Abstract
Background
Severe malnutrition is frequently complicated by sepsis, leading to high case fatality. Oral ciprofloxacin is a potential alternative to the standard parenteral ampicillin/gentamicin combination, but its pharmacokinetics in malnourished children is unknown.
Methods
Ciprofloxacin (10 mg/kg, 12 hourly) was administered either 2 h before or up to 2 h after feeds to Kenyan children hospitalized with severe malnutrition. Four plasma ciprofloxacin concentrations were measured over 24 h. Population analysis with NONMEM investigated factors affecting the oral clearance (CL) and the oral volume of distribution (V). Monte Carlo simulations investigated dosage regimens to achieve a target AUC0–24/MIC ratio of ≥125.
Results
Data comprised 202 ciprofloxacin concentration measurements from 52 children aged 8–102 months. Absorption was generally rapid but variable; Cmax ranged from 0.6 to 4.5 mg/L. Data were fitted by a one-compartment model with first-order absorption and lag. The parameters were CL (L/h) = 42.7 (L/h/70 kg) × [weight (kg)/70]0.75 × [1 + 0.0368 (Na+ – 136)] × [1 – 0.283 (high risk)] and V (L) = 372 × (L/70 kg) × [1 + 0.0291 (Na+ – 136)]. Estimates of AUC0–24 ranged from 8 to 61 mg·h/L. The breakpoint for Gram-negative organisms was <0.06 mg/L with doses of 20 mg/kg/day and <0.125 mg/L with doses of 30 or 45 mg/kg/day. The cumulative fraction of response with 30 mg/kg/day was ≥80% for Escherichia coli, Klebsiella pneumoniae and Salmonella species, but <60% for Pseudomonas aeruginosa.
Conclusions
An oral ciprofloxacin dose of 10 mg/kg three times daily (30 mg/kg/day) may be a suitable alternative antibiotic for the management of sepsis in severely malnourished children. Absorption was unaffected by the simultaneous administration of feeds.
Keywords: quinolone, drug absorption, marasmus, kwashiorkor, Gram-negative
Introduction
Severe malnutrition remains a common cause of admission to hospital in less-developed countries. Many centres, particularly in Africa, report poor outcome despite adherence to recommended treatment guidelines.1–4 The children at the greatest risk of fatal outcome are those with Gram-negative septicaemia, constituting 48%–55% of invasive bacterial pathogens, and those admitted with diarrhoea and/or shock.5,6 Changes in the intestinal mucosal integrity and gut microbial balance occur in severe malnutrition,7 resulting in treatment failure and adverse clinical outcome.8 The higher prevalence of gut barrier dysfunction in children with severe malnutrition may have important effects on the absorption of antimicrobials and their bioavailability, and therefore may limit choices for the delivery of antimicrobial medication.
Children with severe and complicated malnutrition routinely receive broad-spectrum parenteral antibiotics.9 In vitro antibiotic susceptibility testing indicates that up to 85% of organisms are fully susceptible to the first-line treatment, parenteral ampicillin and gentamicin, recommended by the WHO for children with severe and complicated malnutrition.4 Pharmacokinetic studies have demonstrated satisfactory plasma concentrations of these commonly used antibiotics.10 However, in vitro resistance has been associated with later deaths, and the current second-line antibiotic (chloramphenicol) was found to offer little advantage over the ampicillin and gentamicin combination.4
Fluoroquinolones are effective against most Gram-negative organisms and have activity against Gram-positive organisms, especially when given in combination.11 The quinolones are used in the treatment of serious bacterial infections in adults; however, their use in children has been restricted due to concerns about potential cartilage damage.12 Nevertheless, quinolones are increasingly prescribed for paediatric patients. Ciprofloxacin is licensed in children >1 year of age for pseudomonal infections in cystic fibrosis, for complicated urinary tract infections, and for the treatment and prophylaxis of inhalation anthrax.13 When the benefits of treatment outweigh the risks, ciprofloxacin is also licensed in the UK for children >1 year of age with severe respiratory tract and gastrointestinal system infection.14 In its many years of clinical use, ciprofloxacin has been found effective, even with oral administration, owing to its bioavailability of ∼70%.15 However, few studies have evaluated the population pharmacokinetics of ciprofloxacin in children,16–18 and no studies have been conducted in severe malnutrition.
For the treatment of Gram-negative infection, common in severe malnutrition, pragmatic and cost-effective treatments are needed to improve outcome. Since intravenous formulations of fluoroquinolones are very expensive, they are rarely used in resource-poor settings. Oral formulations would appear to be the best option in patients who are able to ingest and adequately absorb medication. The aims of this study were to determine the pharmacokinetic profile of oral ciprofloxacin given at a dose of 10 mg/kg twice daily to children admitted to hospital with severe malnutrition, to develop a population model to describe the pharmacokinetics of ciprofloxacin in this patient group, and to use Monte Carlo simulation techniques to investigate potential relationships between dosage regimens and antimicrobial efficacy.
Methods
Patients
The study was conducted at Kilifi District Hospital, Kenya. All children admitted to the ward were examined by a member of the clinical research team. Between July 2008 and February 2009, children >6 months of age were assessed for eligibility for the study. Eligible children had severe malnutrition, defined as one of the following: weight-for-height z-score (WHZ) of ≤3; mid-upper arm circumference of <11 cm; or the presence of bilateral pedal oedema (kwashiorkor). Children with evidence of intrinsic renal disease (creatinine concentration >300 μmol/L and hypertension or hyperkalaemia) were excluded. No cases had coexisting chronic bone or joint disease, or concurrently prescribed antacids, ketoconazole, theophylline or corticosteroids. The study was explained to the child's parent or guardian in their usual language and written informed consent was obtained. The Kenya Medical Research Institute/National Ethical Review Committee approved the study.
Baseline laboratory tests included a full blood count, blood film for malaria parasites, plasma creatinine, electrolytes, plasma glucose, blood gases, blood culture and HIV rapid antibody test. Blood cultures were processed by a BACTEC 9050 instrument (Becton Dickinson, NJ, USA). Children were treated according to the standard WHO management guidelines for severe malnutrition. These include nutritional support with a special milk-based formula (F75 and F100), multivitamin and multimineral supplementation and reduced sodium oral rehydration solution (RESOMAL) for children with diarrhoea (>3 watery stools/day). At admission, all children were prescribed parenteral ampicillin (50 mg/kg four times a day) and intramuscular gentamicin (7.5 mg/kg once daily) for 7 days. These were revised, when indicated by the child's clinical condition or culture results. Other fluoroquinolones were not prescribed during the study period, since they were not routinely available.
