Abstract
Prokineticin 1 (PROK1) signalling via prokineticin receptor 1 (PROKR1) regulates the expression of several genes with important roles in endometrial receptivity and implantation. This study investigated PROK1 regulation of Dickkopf 1 (DKK1) expression, a negative regulator of canonical Wnt signalling, and its function in the non-pregnant endometrium and first trimester decidua. DKK1 mRNA expression is elevated during the mid-secretory phase of the menstrual cycle and expression increases further in first trimester decidua. DKK1 protein expression is localized to glandular epithelial and stromal cells during the proliferative, early- and mid-secretory phases, whereas expression is confined to the stroma in the late-secretory phase and first trimester decidua. PROK1 induces the expression of DKK1 in endometrial epithelial cells stably expressing PROKR1 and in first trimester decidua explants, via a Gq-calcium-calcineurin-nuclear factor of activated T-cells-mediated pathway. Endometrial epithelial cell proliferation is negatively regulated by PROK1-PROKR1 signalling. We demonstrate that this effect on cell proliferation occurs via DKK1 expression, as siRNA targeted against DKK1 reduces the PROK1-induced decrease in proliferation. Furthermore, decidualization of primary human endometrial stromal cells with progesterone and cyclic adenosine monophosphate is inhibited by miRNA knock down of PROK1 or DKK1. These data demonstrate important roles for PROK1 and DKK1 during endometrial receptivity and early pregnancy, which include regulation of endometrial cell proliferation and decidualization.
Keywords: decidualization, endometrium, proliferation, Dickkopf 1, prokineticin
Introduction
Prokineticin 1 (PROK1) is a secreted protein previously known as endocrine gland vascular endothelial growth factor (LeCouter et al., 2001; Li et al., 2001). PROK1 can regulate diverse biological processes including intestinal smooth muscle contraction (Li et al., 2001), endothelial cell proliferation and angiogenesis (LeCouter et al., 2001; Lin et al., 2002), haematopoiesis (LeCouter et al., 2004; Dorsch et al., 2005) and inflammation (Monnier and Samson, 2008). PROK1 binds to two closely related G protein-coupled receptors, prokineticin receptors 1 and 2 (PROKR1 and 2), and can mediate downstream activation of several signalling molecules including extracellular signal-regulated kinase 1/2 (Evans et al., 2008) and the calcineurin-nuclear factor of activated T-cells (NFAT) pathway (Maldonado-Perez et al., 2009). PROK1 is recognized as an important regulator of female reproductive function (Maldonado-Perez et al., 2007), with described roles in the ovary (Ferrara et al., 2003; Fraser et al., 2005), fallopian tube (Shaw et al., 2010), placenta (Hoffmann et al., 2006; Denison et al., 2008) and uterus (Battersby et al., 2004; Ngan et al., 2006; Evans et al., 2008; Evans et al., 2009).
PROK1 in the non-pregnant endometrium is temporally regulated across the menstrual cycle, with levels increasing in the mid-secretory phase (Battersby et al., 2004; Evans et al., 2008). In addition, levels of PROK1 and PROKR1 are further elevated in decidua of early pregnancy (Evans et al., 2008). Expression of PROK1 and PROKR1 is localized to glandular epithelial cells and the stroma throughout the menstrual cycle and in decidua of early pregnancy (Battersby et al., 2004; Evans et al., 2008). Studies have demonstrated regulation of PROK1 by progesterone and human chorionic gonadotrophin in the endometrium (Battersby et al., 2004; Ngan et al., 2006; Evans et al., 2009). Furthermore, PROK1 signalling through PROKR1 in the endometrium has been shown to regulate the expression of several genes involved in endometrial receptivity and implantation, including cyclooxygenase 2 (Evans et al., 2008), leukaemia inhibitory factor (Evans et al., 2009), interleukin 8 (IL8) (Maldonado-Perez et al., 2009), interleukin 11 (IL11) (Cook et al., 2010) and connective tissue growth factor (Waddell et al., 2010). These findings implicate PROK1 as an important regulator of early pregnancy. Indeed, there is a correlation between recurrent miscarriage and genetic polymorphisms in PROK1 and its receptors (Su et al., 2010). Aberrant elevation in PROK1 expression has also been associated with impaired decidualization and recurrent miscarriage (Salker et al., 2010).
We previously identified Dickkopf 1 (DKK1), a secreted antagonist of the canonical Wnt signalling pathway (Bafico et al., 2001; Mao et al., 2001; Semenov et al., 2001), as a target of PROK1-PROKR1 signalling (Evans et al., 2008). Wnt signalling is considered to play a role in regulating proliferation (Hou et al., 2004; Jeong et al., 2009) and decidualization (Li et al., 2007; Jeong et al., 2009) of the endometrium, and in embryo−endometrial cross talk during implantation (Chen et al., 2009). DKK1 expression is temporally regulated across the menstrual cycle, with levels peaking in the mid-secretory phase (Carson et al., 2002; Kao et al., 2002; Borthwick et al., 2003; Riesewijk et al., 2003; Tulac et al., 2003; Horcajadas et al., 2004; Talbi et al., 2006), and increases in response to decidualization in vitro (Kao et al., 2002; Tulac et al., 2006; Kane et al., 2008).
Thus, this study investigated the effect of PROK1 on DKK1 expression and the role of the PROK1-DKK1 pathway in endometrial function. Our data demonstrate that PROK1 and DKK1 regulate endometrial processes including epithelial cellular proliferation and differentiation of the stroma during early pregnancy.
Materials and Methods
Reagents
DMEM/F-12 GlutaMAX and RPMI 1640 culture media were obtained from Invitrogen (Paisley, UK). G418 was obtained from InvivoGen (San Diego, CA, USA). Recombinant PROK1 was purchased from Peprotech (London, UK). Medroxyprogesterone acetate (MPA), cyclic adenosine monophosphate (cAMP), ethylene glycol tetra-acetic acid (EGTA; extra-cellular calcium chelator, used at 1.5 mM), collagenase and DNAse were purchased from Sigma (Poole, UK). YM-254890 (Gq inhibitor, used at 1 µM) was kindly supplied by Dr Jun Takasaki (Molecular Medicine Laboratories, Yamanouchi Pharmaceutical Co. Ltd, Tokyo, Japan). Bapta-AM (intra-cellular calcium chelator, used at 50 µM), cyclosporin A (CSA; calcineurin inhibitor, used at 1 µM) and Inhibitor of NFAT-Calcineurin Association-6 (INCA-6, used at 40 µM) were purchased from Calbiochem (Nottingham, UK). SN50 (nuclear factor-κB (NFκB) cell-permeable inhibitory peptide, used at 100 µg/ml) was obtained from Biomol International (Exeter, UK). The specific doses of the inhibitors used were established previously (Sales et al., 2009). DKK1 monoclonal antibody (M11, clone 2A5) and recombinant protein were purchased from Abnova Corp. (Taipei, Taiwan).
Tissue collection
Endometrial tissue was collected from women aged 21–39 with no underlying endometrial pathology and regular menstrual cycles (25–35 days) who had not received any hormonal preparation for 3 months preceding biopsy collection. Biopsies were dated according to Noyes criteria by a pathologist. Circulating oestradiol and progesterone concentrations were measured and were consistent with the histological assessment. First trimester decidua tissue (7–10 weeks gestation) was collected from women undergoing elective surgical termination of pregnancy with gestation confirmed by ultra-sound scan. Ethical approval was obtained from Lothian Ethics Research Committee, and written informed consent obtained before tissue collection.
