Abstract
Despite extensive research on the emergence of and treatments for methicillin-resistant Staphylococcus aureus (MRSA), prior studies have not rigorously evaluated the impact of methicillin resistance on the overall incidence of S. aureus infections. Yet, there are direct clinical and research implications of determining whether methicillin-susceptible S. aureus (MSSA) infection rates remain stable in the face of increasing MRSA prevalence or whether MSSA will be replaced over time. A synthesis of prior studies indicates that the emergence of healthcare-associated MRSA (HA-MRSA) and community-associated MRSA (CA-MRSA) has led to an increase in the overall incidence of S. aureus infections, with MRSA principally adding to, rather than replacing, MSSA. However, colonization with CA-MRSA may at least partially replace colonization with MSSA. So far, evidence indicates that MSSA still accounts for many infections. Therefore, eradication of MRSA alone is not sufficient to address the public health burden of S. aureus.
Keywords: bacterial resistance, staphylococcal infections, healthcare-associated infections, community-associated infections
Introduction
Staphylococcus aureus is a ubiquitous member of the human microbiological flora, with up to 20%–30% of humans persistently asymptomatically colonized and 50%–60% intermittently colonized.1,2 It is the most commonly isolated human bacterial pathogen, and it is the cause of many skin and soft tissue infections (SSTIs) and invasive diseases, such as sepsis, endocarditis, pneumonia and osteomyelitis.3 The emergence of healthcare-associated methicillin-resistant S. aureus (HA-MRSA) has posed a major public health problem since the 1960s and the rapid rise of community-associated MRSA (CA-MRSA) in the late 1990s has further directed attention towards the burden of MRSA infections. To date, CA-MRSA and HA-MRSA have each been associated with a limited number of bacterial genetic backgrounds, combined with specific resistance elements; thus, MRSA as a group have remained less genetically diverse than the background methicillin-susceptible S. aureus (MSSA) lineages from which they are thought to be descended.4,5 Recent attention has focused on the increasing severity and frequency of infections caused by MRSA, especially with regard to its greater clinical and economic impact compared with MSSA.6 Since empirical regimens for sepsis are often not effective for MRSA infections, and vancomycin is generally considered to be a suboptimal antistaphylococcal agent, drug development has focused on identifying effective treatments for MRSA infections. However, MSSA strains continue to cause a considerable number of S. aureus infections.7
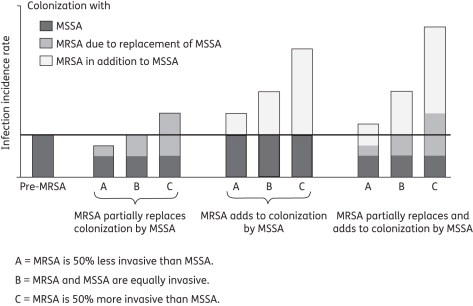
Despite extensive research on the emergence of MRSA, its clinical course and treatment, few studies have rigorously evaluated the impact of methicillin resistance on the overall incidence of S. aureus infections. The treatment of susceptible strains may have created an ecological niche for resistant bacteria that would otherwise not be available. Thus, one may expect that treatment with cloxacillin and other antistaphylococcal β-lactams may result in selective killing of the susceptible strains in the population and allow for replacement by resistant strains.8 As a result, increased MRSA rates over time should correlate with a compensatory decline in MSSA.9 This hypothesis is concordant with bacterial interference studies showing that colonization with one strain of S. aureus prevents subsequent colonization with other strains of S. aureus.10 Alternatively, it is plausible that MRSA and MSSA do not compete, and, thus, increasing MRSA incurs an additive burden of morbidity and mortality. Lastly, there may be both replacement and additive effects, with the magnitude of increased S. aureus infection depending on the colonization capacity and invasiveness of different strains (see Figure 1).
Figure 1.
Schematic representing the impact of MRSA on the infection incidence rate for S. aureus, depending on both the colonization and infectiveness of MRSA and MSSA. The schematic shows that even if the infection incidence rate of total S. aureus is increasing, it may be due to replacement of MSSA, MRSA incidence in addition to MSSA or a combination of the two processes. Furthermore, the magnitude of the increased S. aureus infection rates depends on the colonization capacity and invasiveness of different strains. The greater the invasiveness of MRSA relative to MSSA, the greater the impact of MRSA on the total S. aureus burden. The horizontal line indicates the S. aureus infection incidence rate before the emergence of MRSA.
Because we reviewed observational studies, it is difficult to draw firm causal conclusions about the biological mechanisms involved. Ideally, we would compare the incidence and prevalence of MSSA in the presence of MRSA to the same quantities for MSSA in the same population at the same time in the complete absence of MRSA. However, the latter scenario is counterfactual and not observed.11 Therefore, if the studies show that both MRSA and MSSA are increasing, this is consistent with the hypothesis that the two strains do not compete. However, it is also consistent with the possibility that MRSA may partially replace MSSA, yet MSSA continues to increase due to other factors, such as lapses in infection control or an increase in the number of sicker patients vulnerable to infection. Similarly, if the studies show that increasing MRSA is associated with decreasing MSSA, this may suggest that the two strains compete for the same ecological niche. However, it may alternatively be due to a decline in MSSA resulting from improved infection control efforts that are more effective against MSSA than MRSA, combined with the selective emergence of MRSA. At present, we cannot conclusively address these causal questions, though observational studies can provide suggestive evidence. Throughout this review, we therefore emphasize the descriptive question of the absolute incidence of MSSA infections in the presence of MRSA, while commenting on possible causal mechanisms.
Notwithstanding the causal question, there are direct clinical implications of determining whether MSSA infection rates remain stable in the face of increasing MRSA prevalence or whether MSSA is declining in absolute incidence over time. Since MRSA is not susceptible to β-lactam antibiotics, vancomycin is widely used for the empirical treatment of suspected Gram-positive bloodstream infections (BSIs) in patients with MRSA risk factors. However, vancomycin treatment of invasive MSSA infections is associated with higher microbiological and clinical failure rates, and increased toxicity relative to β-lactam antibiotics.12,13 Therefore, if MRSA is expected to replace most or all of MSSA infections, perhaps empirical treatment with vancomycin is correct and research efforts should focus on identifying more effective antimicrobials for MRSA. However, if MSSA is expected to remain prevalent, perhaps investigators should focus on developing rapid diagnostic tests that will distinguish between MRSA and MSSA, and thereby enable immediate therapy with the optimal anti-infective. Additionally, the potential finding that MSSA is expected to remain prevalent indicates that health services must concentrate efforts on preventing all kinds of S. aureus infections, and highlights the importance of understanding the factors that promote the coexistence of MRSA and MSSA, at the biological level and at the population level.9,14 Understanding the mechanisms underlying the potential coexistence of drug-susceptible and drug-resistant strains may provide insight into the population dynamics of pathogens with drug resistance, and help to identify the distinctive features of MSSA in this context. This review presents data from the relevant epidemiological studies on S. aureus to provide insight into the changing epidemiology of S. aureus and point to various limitations that should be addressed in future analyses.
Search strategy and selection criteria
Studies for this review were identified by searches of Medline and the references from relevant articles. The search terms were ‘methicillin’, ‘MRSA’, ‘MSSA’, ‘resistance’, ‘trends’, ‘replacement’, ‘addition’, ‘selective pressure’ and ‘competition’. Only English language papers were reviewed. All studies reporting changes in both MRSA and MSSA in human subjects are included in Table 1. The search was limited to publications in English up to 9 February 2011.
Table 1.
