Abstract
Objectives
Mycoplasma pneumoniae respiratory infection is a common cause of acute respiratory infection in children and adults. We evaluated the efficacy of increasing dosages of clarithromycin for the optimized therapy of M. pneumoniae respiratory infection in a mouse model.
Methods
BALB/c mice were intranasally inoculated once with M. pneumoniae or SP4 broth (control). Groups of mice were treated with increasing dosages of clarithromycin (10, 25 or 75 mg/kg/day) or placebo subcutaneously daily. Groups of mice were evaluated after 1, 2, 3, 6 and 12 days of therapy. Outcome variables included quantitative M. pneumoniae culture, histopathological score of the lungs, bronchoalveolar lavage (BAL) cytokine/chemokine/growth factor concentrations and plethysmography after aerosolized methacholine to assess airway hyperresponsiveness.
Results
Elevated dosages of clarithromycin resulted in greater antimicrobial efficacy with significantly reduced M. pneumoniae quantitative cultures (P < 0.05), as well as greater improvement in markers of disease severity with significantly reduced lung histopathology scores, BAL cytokine concentrations and airway hyperresponsiveness (P < 0.05).
Conclusions
Escalated dosing of clarithromycin resulted in significantly greater therapeutic efficacy in the treatment of experimental M. pneumoniae respiratory infection.
Keywords: macrolides, cytokines, asthma, pharmacokinetics, pharmacodynamics
Introduction
Mycoplasma pneumoniae infection presents as various acute respiratory tract illnesses, including pharyngitis, tracheobronchitis, wheezing and community-acquired pneumonia. Of greatest clinical significance is the prominent role of M. pneumoniae in community-acquired pneumonia and exacerbations of wheezing in both children and adults. Randomized, double-blind, placebo-controlled clinical trials have demonstrated that appropriate antimicrobial therapy significantly decreases the duration of fever, cough, malaise, hospitalization and radiological abnormalities in M. pneumoniae pneumonia.1–4 Of note, even though treatment with macrolide, ketolide, tetracycline or peptide deformylase inhibitor antimicrobials significantly reduces pulmonary inflammation in animal M. pneumoniae pneumonia investigations, M. pneumoniae was not eradicated from the lungs in these in vivo investigations.3 In addition, a recent human investigation found that persistent M. pneumoniae carriage following acute infection is not affected by antibiotic therapy.5 In terms of pathogenesis, M. pneumoniae is thought to principally act as an extracellular pathogen by attaching to the surface of the ciliated respiratory epithelium.3 This site of pathogenesis may have important implications in the treatment of M. pneumoniae infection.
Macrolide antimicrobials, such as clarithromycin, are an important treatment option for acute M. pneumoniae pneumonia. It is known that macrolides accumulate in the respiratory epithelial lining fluid in close proximity to the site where M. pneumoniae is hypothesized to exert its pathogenic effect.6,7 The treatment effect of macrolides on M. pneumoniae at the site of active infection, specifically the respiratory epithelium, has not been completely characterized. Given the exceedingly low macrolide MIC for M. pneumoniae (in the range of micrograms per litre), it is unclear if antimycoplasma therapy can be optimized by increasing the macrolide dosage. In addition, the effect of escalated dosages of macrolide on markers of M. pneumoniae disease severity, such as pulmonary inflammation, is unknown. While investigations of a similar nature have been reported for organisms such as Streptococcus pneumoniae, the ‘atypical’ nature of M. pneumoniae warrants specific investigation.
Our laboratory has previously studied various agents and treatment regimens for experimental M. pneumoniae pneumonia.8–15 In the present study, we investigated the effect of clarithromycin given at increasing dosages for the therapy of M. pneumoniae pneumonia in our established murine model. Specifically, we evaluated pulmonary histopathological inflammation, bronchoalveolar lavage (BAL) cytokine/chemokine/growth factor concentrations, airway hyperresponsiveness and quantitative BAL M. pneumoniae culture during the course of treatment with increasing dosages of clarithromycin. Clarithromycin concentrations in serum and BAL (a surrogate marker for respiratory epithelium clarithromycin exposure) were also determined to estimate pharmacokinetic/pharmacodynamic (PK/PD) parameters for the clarithromycin therapy.
