-box activity. Consistent with this hypothesis, examination of Wnt3a homozygotes at later stages (5–6 somites) reveals that both Wnt3a (Fig. 4A,B), and T (Fig. 2J) are coexpressed weakly in the mutant streak. Proof of a reciprocal autoregulatory loop that maintains both T and Wnt3a expression in the streak will require a thorough characterization of the Wnt3a regulatory region and the analysis of compound mutants in T-box genes.
Figure 4.
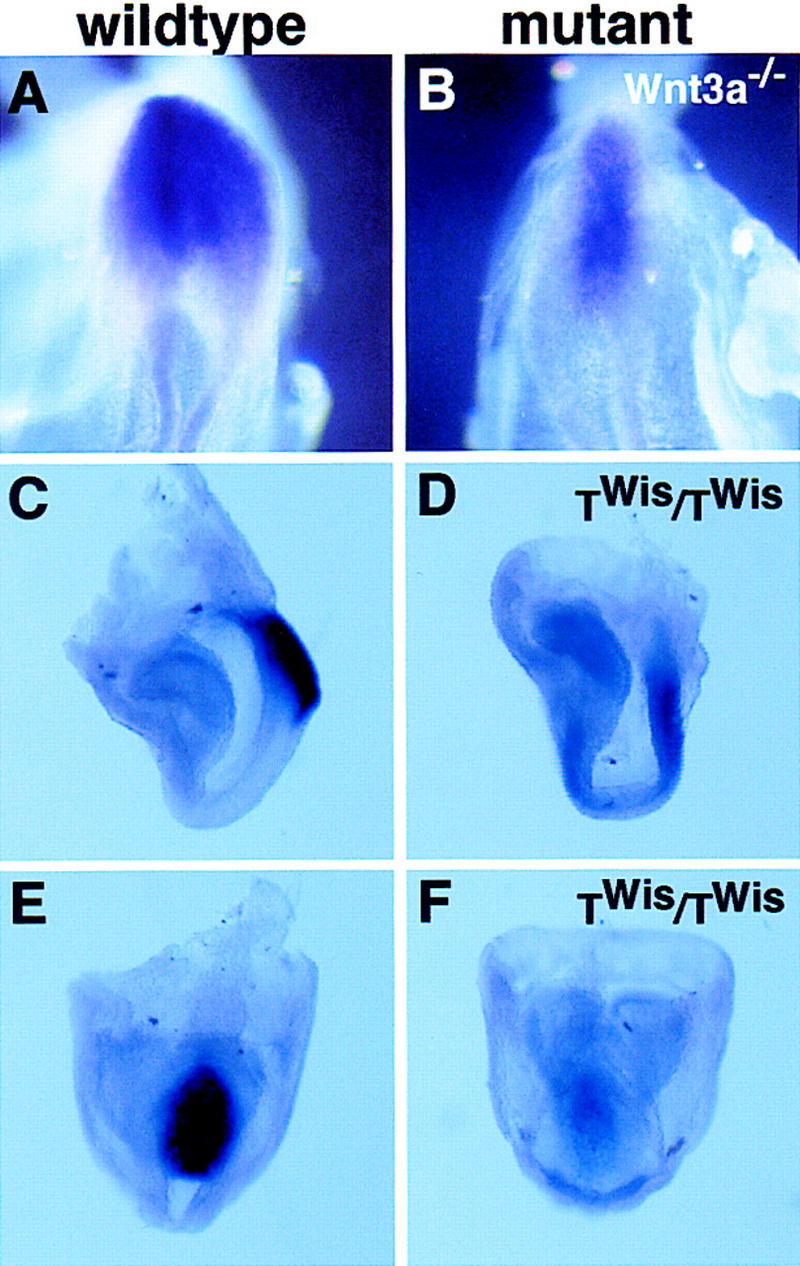
Analysis of Wnt3a expression in Wnt3a−/− and TWis mutant embryos. Dorsal views of the primitive streak region of 5- to 6-somite stage wild-type (A) and Wnt3a−/− (B) embryos. Lateral views of Wnt3a expression in the primitive streak of a 3-somite wild-type (C) and the equivalent of a 2-somite TWis homozygote (staging based on headfold morphology) (D). The reduced expression of Wnt3a in the TWis mutant primitive streak (F) compared to the wild-type (E) is more evident in a ventral–posterior view of the same embryos depicted in C and D.
