Abstract
Purpose
To assess associations of soluble IL-2 receptor alpha (sIL-2rα) concentration with outcomes in pediatric acute myeloid leukemia in a phase 3 trial of IL-2 therapy.
Procedures
We randomized 289 children with AML in first remission after intensive chemotherapy to receive IL-2 infused on days 0-3 and 8-17 (IL-2 group) or no further therapy (AML control group). We measured sequential serum sIL-2rα concentrations in both groups before, during and after therapy in both groups and in reference controls without AML.
Results
Before treatment, mean sIL-2rα concentrations were similar in the IL-2 group and AML controls, but significantly higher than in reference controls. Both AML groups experienced reduction in sIL-2rα concentration after chemotherapy. Thereafter in the IL-2 group, mean sIL-2rα concentration increased from 2669 pg/ml before IL-2 to 15,534 pg/ml on day 4 (p<0.001) and 10,585 pg/ml on day 18 (p<0.001). In the control group sIL-2rα concentration did not change after 28 days of follow-up. Five-year disease-free survival (DFS) was 51% in the IL-2 group and 58% in the controls (p=0.489) and overall survival was 70% and 73% respectively (p=0.727).
Conclusion
SIL-2r α concentration was elevated in AML at diagnosis and tended to normalize after chemotherapy. IL-2 infusion significantly increased sIL-2rα concentration, but did not improve DFS or survival in pediatric AML. Furthermore, sIL-2rα concentration was not predictive of outcome before, during or after treatment for AML.
Keywords: acute myeloid leukemia, interleukin-2, soluble interleukin-2 receptor alpha
INTRODUCTION
The graft vs. leukemia effect of allogeneic hematopoietic stem cell transplantation in acute myeloid leukemia (AML) is mediated by the immune system.1-3 IL-2 has substantial anti-tumor activity in several cancers in which the immune system contributes to disease control.4-8 In AML, IL-2 can stimulate proliferation of antigen-specific T-cells, can enhance cytolytic activity of natural killer cells against AML targets, and induces activated lymphocytes to release interferon gamma and tumor necrosis factor alpha.4 Responses to IL-2 are associated with release of the α chain of the tri-molecular αβγ high affinity IL-2 receptor from the surface of responding lymphocytes.9-12 The serum concentration of soluble α chain reflects the extent of in vivo lymphocyte activation by IL-2 and in most cases elevated sIL-2rα is associated with poor prognosis.9,10.
These biologic activities of IL-2 led to many clinical trials of IL-2 therapy in AML and other cancers. Uncontrolled trials of single agent IL-2 with or without autologous stem cell transplantation (ASCT) sometimes reduced tumor burden, occasionally induced remissions and rarely achieved long-term survival13-15. While contribution of IL-2 in these uncontrolled studies was not clear, historically controlled studies of IL-2 therapy in adults with AML in remission after chemotherapy or ASCT suggested improvements in outcomes.16-21 More recent randomized trials in adults with AML have not found any clinical benefits.22-23 However, none of these trials systematically documented the in vivo immunological activation resulting from IL-2 administration.
The Children’s Cancer Group (CCG) conducted a pilot study that demonstrated feasibility of IL-2 infusion following completion of chemotherapy in children in first remission AML and showed correlative increases in sIL-2rα concentration and NK cell numbers.24 The subsequent Phase 3 trial for newly diagnosed AML patients (CCG-2961),24 randomized patients in remission after 3 courses of chemotherapy to IL-2 therapy or observation. Serial sIL-2rα concentrations were measured from before treatment, after chemotherapy and after IL-2 or follow-up.