Materials and methods
Organism and growth conditions
M. pneumoniae (San Antonio strain S1; isolated 1993; M. pneumoniae subtype 2) was reconstituted in SP4 broth and subcultured after 24–48 h in flasks containing 20 mL of SP4 media at 37°C. When the broth turned an orange hue (∼72 h), the supernatant was decanted and 2 mL of fresh SP4 broth was added to each flask. A cell scraper was used to harvest the adherent mycoplasmas from the bottom of individual flasks. This achieved an M. pneumoniae concentration in the range of 108 cfu/mL. Aliquots were stored at −80°C. All SP4 media contained nystatin (50 U/mL) and ampicillin (1.0 mg/mL) to inhibit the growth of potential contaminants.
Animals and inoculation
Mice were obtained from commercial vendors (Jackson Labs), who confirmed their mycoplasma- and murine virus-free status. The Animal Resource Center at the University of Texas Southwestern Medical Center performed quarterly health surveillance on sentinel mice housed in the mouse storage room. Antibodies against mouse hepatitis virus, Sendai virus, pneumonia virus of mice, reo-3 virus, mouse encephalitis virus (GD-7), mouse rotavirus (EDIM), minute virus of mice and Mycoplasma pulmonis were analysed. Sentinel mice were also screened for pinworm and mites. The sentinel mice tested negative for these pathogens. Mice were housed in filter-top cages and allowed to acclimatize to their new environment for 1 week. Isoflurane, an inhaled anaesthetic, was used for sedation during inoculation. BALB/c mice (female, 9–12 weeks old) were intranasally inoculated once with 50 μL of 108 cfu/mL M. pneumoniae. All mice were housed in the same animal room and received identical daily care. Animal guidelines were followed in accordance with the Institutional Animal Care and Research Advisory Committee at the University of Texas Southwestern Medical Center at Dallas.
Treatment regimen
Groups of mice were treated with clarithromycin (10, 25 or 75 mg/kg) or placebo subcutaneously daily for 12 days, starting 1 day after inoculation. Clarithromycin was reconstituted in sterile 5% dextrose water. Placebo groups received sterile 5% dextrose water.8
Experimental design and sample collection
Mice were evaluated after 1, 2, 3, 6 and 12 days of therapy. Samples were obtained from 7–12 mice per treatment group (4 groups: 10 mg/kg clarithromycin; 25 mg/kg clarithromycin; 75 mg/kg clarithromycin; and placebo therapy) at each timepoint from repeated experiments. Mice were anaesthetized with an intraperitoneal injection of 75 mg/kg ketamine and 5 mg/kg acepromazine before cardiac puncture. Blood was centrifuged at 3500 g for 10 min and the serum was stored at −80°C. BAL samples were obtained by instilling 500 μL of SP4 broth through a 25 gauge needle into the lungs, via the trachea, followed by aspiration of this fluid into a syringe. Lung specimens, including the trachea, were collected and fixed for histological evaluation.
Culture
Twenty-five microlitres of undiluted BAL sample and serial 10-fold dilutions of BAL sample in SP4 broth (50 μL of undiluted BAL sample was used for the initial dilution) were immediately cultured on SP4 agar plates at 37°C, whereas the remaining undiluted BAL sample was stored at −80°C. Quantification was performed by counting colonies on plated specimens and expressed as log10 cfu/mL.
Histopathology
The histopathological score was determined by a single pathologist who was unaware of the treatment status of the animals from which specimens were taken. The histopathological score was based on the grading of the peribronchiolar/bronchial infiltrate, the bronchiolar/bronchial luminal exudate, the perivascular infiltrate and parenchymal pneumonia (the neutrophilic alveolar infiltrate). This histopathological scoring system assigned values from 0 to 26 (the greater the score, the greater the inflammatory changes in the lung).16
Plethysmography
Whole-body, unrestrained, non-sedated plethysmography (Buxco, Troy, NY, USA) was used to monitor the respiratory dynamics of mice in a quantitative manner after methacholine exposure (measurement of airway hyperresponsiveness). After mice were allowed to acclimatize to the plethysmography chamber, mice were exposed once to aerosolized methacholine (25 mg/mouse); after exposure, plethysmography readings were again recorded. Enhanced pause (Penh) is a dimensionless value that represents a function of the ratio of peak expiratory flow to peak inspiratory flow and a function of the timing of expiration. Penh correlates with pulmonary airflow resistance or obstruction. Penh, as measured by plethysmography, has been previously validated in animal models of airway hyperresponsiveness.17–21