Studies reporting the impact of HA-MRSA and CA-MRSA on the overall burden of S. aureus in hospitals and communities
| Reference (first author, year) | Study period | Location | Setting | Case detection | Case definition | Measurea | Main findings |
|---|---|---|---|---|---|---|---|
| Impact of MRSA on MSSA in hospitals | |||||||
| Thompson, 198222 | 1979–80 | Virginia, USA | hospital-wide | microbiology results, surveillance of HR patients | SAB, post-operative wound infection, pneumonia | 2, 4 | no change in incidence of S. aureus infections (from 0.20 to 0.25 S. aureus infections per 100 admissions); ↑ in % S. aureus infections due to MRSA (SAB: from 13% to 40%; post-operative wound infections: from 27% to 49%; pneumonia: from 19% to 24%) |
| Linnemann, 198223 | 1977–80 | Cincinnati, USA | burn and surgical ICU | microbiology results, surveillance of HR patients | SAB | 2, 5b | incidence of SAB constant (from ∼4.5 to 5.0 cases per 1000 admissions); as MRSA ↑ to 1.2 per 1000 admissions in 1979, MSSA ↓ to 3.5 per 1000; when MRSA ↓ in 1980, MSSA ↑ |
| Pavillard, 198226 | 1978–80 | Melbourne, Australia | population-based | questionnaire administered to hospital directors | S. aureus colonization or infection | 1, 4 | MRSA ↑ from 2 patients in 1978 (<2% of S. aureus) to 46 patients in 1979 (30% of S. aureus); MSSA frequency varied with no systematic trend |
| Boyce, 198327 | 1979–83 | Mississippi, USA | burn unit | microbiology results | SAB | 2, 4b | ↑ in % infections due to S. aureus (from 11% to 15%) and % S. aureus infections due to MRSA (from 11% to 32%) |
| Mylotte, 198728 | 1977–85 | New York, USA | hospital-wide | microbiology results | SAB | 1, 2 | ↑ in SAB (16.1 per 10 000 discharges to 28.5 per 10 000 discharges; from n = 20 to 41), including ↑ in MRSA (from n = 0 to 12) and MSSA (from n = 20 to 29) |
| Tam, 198829 | 1976–85 | Hong Kong, China | neonatal unit | microbiology results | severe S. aureus infection | 1, 5b | in 1976–80 versus 1981–85, ↑ in # of MRSA (from 7 to 35) and 4.5-fold ↑ in MRSA per 1000 admissions (from 2.5 to 11.2), # of MSSA constant (∼1 or 2 per year) |
| Law, 198837 | 1985–86 | London, England | acute medical and surgical wards | microbiology results | SAB and urinary tract infections | 1b | in response to infection control efforts, MRSA infections ↓ while MSSA infections remained fairly constant |
| Stamm, 199330 | 1986–91 | Alabama, USA | hospital-wide | microbiology results | SAB | 2, 5 | ↑ in total S. aureus (from 1.4 to 1.7 infections per 100 admissions), including ↑ in MRSA (from 0 to 0.3 infections per 100 admissions) and MSSA (from 0.5 to 0.7 infections per 100 admissions) |
| Pujol, 199431 | 1990–91 | Barcelona, Spain | hospital-wide | microbiology results, screening of HR patients | S. aureus colonization or SAB | 1b | ↑ in # of total S. aureus, including ↑ in both MRSA and MSSA |
| Jernigan, 199532 | 1986–93 | Virginia, USA | hospital-wide | microbiology results, surveillance of HR patients | S. aureus colonization or infection | 4, 5b | % S. aureus due to MRSA was constant in 1986–89 (5.4%), but ↑ to 17.7% thereafter; no correlation between the # of MRSA and MSSA infections per 1000 admissions |
| Spindel, 199524 | 1987–91 | Vancouver, Canada | nursing home | microbiology results | S. aureus infection | 1, 2, 6 | stable # and rate per 1000 resident-days of total S. aureus (∼12 cases per year, from 0.29 to 0.33 per 1000 resident-days); ↑ in # and rate of MRSA (from 0 to 5 cases, from 0 to 0.14 per 1000 resident-days), ↓ in # and rate of MSSA (from 12 to 7 cases, from 0.29 to 0.19 per 1000 resident-days) |
| Speller, 199733 | 1989–95 | England and Wales | population-based | microbiology results | SAB | 1, 2, 4b | ↑ in # of total S. aureus (from 3526 to 5770), representing constant proportion of all isolates; ↑ in # of MRSA (from 56 to 762) and % S. aureus due to MRSA (from 1.6% to 13.2%), ↑ in # of MSSA (from 3470 to 5008), ↓ in % S. aureus due to MSSA (from 98.4% to 86.8%) |
| Morgan, 199934 | 1993–97 | Wales | population-based | microbiology results | S. aureus colonization or infectionc | 1, 4 | ↑ in # of total S. aureus (from 323 to 770), ↑ in # of MRSA and % isolates due to MRSA (from 11/2059 = 0.5% to 292/3924 = 7.4%), ↑ in # of MSSA but ↓ in % isolates due to MSSA (from 252/2059 = 12.2% to 379/3924 = 9.7%) |
| Albertini, 200225 | 1996–2000 | France | population-based | microbiology results | S. aureus colonization or infection | 1, 4, 6 | no change in # of total S. aureus (from 6834 to 6824); ↑ in # of MRSA (from 2433 to 2798), % S. aureus due to MRSA (from 35.6% to 41.0%) and rate of MRSA (from 0.71 to 0.96 per 1000 hospital-days); ↓ in # of MSSA (from 4401 to 4026) and % S. aureus due to MSSA (from 64.4% to 59.0%) |
| Assadian, 200335 | 1994–98 | Austria | population-based | questionnaire administered to hospital directors | S. aureus colonization or infectionc | 1, 2, 4, 5 | ↑ in # of total S. aureus (from 3012 to 22 179), including ↑ in # of MRSA (from 476 to 1825) and MSSA (from 2536 to 20 354); ↑ in # per 1000 hospital admissions with any S. aureus (from 7.07 to 15.73), MRSA (from 0.85 to 1.29) and MSSA (from 6.22 to 14.44); ↓ in % S. aureus due to MRSA (from 15.8% to 8.2%) and ↑ in % S. aureus due to MSSA (from 84.2% to 91.8%) |
| Seal, 200336 | 1986–2000 | Chicago, USA | hospital-wide | microbiology results | S. aureus clinical isolatesd | 1, 4b | ↑ in # of total S. aureus (from 874 to 1176), including ↑ in # of MRSA (from 114 to 329) and MSSA (from 760 to 847); ↑ in % S. aureus due to MRSA (from 13% to 28%) and ↓ in % S. aureus due to MSSA (from 87% to 72%) |
| Whyte, 200540 | 2002–04 | Limerick, Ireland | population-based | microbiology results | SABc | 1, 4, 6 | # of total S. aureus was 96, 70 and 79; % S. aureus due to MRSA was 44%, 56% and 48%; rate of MRSA per 1000 bed-days was 0.176, 0.159 and 0.152 |
| Wyllie, 200641 | 1997–2003 | Oxford, England | medical, surgical and trauma specialties | microbiology results | SAB | 2, 5b | ↑ in # of S. aureus per 100 000 admissions and # of MRSA per 100 000 admissions; # of MSSA per 100 000 admissions did not alter; after adjustment for case mix, ↑ in # of S. aureus per 100 000 admissions (1.06 per year), ↑ in # of MRSA per 100 000 admissions (1.22 per year) and ↓ in # of MSSA per 100 000 admissions (0.93 per year) |
| Anderson, 200742 | 2000–05 | south-eastern USA | population-based | microbiology results, screening of surgical patients, clinical rounds, questionnaire to surgeons | severe SSI | 1, 2, 5b | ↑ in # of S. aureus per 100 procedures (from 0.39 to 0.49), ↑ in # of MRSA (from 24 to 56) and ↑ in # of MRSA per 100 procedures (from 0.12 to 0.23); no change in MSSA |
| van der Mee-Marquet, 200743 | 2004–06 | France | population-based | microbiology results | SABc | 1, 4, 6 | ↑ in # of S. aureus (from 122 to 157), including ↑ in # of MRSA (from 33 to 46) and MSSA (from 89 to 111), but corresponds to stable % S. aureus due to MRSA (from 33/122 = 27% to 46/157 = 29%) and MSSA (from 89/122 = 73% to 111/157 = 71%); ↑ in rate of total S. aureus (from 0.202 to 0.234 per 1000 patient-days), MRSA (from 0.055 to 0.068 per 1000 patient-days) and MSSA (from 0.147 to 0.165 per 1000 patient-days) |
| Allard, 200844 | 1991–2005 | Quebec, Canada | hospital-wide | microbiology results | SABd | 3 | in 1997–99 versus 2003–05, ↑ in MRSA (from 0 to 7.4 per 100 000 inhabitants) and MSSA stable (from 24.1 to 25.0 per 100 000 inhabitants) |
| Robicsek, 2008101 | 2003–07 | Illinois, USA | hospital-wide | microbiology results, screening of all ICU admissions | SAB | 6 | in response to infection control efforts, ↓ in rate of total S. aureus (from 8.9 to 3.9 per 10 000 patient-days), including ↓ in rate of MRSA (from 2.1 to 1.1 per 10 000 patient-days) and ↓ in rate of MSSA (from 2.1 to 1.6 per 10 000 patient-days) |
| Burton, 200945 | 1997–2007 | USA | population-based: ICUs | National Nosocomial Infection Surveillance System | central line SAB | 4, 6 | 25.8% ↑ in % S. aureus due to MRSA (from 47.9% to 64.5%), 49.6% ↓ in rate of MRSA (from 0.43 to 0.21 per 1000 central line-days) and 70.1% ↓ in rate of MSSA (from 0.31 to 0.09 per 1000 central line-days) |
| Hacek, 200946 | 2002–07 | Illinois, USA | hospital-wide | screening of all patient admissions | S. aureus clinical isolatesc | 1 | in response to infection control efforts, ↓ in # of total S. aureus (from 842 to 649) and # of MRSA (from 434 to 255); # of MSSA remained constant (∼400 per year) |
| Pearson, 200947 | 2004–08 | England | population-based | microbiology results | voluntary: clinically significant SAB mandatory: microbiology resultsc | 1b | voluntary: ↓ in # of total S. aureus, 53% ↓ in # of MRSA, # of MSSA stable in 2004–06, then 6% ↑ in 2007 mandatory: ↓ in # of total S. aureus, 56% ↓ in # of MRSA, ↓ in # of MSSA |
| Chuang, 201065 | 1981–2007 | Taipei, Taiwan | hospital-wide | microbiology results | S. aureus infection and SABc | 5b | ↑ in S. aureus infections (from 0.199 to 0.404 per 100 discharges), ↑ in MRSA infections (from 0.028 to 0.272 per 100 discharges), ↓ in MSSA infections (from 0.164 to 0.132 per 100 discharges); ↑ in SAB (0.024 to 0.203 per 100 discharges), ↑ in MRSA SAB (from 0.000 to 0.138 per 100 discharges), ↑ in MSSA SAB (from 0.020 to 0.066 per 100 discharges) |