METHODS
Subjects
CCG-2961 (ClinicalTrials.gov NCT0002798, Combination Chemotherapy With or Without Bone Marrow Transplantation in Treating Children With Acute Myelogenous Leukemia or Myelodysplastic Syndrome) was a phase 3 trial for previously untreated AML.25 Eligible patients were age one day to less than 21 years with French-American and -British (FAB) AML subtypes M0-M2 and M4-M7.26 CCG-2961 opened in August 1996 and closed in December 2002. Institutional Review Boards approved the study. Written informed consent was required. CCG-B972 was a correlative biology study that provided for acquisition and banking of serum specimens for sIL-2rα assessment from these same patients. Participation in CCG-B972 was not required for participation in the CCG-2961 study.
Four groups served as non-AML reference controls for the sIL-2rα assay: 55 healthy adults described in the manufacturer’s brochure of the DuoSet ELISA Development Kit, R&D Systems, Minneapolis, MN; 15 healthy adult volunteers; 14 adult patients with malignant melanoma prior to receiving immunotherapy; and 36 children with neuroblastoma 56-100 days following autologous HSCT.
IL-2 Treatment and sIL-2rα Specimen Processing
Figure 1 shows the treatment schema, and supplementary figure 1 is the Consort diagram showing enrolled patients. After completion of chemotherapy, patients in remission were invited to participate in randomization to IL-2 therapy or observation. IL-2 randomization was performed centrally using fixed block allocation. The IL-2 group received IL-2 at 9 × 106 IU/m2/day by continuous infusion (CI) on days 0-3 and 1.6 × 106 IU/m2/day CI on days 8-17 as previously described (Inset, Figure 1).23,15 Chiron, Inc. provided IL-2 to the National Cancer Institute, which distributed it to institutions. Serum samples were obtained at study entry and following completion of all chemotherapy and in the IL-2 group on days 4 and 18 of IL-2 of infusion therapy and in the control group, on days 0 and 28 of follow-up.
Figure 1.
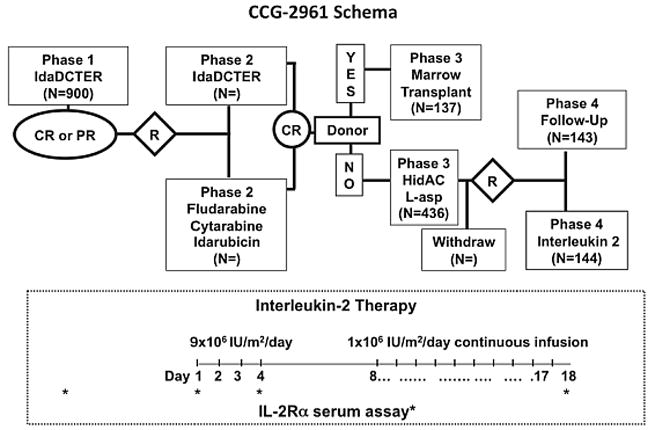
Treatment schema for CCG-2961 and schedule of interleukin-2 (IL-2) infusions (inset) and sampling for serum IL-2 receptor (block arrows). IdaCTER is idarubicin, cytarabine, thioguanaine, etoposide, and rubidomycin (daunorubicin), HidAC is High dose Ara-C, L-asp is L-asparaginase and R is randomization.