Cytokines/chemokines/growth factors
Concentrations of cytokines, chemokines and growth factors in BAL specimens were assessed using Multiplex Bead Immunoassays (Bio-Rad Laboratories) in conjunction with the Luminex LabMAP system, following the manufacturer's instructions. The assay limits of detection, as per Bio-Rad Laboratories guidelines, are as follows: interleukin (IL)-1α, 1.1 pg/mL; IL-1β, 0.8 pg/mL; IL-2, 1.1 pg/mL; IL-4, 0.5 pg/mL; IL-5, 0.8 pg/mL; IL-6, 1.1 pg/mL; IL-8, 0.5 pg/mL; IL-9, 0.7 pg/mL; IL-10, 0.9 pg/mL; IL-12p70, 0.5 pg/mL; IL-13, 2.1 pg/mL; IL-17, 0.2 pg/mL; eotaxin, 14.6 pg/mL; granulocyte colony-stimulating factor, 1.1 pg/mL; granulocyte macrophage colony-stimulating factor, 4.5 pg/mL; interferon (IFN)-γ, 19.3 pg/mL; monocyte chemotactic protein 1, 6.7 pg/mL; macrophage inflammatory protein (MIP)-1α, 1.1 pg/mL; MIP-1β, 1.1 pg/mL; platelet-derived growth factor, 1.0 pg/mL; regulated on activation, normal T cell expressed and secreted, 1.2 pg/mL; tumour necrosis factor α, 3.0 pg/mL; and vascular endothelial growth factor, 0.5 pg/mL. For statistical analysis, samples with readings below the limit of the standard curve of the assay were assigned a value one-half that of the lowest detectable value.
Clarithromycin concentration
Clarithromycin concentrations were measured in infected mouse serum and BAL samples on the third day of treatment at 1, 2, 4, 10 and 24 h after the third daily administration of clarithromycin in three or four mice per dosage at each timepoint. Clarithromycin concentrations were measured using a Shimadzu HPLC system interfaced with a tandem, triple quadrupole mass spectrometer (API 3000). Serum or BAL fluid samples (100 μL) and an internal standard (roxithromycin) were extracted. The organic phase was evaporated and reconstituted with 200 μL of acetonitrile for injection. Following injection (10 μL), chromatographic separation was performed using isocratic elution on a C18 column at 40°C and with a 5 min run time. Clarithromycin and the internal standard were analysed using positive electrospray ionization combined with multiple reaction monitoring. The standard curve was linear (r = 0.9995). The lower limit of quantification was 2 ng/mL. The macrolide MIC for the M. pneumoniae strain used in the experiment was ≤0.008 mg/L (The University of Alabama at Birmingham Diagnostic Mycoplasma Laboratory, Birmingham, AL, USA).22,23
Statistics
One-way analysis of variance (ANOVA) was used to compare the treatment groups at each timepoint, if the data were normally distributed. In the instances where the data were not normally distributed, the Kruskal–Wallis test was used for comparisons. If a difference was found between groups, then a pairwise multiple comparison procedure was performed. A comparison was considered statistically significant if the P value was ≤0.05.
Results
BAL culture
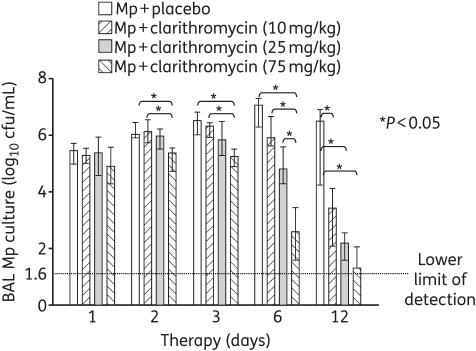
After 1 day of therapy, there were no significant differences in the quantitative M. pneumoniae BAL cultures between the placebo and the 10, 25 or 75 mg/kg clarithromycin treatment groups (Figure 1). After 2, 3 and 6 days of therapy, significant differences were observed between the placebo and clarithromycin treatment groups, with 75 mg/kg clarithromycin resulting in significantly reduced BAL cultures compared with all the other groups after 6 days of therapy (Figure 1). After 12 days of therapy, all three clarithromycin treatment regimens had significantly lower BAL cultures compared with placebo (Figure 1).
Figure 1.
Quantitative M. pneumoniae (Mp) cultures of bronchoalveolar lavage (BAL) fluid samples from mice inoculated with M. pneumoniae and treated with clarithromycin at 10, 25 or 75 mg/kg/day, or with placebo for 12 days (treatment began 1 day after inoculation). Bars represent results from 7–12 mice per treatment group at each timepoint from repeated experiments. Values shown are the medians and 25th to 75th percentiles (error bars). *P < 0.05 between the two specified treatment groups at the timepoint by Kruskal–Wallis test followed by pairwise multiple comparisons.