| Chen, 201048 | 1994–2008 | Beijing, China | hospital-wide | microbiology results | S. aureus clinical isolatesc | 1 | ↑ in # of MRSA (from 33 to 60) and # of MSSA (from 17 to 40) |
| Rodríguez-Baño, 201039 | 1995–2008 | Seville, Spain | hospital-wide, patients and healthcare workers | microbiology results | S. aureus colonization or infection | 1, 4, 6 | in response to infection control efforts, ↓ in # of MRSA colonization or infection (from 190 to 65), ↓ in rate of MRSA colonization (from 0.56 to 0.07 per 1000 patient-days) and ↓ in % S. aureus due to MRSA (from 47 to 11); stable rate of MSSA (0.12 per 1000 patient-days), though intervention aimed specifically at MRSA (decolonization of MRSA at admission) |
| Wilson, 201149 | 2004–08 | England | population-based | microbiology results | SABc | 1 | ↓ in # of total S. aureus (from 10 609 to 8255), ↓ in # of MRSA (from 4216 to 1893), # of MSSA constant (from 6393 to 6362) |
| Impact of MRSA on MSSA in the community | |||||||
| Wyllie, 200568 | 1997–2003 | Oxford, England | medical, surgical and trauma specialties | microbiology results | SAB | 1, 4 | # of S. aureus infections fairly stable, but ↑ in % S. aureus due to MRSA from 14% (16/115) in 1997–98 to 24% (25/105) in 2003 |
| Kaplan, 200569 | 2001–04 | Texas, USA | children's hospital | microbiology results | SAB (95.6% SSTI) | 1, 4b | 2.2-fold ↑ in # of CA-MRSA and 1.7-fold ↑ in # of CA-MSSA isolates; % S. aureus due to MRSA ↑ from 71.5% (551/771) to 76.4% (1193/1562) |
| McCaig, 200670 | 1992–2003 | USA | population-based | National Ambulatory Medical Care Surveys and National Hospital Ambulatory Medical Care Surveys | physician office, outpatient and ED visits for SSTI | 3b | no difference in # of overall and physician office visits for SSTIs per 10 000 inhabitants in 1992–94 versus 2001–03, but 59% ↑ in # of outpatient SSTI visits per 10 000 inhabitants and 31% ↑ in # of ED SSTI visits per 10 000 inhabitants |
| Arnold, 200671 | 2000–04 | Tennessee, USA | children's hospital | microbiology results | acute osteomyelitis or septic arthritis | 1, 5b | ↑ in % infections due to MRSA (from 4% to 40%), % infections due to MSSA remained constant (from 10% to 13%) |
| Fortunov, 200672 | 2001–05 | Texas, USA | neonatal unit | microbiology results | SAB | 1, 4b | ↑ in # of S. aureus infections and % S. aureus due to MRSA from 50% (10/20 infections) in 2002 to 83% (30/36 infections) in 2004; absolute # of MSSA isolates remained steady or ↓ slightly |
| Manzur, 200773 | 1991–2003 | Barcelona, Spain | hospital-wide | microbiology results | SAB | 5 | the incidence of MRSA BSIs at hospital admission increased from 0.08 to 0.37 cases per 1000 admissions; the incidence of MSSA BSIs at hospital admission remained constant, with an average of 2.45 cases per 1000 admissions |
| Klein, 20077 | 1999–2005 | USA | population-based | National Hospital Discharge Survey | S. aureus-related hospitalizationsd | 1, 2, 5 | ↑ in # of S. aureus (from 294 570 to 477 927) and # of S. aureus per 1000 hospital admissions (from 9.17 to 13.79); ↑ in # of MRSA (from 127 036 to 278 203), % S. aureus due to MRSA (from 43% to 58%) and # of MRSA per 1000 hospital admissions (from 3.95 to 8.02); ↑ in # of MSSA (from 167 534 to 199 724), ↓ in % S. aureus due to MSSA (from 57% to 42%), ↑ in # of MSSA per 1000 hospital admissions (from 5.21 to 5.76) |
| Hota, 200774 | 2000–05 | Chicago, USA | ED, clinics and inpatient wards | microbiology results | SSTI | 1, 3, 4b | ↑ in # of CA-MRSA and CA-MSSA isolates, and in % S. aureus due to CA-MRSA; ↑ in CA-MRSA infections from 24.0 to 164.2 per 100 000 inhabitants; ↑ in CA-MSSA infections from 90.7 to 121.9 per 100 000 inhabitants |
| Hersh, 200875 | 1997–2005 | USA | population-based | National Hospital Ambulatory Medical Care Survey | SSTI ED visits and hospitalizations | 3b | ↑ in # of SSTIs per 1000 inhabitants (from 32.1 to 48.1); ↑ in # of abscess/cellulitis (from 17.3 to 32.5 per 1000 inhabitants) |
| Pallin, 200876 | 1993–2005 | USA | population-based | National Hospital Ambulatory Medical Care Survey | ED visits for SSTI | 1 | ↑ in # of SSTIs (from 1.2 million to 3.4 million, from 1.35% to 2.98% of ED visits); antibiotics against CA-MRSA rarely used in 1993–2001, but ↑ to 38% by 2005 |
| Laupland, 200877 | 2000–06 | Calgary, Canada | population-based | microbiology results | SAB | 3b | # of total SAB per 100 000 inhabitants with HA-MRSA and HA-MSSA were similar throughout the study, but CA-MRSA ↑ as CA-MSSA ↓ |
| Gorwitz, 200815 | 2001–04 | USA | population-based | National Health & Nutrition Examination Survey | nasal colonization with S. aureus | 1, 3 | in 2001–02 versus 2003–04, MRSA ↑ from 0.8% (2.3 million people) to 1.5% (4.1 million people) of the US population and S. aureus ↓ from 32.4% (89.4 million people) to 28.6% (78.9 million people) of the US population |
| Dailiana, 200878 | 2003–06 | Thessalia, Greece | orthopaedic surgery unit and outpatient clinics | microbiology results | ulcerative upper extremity infections | 1 | ↓ in # of total S. aureus (from 66 to 34), ↑ in # of MRSA (from 13 to 20), ↓ in # of MSSA (from 53 to 14) |
| Price, 200879 | 2003–05 | ‘urban hospital’ | orthopaedic surgery unit | screening of pre-operative outpatients | nasal colonization with S. aureus | 1, 2, 5 | ↑ in # of total S. aureus and % of patients with S. aureus (from 25/78 = 32% to 37/97 = 38%), ↑ in # of MRSA and % of patients with MRSA (from 0/78 = 0% to 4/97 = 4%), no change in # of MSSA and % of patients with MSSA (from 25/78 = 32% to 33/97 = 34%) |
| Tattevin, 200980 | 1997–2006 | California, USA | hospital-wide | microbiology results | S. aureus clinical isolates | 1, 5 | ↓ in # of total S. aureus, including ↑ in # of patients with MRSA (from 56/147 = 38.1% to 99/137 = 72.3%) and ↓ in # of patients with MSSA (from 91/147 = 61.9% to 38/137 = 27.7%) |
| Orscheln, 200981 | 1999–2007 | Missouri, USA | children's hospital | microbiology results | SSTI | 1b | 50-fold ↑ in # of MRSA abscess cultures and 5-fold ↑ in # of MSSA abscess cultures |
#, number; ↑, increase; ↓, decrease.
SAB, S. aureus bacteraemia (i.e. BSIs); SSI, surgical site infection; HR, high risk; ICU, intensive care unit.
a1 = number of S. aureus or MRSA or MSSA (frequency); 2 = number of S. aureus among all isolates/infections/procedures/admissions (proportion); 3 = number of S. aureus or MRSA or MSSA among all inhabitants (proportion); 4 = number of MRSA or MSSA among all S. aureus isolates/infections/procedures/admissions (proportion); 5 = number of MRSA or MSSA among all hospital admissions/procedures (proportion); 6 = number of S. aureus or MRSA or MSSA per person-days (rate).
bSome measures were presented in a figure, so point estimates cannot be reported here.
cSome studies did not distinguish between isolates.
dSome studies reported that both HA-MRSA and CA-MRSA isolates are included in the analysis.
In this systematic review, we present details of observational studies on CA-MRSA, HA-MRSA and MSSA (see Table 1). Since these studies are based on different populations and settings, we did not conduct a meta-analysis of the findings. Only one study15 focused specifically on changes in colonization, so it is unclear whether MRSA is replacing or adding to the frequency of MSSA colonization, or whether colonization rates are similar but MRSA is associated with greater invasiveness, reduced susceptibility to host immune responses and more severe infections. Whether increased MRSA impacts total S. aureus levels via changes in colonization or infection or both, the objective of this review is to assess the ultimate impact on disease burden.
Impact of MRSA on MSSA in hospitals
Since MRSA was first identified in nosocomial isolates of S. aureus in 1961,16 it has become endemic in hospitals and intensive care units around the world.6 Reports from the National Nosocomial Infection Surveillance System indicate that in the USA, the proportion of methicillin resistance among nosocomial S. aureus isolates has increased from 2.4% in 197517 to 29% in 199118 and 64.4% in 2003.19 In the UK, the proportion of S. aureus due to MRSA increased from 2% in 1990 to >40% in the early 2000s.20
Many anticipated that MRSA would ultimately replace MSSA in a process similar to what happened in the 1960s when penicillin-susceptible S. aureus was replaced by resistant strains.21 Two early studies lent support to this hypothesis. Between 1979 and 1980, the number of hospital-acquired S. aureus BSIs, pulmonary infections and post-operative wound infections at the University of Virginia Medical Center remained unchanged, but there was a statistically significant increase in the proportion of S. aureus BSIs (13% versus 40%) and post-operative wound infections (27% versus 49%) that were methicillin resistant.22 In a similar study,23 there was a stable incidence of nosocomial S. aureus BSIs in 1977–80, ranging from 4.5 to 5.0 cases per 1000 admissions during the 4 years of the study, but as MRSA BSIs increased to 1.2 per 1000 admissions in 1979, MSSA BSIs decreased to 3.5 per 1000; when MRSA BSIs decreased in 1980, MSSA BSIs increased.