Figure 1 inset shows the timing of sIL-2rα specimen acquisition in relation to IL-2 administration. Specimens stripped of identifiers were shipped overnight to the COG Immunology Reference Laboratory at the University of Wisconsin. Clinical data were sent to the COG Operations office. Samples were frozen at -20° C. For analysis all samples were thawed and spun down. One ml of supernatant was preserved with 1 μL 1% thimerosal at 4° C. Samples designated as “on study”, “pre IL-2 Day 0”, “no IL-2 Day 0”, and “no IL-2 day 28” were diluted 1/5; samples labeled “IL-2 Day 4”, and “IL-2 Day 18” were diluted 1/20 to fit in the optimal assay range (0-2000 pg/ml) according to manufacturer’s specifications for the sIL-2rα enzyme-linked immunosorbent assay (ELISA) (DuoSet ELISA Development Kit, R&D Systems, Minneapolis, MN). Standards and serum samples diluted in PBS/BSA and 100 μL aliquots were added in duplicates to micro wells coated with mouse anti-human IL-2rα antibody in PBS overnight at room temperature, washed with PBS, blocked with PBS + 1% BSA for 3 hrs at room temperature, rewashed with PBS + 0.05% Tween-20, incubated overnight at 4°C and rewashed with PBS/Tween. Biotinylated-goat anti-human IL-2Rα antibody was added. Wells were incubated for 3 hrs at room temperature on an orbital shaker. After washing, streptavidin-peroxidase (HRP) was added for 20 minutes. Wells were washed, and tetramethylbenzidine (TMB) substrate was added to initiate the enzymatic reaction. Color development was stopped after 10 minutes with 2N H2SO4. Microplates were evaluated at 450 nm (with a 570 nm reference) using a Spectra microelisa reader (TECAN, Austria). Soluble IL-2rα values were calculated against a standard curve based on a NS0-expressed recombinant human IL-2rα protein provided in the kit and with WinSelect data processing software. Results were sent to the COG statistician.
Statistics and Analysis
Demographic and clinical variables collected were age, gender, ethnicity, white blood cell count at diagnosis, cytogenetic subset, day 14 marrow response, availability of a matched familial marrow donor, and randomized regimen in phases 2 and 4 (Figure 1). Outcome measures include disease-free survival (DFS) and overall survival (OS) defined as follows: DFS, time from the end of phase 3 to relapse or death; and OS, time from study entry to death. The study was powered to show a 10% difference in DFS between IL-2 and control groups. The Kaplan-Meier method27 was used to calculate estimates of OS and DFS. Differences between groups of patients were tested for significance using the log-rank statistic.28 Toxicities from primary toxicity categories are reported from phase 4 (IL-2 phase) as rates.
Children lost to follow-up were censored at date of last known contact or at a cutoff 6 months before October 2006. Patients who withdrew were followed for events and survival. This report analyzes data collected up to October 30, 2006 with a median follow-up of 56 months. All analyses are based on intention-to treat. A p-value of < 0.05 was set as a threshold for significance.
On-study AML sIL-2rα values were compared with single specimens from non-AML reference controls. AML patients provided specimens for sIL-2rα analyses at one or more time points. Correlations of on-study sIL-2rα concentration with outcome and correlations of the serial post-treatment specimens with outcome for randomized patients were analyzed. Mean values of sIL-2rα concentration were compared using an unpaired t-test; median values, using the Mann-Whitney test.29 All authors had access to the complete data sets for phase 4 of the CCG-2961 and for the B972 studies.
RESULTS
Outcomes of IL-2 Randomization
Table I shows demographic and clinical characteristics and outcomes of the eligible patients. At the end of Phase 3, of 385 eligible patients, 96 (25%) withdrew from the study, 144 were randomized to the IL-2 group and 145 to the control group. Those who withdrew were significantly older and experienced significantly inferior OS (Table I). There was a significantly higher proportion of males randomized to the IL-2 group. At five years DFS is 51% + 9% in the IL-2 group and 58% + 8% in the control group (p value=0.489) (Figure 2). OS is also not different for the IL-2 and control groups, 70% ± 8% and 73% ± 8% respectively (Figure 2)(p value=0.727). There is no trend for better or worse outcomes among those who received fludarabine/ara-C/idarubicin or the 5 drug IDA-DCTER regimens in Phase 2 in either the IL-2 or control arms of the study (Figure 3) (p value = 0.709).
Table I.