Lung histopathology
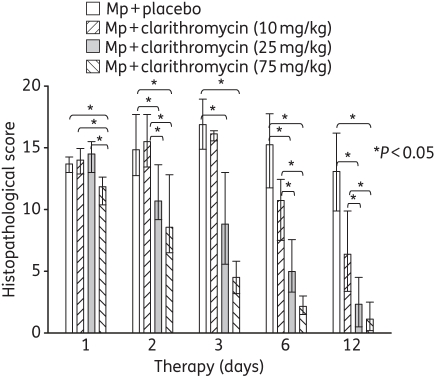
Significant differences in the lung histopathological score were found between the treatment groups at all the timepoints, as illustrated in Figure 2. In contrast to BAL cultures after 1 day of therapy, 75 mg/kg clarithromycin resulted in a significantly reduced histopathological score compared with placebo, 10 mg/kg clarithromycin and 25 mg/kg clarithromycin (Figure 2). While significant reductions in the histopathological score were found at all five timepoints with 75 mg/kg clarithromycin, the 25 mg/kg clarithromycin dose was observed to reduce the histopathological score after 2, 3, 6 and 12 days of therapy (Figure 2). Compared with placebo, 10 mg/kg clarithromycin did not have a significant effect on the histopathological score.
Figure 2.
Lung histopathology score from mice inoculated with M. pneumoniae (Mp) and treated with clarithromycin at 10, 25 or 75 mg/kg/day, or with placebo for 12 days (treatment began 1 day after inoculation). Bars represent results from 7–12 mice per treatment group at each timepoint from repeated experiments. Values shown are the medians and 25th to 75th percentiles (error bars). *P < 0.05 between the two specified treatment groups at the timepoint by Kruskal–Wallis test followed by pairwise multiple comparisons.
Airway hyperresponsiveness
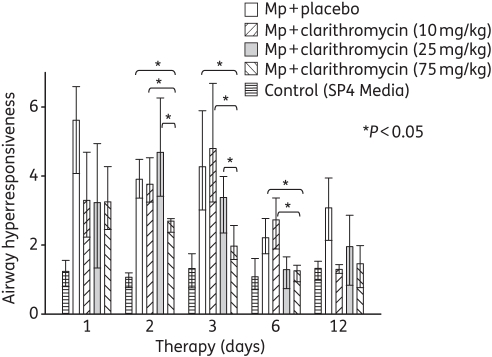
Only treatment with 75 mg/kg clarithromycin resulted in a significant reduction in airway hyperresponsiveness compared with placebo (Figure 3). Airway hyperresponsiveness was also significantly reduced by 75 mg/kg clarithromycin compared with 10 and 25 mg/kg clarithromycin (Figure 3).
Figure 3.
Airway hyperresponsiveness was assessed by whole-body plethysmography by measuring Penh after methacholine exposure in mice inoculated with M. pneumoniae (Mp) and treated with clarithromycin at 10, 25 or 75 mg/kg/day, or with placebo for 12 days (treatment began 1 day after inoculation). Bars represent results from 7–12 mice per treatment group at each timepoint from repeated experiments. Values shown are the medians and 25th to 75th percentiles (error bars). *P < 0.05 between the two specified treatment groups at the timepoint by Kruskal–Wallis test followed by pairwise multiple comparisons.
Cytokines/chemokines/growth factors
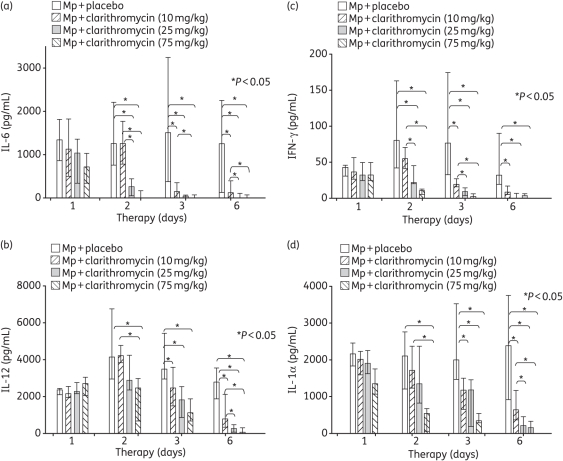
BAL concentrations of IL-1α, IL-6, IL-12 and IFN-γ were significantly reduced by treatment with 10, 25 and 75 mg/kg clarithromycin compared with placebo (Figure 4). In addition, higher dosages of clarithromycin significantly reduced BAL concentrations of IL-1α, IL-6, IL-12 and IFN-γ compared with lower dosages of clarithromycin (Figure 4). No significant differences were found for the other cytokines, chemokines or growth factors investigated.