Cell and tissue culture and treatment
Stable PROKR1 expressing Ishikawa cells were produced and characterized as described (Evans et al., 2008). PROKR1 Ishikawa cells were cultured in DMEM/F-12 culture medium supplemented with 10% foetal bovine serum, 100 IU penicillin and 100 µg streptomycin at 37°C and 5% CO2, with addition of 200 µg/ml G418. Decidua tissue for explant studies was chopped finely, maintained in serum-free DMEM/F-12 medium and divided into equal portions for experimental procedures. Cells and tissue were incubated overnight in serum-free medium before treatment with 40 nM PROK1. In signalling pathway inhibitor experiments, cells or tissue were pre-treated with inhibitors for 1 h before the addition of PROK1. Regulator of calcineurin 1 isoform 4 (RCAN1-4) adenovirus was produced as described (Maldonado-Perez et al., 2009). Ishikawa PROKR1 cells were incubated with five adenovirus plaque-forming units (pfu)/cell for 24 h to induce over-expression of RCAN1-4, and then serum-starved overnight before treatment with 40 nM PROK1 for 8 h. Decidua explants were incubated with 2.5 × 106 adenovirus pfu/piece of tissue (5 pfu/cell, estimating 5 × 105 cells/piece of tissue) for 24 h, and then treated with 40 nM PROK1 for 24 h. Cells and tissue were harvested with conditioned medium collected for enzyme-linked immunosorbent assay (ELISA) and RNA extracted for reverse transcriptase-polymerase chain reaction (RT–PCR) analysis.
Primary human endometrial stromal cell isolation, transduction and decidualization
Non-pregnant endometrial tissue was digested in a solution of 1 mg/ml collagenase and 0.1 mg/ml DNAse in 2 ml phosphate-buffered saline (PBS) for 80 min at 37°C. Tissue was further dissociated by passing through an 18 G needle, and after addition of 10 ml RPMI culture medium supplemented with 10% fetal bovine serum, 100 IU penicillin and 100 µg streptomycin, was passed through a 70 µm filter. The resulting filtrate containing stromal cells was centrifuged at 1700g for 3 min and resuspended in 10 ml complete RPMI medium and plated in a 75 cm2 tissue culture flask.
Lentiviral miRNA constructs were used to knock down the expression of PROK1 or DKK1 in primary stromal cells. Cells were transduced with Lv-cppt-EmGFP-PROK1-72_287 (emerald green fluorescent protein (GFP) denoted by EmGFP), which targets two regions of PROK1 (Evans et al., 2009), or two separate miRNA sequences targeting DKK1 (Lv-cppt-EmGFP-DKK1-92 or -DKK1-487), at five transduction units/cell. All numbers correspond to annealing site in basepairs downstream of the start codon. Oligonucleotides encoding DKK1 miRNA constructs were obtained from Invitrogen and inserted into the pcDNA6.2-GW/EmGFP-miR vector. These were recombined to create pLenti6/V5-cppt-EmGFP-hum-DKK1-92 and -487. Lentivirus was produced with a Block-iT lentiviral Pol II miR RNAi expression system (Invitrogen) according to the manufacturer's instructions.
In decidualization experiments, cells were treated with 1 µM MPA and 0.2 mg/ml cAMP in RPMI supplemented with 2% foetal bovine serum and antibiotics for 5 days. The medium was refreshed every 2 days. Cells were harvested with RNA extracted for RT–PCR analysis.
Taqman quantitative PCR
Total RNA was extracted from cells using QIAzol lysis reagent and MaXtract phase lock tubes from Qiagen (Crawley, UK), according to the manufacturer's guidelines. Total RNA was extracted from tissue using the RNeasy mini kit from Qiagen. RNA samples were quantified and reverse transcribed using the SuperScript VILO cDNA synthesis kit from Invitrogen. PCR reactions were carried out using an Applied Biosystems ABI 7500 system. Primer and FAM (6-carboxyfluorescein)-labelled probe sequences are listed in Table I and were designed to span an intron. The expression of analysed genes was normalized for RNA loading using 18S ribosomal RNA primers and probe (Applied Biosystems, UK). Results were calculated relative to a standard included in all plates (endometrial tissue cDNA). Tissue DKK1 expression levels are shown as relative to the endometrial tissue cDNA standard, and experimental data are expressed as fold increase compared with vehicle-treated control cells or tissue.
Table I.
Taqman primer and probe sequences for DKK1, IGFBP1, PRL and IL11.
| Gene | Primer/probe sequence (5′–3′) |
|---|---|
| DKK1 forward | GCGGGAATAAGTACCAGACCAT |
| DKK1 reverse | GGGACTAGCGCAGTACTCATCAGT |
| DKK1 probe | TACCAGCCGTACCCGTGCGCAG |
| IGFBP1 forward | CACAGGAGACATCAGGAGAAGAAA |
| IGFBP1 reverse | ACACTGTCTGCTGTGATAAAATCCAT |
| IGFBP1 probe | TTCCAAATTTTACCTGCCAAACTGCAACAA |
| PRL forward | CGGAAGTACGTGGTATGCAAGA |
| PRL reverse | TCAGGATGAACCTGGCTGACT |
| PRL probe | CCCCGGAGGCTATCCTATCCAAAGCT |
| IL11 forward | CCCAGTTACCCAAGCATCCA |
| IL11 reverse | AGACAGAGAACAGGGAATTAAATGTGT |
| IL11 probe | CCCCAGCTCTCAGACAAATCGCCC |
Enzyme-linked immunosorbent assay
Conditioned medium was assayed for DKK1 using an ELISA kit from R&D Systems (Abingdon, UK), according to the manufacturer's instructions. Briefly, medium or standards were applied to a microplate coated with mouse anti-human DKK1 antibody for 2 h at room temperature. Biotinylated goat anti-human DKK1 antibody was then applied for 2 h, followed by streptavidin-horseradish peroxidise (HRP) for 20 min. A colour development solution (1:1 mix of hydrogen peroxide and tetramethylbezidine) was applied for 20 min and colour development stopped by addition of sulphuric acid. The optical density was measured at 450 nm and DKK1 levels in pg/ml were calculated from a standard curve.
Immunohistochemistry
Localization of DKK1 was performed by immunohistochemistry in 5 µm paraffin-embedded sections. Briefly, sections were dewaxed and then rehydrated, and antigen retrieval was performed by boiling in 0.01 M citrate buffer for 20 min. Endogenous peroxidise activity was quenched using 3% H2O2/PBS for 30 min at room temperature and non-specific protein binding was blocked using 20% normal goat serum. Sections were incubated with mouse anti-human DKK1 monoclonal antibody (3 µg/ml) overnight at 4°C. A goat anti-mouse biotinylated secondary antibody was applied for 1 h at room temperature, followed by streptavidin-HRP for 1 h. HRP activity was detected by incubation with diaminobenzidine for 5 min and the reaction was stopped in tap water. Sections were then counterstained, dehydrated, cleared and mounted before photographs were taken using a Zeiss AxioCam HRc coupled to an Olympus AX70 microscope. Negative controls were performed using primary antibody pre-absorbed with a 10 times excess of DKK1 protein (30 µg/ml) overnight at 4°C.