Clinical characteristics and outcomes of patients eligible for phase 4 randomization
| Time | Phase 4 Randomization | None | P value | |||||
|---|---|---|---|---|---|---|---|---|
| Group | A | B | C | |||||
| IL-2 | No IL-2 | Withdrawal | A vs. B | AB vs. C | ||||
| N | % | N | % | N | % | |||
| 144 | 37% | 145 | 38% | 96 | 25% | |||
| Variable | ||||||||
| Age (yrs) | ||||||||
| Median | 6.9 | 7.6 | 11.0 | 0.729 | 0.008 | |||
| (Range) | (0.04-17.9) | (0.01-19) | (0.11-19.8) | |||||
|
| ||||||||
| Male Gender | 89 | 62% | 67 | 46% | 45 | 47% | 0.011 | 0.276 |
|
| ||||||||
| Ethnicity | ||||||||
| White | 92 | 67% | 96 | 66% | 59 | 61% | 0.965 | 0.447 |
| Black | 10 | 7% | 16 | 11% | 16 | 17% | 0.370 | 0.067 |
| Asian | 24 | 17% | 24 | 17% | 15 | 16% | 0.976 | 0.885 |
| Hispanic | 7 | 5% | 0 | 0% | 2 | 2% | 0.006 | 0.864 |
| Other | 5 | 4% | 9 | 6% | 4 | 4% | 0.467 | 0.974 |
| Unknown | 6 | 0 | 0 | |||||
|
| ||||||||
| WBC×109/L | ||||||||
| Median | 22.5 | 17.6 | 20 | 0.126 | 0.686 | |||
| Range | (1.0-860) | (0.8-378) | (0.7-269) | |||||
|
| ||||||||
| Karyotype | ||||||||
| Favorable | 26 | 29% | 26 | 28% | 16 | 29% | 0.945 | 0.924 |
| Standard | 64 | 70% | 61 | 69% | 41 | 70% | 0.921 | 0.876 |
| Unfavorable | 1 | 1% | 2 | 2% | 2 | 1% | 0.619 | 0.599 |
| Unknown | 53 | 56 | 37 | |||||
|
| ||||||||
| DFS±2SD | 53% ± 9% | 58% ± 8% | 48% ± 11% | 0.489 | 0.230 | |||
| OS±2SD | 70% ± 8% | 73% ± 8% | 56% ± 11% | 0.727 | 0.021 | |||
Legend: Favorable karyotypes are t(8;21), inv(16) or t(16;16); unfavorable, del(7), 7q-, del (5), 5q-and complex (≥3 abnormalities); and standard, all others. P values compare the two randomized groups (A vs. B) and both randomized groups to the withdrawal group (AB vs. C).
Figure 2.
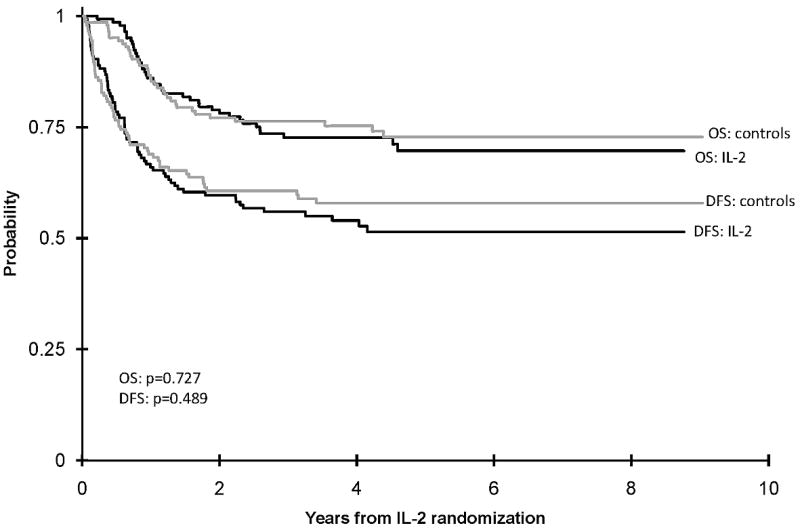
Actuarial five-year disease-free survival and overall survival of randomized patients.
Figure 3.