Figure 4.
Cytokine and chemokine concentrations in bronchoalveolar lavage (BAL) fluid specimens in mice inoculated with M. pneumoniae (Mp) and treated with clarithromycin at 10, 25 or 75 mg/kg/day, or with placebo for 12 days (treatment began 1 day after inoculation). (a) Interleukin (IL)-6, (b) IL-12, (c) IFN-γ and (d) IL-1α. Bars represent results from 7–12 mice per treatment group at each timepoint from repeated experiments. Values shown are the medians and 25th to 75th percentiles (error bars). *P < 0.05 between the two specified treatment groups at the timepoint by Kruskal–Wallis test followed by pairwise multiple comparisons.
Clarithromycin PK/PD parameters
Table 1 lists the estimated PK/PD parameters from the clarithromycin concentrations measured in serum and BAL samples from infected mice on the third day of treatment.
Table 1.
Estimated pharmacokinetic/pharmacodynamic parameters from clarithromycin concentrations measured in infected mice serum and bronchoalveolar lavage on the third day of treatment in three or four mice per dosage at 1, 2, 4, 10 and 24 h after the third daily administration of clarithromycin
| Clarithromycin dose |
||||||
|---|---|---|---|---|---|---|
| 10 mg/kg |
25 mg/kg |
75 mg/kg |
||||
| PK/PD parameter | serum | BAL | serum | BAL | serum | BAL |
| 1 h concentration (mg/L) | 0.988 | 0.047 | 2.222 | 0.146 | 8.875 | 0.602 |
| AUC0–24 (mg·h/L) | 2.41 | 0.12 | 7.57 | 0.41 | 30.03 | 2.08 |
| 1 h concentration/MIC | 124.8 | 5.9 | 277.8 | 18.3 | 1109.3 | 75.3 |
| Time above MIC | 92.1% | 26.3% | 97.1% | 35.8% | 99.6% | 58.3% |
| AUC0–24/MIC | 301.1 | 14.5 | 947.0 | 50.6 | 3753.8 | 259.6 |
PK/PD, pharmacokinetic/pharmacodynamic; BAL, bronchoalveolar lavage.
MIC for the M. pneumoniae strain used in the experiment was ≤0.008 mg/L (0.008 mg/L used for MIC value for calculations).
Discussion
Macrolide antimicrobials, such as clarithromycin, are often considered the treatment of choice for M. pneumoniae infection. Owing to the fact that clarithromycin MICs for M. pneumoniae are very low, with the clarithromycin MIC90 for M. pneumoniae being ≤0.001 mg/L, it may be predicted that escalating dosages of clarithromycin would be unlikely to result in greater treatment efficacy, since favourable serum PK/PD parameters can be attained with relatively small dosages of clarithromycin.24 However, we found that elevating the dosage of clarithromycin used to treat experimental M. pneumoniae pneumonia did result in greater therapeutic efficacy. Elevated dosages of clarithromycin resulted in significantly greater antimicrobial efficacy (reduced quantitative cultures), as well as significantly greater improvement in markers of disease severity (reduced lung histopathological score, cytokine concentrations and airway hyperresponsiveness). Of note, macrolides have been postulated to have host immunomodulating activity; however, past investigations in our laboratory have indicated that the beneficial activity of macrolides in the treatment of M. pneumoniae respiratory tract infection is antimicrobial in nature, as opposed to resulting from a primary host immunomodulation mechanism.8,9 While we found greater efficacy with increased dosages of clarithromycin in our investigation, it is unknown if even larger dosages would result in further improved efficacy or would reach an upper limit of efficacy for the treatment of M. pneumoniae.