Study procedures
Ciprofloxacin concentration–time profiles were obtained during two study periods. In the initial study period, children were given the ciprofloxacin either 2 h before or 2 h after they had their nutritional milks or meal. In this first period of the study (n = 36), an equal number of patients were included in three subgroups: low risk; intermediate risk; and high risk of fatality. The stratification of risk was based on a previous report.4 High risk included children presenting with one of the following: depressed conscious state; bradycardia (heart rate <80 beats per minute); evidence of shock (capillary refill time ≥2 seconds, temperature gradient or weak pulse); or hypoglycaemia (blood glucose <3 mmol/L). Intermediate risk included any one of: deep ‘acidotic’ breathing; signs of severe dehydration (plus diarrhoea); lethargy; hyponatraemia (sodium <125 mmol/L); or hypokalaemia (potassium <2.5 mmol/L). Children with low risk had none of these factors. In the second study period (n = 16), the drug was administered at the time they received nutritional feeds and did not have any risk stratification.
Drug administration and blood sampling
Participants received witnessed doses of oral ciprofloxacin (10 mg/kg body weight), i.e. a standard treatment dose, every 12 h for 48 h, starting on the day of admission. Routine antimicrobials were given simultaneously as the empirical treatment for invasive infections. Since oral suspensions are not available locally, ciprofloxacin tablets (Bactiflox™ 250 mg, Mepha Pharma AG, Switzerland) were reformulated into an aqueous suspension by the study pharmacist. A separate tablet was used to prepare each individual dose and the suspension was then used immediately. Individual doses were measured by the pharmacist under the observation of the trial nurse, according to the patient's body weight. This approach has been used and reported previously.19
Children were allocated, using a computer-generated randomization list, to one of three blood sampling schedules: Group A at 2, 4, 8 and 24 h; Group B at 3, 5, 9 and 12 h; and Group C at 1, 3, 6 and 10 h. Children in each of the risk categories were evenly allocated across the sampling schedules. To minimize discomfort to the child, blood sampling for the measurement of ciprofloxacin concentrations was through an in situ cannula, which was only used for this purpose. At each timepoint, a venous blood sample (0.5 mL) was collected into a lithium heparin tube, centrifuged, and the plasma separated and stored in cryovials at −20°C until analysed. A rapid, selective and sensitive HPLC method coupled with fluorescence detection was used to determine the concentration of ciprofloxacin in the plasma samples.20 The intra- and interassay imprecisions of the HPLC method were <8.0%, and accuracy values ranged from 93% to 105% for quality control samples at 0.2, 1.8 and 3.6 mg/L. Calibration curves of ciprofloxacin were linear over the concentration range of 0.02–4 mg/L, with correlation coefficients ≥0.998.
Pharmacokinetic analysis
Population pharmacokinetic parameter estimates were obtained with NONMEM version VI using first-order conditional estimation with interaction.21 Post-processing of the NONMEM results was performed with Xpose version 4 programmed in R 2.9.2.22,23 Preliminary analyses found that a one-compartment elimination model adequately described the concentration–time profiles; absorption was described using first- and zero-order models with an absorption lag and with a transit compartment model.24 Relationships between the oral clearance of ciprofloxacin (CL) and weight, and between the oral volume of distribution (V) and weight were described using an allometric approach,25 i.e.
Between-subject variabilities (BSV) in the pharmacokinetic parameters were assumed to be log-normally distributed and covariance between the variabilities in the pharmacokinetic parameters was investigated. A combined proportional and additive error model was used to describe the residual error.
A wide range of demographic, biochemical and haematological data were collected in the course of the study. In addition, creatinine clearance estimates for each patient, according to age range, were calculated using the equations of Schwarz et al.26,27 Potential relationships between the clinical and demographic factors and empirical Bayes' estimates (individual estimates) of ciprofloxacin oral CL, oral V and absorption rate were initially examined visually using scatter plots and by general additive modelling within Xpose.22 Factors identified as potential covariates that might explain variability in ciprofloxacin pharmacokinetics were then added individually to the population model. A statistically significant improvement in the fit of the model to the data was defined as a reduction in the objective function value (OFV) of ≥3.84 (P <0.05) in the forward stepwise analysis. The covariate that produced the greatest fall in OFV was included first, and then other covariates were added to and removed from the model in a stepwise manner that included changing the order of inclusion and removal. Statistical significance was defined as an increase in OFV of ≥6.63 (P < 0.01) when a covariate was removed. Additional criteria, such as goodness-of-fit plots, the precision of the parameter estimates, and the ability of covariates to explain variability in the oral CL, oral V and absorption rate, were also considered.
The final population model was evaluated in three ways: a bootstrap sampling procedure with 1000 samples; a prediction-corrected visual predictive check (pcVPC) based on 1000 simulations; and by examination of normalized prediction distribution errors (npde) from 1000 simulations. Both the bootstrapping procedure and the pcVPC were performed using PsN toolkit;28,29 npde were computed using the software developed by Brendel et al.30
Time to maximum (Tmax) and maximum concentrations of ciprofloxacin (Cmax) for each patient were obtained from the raw data and estimated using the individual pharmacokinetic parameter values. Estimates of steady-state AUC within each dosage interval (AUC12) and each day (AUC24) were calculated from 12 hourly dose/CLi and daily dose/CLi, respectively, where CLi are the individual estimates of oral CL. Individual estimates of the elimination half-life (t½) were calculated from 0.693 × Vi/CLi, where Vi are the individual estimates of oral V.
Monte Carlo simulations
The final parameters of the population pharmacokinetic model were used to simulate estimates of oral CL for 10 000 patients using NONMEM version VI.21 Relevant clinical characteristics (weight and sodium concentration) were assumed to arise from log-normal distributions with outer limits set to the values observed in the raw data. The incidence of ‘high risk’ in the simulated dataset was 31%. AUC24 estimates were then determined for each simulated patient from daily dose/oral CL. Simulations were performed for three dosage regimens: 20 mg/kg/day (current daily dosage regimen); 30 mg/kg/day; and 45 mg/kg/day. For evaluation of these dosage regimens, MIC values were chosen across the range 0.03–8 mg/L. The probability of target attainment (PTA) was defined as the probability that the target AUC0–24/MIC ratio was achieved at each MIC. Target AUC0–24/MIC ratios of ≥125 were used.31 For each ciprofloxacin regimen, the highest MIC at which the PTA was ≥90% was defined as the pharmacokinetic/pharmacodynamic susceptible breakpoint.
A second analysis was conducted using the MIC distributions for Escherichia coli, Pseudomonas aeruginosa, Salmonella spp. and Klebsiella pneumoniae derived from the database of the European Committee on Antimicrobial Susceptibility Testing.32 These MIC distributions were extracted from 17 877 strains of E. coli, 27 825 strains of P. aeruginosa, 5898 strains of K. pneumoniae and 1733 strains of Salmonella spp. The cumulative fraction of response (CFR) was used to estimate the overall response of pathogens to ciprofloxacin with each of the three dosage regimens. This estimate accounts for the variability of drug exposure in the population and the variability in the MIC combined with the distributions of MICs for the pathogens. For each MIC, the fraction of simulated patients who met the pharmacodynamic target (AUC0–24/MIC ≥ 125) was multiplied by the fraction of the distribution of microorganisms for each MIC. The CFR was calculated as the sum of fraction products over all MICs.