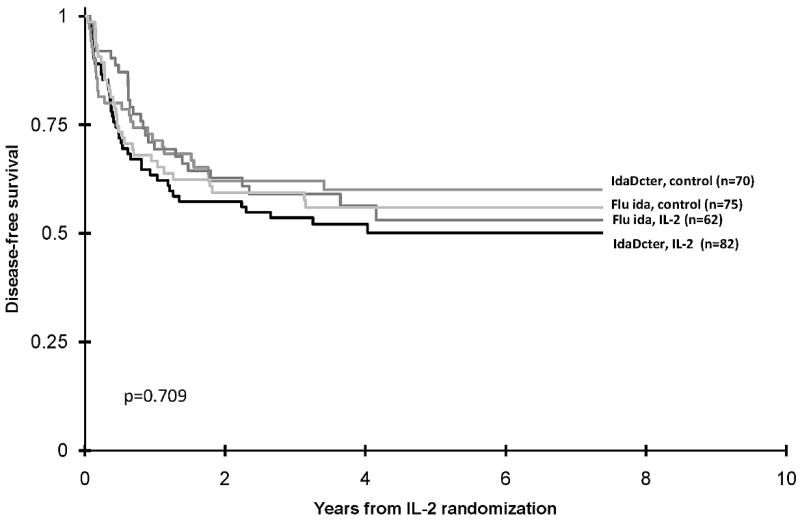
Actuarial disease-free survival of Interleukin-2 group and control group according to Phase 2 randomization to IDADCTER (idarubicin, cytarabine, thioguanine, etoposide, and daunorubicin) and FLU IDA (fludarabine monophosphate, cytarabine and idarubicin)
Among the 144 patients in the IL-2 group, 28% experienced grade 3 toxicity and 14% grade 4 toxicity. The most common toxicity was blood pressure changes that were not otherwise classified. Fifteen percent had hepatic toxicity, most often enzyme elevation. In contrast to the CCG-2941 pilot study,21 fever and rash were not noted in this study which did not capture grade 1 or 2 toxicity. One patient discontinued therapy after the high-dose IL-2 infusion. ICU admissions were rare.
Sequential Changes in sIL-2ra Concentration
Table II compares the on-study sIL-2rα concentrations for 134 AML patients on CCG-2961 who contributed on-study specimens to those of four non-AML reference control groups. Mean on-study sIL-2Rα concentrations of the AML patients were notably higher than the reported range of values from the healthy volunteers provided by the manufacturer and significantly higher than the values obtained in the reference laboratory from healthy adults, melanoma patients prior to immunotherapy, and neuroblastoma patients 56-100 days following AHSCT.
Table II.
a Comparison of serum sIL-2Rrα concentration of children with AML with those of healthy adults and children and adults with other cancers
| Group | AML Patients On study | non AML Controls Reference | P Value |
|---|---|---|---|
| AML vs. Reference | |||
| N | 134 | 55 | |
| Mean sIL-2Rα2 | 3668 | 1055 | * |
| Median | 2732 | na | * |
| Range | 239-11279 | 458-1997 | |
| Std Dev | 2736 | na | |
| Std Err | 236 | na | |
| Volunteers | AML vs. Volunteers | ||
| N | 15 | ||
| Mean sIL-2Rα | 826 | <0.001 | |
| Median | 739 | <0.001 | |
| Range | 294-1712 | ||
| Std Dev | 395 | ||
| Std Err | 102 | ||
| Melanoma | AML vs. Melanoma | ||
| N | 14 | ||
| Mean sIL-2Rα | 941 | <0.001 | |
| Median | 884 | <0.001 | |
| Range | 628-2173 | ||
| Std Dev | 379 | ||
| Std Err | 101 | ||
| Neuroblastoma | AML vs. Neuroblastoma | ||
| N | 36 | ||
| Mean sIL-2Rα | 1772 | <0.001 | |
| Median | 1660 | <0.001 | |
| Range | 759-3415 | ||
| Std Dev | 727 | ||
| Std Err | 121 | ||
| ALL non-AML | AML vs. non AML | ||
| N | 65 | ||
| Mean sIL-2Rα | 1376 | <0.001 | |
| Median | 1133 | <0.001 | |
| Range | 294-3415 | ||
| Std Dev | 744 | ||
| Std Err | 92 |
Table IIa Legend: On study sIL-2rα values were obtained for 134 AML patients in this CCG2961 study. These are compared to values for the 4 indicated non-AML control groups.