While M. pneumoniae is generally associated with mild-to-moderate community-acquired pneumonia that is self-limited and/or responds well to appropriate antimicrobial therapy, M. pneumoniae pneumonia may also be severe, refractory or, rarely, even fatal. Our group previously investigated the impact of steroids given with macrolide therapy for the treatment of experimental M. pneumoniae respiratory tract infection; we found that the addition of steroid therapy to macrolides significantly improved the outcome of disease parameters.14 In the current investigation, we identify another therapeutic option (escalating dosages of macrolides) that may also improve the outcome of difficult-to-manage clinical M. pneumoniae infection. Escalated doses of clarithromycin have been advocated for other atypical infections, e.g. clarithromycin at double the standard dosage is sometimes advocated in paediatric non-tuberculosis mycobacterial infections.25 Of note, we also previously utilized this animal model to evaluate the efficacy of azithromycin therapy given as either a single high dose or divided over 5 days; although both azithromycin regimens significantly reduced the quantitative cultures, lung histopathological score, and pulmonary cytokines and chemokines, there were no significant differences between the two azithromycin regimens.10
Clarithromycin concentrations were measured in the serum of mice so that comparisons could be made with standard clinical dosages of clarithromycin in humans. Compared with the serum concentrations attained in the mice in this investigation (Table 1), the maximum serum concentration in adults for an oral clarithromycin dosage of 500 mg twice daily is ∼2.67 mg/L, with an area under the concentration–time curve over 24 h (AUC0–24) of 39.18 mg·h/L.7 Hence, in this investigation, the maximum clarithromycin serum concentration attained in the mice was ∼3.3 times greater, while the AUC0–24 was ∼77% (lower) compared with that found in adults with an oral clarithromycin dosage of 500 mg twice daily. An oral clarithromycin dosage of 250 mg twice daily yields an AUC0–24 of 12.88 mg·h/L in adults. So the AUC0–24 of 30.03 mg·h/L for the 75 mg/kg/day dosage in mice is between the AUC0–24 values of oral clarithromycin dosages of 250 mg twice daily and 500 mg twice daily in adults.7 Hence, the equivalent adult human oral dosage to the 75 mg/kg/day mouse dosage would be between 250 mg twice daily and 500 mg twice daily, based on AUC0–24. A limitation of the majority of animal antimicrobial studies is the inability to exactly replicate human antimicrobial kinetics; however, the clarithromycin values attained in this investigation are within a range comparable with human clarithromycin values. In addition, the degree of protein binding of clarithromycin in mice is comparable with that in humans, making direct comparison of the concentration values between species useful.6 Importantly, clarithromycin is metabolized in humans to an active antimicrobial metabolite (14-OH clarithromycin) in significant concentrations; this active metabolite is absent in mice.26 This is a notable limitation of this animal antimicrobial study.
Novelli et al.27 administered various clarithromycin dosing frequency regimens to mice with S. pneumoniae peritonitis and thigh infection, and demonstrated that, contrary to what happens when erythromycin is used, the efficacy of clarithromycin is higher when the drug is administered less frequently and the highest maximum concentration to MIC ratio is achieved. Other investigations of clarithromycin have found that serum AUC0–24/MIC is the most accurate predictor of clarithromycin antimicrobial efficacy.6,7 As mentioned previously, macrolides accumulate in the respiratory epithelial lining fluid where M. pneumoniae have a localized pathogenic effect. We measured clarithromycin concentrations in mouse BAL samples as a surrogate marker for respiratory epithelium clarithromycin exposure for the increasing clarithromycin dosages. The BAL clarithromycin levels offer a pharmacological comparison between the dosages; however, due to the dilutional nature of these measurements, it is not possible to estimate actual correlative PK/PD parameters for the epithelial lining fluid. Comparative BAL clarithromycin levels in humans are not available.
Although, currently, macrolides are considered a drug of choice for the treatment of M. pneumoniae infection, macrolide-resistant M. pneumoniae is now a clinical reality in parts of the globe. Macrolide-resistant M. pneumoniae appears to be emerging in the USA as well.28,29
In conclusion, despite extremely low clarithromycin MICs for M. pneumoniae and resultant favourable PK/PD parameters at relatively low dosages, escalated dosing of clarithromycin resulted in significantly greater therapeutic efficacy in the treatment of M. pneumoniae respiratory infection. Optimizing macrolide therapy for the clinical treatment of severe or refractory M. pneumoniae infection must be balanced with the possible toxicities that may accompany higher dosages of macrolides.
Funding
This work was supported by NIH/NIAID/Asthma and Allergic Diseases Cooperative Research Centers Grant U19AI070412 and NIH/NIAID/K08 AI052262, as well as the Kleberg Foundation.
Transparency declarations
None to declare.
Acknowledgements
This work was previously presented as a poster at the Forty-eighth Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, 25–28 October 2008 (abstract B-60).