With few exceptions,24,25 later studies typically found that the incidence of MSSA infections did not decrease in the face of rising MRSA prevalence.26–36 For instance, Stamm et al.30 found that in 1986–91, the total risk of S. aureus infection increased from 14 to 17 cases per 1000 admissions, including an increased risk of MRSA infections (from 0 to 3 per 1000 admissions) and MSSA infections (from 5 to 7 per 1000 admissions). Other studies37–39 showed that infection control efforts led to declines in MRSA with no reciprocal increase in MSSA infections. In recent years, additional studies have accumulated, demonstrating that HA-MRSA does not simply replace MSSA, but rather it has led to an increase in the total number of S. aureus infections.40–49
Rather than indicating a causal mechanism of independent ecological niches instead of strain replacement, this may be due to a changing case mix, such as increases in length of stay. In a study of two Oxfordshire hospitals, Wyllie et al.41 reported that between 1997 and 2003, there was an increase in the number of nosocomial BSIs among hospital admissions, including a constant proportion due to MSSA BSIs and an increase in the proportion due to MRSA. However, although the unadjusted incidence of MSSA BSIs remained constant over time, when factors related to increased risk, including aging of the hospital population and increases in length of stay, are accounted for in a multivariable analysis, the incidence of MSSA BSIs appears to have decreased as MRSA BSIs increased. This discrepancy highlights the distinction between questions regarding clinical implications and causal mechanisms; when considering the overall burden of MRSA and MSSA in the total population irrespective of age and other risk factors, the unadjusted measures are relevant. However, when attempting to understand the causal association between MRSA and MSSA, adjusted measures are used to remove differences in the composition of the populations at the different timepoints to allow for comparisons independent of these extraneous factors.
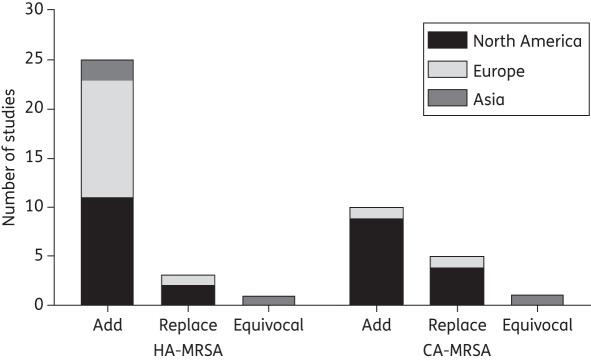
In summary, most observational studies suggest that the growing disease burden of HA-MRSA has not been temporally associated with a decline in the burden of MSSA. Although most of the studies reported frequencies or proportions and not rates, and some of the studies only included 1 year of observation22,31 or consisted of small sample sizes,27,30 this finding appears to be consistent across studies (see Figure 2).
Figure 2.
Number of studies identified from North America, Europe and Asia reporting evidence that MRSA is replacing MSSA, adding to MSSA or the study results are equivocal. The figure represents the conclusions from the 45 studies identified for this review. The figure does not account for differences in study quality or size, and it does not distinguish between colonization and infection.
Impact of MRSA on MSSA in the community
In the past, MRSA was solely a nosocomial pathogen. However, in the late 1990s, MRSA infections were reported in young otherwise healthy people, causing infections with worse clinical outcomes than are seen with infections caused by HA-MRSA strains. Some of the earliest cases of CA-MRSA infection occurred among Western Australian aborigines who lived in a remote community with no access to healthcare centres.50 In the USA, the CDC reported that between 1997 and 1999, four previously healthy children from the upper midwestern USA with no previous contact with healthcare facilities rapidly died of MRSA infections.51 Soon thereafter, CA-MRSA infections were identified among men who have sex with men and among incarcerated people in other areas.52 Since these initial reports, CA-MRSA has become epidemic in the USA53,54 and other countries,55,56 causing diseases ranging from mild and severe skin infections to fatal necrotizing pneumonia.57 According to a 2006 study, it is the leading identifiable cause of SSTIs in US emergency department (ED) patients.58
Initially, investigators distinguished between HA-MRSA and CA-MRSA by their different genetic characteristics, which result in important phenotypic effects.59,60 HA-MRSA strains are usually multiresistant to non-β-lactam antibiotics and contain staphylococcal cassette chromosome mec (SCCmec) type I, II or III. In contrast, CA-MRSA strains were initially susceptible to most antistaphylococcal antimicrobials, they have low methicillin MICs, they usually carry the smaller genetic island SCCmec type IV or V element and they have distinct genetic determinants of virulence, including Panton–Valentine leucocidin, a cytotoxin that causes leucocyte destruction and tissue necrosis. Although these characteristics initially distinguished HA-MRSA from CA-MRSA, there has been an increasing blurring of the two categories over time.61,62 Since asymptomatic colonization can persist for months to years,38 the setting of organism acquisition may differ from that of disease onset. Thus, there are a few reports of community-onset HA-MRSA infections63 and several reports of nosocomial CA-MRSA outbreaks.64 Several of the studies identified for this review did not distinguish between HA-MRSA and CA-MRSA34,35,39,40,43,46–49,65 or stated that both types were included in their analysis.7,36,44
Otter and French56 note reasons why CA-MRSA colonization and infection may be underestimated. First, most carriers are not infected and therefore are likely to remain undetected. Second, although most patients with HA-MRSA infections have nasal colonization,2 there have been reports of CA-MRSA colonization of non-nasal sites and infection without colonization at usual sites of colonization.66,67 Third, even when infections are present, patients are often treated in community or outpatient settings, where cultures for S. aureus might not be performed. Fourth, since community-associated strains are increasingly isolated in patients with healthcare contact and are gaining multidrug resistance, they might be misclassified as HA-MRSA.64 On the other hand, reports of increasing CA-MRSA over time may be due to increased reporting instead of a true increase in disease incidence. For instance, as public concern over MRSA increases, patients who may have treated infections at home or waited to see their physician may be more likely to visit the ED and physicians may be more likely to diagnose non-infectious conditions as SSTIs.
Despite these challenges, researchers have evaluated whether increased CA-MRSA is associated with a decline in MSSA in the community.7,15,68–81 In these studies, organisms were considered to be community acquired if the isolate was obtained from a patient with no underlying illnesses predisposing to frequent hospitalizations and if it was isolated either in an outpatient setting or in a hospital within 48 h of admission. In a study of CA-MRSA infection in children hospitalized in 2001–04,69 the proportion of community-acquired S. aureus isolates that were methicillin resistant increased from 71.5% to 76.4%; the number of CA-MRSA isolates increased 2.2-fold and the number of CA-MSSA isolates increased 1.7-fold. Similarly, Hota et al.74 reported that from 2000 to 2005, the incidence of CA-MRSA SSTIs increased from 24.0 to 164.2 cases per 100 000 inhabitants and the incidence of CA-MSSA SSTIs increased from 90.7 to 121.9 cases per 100 000 inhabitants.
Some studies evaluated trends in outpatient visits for SSTIs as a proxy for changes in CA-MRSA.70,75,76 Pallin et al.76 analysed data from the National Hospital Ambulatory Medical Care Survey for 1993–2005 to examine whether the frequency of ED visits for SSTIs increased contemporaneously with the emergence of CA-MRSA or whether this organism merely replaced others, with the underlying disease incidence remaining the same. Diagnoses of SSTIs increased from 1.2 million visits in 1993 (1.35% of all ED visits) to 3.4 million visits in 2005 (2.98% of all ED visits). Although data on the cause of the observed infections were not available, the results may suggest that ED utilization for SSTIs is increasing both in absolute terms and relative to all other conditions, and that CA-MRSA may be causing more disease, rather than displacing other organisms from pre-existing ecological niches.
In contrast to the studies suggesting that CA-MRSA adds to the total burden of S. aureus, three recent studies have reported that CA-MRSA replaces MSSA in the community.15,77,80 In a population-based study of S. aureus infections between 2000 and 2006,77 the incidence of healthcare-associated and nosocomial MSSA BSIs was similar throughout the study. However, as the incidence of CA-MRSA BSIs increased, the incidence of community-acquired MSSA BSIs decreased. One study15 focused on colonization rather than infection, reporting that CA-MRSA has in fact started replacing MSSA to establish itself as commensal flora, just as penicillin-resistant S. aureus replaced its penicillin-susceptible predecessor in the 1940s. In a nationally representative survey of nasal S. aureus colonization between 2001 and 2004,15 the estimated proportion of the US population colonized with CA-MRSA increased from 0.8% (2.3 million people) to 1.5% (4.1 million people), but the proportion colonized with any S. aureus decreased from 32.4% (89.4 million people) in 2001–02 to 28.6% (78.9 million people) in 2003–04. The authors hypothesized that the decline in MSSA colonization is due to increased population-level exposure to antimicrobials that may suppress MSSA and promote colonization with MRSA. However, this study reports colonization rather than infection, so it is not necessarily comparable to other studies addressing the impact of MRSA on disease burden; whereas the estimated number of S. aureus-related hospitalizations increased 62% between 1999 and 2005,7 this study indicates that total S. aureus colonization is decreasing. The reason for such discrepant findings on the impact of CA-MRSA on total S. aureus is unclear. The results suggesting MSSA replacement, however, cannot be ignored, as both analyses involved large well-designed population-based cohorts. In summary, so far, the research suggests that CA-MRSA has most likely increased the total burden of S. aureus infections rather than simply displacing the problem of MSSA, though one colonization study suggests that colonization with CA-MRSA has coincided with reduced MSSA colonization.
Limitations of current research
There is an urgent need to clarify the prevalence and epidemiology of S. aureus infections and the relationship between susceptible and resistant strains. Since resistance development is an evolutionary process, continuing surveillance is essential to identify emerging pathogens at national and global levels, to create and refine strategies for controlling antimicrobial resistance and to guide clinical decisions regarding appropriate treatment. In order to improve the quality of these efforts, limitations of previous studies should be remedied in future analyses.