Proliferation assay
Transient transfections of PROKR1 Ishikawa cells were performed using commercially supplied DKK1 targeting siRNA (Invitrogen) with Superfect transfection reagent (Qiagen), according to the manufacturer's instructions. For RCAN1-4 adenovirus over-expression studies, Ishikawa PROKR1 cells were incubated with five adenovirus pfu/cell of either control adenovirus or RCAN1-4 adenovirus for 24 h. Proliferation of cells was then determined using a CellTitre 96 Aqueous One Solution cell proliferation assay (Promega, Southampton, UK). Briefly, cells were seeded at 5 × 103 cells per well in a 96-well plate and serum-starved overnight before treatment with 40 nM PROK1. After 72 h, proliferation was measured by addition of the CellTitre 96 Aqueous One Solution reagent, according to the manufacturer's protocol. Cells were incubated for 4 h at 37°C and 5% CO2 to reduce the tetrazolium compound to a 490 nm absorbing formazan compound. Relative cell number is expressed as the absorbance value measured at 490 nm.
Statistics
T-test was used for all paired analyses, two-way analysis of variance (ANOVA) with Bonferroni post-hoc test for time course treatment analyses and one-way ANOVA with Tukey`s post-hoc test for analysis of three groups or more. Data are shown as mean ± SEM.
Results
Expression and localization of DKK1 in the human endometrium and first trimester decidua
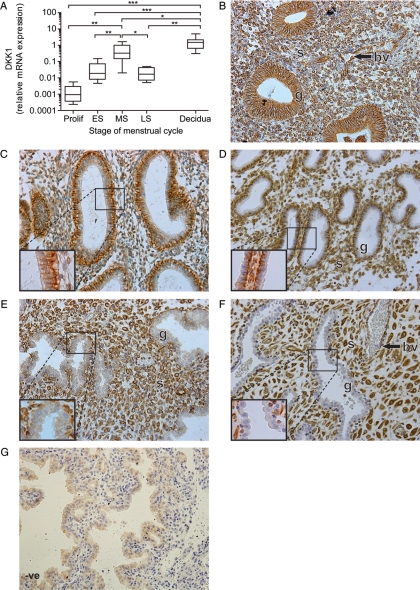
We investigated the temporal expression of DKK1 mRNA across the menstrual cycle and in decidua of early pregnancy using quantitative RT–PCR analysis. DKK1 mRNA expression was significantly elevated in the mid-secretory phase of the menstrual cycle (mean fold change 328.6 compared with proliferative phase, Fig. 1A). DKK1 expression was further elevated in first trimester decidua tissue compared with mid-secretory endometrium (mean fold change 2.9, Fig. 1A).
Figure 1.
Temporal expression and localization of DKK1 in the human endometrium and first trimester decidua. DKK1 mRNA expression levels in human endometrium across the menstrual cycle (Prolif; n= 10, ES; n= 10, MS; n= 7, LS; n= 4) and first trimester decidua tissue (n= 33) are shown in (A). mRNA levels are expressed relative to a standard endometrial cDNA sample. Boxes represent data lying within the fifth to the 95th percentile and whiskers represent the minimum and maximum values. *, ** and *** represent significance at P< 0.05, P< 0.01 and P< 0.001, respectively. Localization of DKK1 protein across the menstrual cycle and during early pregnancy is shown (for each stage of the cycle and early pregnancy three serial sections from 3–5 different tissue samples were examined, representative sections are shown in B–F). Expression in the glandular epithelium (g) during the proliferative phase (B) became restricted to the basal and basolateral region of the glandular cells in the early- (C) and mid-secretory (D) phases. In the late-secretory phase (E) and first trimester decidua (F), expression was only present in the stroma (s) and blood vessels (bv). Negative control (-ve) is shown for decidua tissue (G).
The localization of DKK1 protein was determined in human endometrium and decidua by immunohistochemistry. DKK1 protein localized to the stromal compartment, blood vessels and glandular epithelium in proliferative phase endometrium (Fig. 1B). In the early- (Fig. 1C) and mid-secretory phases (Fig. 1D), DKK1 expression was present in the stroma, and became restricted to the basal and basolateral region of the glandular epithelium. In late-secretory phase endometrium (Fig. 1E) and decidua tissue (Fig. 1F), DKK1 expression was only seen in the stroma and blood vessels and was absent from the glandular epithelium. Tissue sections incubated with primary antibody pre-absorbed with DKK1 protein was used as a negative control (Fig. 1G).
PROK1 increases DKK1 expression in PROKR1 Ishikawa cells via the calcineurin-NFAT pathway
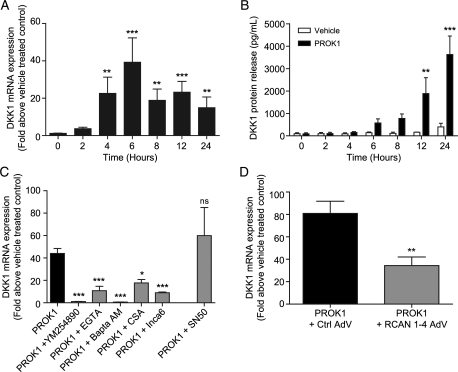
We previously identified DKK1 as a target of PROK1-PROKR1 signalling using microarray profiling (Evans et al., 2008). The expression levels of DKK1 across the menstrual cycle and in early pregnancy seen in the present study mirror the expression profile of PROK1 reported previously (Evans et al., 2008). Therefore, we sought to determine whether the observed DKK1 expression in endometrial epithelial cells is induced by autocrine or paracrine PROK1 stimulation as suggested by the reported PROK1 immunolocalization to both epithelial and stromal cells. Studies were performed on an endometrial epithelial cell line (Ishikawa) engineered to express levels of PROKR1 similar to that observed in the non-pregnant endometrium (Evans et al., 2008). Treatment of PROKR1 Ishikawa cells with 40 nM PROK1 resulted in a time-dependent increase in DKK1 mRNA expression (Fig. 2A) and protein release into the culture medium (Fig. 2B), which peaked at 6 h (P< 0.001 versus 0 h) and 24 h (P< 0.001 versus vehicle), respectively. The molecular mechanism whereby PROK1 regulates DKK1 expression via PROKR1 was investigated using a panel of small-molecule chemical inhibitors of intracellular signalling (Fig. 2C). This indicated that PROK1-induced DKK1 mRNA expression after 6 h of agonist stimulation was significantly inhibited by the use of a Gq inhibitor (YM254890; P< 0.001), extra- and intra-cellular calcium chelators (EGTA and Bapta-AM, respectively; P< 0.001), a calcineurin inhibitor (CSA; P< 0.05) and an inhibitor of NFAT (INCA-6; P< 0.001). In contrast, inhibition of NFkB (inhibitory peptide SN50), which shares an overlapping consensus sequence with NFAT, had no effect on PROK1-induced DKK1 mRNA expression. Calcineurin–NFAT signalling induced by PROK1 is negatively regulated by regulator of calcineurin 1 isoform 4 (RCAN1-4) (Maldonado-Perez et al., 2009; Cook et al., 2010). We investigated whether RCAN1-4 also acts as a negative regulator of PROK1-induced DKK1 expression. Adenoviral over-expression of RCAN1-4 in PROKR1 Ishikawa cells significantly decreased PROK1-induced DKK1 expression (P< 0.01 versus control vector adenovirus, Fig. 2D). Collectively, these data demonstrate a role for the calcineurin-NFAT pathway as a mediator of PROK1-induced DKK1 mRNA expression in endometrial epithelial cells.
Figure 2.