Results
Participants and admission clinical characteristics
Of the 90 children with severe malnutrition admitted during the study period, 52 were enrolled into the study. Twelve who fulfilled the entry criteria declined to give consent, in four intravenous access was not possible and 22 were excluded due to completion of recruitment to a specific subgroup. The demographic and clinical characteristics of the patients included in the study are summarized in Table 1. Median age [interquartile range (IQR)] was 23 months (15–33 months), 29 (56%) were male, oedema (defining kwashiorkor) was present in 24 patients (46%) and eight (15%) children were HIV antibody positive. The median weight (IQR) was 6.9 kg (6.1–8.4 kg). The estimated creatinine clearance ranged from 5 to 128.7 mL/min/1.73 m2; only one patient had severe renal impairment. According to the risk stratification, mortality was 21%, 12% and 0% in the high-risk, intermediate-risk and low-risk groups, respectively. Bacteraemia was present in six children; three had E. coli, two had S. pneumoniae and one had K. pneumoniae. The isolates were tested against ampicillin, gentamicin, ciprofloxacin, chloramphenicol and ceftriaxone. The K. pneumoniae isolate was resistant to ampicillin only, while one E. coli isolate was resistant to all of the tested antibiotics. One S. pneumoniae isolate was resistant to ampicillin and had intermediate susceptibility to ciprofloxacin. Ciprofloxacin was well tolerated and there were no adverse events reported with its use.
Table 1.
Summary of the demographic and clinical characteristics of the patients who participated in the study
| Characteristic | Number (%)/median (range) | Interquartile range |
|---|---|---|
| Male/female | 29/23 (56/44) | |
| Oedema | 24 (46) | |
| HIV positive | 8 (15) | |
| Age (months) | 23 (8–102) | 15–33 |
| Weight (kg) | 6.9 (4.1–14.5) | 6.1–8.4 |
| Height (cm) | 75.4 (58.5–114.4) | 70.1–81.4 |
| MUAC (cm) | 11 (7.7–14.3) | 10–12 |
| WHZ | −3.26 (−5.69–0.04) | −3.67 to −2.48 |
| Vomiting | 15 (29) | |
| Shock | 7 (13) | |
| Dehydration | 25 (48) | |
| Low risk | 22 (42) | |
| Intermediate risk | 14 (27) | |
| High risk | 16 (31) | |
| White blood cell (×106/L) (6–17.5) | 13.3 (5.5–84.4) | 10.3–17.1 |
| Haemoglobin (g/dL) (9–14) | 9.0 (2.1–12.8) | 7.2–9.8 |
| Platelets (×106/L) (150–400) | 309 (16–1369) | 210–451 |
| Sodium (mmol/L) (138–145) | 136 (120–160) | 131–138 |
| Potassium (mmol/L) (3.5–5) | 3.1 (1.2–5.1) | 2.3–4.1 |
| Glucose (mmol/L) (2.8–5) | 4 (0.4–11.4) | 2.8–4.7 |
| Bicarbonate (mmol/L) (22–29) | 15.4 (4.7–26) | 11.7–18.5 |
| Base excess (−4 to +2) | −8.0 (−26.9–2.1) | −13.1 to −4.5 |
| Serum creatinine (μmol/L) (44–88) | 44 (27–676) | 36.8–51.9 |
| Creatinine clearancea (mL/min/1.73 m2) | 85.8 (5.0–128.7) | 70.7–101.4 |
Pharmacokinetic data analysis
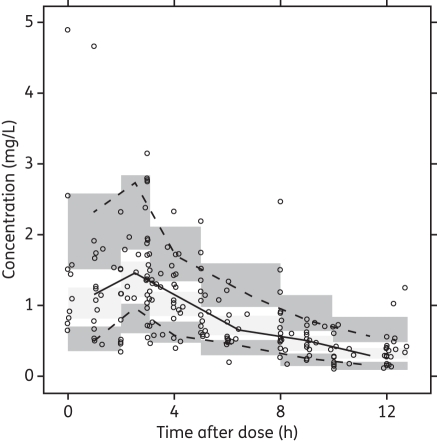
A total of 202 plasma ciprofloxacin concentration measurements were available, with a median of 4 (range 2–4) measurements per patient. Individual concentration–time profiles are presented in Figure 1. Tmax ranged from 1 to 5 h (median 3 h) after the dose, and in 69% of patients Cmax was observed within 1–3 h. Cmax ranged from 0.6 to 4.5 mg/L with a median of 1.7 mg/L. Cmax was >1 mg/L and >2 mg/L in 77% and 31% of patients, respectively. Trough concentrations at 12 h after the first dose were available from 17 patients and ranged from 0.1 to 0.7 mg/L with a median of 0.3 mg/L.
Figure 1.
Ciprofloxacin concentration measurements in 52 children with malnutrition following oral doses of 10 mg/kg. Samples were measured after the first dose in all patients and after a second dose 12 h later in 16 patients.
Both the first-order with lag and the transit compartment absorption models provided good fits of the population and individual concentration–time profiles. Since the transit compartment model was unstable during validation procedures and provided little improvement in the overall fit of the data, the first-order model with lag was used for covariate model development. The transit compartment model was tested again with the final covariate model, but offered no clear advantage.
Plots of individual estimates of oral CL and oral V against the measured and derived clinical and demographic data identified potential relationships between oral CL and risk category, serum sodium concentration, serum potassium concentration, feeding status, shock and dehydration, and between oral V and risk category, serum sodium concentration, shock, dehydration, diarrhoea and vomiting. These factors all achieved small, but statistically significant, reductions in the OFV when included individually in the population model for oral CL. The biggest reductions occurred with sodium concentration (6.24) and the high-risk category (6.05). When these factors were combined, the OFV fell by a further 14.46 points. BSV in oral CL fell from 49.8% with the base model to 38.1% with the covariate model. No additional factors reduced the variability in oral CL, but a further reduction in OFV and BSV in oral V (from 48.4% to 43.0%) was obtained when sodium concentration was added to the model for oral V. Although the inclusion of bicarbonate concentration as a factor influencing the absorption rate constant (ka) produced a statistically significant reduction in OFV, it did not reduce BSV in ka and was therefore excluded. The final population model parameters and bootstrap estimates are presented in Table 2 and summarized below.
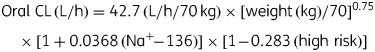 |
Table 2.