In conclusion, we have demonstrated that Wnt3a regulates paraxial mesoderm development in the anterior primitive streak, at least in part, through the direct regulation of the mesodermal determinant T. Analyses of the Wnt3a and T mutant phenotypes demonstrate that specification of mesodermal cell fates and extension of the A-P body axis are intimately linked processes during embryogenesis. Animals heterozygous for null alleles of either Wnt3a or T display kinked or shortened tail phenotypes due to haploinsufficiency (Dobrovolskaia-Zavadskaia 1927; Greco et al. 1996). Examination of ordered allelic series of mutations in either Wnt3a or T demonstrates a correlation between the severity of the axial truncation and gene dosage (MacMurray and Shin 1988; Greco et al. 1996) and suggests that Brachyury and Wnt3a participate in the development of the entire A-P axis. Our demonstration that T is a transcriptional target of the Wnt3a signaling pathway is consistent with this genetic data and suggest that a primary function of Wnt3a may be to tightly regulate the dosage of T during embryogenesis.
In wild-type embryos, pluripotent epithelial epiblast cells of the anterior primitive streak ingress through the streak and become mesenchymal upon exposure to mesoderm-promoting factors such as Wnt3a. In the absence of either Wnt3a or T, epiblast cells retain their epithelial character and assume a neural fate. Nevertheless, these putative neural epithelial cells continue to ingress through the primitive streak to form an ectopic tube that ultimately lies ventral to the primary neural tube. Thus, continued expression of Wnt3a and T is essential for mesodermal progenitors to adopt specific mesodermal fates and to promote mesenchymal morphology but neither gene appears essential for ingression of cells through the streak. How exactly Wnt3a and Brachyury control these cellular processes is currently unclear.
One demonstrated function for T is in the regulation of morphogenetic cell movements (Wilson et al. 1995). Experimentally increasing the levels of Brachyury in streak cells led to increased movement of these cells away from the primitive streak (Wilson and Beddington 1997). Interestingly, different cells in the wild-type streak appear to express different levels of Brachyury (Kispert and Herrmann 1994; Wilson and Beddington 1997). Because we have demonstrated that Wnt3a regulates T transcription directly, it seems likely that Wnt3a modulates the proportion of cells that stay in the streak versus cells that exit the streak as paraxial mesoderm progenitors. It is tempting to speculate that Wnt3a specifies the fate of a pluripotent primitive streak stem cell (Tam and Beddington 1987; Nicolas et al. 1996; Wilson and Beddington 1996) to give rise to a paraxial mesodermal daughter cell that expresses high levels of T and exits the streak to contribute to trunk somites. The suggestion has been made from amphibian studies that neural development is a default state, which, in contrast to mesoderm development, does not require a specific inductive process. Our results here are consistent with this general model in that Wnt3a is required for paraxial mesoderm development; its absence leads to ectopic neural development. Thus, Wnt3a ultimately modulates a balance between mesodermal and neural cell fates in the primitive streak during gastrulation and A-P axis development.
Materials and methods
Embryo collection and analysis
Embryos were dissected into PBS and yolk sacs were biopsied for PCR genotyping. The targeted Wnt3a allele was genotyped by PCR as described (Takada et al. 1994). Embryos homozygous for TWis were generated by intercrossing TWis heterozygotes (easily identified by their lack of tails) and were identified morphologically by their lack of somites. Homozygous T embryos were also generated by intercrossing T heterozygotes. Experiments performed on T and TWis homozygous embryos were repeated four times. Embryos were fixed in 4% paraformaldehyde for whole-mount RNA in situ hybridization analysis according to the method of Wilkinson and Nieto (1993). Embryos dissected for β-galactosidase staining were fixed in 1% formaldehyde/0.2% glutaraldehyde and processed following the method of Whiting et al. (1991). Whole-mount stained embryos were processed for paraffin embedding and sectioned as described (Takada et al. 1994; Yoshikawa et al. 1997).