Many studies reported the proportion of isolates collected that are resistant to methicillin without reporting the total number of isolates or person-time used in the denominator. Though an increase in the proportion of isolates that are methicillin resistant inevitably results in a corresponding decreasing proportion due to MSSA, the absolute frequency of MSSA may decrease, remain stable or increase.82 Additionally, comparisons between proportions are vulnerable to the effects of chance variability, regression to the mean and low power to detect genuine underlying changes.83 Therefore, it is preferable to report changes in the incidence rate of resistant and susceptible infections.40,84,85 Due to confusion in the terminology, many authors claimed to report rates of resistance, when in fact they had calculated proportions or vice versa. Several authors7,34 have provided examples showing why it is misleading to report trends using proportions without presenting absolute frequencies. Between 1999 and 2005, there was an increase in the number of MRSA-related hospitalizations, which represented an increase in the proportion of all S. aureus-related hospitalizations (127 036/294 570 = 43% to 278 203/477 927 = 58%). The number of MSSA-related hospitalizations increased, but this represented a decrease in the proportion of all S. aureus-related hospitalizations (167 534/294 570 = 57% to 199 724/477 927 = 42%).7 Similarly, Burton et al.45 underscored the discrepancy between measures using data from US intensive care units in 1997–2007. While the overall proportion of S. aureus central line-associated BSIs due to MRSA increased by 25.8%, the rate of MRSA and MSSA BSIs per 1000 central line-days declined by 49.6% and 70.1%, respectively.
It is also difficult to compare results within and between studies when baseline rates differ, because these differences result in different levels of statistical power to detect these changes. When the MRSA or MSSA case count is low at baseline, a small incremental increase may represent a large proportional increase, but when case counts are higher, small incremental changes represent a smaller proportion of the sample. For instance, imagine a study that reports a baseline incidence of 1 MRSA case per 1000 admissions and 20 MSSA cases per 1000 admissions. At the end of the study, the incidence of MRSA has increased to 2 per 1000 admissions and the incidence of MSSA has decreased to 19 per 1000 admissions. Whereas a study may have the power to detect the 2-fold increase in MRSA, a similar one-unit change in MSSA represents a smaller proportional change and hence may not be detected in a statistical test.
Based on the published studies, it is unclear whether changes in the rates of MSSA and total S. aureus BSIs are causally related to changes in the rates of MRSA, or whether they are due to changes in screening and reporting protocols, population characteristics, healthcare practices, infection control efforts, geographical and temporal trends in microbial use, other sources of antimicrobial exposure in the community or a combination of factors.86,87 Trends in the number of MRSA infections may be due to changes in the number of total hospital admissions and in risk factors, such as co-morbidities and length of stay. Case definitions may vary between surveillance studies and over time, especially with the blurring of CA-MRSA and HA-MRSA. In response to increasing concerns about MRSA, clinicians may have obtained more specimens for culture, they may have attributed more non-infectious inflammatory conditions to MRSA or they may have been more likely to ask patients to return to the hospital for follow-up reassessments. Thus, ascertainment bias may have led to an inflated number of visits for resistant strains and under-reporting of infections caused by susceptible strains.
Many of the earlier studies constructed pre–post comparisons or tests for trend over several time periods. However, the statistical tests used in such studies assume that outcomes are independent, which is untenable in the setting of transmissible pathogens.88 The use of time-series methods to study trends in S. aureus infections may improve the quality of these analyses, by accounting for the non-independence between cases. However, in attempting to assess causality from trends detected in this way, several difficulties arise. Proper statistical modelling of time-varying confounding factors can be challenging in time-series models. Furthermore, while accounting for changes in population risk factors may be possible by collecting demographic information and medical histories, it is far more challenging to prevent bias arising from changes in whether or not a patient seeks medical care, whether the treating physician obtains a bacteriological sample, and, if so, the method used to collect and process samples and the interpretation and reporting of the culture results. At the very least, guidelines for when cultures should be obtained and automated reporting of laboratory results may help minimize differences in the decision-making process, and help to standardize the process of collection and reporting between physicians and over time.
With the constant exchange of pathogens between community and nosocomial settings, surveillance systems that track carriers through repeated transitions between healthcare and community settings89 may be beneficial. Also, since resistant organisms may be more likely to be isolated multiple times,90 it would be useful to conduct a longitudinal study following the same people over time, instead of cross-sectional estimates on different people at different timepoints. Pooling data from multiple surveillance programmes may help to identify reasons for discrepancies in infection rates and help elucidate potential interventions to mitigate or perhaps prevent further antimicrobial resistance.91 However, since hospitals may differ in their screening intensity and diagnostic practices, and they may include different case mixes due to variation in the care provided in each hospital and differences in referral practices, caution must be exercised when aggregating data from different populations.82,92 Additionally, most of the prior studies examined the increased burden of MRSA over MSSA in terms of in-hospital outcomes or cross-sectional surveillance of colonization levels and not long-term effects, which may indicate a different trend. For instance, despite an increase in the proportion of MRSA-related hospitalizations over time, the proportion of MRSA-related hospitalizations that resulted in death decreased over the study period.7,44,93 Therefore, use of mortality data and other measures of long-term outcomes deserve attention.
Combining efforts for epidemiological investigation with microbiological typing is essential for accurately documenting the evolving epidemiology of S. aureus, and may help focus infection control efforts and define reservoirs of transmission. The prevalence of methicillin resistance among S. aureus isolates varies widely between countries, regions, hospitals and different wards within the same hospital.94 The rates of HA-MRSA vary within the USA, with more HA-MRSA infections seen in the south.95 In Europe, the proportion of S. aureus isolates that are resistant to methicillin is only 0.5% in Iceland and <1% in Finland, but 10%–30% in Denmark and 44% in Greece.94,96 This variability may be due to different MRSA strains with different colonization and/or virulence characteristics, differences in infection control practices97 or differences in population characteristics related to increased risk of MRSA infection.98 The genotype distribution of CA-MRSA also varies by geographical region. In North America, USA300 (ST8; where ST stands for sequence type) and USA400 (ST1) are the predominant clones, whereas the most common strain in Europe is ST80, in Taiwan it is ST59 and in eastern Australia it is ST30.54 The reason for this geographical variation remains a mystery. Molecular studies are therefore needed to further evaluate potential differences between regions and changes over time in virulence factors of S. aureus. Additionally, further studies are necessary to clarify the role of colonization in the pathogenesis of CA-MRSA and other S. aureus infections, and the role that non-nasal colonization plays as a reservoir for CA-MRSA transmission and re-infection.
Discussion
In summary, research suggests that MRSA need not replace MSSA. Rather, MRSA has in most cases added to the total burden of S. aureus infection in the population. It is not clear why MSSA remains prevalent in the presence of MRSA. Previous studies have shown that some MSSA lineages have a genetic background that is not common to the endemic MRSA clones.4 These MSSA lineages may not provide a stable genomic environment for the integration of SCCmec and, hence, they are not replaced by MRSA. Alternatively, they may have a selective advantage in a subset of hosts (especially outside of hospitals) who are not exposed to much antimicrobial pressure and may be able to persist by circulating within this group. Yet, they probably possess characteristics that favour their persistence in the host as well as the transfer between hosts.99 Thus, MSSA infection remains prevalent despite the increasing incidence of MRSA infection.
A mathematical model9 has suggested that improvements in infection control, with all else equal, may reduce drug-resistant infections while leaving drug-susceptible nosocomial infection incidence approximately unchanged; conversely, worsening infection control will disproportionately favour resistant strains. This counterintuitive result (counterintuitive because at the individual level, infection control can be effective against any infection, regardless of drug resistance) depends on a model assumption that patients admitted into the hospital are more likely to be already colonized with susceptible strains, while resistant strains tend to be more commonly acquired in the hospital. Thus, at the population level, resistant strains are more dependent on nosocomial transmission and, hence, are more sensitive to changes in infection control. A consequence of this finding is that (under the model's assumptions) it is possible that MRSA and MSSA compete to colonize hosts, but that increases in MRSA may occur without corresponding declines in MSSA.
Whether HA-MRSA and CA-MRSA partially replace MSSA infections or add to the total burden of S. aureus, perhaps the key conclusion from this review is that all studies reported that MSSA persisted, if not increased, in both hospitals and in the community. Therefore, since MSSA remains responsible for many S. aureus infections, efforts to reduce MSSA and not only MRSA deserve attention. Vancomycin is effective against MRSA, but β-lactams are more effective for MSSA infections. Consequently, since our review suggests that many S. aureus cases are attributable to MSSA, clinicians should be encouraged to obtain cultures from soft tissue infections before prescribing antimicrobial therapy. Instead of assuming that all cases are MRSA and, hence, should be treated with vancomycin, distinguishing between MRSA and MSSA infections would assure that patients receive optimal treatment. In order to meet this need, researchers should be encouraged to develop reliable and rapid techniques for the identification and characterization of clinical isolates of S. aureus.
As evidence continues to accumulate showing the massive clinical and economic burden of MRSA, research and health services efforts have largely focused on developing effective MRSA screening techniques, treatments and infection control programmes, and on preventing further resistant strains. While these objectives should remain a priority, the importance of preventing MSSA infections should not be dismissed. MSSA infections are usually not resistant to multiple drugs and basic infection control efforts have proven to be effective.100 Therefore, as a modifiable public health problem with significant clinical and economic costs, efforts to prevent and effectively treat both MSSA and MRSA should be a high priority. Further research is necessary to develop more accurate and rapid diagnostics, to gain a better understanding of the pathogenesis of staphylococcal colonization and infection, and to identify new antimicrobials and non-antimicrobial methods of S. aureus prevention and treatment. In the meantime, continued surveillance of emerging S. aureus strains and aggressive efforts to minimize excessive antimicrobial use and increase compliance with infection control programmes is necessary to reduce S. aureus infections due to both resistant and susceptible strains.
Funding
This work was supported by the Models of Infectious Disease Agents Study Program of the US NIH through cooperative agreements 5U01GM076497 and 1U54GM088558 to M. L. and T32 AI007535-12. M. L. has received honoraria and/or consulting fees from Pfizer, Novartis, i3 Innovus, AIR Worldwide and the Avian Pandemic Flu Registry (Outcome Sciences), funded by Roche. The funding sources had no involvement in the preparation of this paper or the decision to submit this paper for publication.
Transparency declarations
None to declare.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the NIH.