Because the manufacturer did not provide median, standard deviation or standard error, the statistical comparisons do not include comparisons for the 55 healthy controls.
Table III shows the serial changes in the sIL-2rα concentration in AML patients following chemotherapy and before, during and after IL-2 in the IL-2 group or at comparable times in the control group. Of the 289 patients randomized to IL-2 or control, 64 provided pre-chemotherapy serum samples, and 80 provided samples at the later time points. There were no differences in mean on-study sIL-2Rα concentrations between the 64 AML patients who provided on study serum that participated in the Phase 4 randomization and the 70 AML patients that provided on study serum samples who did not participate (p=0.784). Of the 64 randomized patients with on study specimens, the 34 patients randomized to IL-2 showed no difference in on study sIL-2Rα concentrations compared to the 30 randomized to observation (Table III, Time 1). There was also no significant difference in mean sIL-2Rα concentration between the 2 groups after 3 courses of chemotherapy (Table III, Time 2). However, comparison of pre- and post- chemotherapy samples within each group showed significant reduction in median sIL-2Rα concentration (Figure 4, Time 1 versus Time 2 ) (p=0.001): the elevated sIL-2Rα before treatment (time 1) was attenuated by 3 courses of chemotherapy (time 2). Of note, sIL-2Rα concentration of the AML patients after chemotherapy are similar to those of the treated neuroblastoma patients (Tables II and III).
Table III.
Sequential sIL-2rα values for AML patients randomized to IL-2 group or control group
| N | Mean sIL-2Rα | Median sIL-2Rα | Range | p-value (test of means) | ||
|---|---|---|---|---|---|---|
| On-study | ||||||
| Time 1 | IL-2 | 34 | 3342 | 2447 | 475-8691 | 0.439 |
| Control | 30 | 3891 | 2221 | 524-11279 | ||
| Post chemo | ||||||
| Time 2 | IL-2: Day 0 F/U | 39 | 2669 | 1353 | 364-30567 | 0.276 |
| Control: Pre-IL-2 | 30 | 1594 | 1440 | 547-3630 | ||
| Time 3 | IL-2: Day 4 | 39 | 15534 | 13659 | 4125-40253 | - |
| Time 4 | IL-2: Day 18 | 38 | 10585 | 8368 | 773-32310 | <0.001 |
| Control: Day 28 | 31 | 1713 | 1586 | 699 -3966 |
Figure 4.

Comparison of sequential changes in median concentration of serum soluble IL-2 receptor alpha over time in the IL-2 group and control group. Time 1 is on study); time 2 is post chemotherapy (pre-IL-2 for IL-2 group and day 0 for control); time 3 = day 4 of IL-2 for IL-2 group; Time 4 is day 18 of IL-2 for IL-2 group and d 28 follow-up for controls.
In the IL-2 group during phase 4, the mean sIL-2R α concentration increased from 2669 pg/ml on day 0 to 15,534 pg/ml on day 4 (p<0.001) and 10,585 pg/ml on day 18 (p<0.001 whereas in the control group sIL-2Rα did not change between days 0 and 28 (p=0.521). These data confirm that administration of IL-2 caused significant induction of lymphocyte activity. The higher sIL-2Rα concentration on day 4 than day 18 in the IL-2 group is consistent with this dose-dependent activation. Furthermore, the striking differences seen at Time 4 between these 2 groups (Figure 4, p-value <0.001) confirms that the IL-2 group of patients did receive the IL-2, and that despite their prior aggressive multi-agent chemotherapy, their lymphocytes were able to respond to the IL-2.