Parameter estimates arising from the final population model describing the pharmacokinetics of oral ciprofloxacin in malnourished children
| Parameter | Population estimate | Bootstrap estimate | Bootstrap 95% CI |
|---|---|---|---|
| θ1 | 42.7 | 42.5 | 37.0–49.3 |
| θ2 | 0.0368 | 0.0361 | 0.0217–0.0446 |
| θ3 | −0.283 | −0.285 | −0.412 to −0.118 |
| θ4 | 372 | 367 | 316–429 |
| θ5 | 0.0291 | 0.0282 | 0.0155–0.0388 |
| θ6 | 2.97 | 3.44 | 1.32–8.86 |
| θ7 | 0.742 | 0.792 | 0.168–0.924 |
| BSV CL | 38.1 | 37.8 | 28.7–45.8 |
| BSV V | 43.0 | 42.9 | 32.4–51.8 |
| BSV ka | 102 | 110 | 56–159 |
| Residual error Additive (SD) | 0.0273 | 0.0278 | 0.0041–0.0438 |
| Proportional (%CV) | 18.6 | 17.8 | 14.0–22.6 |
Structural model: TVCL = θ1 × (WT/70)0.75 × [1 + θ2 × (Na+ – 136)] × [1 + θ3 × (high risk)]; TVV = θ4 × (WT/70) × [1 + θ5 × (Na+ – 136)]; TVKA = θ6; ALAG = θ7.
Abbreviations: BSV, between-subject variability expressed as a % coefficient of variation; CL, oral clearance; V, oral volume of distribution; TVCL, typical value of oral clearance (L/h); TVV, typical value of oral volume of distribution (L); TVKA, typical value of the absorption rate constant (ka) (1/h); ALAG, absorption lag time (h); WT, body weight (kg); Na+, serum sodium concentration (mmol/L).
The population model therefore identified a standardized oral CL in an adult weighing 70 kg of 42.7 L/h, and found that oral CL was linearly and positively related to sodium concentration. The model described an increase (or decrease) in oral CL by 3.7% for every 1 mmol/L increase (or decrease) in the serum sodium concentration from the median value of 136 mmol/L. The standardized oral CL fell by 28% to 30.6 L/h/70 kg in patients in the ‘high-risk’ category. The standardized oral V estimate was 372 L/70 kg and changed by 2.9% for every 1 mmol/L change in the serum sodium concentration from 136 mmol/L.
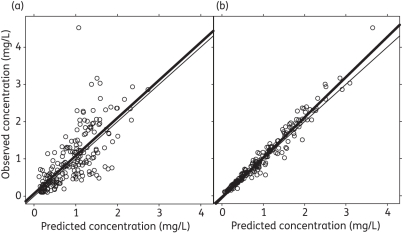
All three validation methods indicated that the model provided a satisfactory description of the data. Figure 2 shows good agreement between the measured concentrations and the concentrations predicted by both the population pharmacokinetic model (r2 = 0.659) and the individual parameter estimates (r2 = 0.971). The pcVPC presented in Figure 3 shows that the population model was able to describe the distribution of the raw concentration data, and the npde check confirmed a normal distribution around each individual observation within the simulated dataset.
Figure 2.
Observed versus population (a) and individual (b) predicted concentrations of ciprofloxacin in malnourished infants based on the final population model. The thin line represents the line of identity; the thick line represents the linear regression line.
Figure 3.
Prediction-corrected visual predictive check of the final model describing the pharmacokinetics of oral ciprofloxacin in infants with malnutrition. The solid line represents the median of the raw data, the dotted lines are the 10th and 90th percentiles of the raw data, and the shaded areas are the 90% confidence intervals of the 10th, 50th and 90th percentiles of the 1000 simulations based on the final model.
Table 3 summarizes the individual Bayes' estimates of the pharmacokinetic parameters and derived estimates of Cmax, Tmax, t½ and AUC0–24. Oral CL had a median of 0.98 L/h/kg in patients in the low and intermediate categories, and 0.67 L/h/kg in high-risk patients. There was a wide variability in the individual estimates of AUC0–24, which ranged from 7.9 to 61 mg·h/L. Median estimates of AUC0–24 were higher in patients in the high-risk category at 29.7 mg·h/L compared with 20.5 mg·h/L in the low and intermediate categories.
Table 3.
Summary of individual ciprofloxacin pharmacokinetic parameter estimates obtained in 52 children with severe malnutrition
| Variable | n | Mean | SD | Median | Minimum | Maximum |
|---|---|---|---|---|---|---|
| CL(L/h) | 52 | 7.43 | 3.54 | 7.19 | 1.83 | 17.1 |
| CL(L/h/kg) | 52 | 1.02 | 0.52 | 0.87 | 0.32 | 2.54 |
| CL (L/h/kg) low/intermediate risk | 36 | 1.14 | 0.53 | 0.98 | 0.46 | 2.54 |
| CL (L/h/kg) high risk | 16 | 0.77 | 0.41 | 0.67 | 0.32 | 1.53 |
| V (L/kg) | 52 | 5.47 | 2.69 | 4.49 | 2.14 | 14.2 |
| t½ (h) | 52 | 3.97 | 1.38 | 3.78 | 2.01 | 9.04 |
| Observed Tmax (h) | 52 | 2.77 | 1.08 | 3.00 | 1.00 | 5.17 |
| Model-predicted Tmax (h) | 52 | 1.91 | 0.58 | 1.79 | 1.09 | 3.85 |
| Observed Cmax (mg/L) | 52 | 1.68 | 0.79 | 1.71 | 0.58 | 4.52 |
| Model-predicted Cmax (mg/L) | 52 | 1.51 | 0.60 | 1.50 | 0.61 | 3.56 |
| AUC0–24 (mg·h/L) | 52 | 24.8 | 12.4 | 22.4 | 7.9 | 61.3 |
| AUC0–24 (mg·h/L) low/intermediate risk | 36 | 21.1 | 8.8 | 20. 5 | 7.9 | 43.4 |
| AUC0–24 (mg·h/L) high risk | 16 | 32.9 | 15.5 | 29.7 | 13.1 | 61.3 |
Abbreviations: CL, oral clearance; V, oral volume of distribution; t½, elimination half-life; Tmax, the time of the maximum concentration; AUC0–24, the steady-state 24 h AUC.
Monte Carlo simulations
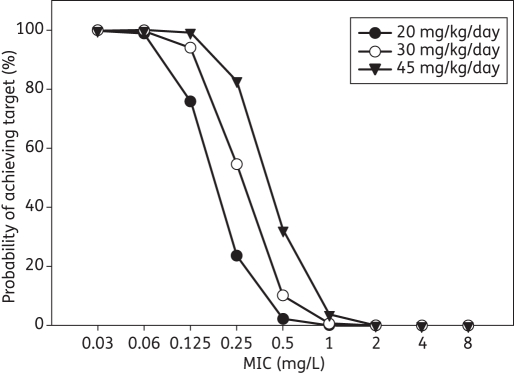
The percentage of simulated patients who achieved an AUC0–24/MIC ratio of ≥125 at each MIC value with the three ciprofloxacin daily dosage regimens is presented in Figure 4. With 20 mg/kg/day, only 76% of patients would be expected to achieve the target AUC0–24/MIC ratio if the MIC was 0.125 mg/L. However, with daily doses of 30 and 45 mg/kg, the percentages increased to 95% and 99%, respectively. Consequently, the pharmacokinetic/pharmacodynamic breakpoint for Gram-negative organisms was <0.06 mg/L for the study dose of 20 mg/kg/day, and <0.125 mg/L for doses of 30 and 45 mg/kg/day. The target AUC0–24/MIC ratio was achieved in <5% of patients with all dosage regimens if the MIC was >1 mg/L. When the results were integrated with the MIC distribution for each organism, the CFR was >80% with all three daily dosage regimens for E. coli and Salmonella spp., and was 80% for K. pneumoniae with a dose of 30 mg/kg/day (Table 4). CFR values for P. aeruginosa were <70% for all doses tested.