Acknowledgements
We thank Susan Huang for drawing our attention to the question posed in this manuscript, and we thank Murray A. Mittleman for critical review of this manuscript.
References
- 1.Williams RE. Healthy carriage of Staphylococcus aureus: its prevalence and importance. Bacteriol Rev. 1963;27:56–71. doi: 10.1128/br.27.1.56-71.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wertheim HF, Melles DC, Vos MC, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5:751–62. doi: 10.1016/S1473-3099(05)70295-4. doi:10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 3.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–32. doi: 10.1056/NEJM199808203390806. doi:10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 4.Deurenberg RH, Stobberingh EE. The evolution of Staphylococcus aureus. Infect Genet Evol. 2008;8:747–63. doi: 10.1016/j.meegid.2008.07.007. doi:10.1016/j.meegid.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Strommenger B, Braulke C, Heuck D, et al. spa typing of Staphylococcus aureus as a frontline tool in epidemiological typing. J Clin Microbiol. 2008;46:574–81. doi: 10.1128/JCM.01599-07. doi:10.1128/JCM.01599-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grundmann H, Aires-de-Sousa M, Boyce J, et al. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet. 2006;368:874–85. doi: 10.1016/S0140-6736(06)68853-3. doi:10.1016/S0140-6736(06)68853-3. [DOI] [PubMed] [Google Scholar]
- 7.Klein E, Smith DL, Laxminarayan R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Dis. 2007;13:1840–6. doi: 10.3201/eid1312.070629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dall'Antonia M, Coen PG, Wilks M, et al. Competition between methicillin-sensitive and -resistant Staphylococcus aureus in the anterior nares. J Hosp Infect. 2005;61:62–7. doi: 10.1016/j.jhin.2005.01.008. doi:10.1016/j.jhin.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Lipsitch M, Bergstrom CT, Levin BR. The epidemiology of antibiotic resistance in hospitals: paradoxes and prescriptions. Proc Natl Acad Sci USA. 2000;97:1938–43. doi: 10.1073/pnas.97.4.1938. doi:10.1073/pnas.97.4.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shinefield HR, Ribble JC, Boris M, et al. Bacterial interference between strains of S. aureus. Ann NY Acad Sci. 1974;236:444–55. doi: 10.1111/j.1749-6632.1974.tb41509.x. doi:10.1111/j.1749-6632.1974.tb41509.x. [DOI] [PubMed] [Google Scholar]
- 11.Maldonado G, Greenland S. Estimating causal effects. Int J Epidemiol. 2002;31:422–9. doi:10.1093/ije/31.2.422. [PubMed] [Google Scholar]
- 12.Chang FY, Peacock JE, Jr, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore) 2003;82:333–9. doi: 10.1097/01.md.0000091184.93122.09. doi:10.1097/01.md.0000091184.93122.09. [DOI] [PubMed] [Google Scholar]
- 13.Stryjewski ME, Szczech LA, Benjamin DK, Jr, et al. Use of vancomycin or first-generation cephalosporins for the treatment of hemodialysis-dependent patients with methicillin-susceptible Staphylococcus aureus bacteremia. Clin Infect Dis. 2007;44:190–6. doi: 10.1086/510386. doi:10.1086/510386. [DOI] [PubMed] [Google Scholar]
- 14.Colijn C, Cohen T, Fraser C, et al. What is the mechanism for persistent coexistence of drug-susceptible and drug-resistant strains of Streptococcus pneumoniae? J R Soc Interface. 2010;7:905–19. doi: 10.1098/rsif.2009.0400. doi:10.1098/rsif.2009.0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorwitz RJ, Kruszon-Moran D, McAllister SK, et al. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001–2004. J Infect Dis. 2008;197:1226–34. doi: 10.1086/533494. doi:10.1086/533494. [DOI] [PubMed] [Google Scholar]
- 16.Jevons MP. “Celbenin”-resistant staphylococci. Br Med J. 1961;i 124–5. [Google Scholar]
- 17.Panlilio AL, Culver DH, Gaynes RP, et al. Methicillin-resistant Staphylococcus aureus in U.S. hospitals, 1975–1991. Infect Control Hosp Epidemiol. 1992;13:582–6. doi: 10.1086/646432. doi:10.1086/646432. [DOI] [PubMed] [Google Scholar]
- 18.Boyce JM, Jackson MM, Pugliese G, et al. Methicillin-resistant Staphylococcus aureus (MRSA): a briefing for acute care hospitals and nursing facilities. The AHA Technical Panel on Infections within Hospitals. Infect Control Hosp Epidemiol. 1994;15:105–15. doi: 10.1086/646870. doi:10.1086/646870. [DOI] [PubMed] [Google Scholar]
- 19.Klevens RM, Edwards JR, Tenover FC, et al. Changes in the epidemiology of methicillin-resistant Staphylococcus aureus in intensive care units in US hospitals, 1992–2003. Clin Infect Dis. 2006;42:389–91. doi: 10.1086/499367. doi:10.1086/499367. [DOI] [PubMed] [Google Scholar]
- 20.Johnson AP, Pearson A, Duckworth G. Surveillance and epidemiology of MRSA bacteraemia in the UK. J Antimicrob Chemother. 2005;56:455–62. doi: 10.1093/jac/dki266. doi:10.1093/jac/dki266. [DOI] [PubMed] [Google Scholar]
- 21.Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis. 2001;7:178–82. doi: 10.3201/eid0702.010204. doi:10.3201/eid0702.010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson RL, Cabezudo I, Wenzel RP. Epidemiology of nosocomial infections caused by methicillin-resistant Staphylococcus aureus. Ann Intern Med. 1982;97:309–17. doi: 10.7326/0003-4819-97-3-309. [DOI] [PubMed] [Google Scholar]
- 23.Linnemann CC, Jr, Mason M, Moore P, et al. Methicillin-resistant Staphylococcus aureus: experience in a general hospital over four years. Am J Epidemiol. 1982;115:941–50. doi: 10.1093/oxfordjournals.aje.a113381. [DOI] [PubMed] [Google Scholar]
- 24.Spindel SJ, Strausbaugh LJ, Jacobson C. Infections caused by Staphylococcus aureus in a Veterans' Affairs nursing home care unit: a 5-year experience. Infect Control Hosp Epidemiol. 1995;16:217–23. doi: 10.1086/647093. doi:10.1086/647093. [DOI] [PubMed] [Google Scholar]
- 25.Albertini MT, Benoit C, Berardi L, et al. Surveillance of methicillin-resistant Staphylococcus aureus (MRSA) and Enterobacteriaceae producing extended-spectrum β-lactamase (ESBLE) in northern France: a five-year multicentre incidence study. J Hosp Infect. 2002;52:107–13. doi: 10.1053/jhin.2002.1286. doi:10.1053/jhin.2002.1286. [DOI] [PubMed] [Google Scholar]
- 26.Pavillard R, Harvey K, Douglas D, et al. Epidemic of hospital-acquired infection due to methicillin-resistant Staphylococcus aureus in major Victorian hospitals. Med J Aust. 1982;1:451–4. [PubMed] [Google Scholar]
- 27.Boyce JM, White RL, Spruill EY. Impact of methicillin-resistant Staphylococcus aureus on the incidence of nosocomial staphylococcal infections. J Infect Dis. 1983;148:763. doi: 10.1093/infdis/148.4.763. doi:10.1093/infdis/148.4.763. [DOI] [PubMed] [Google Scholar]
- 28.Mylotte JM, McDermott C, Spooner JA. Prospective study of 114 consecutive episodes of Staphylococcus aureus bacteremia. Rev Infect Dis. 1987;9:891–907. doi: 10.1093/clinids/9.5.891. doi:10.1093/clinids/9.5.891. [DOI] [PubMed] [Google Scholar]
- 29.Tam AY, Yeung CY. The changing pattern of severe neonatal staphylococcal infection: a 10-year study. Aust Paediatr J. 1988;24:275–9. doi: 10.1111/j.1440-1754.1988.tb01361.x. [DOI] [PubMed] [Google Scholar]
- 30.Stamm AM, Long MN, Belcher B. Higher overall nosocomial infection rate because of increased attack rate of methicillin-resistant Staphylococcus aureus. Am J Infect Control. 1993;21:70–4. doi: 10.1016/0196-6553(93)90227-u. doi:10.1016/0196-6553(93)90227-U. [DOI] [PubMed] [Google Scholar]
- 31.Pujol M, Pena C, Pallares R, et al. Risk factors for nosocomial bacteremia due to methicillin-resistant Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 1994;13:96–102. doi: 10.1007/BF02026134. doi:10.1007/BF02026134. [DOI] [PubMed] [Google Scholar]
- 32.Jernigan JA, Clemence MA, Stott GA, et al. Control of methicillin-resistant Staphylococcus aureus at a university hospital: one decade later. Infect Control Hosp Epidemiol. 1995;16:686–96. doi: 10.1086/647042. doi:10.1086/647042. [DOI] [PubMed] [Google Scholar]
- 33.Speller DC, Johnson AP, James D, et al. Resistance to methicillin and other antibiotics in isolates of Staphylococcus aureus from blood and cerebrospinal fluid, England and Wales, 1989–95. Lancet. 1997;350:323–5. doi: 10.1016/s0140-6736(97)12148-1. doi:10.1016/S0140-6736(97)12148-1. [DOI] [PubMed] [Google Scholar]
- 34.Morgan M, Salmon R, Evans-Williams D, et al. Resistance to methicillin in isolates of Staphylococcus aureus from blood and cerebrospinal fluid in Wales, 1993–1997. J Antimicrob Chemother. 1999;44:541–4. doi: 10.1093/jac/44.4.541. doi:10.1093/jac/44.4.541. [DOI] [PubMed] [Google Scholar]
- 35.Assadian O, Daxboeck F, Aspoeck C, et al. National surveillance of methicillin-sensitive and methicillin-resistant Staphylococcus aureus in Austrian hospitals: 1994–1998. J Hosp Infect. 2003;55:175–9. doi: 10.1016/s0195-6701(03)00300-1. doi:10.1016/S0195-6701(03)00300-1. [DOI] [PubMed] [Google Scholar]