Within the group of 134 patients with on study sIL-2Rα data, 4 quartiles were defined based on sIL-2Rα concentration. No significant differences in five-year OS or DFS were seen between the four quartiles. A similar quartile analysis was done based on sIL-2Rα data from each time point for both the IL-2 and control groups (Table IV). We saw no significant correlation between OS and the higher or lower quartiles of sIL-2α concentration for either IL-2 treatment or control groups, at any time. Given the fact that these analyses were not corrected for multiple comparisons, we saw no suggestion of any trend (despite occasional p values < 0.05) for correlation between DFS and the higher or lower quartiles of sIL-2α concentration for either IL-2 treatment or control groups at any time.
Table IV.
Phase 4 outcomes according to quartile of sIL-2rα values for the IL-2 and Control Groups.
| 5 Yr Disease-free Survival | 5-Year Overall Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| P Value | P Value | |||||||
| Group IL-2 | N | % | % ± 2SE% | Within IL-2 | IL-2 vs. Control | % ± 2SE% | Within IL-2 | IL-2 vs. Control |
| Pre IL-2 | ||||||||
| All | 39 | 27 | 57±17 | ns | 84±12 | ns | ||
| Quartile 1 | 10 | 26 | 40±39 | ns | ns | 79±27 | ns | ns |
| Quartile 2 | 10 | 26 | 70±29 | ns | ns | 90±19 | ns | ns |
| Quartile 3 | 10 | 26 | 60±31 | ns | ns | 78±28 | ns | ns |
| Quartile 4 | 9 | 23 | 56±33 | ns | ns | 89±21 | ns | ns |
| Day 4 | ||||||||
| All | 39 | 27 | 50±17 | - | ns | 81±13 | - | ns |
| Quartile 1 | 10 | 26 | 53±29 | ns | ns | 79±27 | ns | ns |
| Quartile 2 | 10 | 26 | 40±31 | ns | ns | 89±21 | ns | ns |
| Quartile 3 | 10 | 26 | 50±32 | ns | ns | 70±29 | ns | ns |
| Quartile 4 | 9 | 23 | 56±33 | ns | ns | 89±21 | ns | ns |
| Day 18 | ||||||||
| All | 38 | 26 | 55±16 | - | - | 78±14 | - | - |
| Quartile 1 | 9 | 24 | 67±35 | ns | - | 67±31 | ns | - |
| Quartile 2 | 10 | 6 | 20±25 | 0.006 | - | 76±30 | ns | - |
| Quartile 3 | 10 | 26 | 60±31 | ns | - | 80±25 | ns | - |
| Quartile 4 | 9 | 24 | 78±28 | ns | - | 89±31 | ns | - |
| Within Controls | Within Controls | |||||||
| Control | ||||||||
| End of Chemo | ||||||||
| All | 30 | 21 | 65±19 | - | - | 81±16 | - | - |
| Quartile 1 | 7 | 23 | 86±26 | ns | - | 86±26 | ns | - |
| Quartile 2 | 8 | 27 | 50±35 | ns | - | 75±31 | ns | - |
| Quartile 3 | 8 | 27 | 70±36 | ns | - | 80±36 | ns | - |
| Quartile 4 | 7 | 23 | 57±37 | ns | - | 86±26 | ns | - |
| Day28 | ||||||||
| All | 31 | 21 | 68±18 | - | - | 84±15 | na | - |
| Quartile 1 | 8 | 23 | 86±23 | ns | - | 88±23 | ns | - |
| Quartile 2 | 8 | 27 | 56±40 | ns | - | 83±30 | ns | - |
| Quartile 3 | 8 | 27 | 100±0 | 0.047 | - | 100±0 | ns | - |
| Quartile 4 | 7 | 23 | 29±34 | 0.001 | - | 71±34 | ns | - |
Legend: Serum sIL-2rα values of patients randomized to IL-2 or Control groups were divided into quartiles at the indicated time points. Quartiles were evaluated for associations with DFS or OS; ns refers to non-significant p value, and ”–“ indicates that there are no applicable comparisons.