Figure 4.
Percentage probability of achieving a target AUC0–24/MIC ratio ≥125.
Table 4.
Cumulative fraction of predicted response to achieve the target AUC0–24/MIC ratio for three ciprofloxacin dosage regimens against strains of Salmonella spp., P. aeruginosa, K. pneumoniae and E. coli
| Cumulative fraction of predicted response (%) |
||||
|---|---|---|---|---|
| Organism | Target AUC0–24/MIC ratio | 20 mg/kg/day | 30 mg/kg/day | 45 mg/kg/day |
| Salmonella spp. | 125 | 96 | 98 | 99 |
| P. aeruginosa | 125 | 43 | 55 | 64 |
| K. pneumonia | 125 | 76 | 80 | 83 |
| E. coli | 125 | 85 | 87 | 88 |
Discussion
This study examined the population pharmacokinetics of ciprofloxacin following oral doses of 10 mg/kg given 12 hourly on the day of admission to a group of 52 paediatric patients with severe malnutrition. Since the study was designed purely to investigate ciprofloxacin pharmacokinetics and not efficacy, all patients also received standard therapy for malnutrition and for sepsis on admission. Patient weight, high risk of mortality and serum sodium concentration were the main factors that influenced the concentration–time profile of ciprofloxacin in this patient group. Estimates of AUC24 ranged from 8 to 61 mg·h/L, indicating that an AUC/MIC ratio ≥125 would only be achieved in all patients studied if the MIC was <0.06 mg/L.
Sparse sampling was necessary due to the nature of the population. The sampling windows covered different sections of the 12 h dosage interval; additional trough concentrations measured after the second dose were available from 16 patients. The observed Cmax concentrations ranged from 0.6 to 4.5 mg/L. This range is lower than the values averaging ∼8.4 mg/L reported by Schaefer et al.18 following intravenous doses of 10 mg/kg to paediatric patients with cystic fibrosis, but is consistent, when corrected for dose, with their range of ∼2.5–5 mg/L (mean 3.5 mg/L) achieved with an oral dose of 15 mg/kg. In contrast, despite using a higher dose of 15 mg/kg, Peltola et al.19 observed similar values of Cmax (0.5–5.3 mg/L) when they administered ground tablets in water and mean Cmax values of ∼2–2.7 mg/L following an oral suspension of 10 mg/kg.33 The observed variability in Cmax and Tmax (1–5 h) in the present study may reflect a combination of variable oral bioavailability and the sampling strategy. One limitation of the present study was that most samples were taken ≥2 h after the dose and the Cmax may have already been attained. Peak concentrations were typically observed at 1–2 h in previous studies.19,33 The observed Cmax measurements suggest that MIC values <0.1 mg/L would ideally be necessary to consistently achieve Cmax/MIC ratios of >10, as recommended by MacGowan et al.34
The concentration–time data were adequately described by first-order absorption with lag and a monoexponential decline. Studies following the intravenous administration of ciprofloxacin have identified a biexponential decline with a short distribution half-life of ∼10–30 min,16–18,35,36 but the sparse sampling schedule in the present study precluded the identification of a distribution phase. The population model indicated a rapid absorption of ciprofloxacin with an estimated half-life of 14 min after a lag of ∼45 min. Previous population analyses in paediatric patients reported shorter or similar absorption lag times, but rates of absorption were slower, with half-lives ranging from ∼30 to 96 min.16–18 In contrast, Peltola et al.,19 who also used ground tablets, reported mean absorption half-lives of 24 min in infants up to 14 weeks old and 17 min in children aged 1–5 years. Both the present and previous population studies identified wide variability in the absorption rate, with BSV estimates ranging from 50% to 103%.16,17
Rajagopalan and Gastonguay17 reported a standardized clearance of 30.3 L/h/70 kg in paediatric patients aged 14 weeks to 17 years. Correcting for their bioavailability estimate of 61% gives an oral clearance of 49.7 L/h/70 kg, which is similar to the value of 42.7 L/h/70 kg obtained in the present study. Both results are reasonably consistent with values of ∼40–70 L/h reported in adults with normal renal function.37–39 Individual weight-corrected oral CL estimates were in the range 0.3–2.5 L/h/kg in the present study and were similar to the CL values reported by Rajagopalan and Gastonguay17 (0.2–1.3 L/h/kg), when corrected for bioavailability (0.3–2.2 L/h/kg), and the oral CL observed by Peltola et al.33 (1–1.5 L/h/kg). The population model developed by Payen et al.16 predicts an oral clearance increasing from 2 to 20 L/h over the age range covered in the present study. These values are again consistent with the present observations of 1.8–17 L/h. These findings suggest that oral CL is similar in this population of malnourished children to those reported in children without malnutrition.
Since there was no intravenous ciprofloxacin dose given, oral bioavailability could not be determined directly from this study. However, the similarity between the estimates of oral CL in the present study with the results from previous studies suggests that bioavailability may also be similar. Ciprofloxacin is generally well absorbed, with a typical bioavailability of ∼70% in adults15 and 60%–70% in children.16–18,40 Rubio et al.40 reported a lower bioavailability in children with cystic fibrosis aged 5–12 years (68%) compared with those aged 13–17 years (95%), but this age effect was not observed in the other studies. The formulation that was used in the present study, i.e. tablets reformulated into a suspension with water, has practical advantages over the commercial oral suspension33 as it is much cheaper and easy to prepare on site.
Another major aim of this study was to evaluate the impact of feeding on oral clearance. Both milk and divalent cations (such as magnesium and other minerals included in the nutritional milk) have been reported to chelate quinolones and reduce the oral bioavailability of ciprofloxacin.41–43 Since most children are managed in resource-poor healthcare settings with a limited number of healthcare personnel, it is both impractical and difficult to ensure that the feeding times are synchronized around the drug administration times. Consequently, a significant proportion of children are likely to receive ciprofloxacin concomitantly with food and a reduction in bioavailability could compromise efficacy. Although the preliminary analysis found an increase in oral clearance in patients who received ciprofloxacin with feeding, the objective function value only fell by 4.5 points, indicating a weak effect. In the final model, no influence of feeding was identified. Thirteen of the 16 patients who were given ciprofloxacin with food were in the low/intermediate-risk category, which was associated with higher oral CL and lower AUC estimates. The influence of risk may therefore have confounded the identification of a food effect, if one existed. Conversely, if food was important, it may have enhanced the apparent influence of risk in the model. The lack of a clear influence of feeding on absorption is a positive finding. If a significant interaction had been observed, then the future use of this antimicrobial would have been compromised by the regular feeding patterns required, and possible variability in the gut motility and gastric emptying in children with severe malnutrition.