- 36.Seal JB, Moreira B, Bethel CD, et al. Antimicrobial resistance in Staphylococcus aureus at the University of Chicago Hospitals: a 15-year longitudinal assessment in a large university-based hospital. Infect Control Hosp Epidemiol. 2003;24:403–8. doi: 10.1086/502222. doi:10.1086/502222. [DOI] [PubMed] [Google Scholar]
- 37.Law MR, Gill ON. Hospital-acquired infection with methicillin-resistant and methicillin-sensitive staphylococci. Epidemiol Infect. 1988;101:623–9. doi: 10.1017/s0950268800029496. doi:10.1017/S0950268800029496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robicsek A, Beaumont JL, Peterson LR. Duration of colonization with methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2009;48:910–3. doi: 10.1086/597296. doi:10.1086/597296. [DOI] [PubMed] [Google Scholar]
- 39.Rodríguez-Baño J, Garcia L, Ramirez E, et al. Long-term control of endemic hospital-wide methicillin-resistant Staphylococcus aureus (MRSA): the impact of targeted active surveillance for MRSA in patients and healthcare workers. Infect Control Hosp Epidemiol. 2010;31:786–95. doi: 10.1086/654003. doi:10.1086/654003. [DOI] [PubMed] [Google Scholar]
- 40.Whyte D, Monahan R, Boyle L, et al. The incidence of S. aureus bacteraemia in acute hospitals of the Mid-Western Area, Ireland, 2002–2004. Euro Surveill. 2005;10:pii–538. [PubMed] [Google Scholar]
- 41.Wyllie DH, Crook DW, Peto TE. Mortality after Staphylococcus aureus bacteraemia in two hospitals in Oxfordshire, 1997–2003: cohort study. BMJ. 2006;333:281. doi: 10.1136/bmj.38834.421713.2F. doi:10.1136/bmj.38834.421713.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson DJ, Sexton DJ, Kanafani ZA, et al. Severe surgical site infection in community hospitals: epidemiology, key procedures, and the changing prevalence of methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28:1047–53. doi: 10.1086/520731. doi:10.1086/520731. [DOI] [PubMed] [Google Scholar]
- 43.van der Mee-Marquet N, Epinette C, Loyau J, et al. Staphylococcus aureus strains isolated from bloodstream infections changed significantly in 2006. J Clin Microbiol. 2007;45:851–7. doi: 10.1128/JCM.02178-06. doi:10.1128/JCM.02178-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allard C, Carignan A, Bergevin M, et al. Secular changes in incidence and mortality associated with Staphylococcus aureus bacteraemia in Quebec, Canada, 1991–2005. Clin Microbiol Infect. 2008;14:421–8. doi: 10.1111/j.1469-0691.2008.01965.x. doi:10.1111/j.1469-0691.2008.01965.x. [DOI] [PubMed] [Google Scholar]
- 45.Burton DC, Edwards JR, Horan TC, et al. Methicillin-resistant Staphylococcus aureus central line-associated bloodstream infections in US intensive care units, 1997–2007. JAMA. 2009;301:727–36. doi: 10.1001/jama.2009.153. doi:10.1001/jama.2009.153. [DOI] [PubMed] [Google Scholar]
- 46.Hacek DM, Paule SM, Thomson RB, Jr, et al. Implementation of a universal admission surveillance and decolonization program for methicillin-resistant Staphylococcus aureus (MRSA) reduces the number of MRSA and total number of S. aureus isolates reported by the clinical laboratory. J Clin Microbiol. 2009;47:3749–52. doi: 10.1128/JCM.01223-08. doi:10.1128/JCM.01223-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pearson A, Chronias A, Murray M. Voluntary and mandatory surveillance for methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-susceptible S. aureus (MSSA) bacteraemia in England. J Antimicrob Chemother. 2009;64(Suppl 1):i11–7. doi: 10.1093/jac/dkp260. doi:10.1093/jac/dkp260. [DOI] [PubMed] [Google Scholar]
- 48.Chen H, Liu Y, Jiang X, et al. Rapid change of methicillin-resistant Staphylococcus aureus clones in a Chinese tertiary care hospital over a 15-year period. Antimicrob Agents Chemother. 2010;54:1842–7. doi: 10.1128/AAC.01563-09. doi:10.1128/AAC.01563-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson J, Elgohari S, Livermore DM, et al. Trends among pathogens reported as causing bacteraemia in England, 2004–2008. Clin Microbiol Infect. 2011;17:451–8. doi: 10.1111/j.1469-0691.2010.03262.x. doi:10.1111/j.1469-0691.2010.03262.x. [DOI] [PubMed] [Google Scholar]
- 50.Udo EE, Pearman JW, Grubb WB. Genetic analysis of community isolates of methicillin-resistant Staphylococcus aureus in Western Australia. J Hosp Infect. 1993;25:97–108. doi: 10.1016/0195-6701(93)90100-e. doi:10.1016/0195-6701(93)90100-E. [DOI] [PubMed] [Google Scholar]
- 51.CDC. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus—Minnesota and North Dakota, 1997–1999. JAMA. 1999;282:1123–5. doi:10.1001/jama.282.12.1123. [PubMed] [Google Scholar]
- 52.CDC. Outbreaks of community-associated methicillin-resistant Staphylococcus aureus skin infections—Los Angeles County, California, 2002–2003. MMWR Morb Mortal Wkly Rep. 2003;52:88. [PubMed] [Google Scholar]
- 53.Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436–44. doi: 10.1056/NEJMoa043252. doi:10.1056/NEJMoa043252. [DOI] [PubMed] [Google Scholar]
- 54.Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629–41. doi: 10.1038/nrmicro2200. doi:10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vandenesch F, Naimi T, Enright MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton–Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis. 2003;9:978–84. doi: 10.3201/eid0908.030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Otter JA, French GL. Molecular epidemiology of community-associated meticillin-resistant Staphylococcus aureus in Europe. Lancet Infect Dis. 2010;10:227–39. doi: 10.1016/S1473-3099(10)70053-0. doi:10.1016/S1473-3099(10)70053-0. [DOI] [PubMed] [Google Scholar]
- 57.Jarvis WR, Schlosser J, Chinn RY, et al. National prevalence of methicillin-resistant Staphylococcus aureus in inpatients at US health care facilities, 2006. Am J Infect Control. 2007;35:631–7. doi: 10.1016/j.ajic.2007.10.009. doi:10.1016/j.ajic.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 58.Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–74. doi: 10.1056/NEJMoa055356. doi:10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 59.Zetola N, Francis JS, Nuermberger EL, et al. Community-acquired meticillin-resistant Staphylococcus aureus: an emerging threat. Lancet Infect Dis. 2005;5:275–86. doi: 10.1016/S1473-3099(05)70112-2. doi:10.1016/S1473-3099(05)70112-2. [DOI] [PubMed] [Google Scholar]
- 60.Deleo FR, Otto M, Kreiswirth BN, et al. Community-associated meticillin-resistant Staphylococcus aureus. Lancet. 2010;375:1557–68. doi: 10.1016/S0140-6736(09)61999-1. doi:10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Friedman ND, Kaye KS, Stout JE, et al. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–7. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 62.David MZ, Glikman D, Crawford SE, et al. What is community-associated methicillin-resistant Staphylococcus aureus? J Infect Dis. 2008;197:1235–43. doi: 10.1086/533502. doi:10.1086/533502. [DOI] [PubMed] [Google Scholar]
- 63.Robinson JO, Pearson JC, Christiansen KJ, et al. Community-associated versus healthcare-associated methicillin-resistant Staphylococcus aureus bacteraemia: a 10-year retrospective review. Eur J Clin Microbiol Infect Dis. 2009;28:353–61. doi: 10.1007/s10096-008-0632-1. doi:10.1007/s10096-008-0632-1. [DOI] [PubMed] [Google Scholar]
- 64.Otter JA, French GL. Nosocomial transmission of community-associated methicillin-resistant Staphylococcus aureus: an emerging threat. Lancet Infect Dis. 2006;6:753–5. doi: 10.1016/S1473-3099(06)70636-3. doi:10.1016/S1473-3099(06)70636-3. [DOI] [PubMed] [Google Scholar]
- 65.Chuang YC, Chen YC, Chang SC, et al. Secular trends of healthcare-associated infections at a teaching hospital in Taiwan, 1981–2007. J Hosp Infect. 2010;76:143–9. doi: 10.1016/j.jhin.2010.05.001. doi:10.1016/j.jhin.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang ES, Tan J, Eells S, et al. Body site colonization in patients with community-associated methicillin-resistant Staphylococcus aureus and other types of S. aureus skin infections. Clin Microbiol Infect. 2010;16:425–31. doi: 10.1111/j.1469-0691.2009.02836.x. doi:10.1111/j.1469-0691.2009.02836.x. [DOI] [PubMed] [Google Scholar]
- 67.Mertz D, Frei R, Periat N, et al. Exclusive Staphylococcus aureus throat carriage: at-risk populations. Arch Intern Med. 2009;169:172–8. doi: 10.1001/archinternmed.2008.536. doi:10.1001/archinternmed.2008.536. [DOI] [PubMed] [Google Scholar]
- 68.Wyllie DH, Peto TE, Crook D. MRSA bacteraemia in patients on arrival in hospital: a cohort study in Oxfordshire 1997–2003. BMJ. 2005;331:992. doi: 10.1136/bmj.38558.453310.8F. doi:10.1136/bmj.38558.453310.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaplan SL, Hulten KG, Gonzalez BE, et al. Three-year surveillance of community-acquired Staphylococcus aureus infections in children. Clin Infect Dis. 2005;40:1785–91. doi: 10.1086/430312. doi:10.1086/430312. [DOI] [PubMed] [Google Scholar]