References
- 1.McCracken GH., Jr Current status of antibiotic treatment for Mycoplasma pneumoniae infections. Pediatr Infect Dis J. 1986;5:167–71. doi: 10.1097/00006454-198601000-00054. doi:10.1097/00006454-198601000-00054. [DOI] [PubMed] [Google Scholar]
- 2.Shames JM, George RB, Holliday WB, et al. Comparison of antibiotics in the treatment of mycoplasmal pneumonia. Arch Intern Med. 1970;125:680–4. doi:10.1001/archinte.125.4.680. [PubMed] [Google Scholar]
- 3.Hardy RD. Mycoplasma infections. In: Dale DC, Federman DD, editors. ACP Medicine. New York: American College of Physicians; 2009. pp. 1–11. [Google Scholar]
- 4.Principi N, Esposito S, Blasi F, et al. Role of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with community-acquired lower respiratory tract infections. Clin Infect Dis. 2001;32:1281–9. doi: 10.1086/319981. doi:10.1086/319981. [DOI] [PubMed] [Google Scholar]
- 5.Nilsson AC, Björkman P, Persson K. Polymerase chain reaction is superior to serology for the diagnosis of acute Mycoplasma pneumoniae infection and reveals a high rate of persistent infection. BMC Microbiol. 2008;8:93. doi: 10.1186/1471-2180-8-93. doi:10.1186/1471-2180-8-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tessier PR, Kim MK, Zhou W, et al. Pharmacodynamic assessment of clarithromycin in a murine model of pneumococcal pneumonia. Antimicrob Agents Chemother. 2002;46:1425–34. doi: 10.1128/AAC.46.5.1425-1434.2002. doi:10.1128/AAC.46.5.1425-1434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain R, Danziger LH. The macrolide antibiotics: a pharmacokinetic and pharmacodynamic overview. Curr Pharm Des. 2004;10:3045–53. doi: 10.2174/1381612043383322. doi:10.2174/1381612043383322. [DOI] [PubMed] [Google Scholar]
- 8.Hardy RD, Rios AM, Chavez-Bueno S, et al. Antimicrobial and immunologic activities of clarithromycin in a murine model of Mycoplasma pneumoniae-induced pneumonia. Antimicrob Agents Chemother. 2003;47:1614–20. doi: 10.1128/AAC.47.5.1614-1620.2003. doi:10.1128/AAC.47.5.1614-1620.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fonseca-Aten M, Salvatore CM, Mejías A, et al. Evaluation of LBM415 (NVP PDF-713), a novel peptide deformylase inhibitor, for treatment of experimental Mycoplasma pneumoniae pneumonia. Antimicrob Agents Chemother. 2005;49:4128–36. doi: 10.1128/AAC.49.10.4128-4136.2005. doi:10.1128/AAC.49.10.4128-4136.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ríos AM, Fonseca-Aten M, Mejías A, et al. Microbiologic and immunologic evaluation of a single high dose of azithromycin for treatment of experimental Mycoplasma pneumoniae pneumonia. Antimicrob Agents Chemother. 2005;49:3970–3. doi: 10.1128/AAC.49.9.3970-3973.2005. doi:10.1128/AAC.49.9.3970-3973.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fonseca-Aten M, Rios AM, Mejias A, et al. Treatment of experimental chronic pulmonary mycoplasmosis. Int J Antimicrob Agents. 2006;28:253–8. doi: 10.1016/j.ijantimicag.2006.04.008. doi:10.1016/j.ijantimicag.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Salvatore CM, Fonseca-Aten M, Katz-Gaynor K, et al. Intranasal interleukin-12 therapy inhibits Mycoplasma pneumoniae clearance and sustains airway obstruction in murine pneumonia. Infect Immun. 2008;76:732–8. doi: 10.1128/IAI.00878-07. doi:10.1128/IAI.00878-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salvatore CM, Techasaensiri C, Tagliabue C, et al. Tigecycline therapy significantly reduces the concentrations of inflammatory pulmonary cytokines and chemokines in a murine model of Mycoplasma pneumoniae pneumonia. Antimicrob Agents Chemother. 2009;53:1546–51. doi: 10.1128/AAC.00979-08. doi:10.1128/AAC.00979-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tagliabue C, Salvatore CM, Techasaensiri C, et al. The impact of steroids given with macrolide therapy on experimental Mycoplasma pneumoniae respiratory infection. J Infect Dis. 2008;198:1180–8. doi: 10.1086/591915. doi:10.1086/591915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ríos AM, Mejías A, Chávez-Bueno S, et al. Impact of cethromycin (ABT-773) therapy on microbiological, histologic, immunologic, and respiratory indices in a murine model of Mycoplasma pneumoniae lower respiratory infection. Antimicrob Agents Chemother. 