PROK1 induces the expression of DKK1 via the NFAT signalling pathway in PROKR1 Ishikawa cells. PROKR1 Ishikawa cells treated with 40 nM PROK1 over a 24 h time-course (n= 10 for each time point) showed a significant fold increase in DKK1 mRNA expression, which peaked at 6 h (A). Treatment of PROKR1 Ishikawa cells with 40 nM PROK1 caused a significant increase in the secretion of DKK1 protein into the culture medium compared with vehicle-treated cells at 12 and 24 h (n= 3 for each time point) (B). Induction of DKK1 mRNA expression at 6 h in PROKR1 Ishikawa cells (n= 17) was inhibited by the use of a Gq inhibitor (YM254890) (n= 8), extra- (EGTA) (n= 9) and intra-cellular (Bapta-AM) (n= 4) calcium chelators, a calcineurin inhibitor (CSA) (n= 3) and a NFAT inhibitor (INCA-6) (n= 10). An inhibitor of NFκB (SN50) (n= 3) did not reduce PROK1-induced DKK1 expression (C). PROKR1 Ishikawa cells infected with adenovirus (AdV) to over-express RCAN1-4 (n= 7) showed a significant decrease in PROK1-induced DKK1 expression, when compared with cells infected with control vector (Ctrl) AdV (n= 7) (D). Data are mean ± SEM of at least three independent experiments. *, ** and *** represent significance at P< 0.05, P< 0.01 and P< 0.001, respectively. Fold change was calculated by dividing expression levels in PROK1-treated cells by vehicle-treated control cells.
PROK1 increases DKK1 expression in first trimester decidua tissue via the calcineurin-NFAT pathway
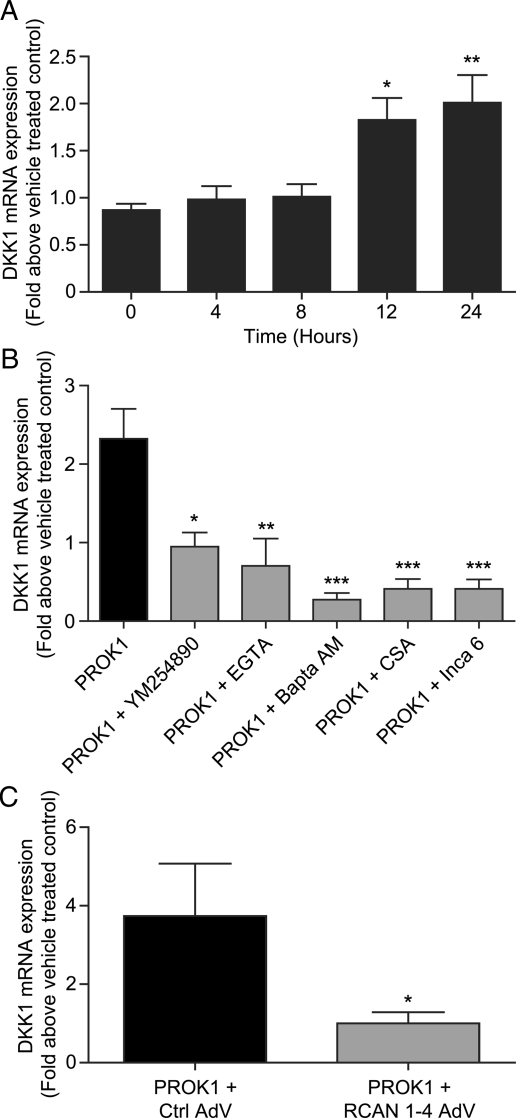
In first trimester decidua tissue, PROK1 and PROKR1 are expressed in both the epithelial cells and stroma (Evans et al., 2008), although DKK1 expression is confined to the stromal compartment. We therefore investigated the molecular pathway whereby PROK1 regulates DKK1 expression in decidualized stromal cells using first trimester decidua explants. Treatment of decidua tissue with 40 nM PROK1 caused a time-dependent increase in DKK1 mRNA expression (after 12 h P< 0.05 versus 0 h, Fig. 3A). PROK1-induced DKK1 expression at 24 h was significantly inhibited by the use of a Gq inhibitor (YM254890; P< 0.05), extra- and intra-cellular calcium chelators (EGTA; P< 0.01 and Bapta-AM; P< 0.001 respectively), a calcineurin inhibitor (CSA; P< 0.001) and an inhibitor of NFAT (INCA-6; P< 0.001, Fig. 3B). Furthermore, PROK1-induced DKK1 expression was also inhibited by adenoviral over-expression of RCAN1-4 in first trimester decidua explants (P< 0.05 versus control vector adenovirus, Fig. 4C). These data indicate that DKK1 is regulated by PROK1 via the Gq-calcium-calcineurin-NFAT pathway in decidualized stromal cells.
Figure 3.
PROK1 induces the expression of DKK1 via the NFAT signalling pathway in cultured first trimester decidua tissue. First trimester decidua tissue treated with 40 nM PROK1 over a 24 h time-course (n= 9 for each time point) displayed a significant fold increase in DKK1 mRNA expression at 12 and 24 h (A). The increase in DKK1 mRNA expression at 24 h (n= 5) was inhibited by the use of a Gq inhibitor (YM254890) (n= 3), extra- (EGTA) (n= 4) and intra-cellular (Bapta-AM) (n= 5) calcium chelators, a calcineurin inhibitor (CSA) (n= 4) and a NFAT inhibitor (INCA-6) (n= 4) (B). Transduction of decidua tissue with RCAN1-4 (n= 5) adenovirus (AdV) caused a significant decrease in PROK1-induced DKK1 expression, when compared with tissue infected with control vector (Ctrl) adenovirus (n= 5) (C). Data are mean ± SEM of at least five independent experiments. *, ** and *** represent significance at P< 0.05, P< 0.01 and P< 0.001 respectively. Fold change was calculated by dividing expression levels in PROK1-treated tissue by vehicle-treated control tissue.
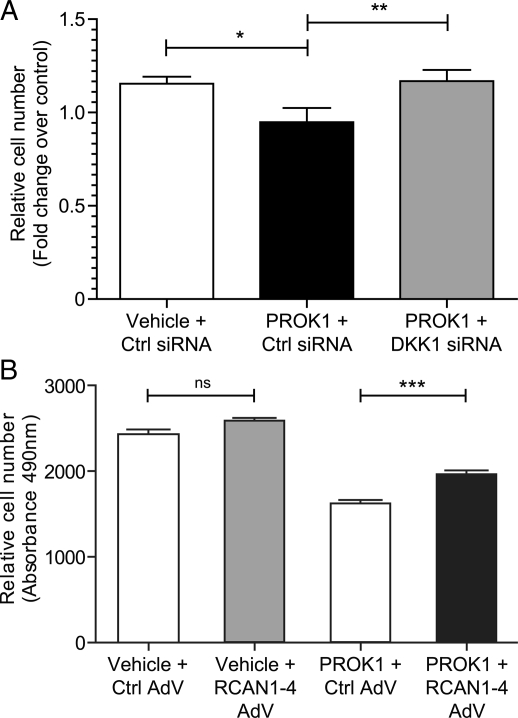
Figure 4.
PROK1 via the NFAT pathway regulates the proliferation of PROKR1 Ishikawa cells. PROK1 caused a reduction in the proliferation of PROKR1 Ishikawa cells transfected with a negative control (Ctrl) siRNA sequence after 72 h; transfection of DKK1 siRNA before treatment with PROK1 prevented this reduction (A). Relative cell number is expressed as fold change over control. RCAN1-4 adenovirus over-expression in PROKR1 Ishikawa cells (PROK1 + RCAN1-4 AdV) reduced the negative effect of PROK1 treatment on proliferation compared with cells expressing control vector AdV (PROK1 + Ctrl AdV) (B). Expression of RCAN1-4 (Vehicle + RCAN1-4 AdV) alone did not have an effect on proliferation compared control (Vehicle + Ctrl AdV). Relative cell number is expressed as the absorbance value measured at 490 nm. Data are mean ± SEM of three independent experiments, n= 8 for each treatment. *, ** and *** represent significance at P< 0.05, P< 0.01 and P< 0.001 respectively.