Transgene construction
A 14.7-kb plasmid (pCTZA) containing 8.3 kb of the murine T promoter fused to lacZ and followed by an SV40 polyadenylation sequence (Stott et al. 1993) was cut with SacII and SalI and subcloned into pBluescript KS(+) (Stratagene). This plasmid was designated pBS0.7TZA and retained ∼500 bp of the proximal T promoter fused to the lacZ reporter. pBS0.7TZA served as the wild-type T promoter control construct and has been shown to contain elements sufficient to drive expression in the primitive streak (Clements et al. 1996). The promoter was isolated further with BamHI and SacII and subcloned into pBluescript to generate pBS0.7T; this promoter construct served as the template for subsequent mutagenesis. Mutagenized promoters were cloned into pBS0.7TZ(S-C), a shuttle vector created by subcloning a 1.4-kb SacII–ClaI fragment that contained the 500-bp promoter and a portion of lacZ into pBluescript. The lacZ reporter was reconstituted by cloning the 1.4-kb fragment containing the mutagenized promoter back into pBS0.7TZA.
The T promoter was mutagenized following the QuikChange site-directed mutagenesis kit (Stratagene) protocol. The 5′ and 3′ Tcf binding sites were mutagenized using the following primers: 2F-5′Lef*, CCAGGGTCCGCCCCGCCGCGAATTCGGAGGTGCAAACATTTGG; 2R-5′Lef*, CCAAATGTTTGCACCTCCGAATTCGCGGCGGGGCGGACCCTGG; 3F-3′Lef*, GGGCAGCTGCTCGGTACTTCCCCGGGTGTCCCGCCCAATCCGCC; and 3R-3′Lef*, GGCGGATTGGGCGGGACACCCGGGGAAGTACCGAGCAGCTGCCC. The 5′ and 3′ E boxes were mutagenized using the following primers: 2F-5′E*, GCGAATTCGGAGGTGCAAAGAAGCTTGGGAGGGCGGGGGTGTCGGG; 2R-5′E*, CCCGACACCCCCGCCCTCCCAAGCTTCTTTGCACCTCCGAATTCGC; 2F-3′E*, GGCCGCGCACCGCCAATGGGTGGCCACTCGGTACTTCCCCGGGTG; 2R-3′E*, CACCCGGGGAAGTACCGAGTGGCCACCCATTGGCGGTGCGCGGCC. All primers were synthesized by Life Technologies and PAGE-purified. All PCR mutagenesis reactions were performed using 18 cycles of denaturing at 95°C for 30 sec, annealing at 55°C for 1 min, followed by an 8-min extension at 68°C.
Generation of transgenic embryos
DNA constructs were prepared for pronuclear injection by digesting with SacII and SalI and purifying by agarose gel to remove vector sequences. DNA was electroeluted in dialysis tubing in 1× TAE and purified using several QIAQuick PCR DNA purification spin columns (Qiagen). Eluants were pooled and ethanol precipitated, and DNA was resuspended in 50 μl of TE at pH 7.5. Concentration was determined by UV spectrophotometry and confirmed by gel electrophoresis using known standards.
Pronuclear injections were performed according to standard published protocols (Hogan et al. 1994). G0 transgenic embryos (8.5 dpc) were dissected and yolk sacs were biopsied for PCR genotyping using primers to the T promoter [T(+7/+20): CCTTTGGCGAATGTGCAGGG] and to lacZ (oligo 1098: AAGGGCGATCGGTGCGGGCC). Samples were cycled 35× at 94°C for 30 sec, 66°C for 30 sec, and 72°C for 1 min.
Acknowledgments
We thank D. Stott for kindly providing the T promoter plasmid pCTZA, and D. Chapman and V. Papiannou for the Tbx6 probe. We also thank E. Robertson for providing TWis mice. T.P.Y. acknowledges the kind support of the Human Frontiers Science Program (HFSP) and the Medical Research Council of Canada. Work in S.T.'s laboratory was supported by grants from the the Ministry of Education, Science, and Culture of Japan, JST, Takeda Science Foundation, and Kato Memorial Bioscience Foundation. Work in A.P.M.'s laboratory was supported by a grant from the National Institutes of Health/National Institute of Child Health and Human Development (HD30249).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL amcmahon@biosun.harvard.edu; FAX (617) 496-3763.