Dehydration and shock were initially found to influence oral CL when examined alone, but both were related to other factors that had a more powerful effect. Of the 16 patients in the high-risk category, 12 had dehydration (of whom 11 had diarrhoea) and 6 had shock. ‘High risk’ therefore accounted for both of these factors and only sodium concentration provided an additional improvement in the fit of the model to the data. An unexpected finding was the lack of an effect of renal function on oral CL, since ciprofloxacin CL is known to decrease in renal impairment.37–39,44 However, the only patient in the group with overt renal failure (creatinine concentration 676 μmol/L) was also categorized as high risk. This individual had one of lowest estimates of oral CL at 1.8 L/h (0.33 L/h/kg). Apart from this one individual, no other patient had severe renal impairment; the highest serum creatinine concentration in other patients was 95 μmol/L. Interestingly, another patient in the high-risk category had a similar and very low oral CL (0.32 L/h/kg), despite a creatinine concentration of 76 μmol/L. The t½ of ciprofloxacin is ∼3–5 h in adults with normal renal function.37,38,44 Similar values have been found in previous studies in infants and young children16,33,45 and in the present study, where t½ had a median of 3.8 h and ranged from 2 to 9 h.
Although children in the high-risk category (including children with impaired consciousness, shock and hypoglycaemia) had significantly lower estimates of oral CL, individual estimates varied widely in both groups (Table 3). ‘High risk’ children represent the most critically ill cases, where both the logistics of administration and gut absorption of oral microbiological agents are likely to be compromised. Importantly, this finding highlights the need for parenteral agents for the most critically ill, for which third-generation cephalosporins are likely to be the most appropriate. We have previously shown delayed uptake and reduced clearance of gentamicin following an intramuscular dose of 7.5 mg/kg in children with severe malnutrition complicated by septic shock.10
The finding that low sodium concentration is associated with a reduced oral CL is also of interest. Hyponatraemia in severe malnutrition has been postulated to be due to ‘adaptive reduction’ where normal homeostatic cellular ATPase and sodium potassium pumps are faulty, resulting in low sodium due increased extracellular water.46,47 The reasons why low sodium is associated with lower estimations of oral CL and oral V are not clear, but although statistically significant, the inclusion of this factor only reduced BSV in oral CL by 6% and BSV in oral V by 4%, so it had a weak effect overall and may be an incidental finding.
The standardized estimate of oral V (372 L/70 kg) was similar to that reported by Forrest et al.37 in adult patients with normal renal function (321 L/1.73 m2), but higher than the estimate of volume of distribution at steady state (Vss) obtained by Rajagopalan and Gastonguay17 in paediatric patients (240 L/70 kg when corrected for bioavailability). Individual estimates of oral V ranged from 2 to 14 L/kg and the median of 4.5 L/kg was more than twice the values of ∼2 L/kg obtained by Schaefer18 and Payen16. Overall, these results indicate that oral V is elevated in patients with malnutrition. Similar results have been observed with gentamicin in malnourished children—both higher mean and a wide interpatient variability.10 As with oral CL, a small but significant relationship between oral V and sodium concentration was identified, with higher sodium concentrations being associated with larger estimates of oral V.
For fluoroquinolones in general, the ratio of the daily AUC to the MIC (AUC/MIC) is likely to be the pharmacodynamic criterion most predictive of clinical outcome.31 Forrest et al.31 found that the probability of clinical cure in a group of seriously ill patients was 80% if the AUC/MIC was ≥125, but was only 42% below this value and that faster eradication rates occurred if the AUC/MIC was >250. The median estimate of steady-state AUC0–12 in the present study (11.2 mg·h/L) is lower than the mean values of ∼13–19 mg·h/L reported by Lipman et al.48 following intravenous doses of 10 mg/kg twice daily to paediatric patients with sepsis. These differences probably reflect absorption, since correcting for a bioavailability of 70% yields similar results to the present study (predicted oral AUC0–12 range 9–13 mg·h/L). Even with the higher AUC, Lipman et al. recommended increasing the dose to 10 mg/kg 8 hourly to obtain AUC/MIC values of 100–150 for organisms with MICs >0.3 mg/L.45 Estimates of AUC0–24 in the present study ranged from 8 to 61 mg·h/L. The individual results indicated that only 12% of the study patients would achieve an AUC/MIC ≥125 if the MIC of the organism was 0.3 mg/L and 0% if it was 0.5 mg/L. Monte Carlo simulations produced slightly higher results (24% and 2%, respectively). Using a range of MIC values, it was found that a dose of ≥30 mg/kg per day would be required to achieve an AUC/MIC of ≥125 in >90% of patients if the MIC was 0.125 mg/L and that an MIC of <0.06 mg/L was necessary to achieve satisfactory AUC/MIC values with a dose of 20 mg/kg/day. Using internationally derived distributions of the MICs of a range of organisms, including the most common isolates in children with severe malnutrition complicated by invasive bacterial disease, a dose of 20 mg/kg/day was sufficient for most isolates of E. coli and Salmonella spp., but 30 mg/kg/day would be necessary to treat Klebsiella infections. Even with higher doses of 45 mg/kg/day, targets for P. aeruginosa could only be achieved in 64% of cases.
Similar problems of underdosing have also been identified in adults. Standard intravenous doses of 400 mg twice daily yielded inadequate AUC/MIC and Cmax/MIC ratios in critically ill patients unless the MIC was <0.25 mg/L.36 Simulations conducted by Montgomery et al.35 demonstrated that for an MIC of 0.5 mg/L, 400 mg 12 hourly would only achieve an AUC/MIC ≥125 in 15% of adults with cystic fibrosis and that an increase to 600 mg 8 hourly would be required to achieve >90% success. If the dose used in the present study was increased to 15 mg/kg 8 hourly and assuming no change in bioavailability, the probability of achieving an AUC/MIC ≥125 would increase to 83% for an MIC of 0.3 mg/L and 32% for an MIC of 0.5 mg/L. However, 37% of patients would then reach daily AUCs >60 mg·h/L. These values are higher than the mean daily AUCs reported following the administration of intravenous high-dose ciprofloxacin (400 mg 8 hourly) to critically ill adults48 and it is possible that such high exposure would increase the risk of toxicity in such patients.
Conclusions
The pharmacokinetics of oral ciprofloxacin in children with severe malnutrition is influenced by weight, serum sodium concentration and the risk of mortality, but there was high variability in the clearance and rate of absorption. Oral ciprofloxacin absorption was unaffected by the simultaneous administration of nutritional feeds. An oral dose of 10 mg/kg twice daily should be effective against E. coli and Salmonella spp., but a higher dose of 10 mg/kg three times a day would be recommended for K. pneumoniae. Oral ciprofloxacin is unlikely to be an effective treatment for P. aeruginosa. Irrespective of the bacterial pathogen, patients with severe illness, at high risk of mortality, should initially receive intravenous antibiotics.