2004;48:2897–904. doi: 10.1128/AAC.48.8.2897-2904.2004. doi:10.1128/AAC.48.8.2897-2904.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cimolai N, Taylor GP, Mah D, et al. Definition and application of a histopathological scoring scheme for an animal model of acute Mycoplasma pneumoniae pulmonary infection. Microbiol Immunol. 1992;36:465–78. doi: 10.1111/j.1348-0421.1992.tb02045.x. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalo JA, Lloyd CM, Wen D, et al. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J Exp Med. 1998;188:157–67. doi: 10.1084/jem.188.1.157. doi:10.1084/jem.188.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamelmann E, Schwarze J, Takeda K, et al. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 1997;156:766–75. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- 19.Schwarze J, Hamelmann E, Bradley KL, et al. Respiratory syncytial virus infection results in airway hyperresponsiveness and enhanced airway sensitization to allergen. J Clin Invest. 1997;100:226–33. doi: 10.1172/JCI119516. doi:10.1172/JCI119516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Schaik SM, Enhorning G, Vargas I, et al. Respiratory syncytial virus affects pulmonary function in BALB/c mice. J Infect Dis. 1998;177:269–76. doi: 10.1086/514208. doi:10.1086/514208. [DOI] [PubMed] [Google Scholar]
- 21.Fonseca-Aten M, Rios AM, Mejias A, et al. Mycoplasma pneumoniae induces host-dependent pulmonary inflammation and airway obstruction in mice. Am J Respir Cell Mol Biol. 2005;32:201–10. doi: 10.1165/rcmb.2004-0197OC. doi:10.1165/rcmb.2004-0197OC. [DOI] [PubMed] [Google Scholar]
- 22.Ben-Mei C, Yi-Zeng L, Xiang C, et al. Quantitative determination of azithromycin in human plasma by liquid chromatography–mass spectrometry and its application in a bioequivalence study. J Pharm Biomed Anal. 2006;42:480–7. doi: 10.1016/j.jpba.2006.05.011. doi:10.1016/j.jpba.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Brewer E, Henion J. Atmospheric pressure ionization LC/MS/MS techniques for drug disposition studies. J Pharm Sci. 1998;87:395–402. doi: 10.1021/js9701059. doi:10.1021/js9701059. [DOI] [PubMed] [Google Scholar]
- 24.Waites KB, Crabb DM, Duffy LB. Comparative in vitro susceptibilities and bactericidal activities of investigational fluoroquinolone ABT-492 and other antimicrobial agents against human mycoplasmas and ureaplasmas. Antimicrob Agents Chemother. 2003;47:3973–5. doi: 10.1128/AAC.47.12.3973-3975.2003. doi:10.1128/AAC.47.12.3973-3975.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cross JT, Jacobs RF. Other mycobacteria. In: Feigin RD, Cherry JD, Demmler GJ, et al., editors. Textbook of Pediatric Infectious Diseases. Philadelphia: Saunders; 2004. pp. 1379–88. [Google Scholar]
- 26.Bedos JP, Azoulay-Dupuis E, Vallee E, et al. Individual efficacy of clarithromycin (A-56268) and its major human metabolite 14-hydroxy clarithromycin (A-62671) in experimental pneumococcal pneumonia in the mouse. J Antimicrob Chemother. 1992;29:677–85. doi: 10.1093/jac/29.6.677. doi:10.1093/jac/29.6.677. [DOI] [PubMed] [Google Scholar]
- 27.Novelli A, Fallani S, Cassetta MI, et al. In vivo pharmacodynamic evaluation of clarithromycin in comparison to erythromycin. J Chemother. 2002;14:584–90. doi: 10.1179/joc.2002.14.6.584. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Atkinson TP, Hagood J, et al. Emerging macrolide resistance in Mycoplasma pneumoniae in children: detection and characterization of resistant isolates. Pediatr Infect Dis J. 2009;28:693–6. doi: 10.1097/INF.0b013e31819e3f7a. doi:10.1097/INF.0b013e31819e3f7a. [DOI] [PubMed] [Google Scholar]
- 29.Matsubara K, Morozumi M, Okada T, et al. A comparative clinical study of macrolide-sensitive and macrolide-resistant Mycoplasma pneumoniae infections in pediatric patients. J Infect Chemother. 2009;15:380–3. doi: 10.1007/s10156-009-0715-7. doi:10.1007/s10156-009-0715-7. [DOI] [PubMed] [Google Scholar]