PROK1-mediated DKK1 expression via the NFAT pathway inhibits PROKR1 Ishikawa cell proliferation
Wnt signalling has been proposed to stimulate cellular proliferation during the menstrual cycle (Hou et al., 2004; Jeong et al., 2009; Wang et al., 2009), and DKK1 is known to inhibit the proliferation of epithelial cells in several tissue types, including the intestine (Pinto et al., 2003; Kuhnert et al., 2004), lung (Pfaff et al., 2011) and thymus (Osada et al., 2010). We hypothesized that one of the functional roles of PROK1-induced DKK1 expression may be as a negative regulator of endometrial epithelial cell proliferation. To examine this, we transfected PROKR1 Ishikawa cells with scrambled control siRNA or DKK1 targeting siRNA. We found that PROK1 reduced cellular proliferation in PROKR1 Ishikawa cells transfected with a negative control siRNA sequence (P< 0.05; Fig. 4A). Silencing DKK1 expression with siRNA prevented this PROK1-mediated reduction in cellular proliferation (P< 0.01; Fig. 4A). To determine whether the NFAT pathway mediates this effect we infected PROKR1 Ishikawa cells with adenovirus over-expressing RCAN1-4, which inhibits the translocation of NFAT to the nucleus. We found that expression of RCAN1-4 reduced the negative effect of PROK1 on cellular proliferation in comparison to PROKR1 Ishikawa cells infected with control vector adenovirus (P< 0.001; Fig. 4B). Expression of RCAN1-4 alone did not affect cellular proliferation (Fig. 4B).These data suggest that PROK1 secreted by epithelial cells or sub-epithelial stromal cells may induce DKK1 release via the NFAT pathway to negatively regulate epithelial cell proliferation in the endometrium.
PROK1 and DKK1 expression influence decidualization in human primary endometrial stromal cells
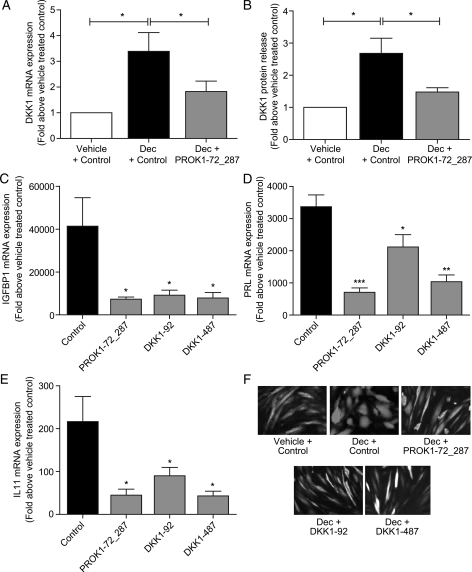
In the current study, DKK1 expression was elevated in first trimester decidua tissue and immunohistochemistry localized DKK1 to the stromal compartment, predominantly to the decidualized stromal cells. We therefore investigated whether PROK1 and DKK1 regulate the decidualization of endometrial stromal cells. Primary human endometrial stromal cells were decidualized by treatment with cAMP and progesterone for 5 days, which induced an increase in DKK1 mRNA expression (P< 0.05, Fig. 5A) and protein release (P< 0.05, Fig. 5B). Lentiviral delivery of miRNA sequences directed against PROK1 (Lv-cppt-GFP-PROK1-72_287) (Evans et al., 2009) significantly reduced DKK1 mRNA expression (P< 0.05 versus negative control sequence, Fig. 5A) and protein release (P< 0.05 versus negative control sequence, Fig. 5B) in decidualized endometrial stromal cells, suggesting that PROK1 contributes to the induction of DKK1 expression during decidualization. Knock down of PROK1 expression prior to decidualization significantly decreased the expression of three markers of decidualization, insulin-like growth factor 1 (IGFBP1; P< 0.05 versus negative control sequence, Fig. 5C), prolactin (PRL; P< 0.001 versus negative control sequence, Fig. 5D) and IL11 (P< 0.05 versus negative control sequence, Fig. 5E). Lentiviral knock down of DKK1 (using Lv-cppt-GFP-DKK1-92 or -DKK1-487) prior to decidualization also significantly decreased the expression of IGFBP1, PRL and IL11 (P< 0.05 versus negative control; Fig. 5C, D and E). We confirmed by fluorescent microscopy that suppression of PROK1 or DKK1 with lentiviral miRNA inhibited changes in cell morphology associated with the decidualization response. When endometrial stromal cells, which had been transduced with a negative control miRNA sequence (Fig. 5F; Vehicle + Control), were treated with cAMP and progesterone for 5 days they became more rounded and cobble stone-like, indicative of decidualization (Fig. 5F; Dec + Control). Transduction of endometrial stromal cells with either PROK1 miRNA (Fig. 5F; Dec + PROK1-72_287) or DKK1 miRNA (Fig. 5F; Dec + DKK1-92 or DKK1-487) prevented this alteration in cellular morphology in response to the decidualizing stimulus.
Figure 5.
PROK1 and DKK1 positively regulate decidualization of primary human endometrial stromal cells. Lentiviral delivery of a chained miRNA construct to knock down PROK1 (PROK1-72_287) significantly reduced DKK1 mRNA expression (A) and protein release (B) in primary human endometrial stromal cells decidualized with 1 µM MPA and 0.2 µg/ml cAMP for 5 days (Dec + PROK1-72_287), when compared with cells transduced with negative control miRNA (Dec + Ctrl). Lentiviral delivery of miRNA constructs to knock down PROK1 (PROK1-72_287) and DKK1 (DKK1-92 or DKK1-487) significantly reduced expression of the markers of decidualization IGFBP1 (C), PRL (D) and IL11 (E) in primary human endometrial stromal cells. Treatments n = 5 in triplicate. Lentiviral GFP expression was used to compare cell morphology in decidualized cells (Dec) with or without (Ctrl) miRNA sequences targeted to PROK1 or DKK1 (F). Decidualized stromal cells transduced with the negative control miRNA sequence (Dec + Ctrl) appeared round in shape. Knock down of PROK1 or DKK1 inhibited this change in cell shape and cells remained spindle-like, similar to undecidualized cells transduced with negative control miRNA (Vehicle + Ctrl). Data are mean ± SEM of six experiments. *, ** and *** represent significance at P< 0.05, P< 0.01 and P< 0.001 respectively. Fold change was calculated by dividing expression levels in cells treated with a decidualizing stimulus by vehicle-treated control cells.
Discussion
PROK1 is a secreted protein expressed in the receptive endometrium and decidua of early pregnancy, which can regulate the expression of numerous genes important in implantation and the establishment of pregnancy (Evans et al., 2008). This study identified that PROK1 signalling via PROKR1 can induce the expression of DKK1, an inhibitor of canonical Wnt signalling. DKK1 acts by antagonizing the Wnt ligand co-receptor low-density lipoprotein receptor-related protein 6 (LRP6) (Bafico et al., 2001; Mao et al., 2001; Semenov et al., 2001) via the formation of a complex with LRP6 and receptors specific to DKK1, Kremen 1 and Kremen 2 (Mao et al., 2002).