- 70.McCaig LF, McDonald LC, Mandal S, et al. Staphylococcus aureus-associated skin and soft tissue infections in ambulatory care. Emerg Infect Dis. 2006;12:1715–23. doi: 10.3201/eid1211.060190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arnold SR, Elias D, Buckingham SC, et al. Changing patterns of acute hematogenous osteomyelitis and septic arthritis: emergence of community-associated methicillin-resistant Staphylococcus aureus. J Pediatr Orthop. 2006;26:703–8. doi: 10.1097/01.bpo.0000242431.91489.b4. doi:10.1097/01.bpo.0000242431.91489.b4. [DOI] [PubMed] [Google Scholar]
- 72.Fortunov RM, Hulten KG, Hammerman WA, et al. Community-acquired Staphylococcus aureus infections in term and near-term previously healthy neonates. Pediatrics. 2006;118:874–81. doi: 10.1542/peds.2006-0884. doi:10.1542/peds.2006-0884. [DOI] [PubMed] [Google Scholar]
- 73.Manzur A, Vidal M, Pujol M, et al. Predictive factors of meticillin resistance among patients with Staphylococcus aureus bloodstream infections at hospital admission. J Hosp Infect. 2007;66:135–41. doi: 10.1016/j.jhin.2007.03.015. doi:10.1016/j.jhin.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 74.Hota B, Ellenbogen C, Hayden MK, et al. Community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections at a public hospital: do public housing and incarceration amplify transmission? Arch Intern Med. 2007;167:1026–33. doi: 10.1001/archinte.167.10.1026. doi:10.1001/archinte.167.10.1026. [DOI] [PubMed] [Google Scholar]
- 75.Hersh AL, Chambers HF, Maselli JH, et al. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med. 2008;168:1585–91. doi: 10.1001/archinte.168.14.1585. doi:10.1001/archinte.168.14.1585. [DOI] [PubMed] [Google Scholar]
- 76.Pallin DJ, Egan DJ, Pelletier AJ, et al. Increased US emergency department visits for skin and soft tissue infections, and changes in antibiotic choices, during the emergence of community-associated methicillin-resistant Staphylococcus aureus. Ann Emerg Med. 2008;51:291–8. doi: 10.1016/j.annemergmed.2007.12.004. doi:10.1016/j.annemergmed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 77.Laupland KB, Ross T, Gregson DB. Staphylococcus aureus bloodstream infections: risk factors, outcomes, and the influence of methicillin resistance in Calgary, Canada, 2000–2006. J Infect Dis. 2008;198:336–43. doi: 10.1086/589717. doi:10.1086/589717. [DOI] [PubMed] [Google Scholar]
- 78.Dailiana ZH, Rigopoulos N, Varitimidis SE, et al. Clinical and epidemiological features of upper-extremity infections caused by Staphylococcus aureus carrying the PVL gene: a four-year study in Greece. Med Sci Monit. 2008;14:CR511–4. [PubMed] [Google Scholar]
- 79.Price CS, Williams A, Philips G, et al. Staphylococcus aureus nasal colonization in preoperative orthopaedic outpatients. Clin Orthop Relat Res. 2008;466:2842–7. doi: 10.1007/s11999-008-0337-x. doi:10.1007/s11999-008-0337-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tattevin P, Diep BA, Jula M, et al. Methicillin-resistant Staphylococcus aureus USA300 clone in long-term care facility. Emerg Infect Dis. 2009;15:953–5. doi: 10.3201/eid1506.080195. doi:10.3201/eid1506.080195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Orscheln RC, Hunstad DA, Fritz SA, et al. Contribution of genetically restricted, methicillin-susceptible strains to the ongoing epidemic of community-acquired Staphylococcus aureus infections. Clin Infect Dis. 2009;49:536–42. doi: 10.1086/600881. doi:10.1086/600881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schwaber MJ, De-Medina T, Carmeli Y. Epidemiological interpretation of antibiotic resistance studies—what are we missing? Nat Rev Microbiol. 2004;2:979–83. doi: 10.1038/nrmicro1047. doi:10.1038/nrmicro1047. [DOI] [PubMed] [Google Scholar]
- 83.Spiegelhalter DJ. Problems in assessing rates of infection with methicillin resistant Staphylococcus aureus. BMJ. 2005;331:1013–5. doi: 10.1136/bmj.331.7523.1013. doi:10.1136/bmj.331.7523.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meyer E, Schwab F, Pollitt A, et al. Resistance rates in ICUs: interpretation and pitfalls. J Hosp Infect. 2007;65:84–5. doi: 10.1016/j.jhin.2006.09.009. doi:10.1016/j.jhin.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 85.Shardell M, Harris AD, El-Kamary SS, et al. Statistical analysis and application of quasi experiments to antimicrobial resistance intervention studies. Clin Infect Dis. 2007;45:901–7. doi: 10.1086/521255. doi:10.1086/521255. [DOI] [PubMed] [Google Scholar]
- 86.Rempel OR, Laupland KB. Surveillance for antimicrobial resistant organisms: potential sources and magnitude of bias. Epidemiol Infect. 2009;137:1665–73. doi: 10.1017/S0950268809990100. doi:10.1017/S0950268809990100. [DOI] [PubMed] [Google Scholar]
- 87.Laupland KB, Ross T, Pitout JD, et al. Investigation of sources of potential bias in laboratory surveillance for anti-microbial resistance. Clin Invest Med. 2007;30:E159–66. doi: 10.25011/cim.v30i4.1777. [DOI] [PubMed] [Google Scholar]
- 88.Cooper B, Lipsitch M. The analysis of hospital infection data using hidden Markov models. Biostatistics. 2004;5:223–37. doi: 10.1093/biostatistics/5.2.223. doi:10.1093/biostatistics/5.2.223. [DOI] [PubMed] [Google Scholar]
- 89.Huang SS, Avery TR, Song Y, et al. Quantifying interhospital patient sharing as a mechanism for infectious disease spread. Infect Control Hosp Epidemiol. 2010;31:1160–9. doi: 10.1086/656747. doi:10.1086/656747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee SO, Cho YK, Kim SY, et al. Comparison of trends of resistance rates over 3 years calculated from results for all isolates and for the first isolate of a given species from a patient. J Clin Microbiol. 2004;42:4776–9. doi: 10.1128/JCM.42.10.4776-4779.2004. doi:10.1128/JCM.42.10.4776-4779.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Verhoef J, Fluit A. Surveillance uncovers the smoking gun for resistance emergence. Biochem Pharmacol. 2006;71:1036–41. doi: 10.1016/j.bcp.2005.10.013. doi:10.1016/j.bcp.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 92.Rosenthal VD, Maki DG, Mehta A, et al. International Nosocomial Infection Control Consortium report, data summary for 2002–2007, issued January 2008. Am J Infect Control. 2008;36:627–37. doi: 10.1016/j.ajic.2008.03.003. doi:10.1016/j.ajic.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 93.Benfield T, Espersen F, Frimodt-Moller N, et al. Increasing incidence but decreasing in-hospital mortality of adult Staphylococcus aureus bacteraemia between 1981 and 2000. Clin Microbiol Infect. 2007;13:257–63. doi: 10.1111/j.1469-0691.2006.01589.x. doi:10.1111/j.1469-0691.2006.01589.x. [DOI] [PubMed] [Google Scholar]
- 94.Tiemersma EW, Bronzwaer SL, Lyytikainen O, et al. Methicillin-resistant Staphylococcus aureus in Europe, 1999–2002. Emerg Infect Dis. 2004;10:1627–34. doi: 10.3201/eid1009.040069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kuehnert MJ, Hill HA, Kupronis BA, et al. Methicillin-resistant-Staphylococcus aureus hospitalizations, United States. Emerg Infect Dis. 2005;11:868–72. doi: 10.3201/eid1106.040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lyytikäinen O, Ruotsalainen E, Jarvinen A, et al. Trends and outcome of nosocomial and community-acquired bloodstream infections due to Staphylococcus aureus in Finland, 1995–2001. Eur J Clin Microbiol Infect Dis. 2005;24:399–404. doi: 10.1007/s10096-005-1345-3. doi:10.1007/s10096-005-1345-3. [DOI] [PubMed] [Google Scholar]
- 97.Bootsma MC, Diekmann O, Bonten MJ. Controlling methicillin-resistant Staphylococcus aureus: quantifying the effects of interventions and rapid diagnostic testing. Proc Natl Acad Sci USA. 2006;103:5620–5. doi: 10.1073/pnas.0510077103. doi:10.1073/pnas.0510077103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Harbarth S, Albrich W, Goldmann DA, et al. Control of multiply resistant cocci: do international comparisons help? Lancet Infect Dis. 2001;1:251–61. doi: 10.1016/S1473-3099(01)00120-7. doi:10.1016/S1473-3099(01)00120-7. [DOI] [PubMed] [Google Scholar]
- 99.Ender M, McCallum N, Adhikari R, et al. Fitness cost of SCCmec and methicillin resistance levels in Staphylococcus aureus. Antimicrob Agents Chemother. 2004;48:2295–7. doi: 10.1128/AAC.48.6.2295-2297.2004. doi:10.1128/AAC.48.6.2295-2297.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Paul J. Surveillance and management of all types of Staphylococcus aureus bacteraemia. BMJ. 2006;333:269–70. doi: 10.1136/bmj.333.7562.269. doi:10.1136/bmj.333.7562.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Robicsek A, Beaumont JL, Paule SM, et al. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Ann Intern Med. 2008;148:409–18. doi: 10.7326/0003-4819-148-6-200803180-00003. [DOI] [PubMed] [Google Scholar]