Funding
This study was supported by Wellcome Trust core funding to KEMRI-Wellcome Trust Research Programme (grant Reference No. 077092). Nahashon Thuo is supported by a Wellcome Trust Masters Training Fellowship (grant reference No. 089353/Z/09/Z). The funding sources had no role in study design, analysis or in the writing of the report.
Transparency declarations
None to declare.
Acknowledgements
This paper is published with the permission of the Director of the Kenya Medical Research Institute (KEMRI). We would like to thank the parents and children who participated in the study, the hospital superintendent, clinicians, nursing staff and field workers of Kilifi District Hospital. We would also like to thank Dr Susan Morpeth, Consultant Microbiologist at KEMRI-Wellcome Trust Research Programme, Kilifi for helpful comments upon an early draft. This study was presented in part as a poster at the PAGE meeting in Athens, Greece, 2011 (www.page-meeting.org/?abstract=2080).
References
- 1.Schofield C, Ashworth A. Why have mortality rates for severe malnutrition remained so high? Bull WHO. 1996;74:223–9. [PMC free article] [PubMed] [Google Scholar]
- 2.Ashworth A, Chopra M, McCoy D, et al. WHO guidelines for management of severe malnutrition in rural South African hospitals: effect on case fatality and the influence of operational factors. Lancet. 2004;363:1110–5. doi: 10.1016/S0140-6736(04)15894-7. doi:10.1016/S0140-6736(04)15894-7. [DOI] [PubMed] [Google Scholar]
- 3.Deen JL, Funk M, Guevara VC, et al. Implementation of WHO guidelines on management of severe malnutrition in hospitals in Africa. Bull WHO. 2003;81:237–43. [PMC free article] [PubMed] [Google Scholar]
- 4.Maitland K, Berkley JA, Shebbe M, et al. Children with severe malnutrition: can those at highest risk of death be identified with the WHO protocol? PLoS Medicine. 2006;3:e500. doi: 10.1371/journal.pmed.0030500. doi:10.1371/journal.pmed.0030500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachou H, Tylleskär T, Kaddu-Mulindwa DH, et al. Bacteraemia among severely malnourished children infected and uninfected with the human immunodeficiency virus-1 in Kampala, Uganda. BMC Infect Dis. 2006;6:160. doi: 10.1186/1471-2334-6-160. doi:10.1186/1471-2334-6-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babirekere-Iriso E, Musoke P, Kekitiinwa A. Bacteraemia in severely malnourished children in an HIV-endemic setting. Ann Trop Paediatrics. 2006;26:319–28. doi: 10.1179/146532806X152845. doi:10.1179/146532806X152845. [DOI] [PubMed] [Google Scholar]
- 7.Welsh FK, Farmery SM, MacLennan K, et al. Gut barrier function in malnourished patients. Gut. 1998;42:396–401. doi: 10.1136/gut.42.3.396. doi:10.1136/gut.42.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brewster DR, Manary MJ, Menzies IS, et al. Intestinal permeability in kwashiorkor. Arch Dis Childhood. 1997;76:236–41. doi: 10.1136/adc.76.3.236. doi:10.1136/adc.76.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Management of Severe Malnutrition: A Manual for Physicians and Other Senior Health Workers. Geneva: WHO; 1999. [Google Scholar]
- 10.Seaton C, Ignas J, Muchohi S, et al. Population pharmacokinetics of a single daily intramuscular dose of gentamicin in children with severe malnutrition. J Antimicrob Chemother. 2007;59:681–9. doi: 10.1093/jac/dkl561. doi:10.1093/jac/dkl561. [DOI] [PubMed] [Google Scholar]
- 11.Cruciani M, Bassetti D. The fluoroquinolones as treatment for infections caused by Gram-positive bacteria. J Antimicrob Chemother. 1994;33:403–17. doi: 10.1093/jac/33.3.403. doi:10.1093/jac/33.3.403. [DOI] [PubMed] [Google Scholar]
- 12.Burkhardt JE, Hill MA, Carlton WW, et al. Histologic and histochemical changes in articular cartilages of immature beagle dogs dosed with difloxacin, a fluoroquinolone. Vet Pathol. 1990;27:162–70. doi: 10.1177/030098589002700303. doi:10.1177/030098589002700303. [DOI] [PubMed] [Google Scholar]
- 13.Committee on Infectious Diseases. The use of systemic fluoroquinolones. Pediatrics. 2006;118:1287–92. doi: 10.1542/peds.2006-1722. doi:10.1542/peds.2006-1722. [DOI] [PubMed] [Google Scholar]
- 14.Paediatric Formulary Committee. British National Formulary for Children. 2009 edn. London: British Medical Association, the Royal Pharmaceutical Society of Great Britain, the Royal College of Paediatrics and Child Health, and the Neonatal and Paediatric Pharmacists Group; 2009. [Google Scholar]
- 15.Drusano GL, Standiford HC, Plaisance K, et al. Absolute oral bioavailability of ciprofloxacin. Antimicrob Agents Chemother. 1986;30:444–6. doi: 10.1128/aac.30.3.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Payen S, Serreau R, Munck A, et al. Population pharmacokinetics of ciprofloxacin in pediatric and adolescent patients with acute infections. Antimicrob Agents Chemother. 2003;47:3170–8. doi: 10.1128/AAC.47.10.3170-3178.2003. doi:10.1128/AAC.47.10.3170-3178.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajagopalan P, Gastonguay MR. Population pharmacokinetics of ciprofloxacin in pediatric patients. J Clin Pharmacol. 2003;43:698–710. [PubMed] [Google Scholar]
- 18.Schaefer H, Stass H, Wedgwood J, et al. Pharmacokinetics of ciprofloxacin in pediatric cystic fibrosis patients. Antimicrob Agents Chemother. 1996;40:29–34. doi: 10.1128/aac.40.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peltola H, Väärälä M, Renkonen OV, et al. Pharmacokinetics of single-dose oral ciprofloxacin in infants and small children. Antimicrob Agents Chemother. 1992;36:1086–90. doi: 10.1128/aac.36.5.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muchohi SN, Thuo N, Karisa J, et al. Determination of ciprofloxacin in human plasma using high-performance liquid chromatography coupled with fluorescence detection: application to a population pharmacokinetics study in children with severe malnutrition. J Chromatog B, Analyt Technol Biomed Life Sci. 2011;879:146–52. doi: 10.1016/j.jchromb.2010.11.032. doi:10.1016/j.jchromb.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beal SL, Sheiner LB, Boeckmann AJ. NONMEM Users Guides (1989–2006) Ellicott City: Icon Development Solutions; [Google Scholar]
- 22.Jonsson EN, Karlsson MO. Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comp Method Prog Biomed. 1999;58:51–64. doi: 10.1016/s0169-2607(98)00067-4. doi:10.1016/S0169-2607(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 23.Venables WN, Smith DM. R version 2.9.2. The R Foundation for Statistical Computing. 2009. R Development Core Team. http://cran.r-project.org. (7 July 2010, date last accessed).