Wnt signalling has been demonstrated to be an important regulator of proliferation, whereas switching off Wnt signalling permits the occurrence of cellular differentiation (Reya and Clevers, 2005; Wang et al., 2009). Wnt signalling has been suggested to contribute to the regulation of endometrial development and differentiation during the normal menstrual cycle (Tulac et al., 2003) and to the events of early pregnancy (Chen et al., 2009; Sonderegger et al., 2010). The ligands, receptors and inhibitors of the Wnt signalling family show unique expression patterns and cellular localizations in the endometrium (Tulac et al., 2003). Evidence suggests that during the normal menstrual cycle, Wnt signalling mediates endometrial proliferation during the proliferative phase (Hou et al., 2004;, Jeong et al., 2009), and that inhibition of Wnt signalling by up-regulation of DKK1 during the mid-secretory phase may allow differentiation of the stroma (Tulac et al., 2006).
In this study, we have demonstrated a temporal pattern of DKK1 expression across the human menstrual cycle, confirming previous reports that DKK1 levels peak in the mid-secretory phase (Carson et al., 2002; Kao et al., 2002; Borthwick et al., 2003; Riesewijk et al., 2003; Tulac et al., 2003; Horcajadas et al., 2004; Talbi et al., 2006). Furthermore, we show for the first time that DKK1 expression is further elevated in first trimester decidua tissue. DKK1 protein expression was observed in proliferative phase endometrium throughout the stroma and glandular epithelium, which is in agreement with a previous study by Yi et al. (2009). During the secretory phase of the menstrual cycle, we observed strong stromal DKK1 expression, in accordance with a study by Tulac et al. (2003). DKK1 localization in the glands during the early- and mid-secretory phases of the menstrual cycle became restricted to the basal and basolateral surfaces of the epithelial cells. In late-secretory endometrium and first trimester decidua tissue, DKK1 protein expression was absent from the glandular epithelial compartment, though present in stromal cells and blood vessels.
This epithelial polarization and alteration in the cellular localization of DKK1 expression across the menstrual cycle and during early pregnancy suggests that it may mediate the switch between cellular proliferation and differentiation needed to promote maturation of the glands and decidualization of the stroma during the secretory phase, via the modulation of Wnt signalling. Indeed, Wnt signalling has been shown to regulate cell polarity in the uterine epithelium during embryonic development (Vandenberg and Sassoon, 2009). It may be the case that DKK1 is expressed on the basal side of the glandular epithelium during the early- and mid-secretory phases of the menstrual cycle to maintain cell polarity, or to promote differentiation as the glands and stroma undergo secretory transformation. The absence of DKK1 expression on the luminal surface of the glands suggests it is not released into the glandular secretions. This would prevent release into the uterine lumen and any potential harmful effects of DKK1 on the developing blastocyst. Canonical Wnt signalling has been shown to be important in promoting blastocyst activation and competency for implantation (Xie et al., 2008).
Previously, PROK1 has been shown to regulate the expression of IL8 (Maldonado-Perez et al., 2009) and IL11 (Cook et al., 2010) via a Gq-calcium-calcineurin-NFAT signalling pathway. We have demonstrated that PROK1 induces DKK1 expression by the same pathway in endometrial epithelial cells and decidualized stromal cells. In this pathway, calcium-dependent activation of calcineurin causes dephosphorylation of NFAT, allowing it to translocate to the nucleus and activate NFAT-regulated gene transcription. The calcinuerin-NFAT signalling pathway is regulated by RCAN1-4, an endogenous negative feedback inhibitor which acts to bind to calcinuerin and prevent its activation of NFAT (Fuentes et al., 2000). We have confirmed that RCAN1-4 is a negative regulator of PROK1-mediated DKK1 expression, similar to our observations for IL8 (Maldonado-Perez et al., 2009) and IL11 (Cook et al., 2010). Therefore, the same signalling cascade is activated by PROK1 to control DKK1 expression in epithelial cells to regulate proliferation, and in the decidua to regulate decidualization of the stroma. The calcineurin-NFAT pathway has previously been shown to be involved in regulating endometrial epithelial cell proliferation (Wallace et al., 2011), and in the endometrial expression of IL11, an important mediator of decidualization (Cook et al., 2010).
Our results indicate that PROK1-induced DKK1 expression via the NFAT pathway can negatively regulate the proliferation of endometrial epithelial cells, since a PROK1-mediated decrease in cellular proliferation was prevented in PROKR1 Ishikawa cells transfected with DKK1 siRNA or infected with adenovirus over-expressing RCAN1-4. As this suggests that PROK1 can cause a decrease in the proliferation of endometrial epithelial cells via DKK1 release; we postulate that this occurs through an inhibition of Wnt signalling. We propose that this represents an important new function of PROK1 in the endometrium across the menstrual cycle, and that DKK1 secretion switches to become basal–basolateral in the uterine glands at the time of implantation so as not to compromise blastocyst development.
Furthermore, we demonstrate a role for PROK1 and DKK1 in mediating decidualization of the endometrial stroma. DKK1 expression is known to be increased upon decidualization of human endometrial stromal cells in culture (Kao et al., 2002; Tulac et al., 2006; Kane et al., 2008). Here we have shown its levels to be elevated in first trimester decidua tissue, where it localizes primarily to the stromal compartment. Recently, PROK1 levels have also been shown to increase in stromal cells decidualized in vitro (Salker et al., 2010; Tiberi et al., 2010), and PROK1 is similarly increased in decidua tissue (Evans et al., 2008). We have found that when the expression of either DKK1 or PROK1 is knocked down in primary endometrial stromal cells, there is a decrease in the expression of the markers of decidualization IGFBP1, PRL and IL11 in response to a decidualizing stimulus. Fluorescent microscopy also demonstrated that after knock down of PROK1 or DKK1, primary stromal cells fail to adopt the characteristic rounded cobble stone-like morphology indicative of decidualization, but rather maintain the long spindle cell-type morphology observed in control undecidualized stromal cells.
Previous studies have indicated the regulation of DKK1 (Tulac et al., 2006) and PROK1 (Battersby et al., 2004) expression by progesterone in the human endometrium. In the current study, progesterone and cAMP in combination induced DKK1 expression in endometrial stromal cells. However, knock down of PROK1 expression in endometrial stromal cells reduces DKK1 expression and protein release upon treatment with progesterone and cAMP, but does not abolish it. Therefore, we propose that both DKK1 and PROK1 lie downstream in the progesterone/cAMP signalling cascade, with potential for DKK1 to be regulated by progesterone directly, and indirectly via progesterone-mediated regulation of PROK1.
In conclusion, we have identified a novel signalling pathway whereby PROK1 can induce the expression of DKK1 in the human endometrium and first trimester decidua. We propose that via negative regulation of cellular proliferation and decidualization, PROK1-mediated DKK1 expression contributes to the generation of a receptive endometrium. Dysregulation of PROK1-mediated expression of DKK1 may be a contributing factor to infertility and recurrent pregnancy loss.
Authors' roles
L.J.M.: acquisition of data, analysis and interpretation of data, writing of manuscript. K.J.S.: analysis and interpretation of data, writing of manuscript. P.B.: design and acquisition of data. H.N.J.: conception and design, critically revising manuscript for important intellectual content. R.D.C.: conception and design, analysis and interpretation of data, writing of manuscript and approval of final version to be published.