References
- Artinger M, Blitz I, Inoue K, Tran U, Cho KW. Interaction of goosecoid and brachyury in Xenopus mesoderm patterning. Mech Dev. 1997;65:187–196. doi: 10.1016/s0925-4773(97)00073-7. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Nusse R. Wnt signaling: A common theme in animal development. Genes & Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- Chapman DL, Papaioannou VE. Three neural tubes in mouse embryos with mutations in the T-box gene Tbx6. Nature. 1998;391:695–697. doi: 10.1038/35624. [DOI] [PubMed] [Google Scholar]
- Chapman DL, Agulnik I, Hancock S, Silver LM, Papaioannou VE. Tbx6, a mouse T-Box gene implicated in paraxial mesoderm formation at gastrulation. Dev Biol. 1996;180:534–542. doi: 10.1006/dbio.1996.0326. [DOI] [PubMed] [Google Scholar]
- Chesley P. Development of the short-tailed mutant in the house mouse. J Exp Zool. 1935;70:429–435. [Google Scholar]
- Clements D, Taylor HC, Herrmann BG, Stott D. Distinct regulatory control of the Brachyury gene in axial and non-axial mesoderm suggests separation of mesoderm lineages early in mouse gastrulation. Mech Dev. 1996;56:139–149. doi: 10.1016/0925-4773(96)00520-5. [DOI] [PubMed] [Google Scholar]
- Dobrovolskaia-Zavadskaia N. Sur la mortification sponta-nee de la queue chez la souris nouveau-nee et sur l'existence d'un caractere hereditaire “non viable.”. C R Hebd Soc Biol. 1927;97:114–116. [Google Scholar]
- Galceran J, Farinas I, Depew MJ, Clevers H, Grosschedl R. Wnt3a−/−-like phenotype and limb deficiency in Lef1−/−Tcf1−/− mice. Genes & Dev. 1999;13:709–717. doi: 10.1101/gad.13.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco TL, Takada S, Newhouse MM, McMahon JA, McMahon AP, Camper SA. Analysis of the vestigial tail mutation demonstrates that Wnt-3a gene dosage regulates mouse axial development. Genes & Dev. 1996;10:313–324. doi: 10.1101/gad.10.3.313. [DOI] [PubMed] [Google Scholar]
- Herrmann BG. Expression pattern of the Brachyury gene in whole-mount TWis/TWis mutant embryos. Development. 1991;113:913–917. doi: 10.1242/dev.113.3.913. [DOI] [PubMed] [Google Scholar]
- Herrmann BG, Labeit S, Poustka A, King TR, Lehrach H. Cloning of the T gene required in mesoderm formation in the mouse. Nature. 1990;343:617–622. doi: 10.1038/343617a0. [DOI] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Constantini F, Lacy E. Manipulating the mouse embryo, a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- Kispert A, Herrmann BG. Immunohistochemical analysis of the Brachyury protein in wild-type and mutant mouse embryos. Dev Biol. 1994;161:179–193. doi: 10.1006/dbio.1994.1019. [DOI] [PubMed] [Google Scholar]
- Latinkic BV, Umbhauer M, Neal KA, Lerchner W, Smith JC, Cunliffe V. The Xenopus Brachyury promoter is activated by FGF and low concentrations of activin and suppressed by high concentrations of activin and by paired-type homeodomain proteins. Genes & Dev. 1997;11:3265–3276. doi: 10.1101/gad.11.23.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMurray A, Shin H-S. The antimorphic nature of the Tc allele at the mouse T locus. Genetics. 1988;120:545–550. doi: 10.1093/genetics/120.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon R, Brown J, Torres M. WNTs modulate cell fate and behavior during vertebrate development. Trends Genet. 1997;13:157–162. doi: 10.1016/s0168-9525(97)01093-7. [DOI] [PubMed] [Google Scholar]