- 24.Savic RM, Jonker DM, Kerbusch T, et al. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodynam. 2007;34:711–26. doi: 10.1007/s10928-007-9066-0. doi:10.1007/s10928-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 25.Holford NH. A size standard for pharmacokinetics. Clin Pharmacokinet. 1996;30:329–32. doi: 10.2165/00003088-199630050-00001. doi:10.2165/00003088-199630050-00001. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz GJ, Feld LG, Langford DJ. A simple estimate of glomerular filtration rate in full-term infants during the first year of life. J Pediatrics. 1984;104:849–54. doi: 10.1016/s0022-3476(84)80479-5. doi:10.1016/S0022-3476(84)80479-5. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz GJ, Gauthier B. A simple estimate of glomerular filtration rate in adolescent boys. J Pediatrics. 1985;106:522–6. doi: 10.1016/s0022-3476(85)80697-1. doi:10.1016/S0022-3476(85)80697-1. [DOI] [PubMed] [Google Scholar]
- 28.Lindbom L, Ribbing J, Jonsson EN. Perl-speaks-NONMEM (PsN)—a Perl module for NONMEM related programming. Comp Method Prog Biomed. 2004;75:85–94. doi: 10.1016/j.cmpb.2003.11.003. doi:10.1016/j.cmpb.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Wallin JR, Bergstrand M, Karlsson MO, et al. Internal and external validation with sparse, adaptive-design data for evaluating the predictive performance of tacrolimus. Abstracts of the Annual Meeting of the Population Approach Group in Europe; Berlin, 2010. PAGE 19 (2010) Abstr 1851. www.page-meeting.org/?abstract=1851. (10 January 2011, date last accessed) [Google Scholar]
- 30.Brendel K, Comets E, Laffont C, et al. Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm Res. 2006;23:2036–49. doi: 10.1007/s11095-006-9067-5. doi:10.1007/s11095-006-9067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forrest A, Nix DE, Ballow CH, et al. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother. 1993;37:1073–81. doi: 10.1128/aac.37.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.European Committee on Antimicrobial Susceptibility Testing. EUCAST MIC- and Zone Diameter Breakpoint Tables. 2011. http://www.eucast.org/eucast_disk_diffusion_test/breakpoints/ (10 January 2011, date last accessed). [Google Scholar]
- 33.Peltola H, Ukkonen P, Saxén H, et al. Single-dose and steady-state pharmacokinetics of a new oral suspension of ciprofloxacin in children. Pediatrics. 1998;101:658–62. doi: 10.1542/peds.101.4.658. doi:10.1542/peds.101.4.658. [DOI] [PubMed] [Google Scholar]
- 34.MacGowan A, Rogers C, Bowker K. The use of in vitro pharmacodynamic models of infection to optimize fluoroquinolone dosing regimens. J Antimicrob Chemother. 2000;46:163–70. doi: 10.1093/jac/46.2.163. doi:10.1093/jac/46.2.163. [DOI] [PubMed] [Google Scholar]
- 35.Montgomery MJ, Beringer PM, Aminimanizani A, et al. Population pharmacokinetics and use of Monte Carlo simulation to evaluate currently recommended dosing regimens of ciprofloxacin in adult patients with cystic fibrosis. Antimicrob Agents Chemother. 2001;45:3468–73. doi: 10.1128/AAC.45.12.3468-3473.2001. doi:10.1128/AAC.45.12.3468-3473.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Zanten ARH, Polderman KH, van Geijlswijk IM, et al. Ciprofloxacin pharmacokinetics in critically ill patients: a prospective cohort study. J Crit Care. 2008;23:422–30. doi: 10.1016/j.jcrc.2007.11.011. doi:10.1016/j.jcrc.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 37.Forrest A, Weir M, Plaisance KI, et al. Relationships between renal function and disposition of oral ciprofloxacin. Antimicrob Agents Chemother. 1988;32:1537–40. doi: 10.1128/aac.32.10.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gasser TC, Ebert SC, Graversen PH, et al. Ciprofloxacin pharmacokinetics in patients with normal and impaired renal function. Antimicrob Agents Chemother. 1987;31:709–12. doi: 10.1128/aac.31.5.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plaisance KI, Drusano GL, Forrest A, et al. Effect of renal function on the bioavailability of ciprofloxacin. Antimicrob Agents Chemother. 1990;34:1031–4. doi: 10.1128/aac.34.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubio TT, Miles MV, Lettieri JT, et al. Pharmacokinetic disposition of sequential intravenous/oral ciprofloxacin in pediatric cystic fibrosis patients with acute pulmonary exacerbation. Pediatr Infect Dis J. 1997;16:112–7. doi: 10.1097/00006454-199701000-00033. discussion 123–6 doi:10.1097/00006454-199701000-00033. [DOI] [PubMed] [Google Scholar]
- 41.Frost RW, Lasseter KC, Noe AJ, et al. Effects of aluminum hydroxide and calcium carbonate antacids on the bioavailability of ciprofloxacin. Antimicrob Agents Chemother. 1992;36:830–2. doi: 10.1128/aac.36.4.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neuvonen PJ, Kivistö KT, Lehto P. Interference of dairy products with the absorption of ciprofloxacin. Clin Pharmacol Ther. 1991;50:498–502. doi: 10.1038/clpt.1991.174. doi:10.1038/clpt.1991.174. [DOI] [PubMed] [Google Scholar]
- 43.Nix DE, Watson WA, Lener ME, et al. Effects of aluminum and magnesium antacids and ranitidine on the absorption of ciprofloxacin. Clin Pharmacol Ther. 1989;46:700–5. doi: 10.1038/clpt.1989.207. doi:10.1038/clpt.1989.207. [DOI] [PubMed] [Google Scholar]
- 44.Drusano GL, Weir M, Forrest A, et al. Pharmacokinetics of intravenously administered ciprofloxacin in patients with various degrees of renal function. Antimicrob Agents Chemother. 1987;31:860–4. doi: 10.1128/aac.31.6.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lipman J, Gous AGS, Mathivha LR, et al. Ciprofloxacin pharmacokinetic profiles in paediatric sepsis: how much ciprofloxacin is enough? Intens Care Med. 2002;28:493–500. doi: 10.1007/s00134-002-1212-y. doi:10.1007/s00134-002-1212-y. [DOI] [PubMed] [Google Scholar]
- 46.Alleyne GA. The effect of severe protein calorie malnutrition on the renal function of Jamaican children. Pediatrics. 1967;39:400–11. [PubMed] [Google Scholar]
- 47.Klahr S, Alleyne GA. Effects of chronic protein-calorie malnutrition on the kidney. Kidney Int. 1973;3:129–41. doi: 10.1038/ki.1973.21. doi:10.1038/ki.1973.21. [DOI] [PubMed] [Google Scholar]
- 48.Lipman J, Scribante J, Gous AG, et al. Pharmacokinetic profiles of high-dose intravenous ciprofloxacin in severe sepsis. The Baragwanath Ciprofloxacin Study Group. Antimicrob Agents Chemother. 1998;42:2235–9. doi: 10.1128/aac.42.9.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]