Funding
This work was supported by Medical Research Council core funding to H.N.J. [grant number: U1276.00.004.00002.03]. Funding to pay the Open Access publication charges for this article was provided by the Medical Research Council.
Acknowledgements
We thank Sharon McPherson, Katie Cairns and Catherine Murray for patient recruitment and assistance with tissue collection; Hilary Critchley, Anne King and Elaine Marshall for primary human endometrial stromal cells; Ted Pinner for graphical assistance.
References
- Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3:683–686. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- Battersby S, Critchley HO, Morgan K, Millar RP, Jabbour HN. Expression and regulation of the prokineticins (endocrine gland-derived vascular endothelial growth factor and Bv8) and their receptors in the human endometrium across the menstrual cycle. J Clin Endocrinol Metab. 2004;89:2463–2469. doi: 10.1210/jc.2003-032012. [DOI] [PubMed] [Google Scholar]
- Borthwick JM, Charnock-Jones DS, Tom BD, Hull ML, Teirney R, Phillips SC, Smith SK. Determination of the transcript profile of human endometrium. Mol Hum Reprod. 2003;9:19–33. doi: 10.1093/molehr/gag004. [DOI] [PubMed] [Google Scholar]
- Carson DD, Lagow E, Thathiah A, Al-Shami R, Farach-Carson MC, Vernon M, Yuan L, Fritz MA, Lessey B. Changes in gene expression during the early to mid-luteal (receptive phase) transition in human endometrium detected by high-density microarray screening. Mol Hum Reprod. 2002;8:871–879. doi: 10.1093/molehr/8.9.871. [DOI] [PubMed] [Google Scholar]
- Chen Q, Zhang Y, Lu J, Wang Q, Wang S, Cao Y, Wang H, Duan E. Embryo-uterine cross-talk during implantation: the role of Wnt signaling. Mol Hum Reprod. 2009;15:215–221. doi: 10.1093/molehr/gap009. [DOI] [PubMed] [Google Scholar]
- Cook IH, Evans J, Maldonado-Perez D, Critchley HO, Sales KJ, Jabbour HN. Prokineticin-1 (PROK1) modulates interleukin (IL)-11 expression via prokineticin receptor 1 (PROKR1) and the calcineurin/NFAT signalling pathway. Mol Hum Reprod. 2010;16:158–169. doi: 10.1093/molehr/gap084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison FC, Battersby S, King AE, Szuber M, Jabbour HN. Prokineticin-1: a novel mediator of the inflammatory response in third-trimester human placenta. Endocrinology. 2008;149:3470–3477. doi: 10.1210/en.2007-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsch M, Qiu Y, Soler D, Frank N, Duong T, Goodearl A, O'Neil S, Lora J, Fraser CC. PK1/EG-VEGF induces monocyte differentiation and activation. J Leukoc Biol. 2005;78:426–434. doi: 10.1189/jlb.0205061. [DOI] [PubMed] [Google Scholar]
- Evans J, Catalano RD, Morgan K, Critchley HO, Millar RP, Jabbour HN. Prokineticin 1 signaling and gene regulation in early human pregnancy. Endocrinology. 2008;149:2877–2887. doi: 10.1210/en.2007-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J, Catalano RD, Brown P, Sherwin R, Critchley HO, Fazleabas AT, Jabbour HN. Prokineticin 1 mediates fetal-maternal dialogue regulating endometrial leukemia inhibitory factor. FASEB J. 2009;23:2165–2175. doi: 10.1096/fj.08-124495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Frantz G, LeCouter J, Dillard-Telm L, Pham T, Draksharapu A, Giordano T, Peale F. Differential expression of the angiogenic factor genes vascular endothelial growth factor (VEGF) and endocrine gland-derived VEGF in normal and polycystic human ovaries. Am J Pathol. 2003;162:1881–1893. doi: 10.1016/S0002-9440(10)64322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser HM, Bell J, Wilson H, Taylor PD, Morgan K, Anderson RA, Duncan WC. Localization and quantification of cyclic changes in the expression of endocrine gland vascular endothelial growth factor in the human corpus luteum. J Clin Endocrinol Metab. 2005;90:427–434. doi: 10.1210/jc.2004-0843. [DOI] [PubMed] [Google Scholar]
- Fuentes JJ, Genesca L, Kingsbury TJ, Cunningham KW, Perez-Riba M, Estivill X, de la Luna S. DSCR1, overexpressed in Down syndrome, is an inhibitor of calcineurin-mediated signaling pathways. Hum Mol Genet. 2000;9:1681–1690. doi: 10.1093/hmg/9.11.1681. [DOI] [PubMed] [Google Scholar]
- Hoffmann P, Feige JJ, Alfaidy N. Expression and oxygen regulation of endocrine gland-derived vascular endothelial growth factor/prokineticin-1 and its receptors in human placenta during early pregnancy. Endocrinology. 2006;147:1675–1684. doi: 10.1210/en.2005-0912. [DOI] [PubMed] [Google Scholar]
- Horcajadas JA, Riesewijk A, Martin J, Cervero A, Mosselman S, Pellicer A, Simon C. Global gene expression profiling of human endometrial receptivity. J Reprod Immunol. 2004;63:41–49. doi: 10.1016/j.jri.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Hou X, Tan Y, Li M, Dey SK, Das SK. Canonical Wnt signaling is critical to estrogen-mediated uterine growth. Mol Endocrinol. 2004;18:3035–3049. doi: 10.1210/me.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JW, Lee HS, Franco HL, Broaddus RR, Taketo MM, Tsai SY, Lydon JP, DeMayo FJ. beta-catenin mediates glandular formation and dysregulation of beta-catenin induces hyperplasia formation in the murine uterus. Oncogene. 2009;28:31–40. doi: 10.1038/onc.2008.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane N, Jones M, Brosens JJ, Saunders PT, Kelly RW, Critchley HO. Transforming growth factor-beta1 attenuates expression of both the progesterone receptor and Dickkopf in differentiated human endometrial stromal cells. Mol Endocrinol. 2008;22:716–728. doi: 10.1210/me.2007-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao LC, Tulac S, Lobo S, Imani B, Yang JP, Germeyer A, Osteen K, Taylor RN, Lessey BA, Giudice LC. Global gene profiling in human endometrium during the window of implantation. Endocrinology. 2002;143:2119–2138. doi: 10.1210/endo.143.6.8885. [DOI] [PubMed] [Google Scholar]
- Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, Nusse R, Kuo CJ. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci USA. 2004;101:266–271. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeCouter J, Kowalski J, Foster J, Hass P, Zhang Z, Dillard-Telm L, Frantz G, Rangell L, DeGuzman L, Keller GA, et al. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature. 2001;412:877–884. doi: 10.1038/35091000. [DOI] [PubMed] [Google Scholar]
- LeCouter J, Zlot C, Tejada M, Peale F, Ferrara N. Bv8 and endocrine gland-derived vascular endothelial growth factor stimulate hematopoiesis and hematopoietic cell mobilization. Proc Natl Acad Sci USA. 2004;101:16813–16818. doi: 10.1073/pnas.0407697101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Bullock CM, Knauer DJ, Ehlert FJ, Zhou QY. Identification of two prokineticin cDNAs: recombinant proteins potently contract gastrointestinal smooth muscle. Mol Pharmacol. 2001;59:692–698. doi: 10.1124/mol.59.4.692. [DOI] [PubMed] [Google Scholar]