- Nicolas JF, Mathis L, Bonnerot C, Saurin W. Evidence in the mouse for self-renewing stem cells in the formation of a segmented longitudinal structure, the myotome. Development. 1996;122:2933–2946. doi: 10.1242/dev.122.9.2933. [DOI] [PubMed] [Google Scholar]
- Nusse R. WNT targets. Repression and activation. Trends Genet. 1999;15:1–3. doi: 10.1016/s0168-9525(98)01634-5. [DOI] [PubMed] [Google Scholar]
- Rashbass P, Wilson V, Rosen B, Beddington RS. Alterations in gene expression during mesoderm formation and axial patterning in Brachyury (T) embryos. Int J Dev Biol. 1994;38:35–44. [PubMed] [Google Scholar]
- Shedlovsky A, King TR, Dove WF. Saturation germ line mutagenesis of the murine t region including a lethal allele at the quaking locus. Proc Natl Acad Sci. 1988;85:180–184. doi: 10.1073/pnas.85.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. T-box genes: What they do and how they do it. Trends Genet. 1999;15:154–158. doi: 10.1016/s0168-9525(99)01693-5. [DOI] [PubMed] [Google Scholar]
- Stott D, Kispert A, Herrmann BG. Rescue of the tail defect of Brachyury mice. Genes & Dev. 1993;7:197–203. doi: 10.1101/gad.7.2.197. [DOI] [PubMed] [Google Scholar]
- Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, McMahon AP. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes & Dev. 1994;8:174–189. doi: 10.1101/gad.8.2.174. [DOI] [PubMed] [Google Scholar]
- Tam PP, Beddington RS. The formation of mesodermal tissues in the mouse embryo during gastrulation and early organogenesis. Development. 1987;99:109–126. doi: 10.1242/dev.99.1.109. [DOI] [PubMed] [Google Scholar]
- Tam PP, Trainor PA. Specification and segmentation of the paraxial mesoderm. Anat Embryol. 1994;189:275–305. doi: 10.1007/BF00190586. [DOI] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- van de Wetering M, Oosterwegel M, Dooijes D, Clevers H. Identification and cloning of TCF-1, a T lymphocyte-specific transcription factor containing a sequence-specific HMG box. EMBO J. 1991;10:123–132. doi: 10.1002/j.1460-2075.1991.tb07928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting J, Marshall H, Cook M, Krumlauf R, Rigby PW, Stott D, Allemann RK. Multiple spatially specific enhancers are required to reconstruct the pattern of Hox-2.6 gene expression. Genes & Dev. 1991;5:2048–2059. doi: 10.1101/gad.5.11.2048. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Nieto MA. Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol. 1993;225:361–373. doi: 10.1016/0076-6879(93)25025-w. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Bhatt S, Herrmann BG. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature. 1990;343:657–659. doi: 10.1038/343657a0. [DOI] [PubMed] [Google Scholar]
- Wilson V, Beddington RS. Cell fate and morphogenetic movement in the late mouse primitive streak. Mech Dev. 1996;55:79–89. doi: 10.1016/0925-4773(95)00493-9. [DOI] [PubMed] [Google Scholar]
- ————— Expression of T protein in the primitive streak is necessary and sufficient for posterior mesoderm movement and somite differentiation. Dev Biol. 1997;192:45–58. doi: 10.1006/dbio.1997.8701. [DOI] [PubMed] [Google Scholar]
- Wilson V, Manson L, Skarnes WC, Beddington RS. The T gene is necessary for normal mesodermal morphogenetic cell movements during gastrulation. Development. 1995;121:877–886. doi: 10.1242/dev.121.3.877. [DOI] [PubMed] [Google Scholar]
- Yoshikawa Y, Fujimori T, McMahon AP, Takada S. Evidence that absence of Wnt-3a signaling promotes neuralization instead of paraxial mesoderm development in the mouse. Dev Biol. 1997;183:234–242. doi: 10.1006/dbio.1997.8502. [DOI] [PubMed] [Google Scholar]