- Li Q, Kannan A, Wang W, Demayo FJ, Taylor RN, Bagchi MK, Bagchi IC. Bone morphogenetic protein 2 functions via a conserved signaling pathway involving Wnt4 to regulate uterine decidualization in the mouse and the human. J Biol Chem. 2007;282:31725–31732. doi: 10.1074/jbc.M704723200. [DOI] [PubMed] [Google Scholar]
- Lin R, LeCouter J, Kowalski J, Ferrara N. Characterization of endocrine gland-derived vascular endothelial growth factor signaling in adrenal cortex capillary endothelial cells. J Biol Chem. 2002;277:8724–8729. doi: 10.1074/jbc.M110594200. [DOI] [PubMed] [Google Scholar]
- Maldonado-Perez D, Evans J, Denison F, Millar RP, Jabbour HN. Potential roles of the prokineticins in reproduction. Trends Endocrinol Metab. 2007;18:66–72. doi: 10.1016/j.tem.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Perez D, Brown P, Morgan K, Millar RP, Thompson EA, Jabbour HN. Prokineticin 1 modulates IL-8 expression via the calcineurin/NFAT signaling pathway. Biochim Biophys Acta. 2009;1793:1315–1324. doi: 10.1016/j.bbamcr.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- Monnier J, Samson M. Cytokine properties of prokineticins. FEBS J. 2008;275:4014–4021. doi: 10.1111/j.1742-4658.2008.06559.x. [DOI] [PubMed] [Google Scholar]
- Ngan ES, Lee KY, Yeung WS, Ngan HY, Ng EH, Ho PC. Endocrine gland-derived vascular endothelial growth factor is expressed in human peri-implantation endometrium, but not in endometrial carcinoma. Endocrinology. 2006;147:88–95. doi: 10.1210/en.2005-0543. [DOI] [PubMed] [Google Scholar]
- Osada M, Jardine L, Misir R, Andl T, Millar SE, Pezzano M. DKK1 mediated inhibition of Wnt signaling in postnatal mice leads to loss of TEC progenitors and thymic degeneration. PLoS One. 2010;5:e9062. doi: 10.1371/journal.pone.0009062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff EM, Becker S, Gunther A, Konigshoff M. Dickkopf proteins influence lung epithelial cell proliferation in idiopathic pulmonary fibrosis. Eur Respir J. 2011;37:79–87. doi: 10.1183/09031936.00142409. [DOI] [PubMed] [Google Scholar]
- Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Riesewijk A, Martin J, van Os R, Horcajadas JA, Polman J, Pellicer A, Mosselman S, Simon C. Gene expression profiling of human endometrial receptivity on days LH + 2 versus LH + 7 by microarray technology. Mol Hum Reprod. 2003;9:253–264. doi: 10.1093/molehr/gag037. [DOI] [PubMed] [Google Scholar]
- Sales KJ, Maldonado-Perez D, Grant V, Catalano RD, Wilson MR, Brown P, Williams AR, Anderson RA, Thompson EA, Jabbour HN. Prostaglandin F(2alpha)-F-prostanoid receptor regulates CXCL8 expression in endometrial adenocarcinoma cells via the calcium-calcineurin-NFAT pathway. Biochim Biophys Acta. 2009;1793:1917–1928. doi: 10.1016/j.bbamcr.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salker M, Teklenburg G, Molokhia M, Lavery S, Trew G, Aojanepong T, Mardon HJ, Lokugamage AU, Rai R, Landles C, et al. Natural selection of human embryos: impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PLoS One. 2010;5:e10287. doi: 10.1371/journal.pone.0010287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- Shaw JL, Denison FC, Evans J, Durno K, Williams AR, Entrican G, Critchley HO, Jabbour HN, Horne AW. Evidence of prokineticin dysregulation in fallopian tube from women with ectopic pregnancy. Fertil Steril. 2010;94:1601–1608. doi: 10.1016/j.fertnstert.2009.10.061. e1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonderegger S, Pollheimer J, Knofler M. Wnt signalling in implantation, decidualisation and placental differentiation–review. Placenta. 2010;31:839–847. doi: 10.1016/j.placenta.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su MT, Lin SH, Lee IW, Chen YC, Hsu CC, Pan HA, Kuo PL. Polymorphisms of endocrine gland-derived vascular endothelial growth factor gene and its receptor genes are associated with recurrent pregnancy loss. Hum Reprod. 2010;25:2923–2930. doi: 10.1093/humrep/deq256. [DOI] [PubMed] [Google Scholar]
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147:1097–1121. doi: 10.1210/en.2005-1076. [DOI] [PubMed] [Google Scholar]
- Tiberi F, Tropea A, Romani F, Apa R, Marana R, Lanzone A. Prokineticin 1, homeobox A10, and progesterone receptor messenger ribonucleic acid expression in primary cultures of endometrial stromal cells isolated from endometrium of healthy women and from eutopic endometrium of women with endometriosis. Fertil Steril. 2010;94:2558–2563. doi: 10.1016/j.fertnstert.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Tulac S, Nayak NR, Kao LC, Van Waes M, Huang J, Lobo S, Germeyer A, Lessey BA, Taylor RN, Suchanek E, et al. Identification, characterization, and regulation of the canonical Wnt signaling pathway in human endometrium. J Clin Endocrinol Metab. 2003;88:3860–3866. doi: 10.1210/jc.2003-030494. [DOI] [PubMed] [Google Scholar]
- Tulac S, Overgaard MT, Hamilton AE, Jumbe NL, Suchanek E, Giudice LC. Dickkopf-1, an inhibitor of Wnt signaling, is regulated by progesterone in human endometrial stromal cells. J Clin Endocrinol Metab. 2006;91:1453–1461. doi: 10.1210/jc.2005-0769. [DOI] [PubMed] [Google Scholar]
- Vandenberg AL, Sassoon DA. Non-canonical Wnt signaling regulates cell polarity in female reproductive tract development via van gogh-like 2. Development. 2009;136:1559–1570. doi: 10.1242/dev.034066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell JM, Evans J, Jabbour HN, Denison FC. CTGF expression is up-regulated by PROK1 in early pregnancy and influences HTR-8/Svneo cell adhesion and network formation. Hum Reprod. 2010 doi: 10.1093/humrep/deq294. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace AE, Catalano RD, Anderson RA, Jabbour HN. Chemokine (C-C) motif ligand 20 is regulated by PGF(2alpha)-F-prostanoid receptor signalling in endometrial adenocarcinoma and promotes cell proliferation. Mol Cell Endocrinol. 2011;331:129–135. doi: 10.1016/j.mce.2010.08.018. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hanifi-Moghaddam P, Hanekamp EE, Kloosterboer HJ, Franken P, Veldscholte J, van Doorn HC, Ewing PC, Kim JJ, Grootegoed JA, et al. Progesterone inhibition of Wnt/beta-catenin signaling in normal endometrium and endometrial cancer. Clin Cancer Res. 2009;15:5784–5793. doi: 10.1158/1078-0432.CCR-09-0814. [DOI] [PubMed] [Google Scholar]
- Xie H, Tranguch S, Jia X, Zhang H, Das SK, Dey SK, Kuo CJ, Wang H. Inactivation of nuclear Wnt-beta-catenin signaling limits blastocyst competency for implantation. Development. 2008;135:717–727. doi: 10.1242/dev.015339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi N, Liao QP, Li T, Xiong Y. Novel expression profiles and invasiveness-related biology function of DKK1 in endometrial carcinoma. Oncol Rep. 2009;21:1421–1427. doi: 10.3892/or_00000370. [DOI] [PubMed] [Google Scholar]