DISCUSSION
The CCG-2961 is the first study to evaluate serial concentrations of SIL-2Rα in the context of a large randomized controlled clinical trial of IL-2 in a relatively homogeneous population. The trial shows sIL-2Rα concentrations are elevated in untreated pediatric AML patients compared to those of healthy controls and some other cancer patients. SIL-2Rα concentrations decline during chemotherapy and show consistent and significant dose-dependent increases in during IL-2 treatment but not during observation in control patients. These findings provide clear evidence of biologic activity of IL-2. Although sIL-2Rα has been reported as elevated at diagnosis in AML and in other cancers and as predictive of response to therapy, typically showing elevated sIL-2Rα as associated with inferior outcome,9,10 our study provides evidence to date that sIL-2Rα concentration is not a predictor of treatment outcome in pediatric AML when administered as per the CCG-2961 protocol.
The 2961 study confirmed that IL-2 given in the dose and schedule used in this trial is tolerable, but is without clinical benefit in children with AML in remission after intensive chemotherapy These findings are consistent with one study in which IL-2 was given at the same dose and schedule as in CCG-2961 after autologous stem cell transplantation for refractory leukemia30 and with two recent randomized trials in adults with AML where post remission IL-2 given more intensively than in CCG-2961 had no impact on DFS or OS and was poorly tolerated.22,23 Some of the differences between the apparent benefits in earlier trials13-21 and the absence of benefit of more recent randomized trials22-24 can be attributed to the larger sample sizes and randomized designs of the latter. Additionally the IL-2 itself is a variable; compared to the Chiron product used in CCG-2961, the Amgen product may effect greater immunological stimulation and greater toxicity.31
One limitation of this study is that the 25% of eligible patients withdrew. Withdrawal was a mixture of patient/parent refusal or physician recommendation. A 25% rate of withdrawal is typical for late randomization where the randomization is something vs. nothing. For example, in CCG-213 the rate of refusal to randomize to continue on AML therapy or stop treatment was 38%.32 Patients who withdrew in our study were significantly older than the randomized patients and had significantly inferior outcomes. In CCG- 2961 older age was significantly associated with inferior outcome.22 However, omission of these patients is unlikely to have affected the clinical or biological response to IL-2 among the randomized patients. A second limitation is that not all patients submitted specimens at each of the requested time points and the comparisons within and between groups over time are not limited to matched pairs. We did not have specimens to compare the sIL-2Rα concentration of untreated AML patients to newly diagnosed children with other malignancies or to age matched healthy children.
Since conception of these studies, understanding of the role of the immune system in controlling cancer has advanced. IL-2 stimulates immune cells and their production of cytokines, some of which stimulate and others of which inhibit leukemic cell growth. Moreover, a population of immunosuppressive T cells [T regulatory cells (Tregs)] is both reactive to IL-2 through high affinity receptors and release sIL-2Rα. In some settings, IL-2 infusions may preferentially enhance proliferation and activity of Tregs.33 More specific targeting of AML reactive immune cells may possibly be more effective. Other approaches to AML immunotherapy in development include myeloid specific antibodies, donor lymphocyte infusions following allogeneic transplant, and redirected autologous effector cells, using genetically engineered chimeric antigen receptors or haploidentical natural killer cells expanded in vitro.34 In these settings, addition of IL-2 appears to be potentially be advantageous. Recent data from a COG trial have shown substantial improvement in both DFS and OS when IL-2 and GM-CSF are used in combination with a tumor reactive (anti-GD2) monoclonal antibody, if applied soon after autologous HSCT for children with high risk neuroblastoma in remission.35, Nevertheless, based on the results of the CCG-2961 trial and other recent trials, there appears to be no role for single agent IL-2 to prevent relapse of AML in remission.
Supplementary Material
Consort Diagram for CCG-2961: IDACTER is idarubicin, cytarabine, thioguanaine, etoposide, and rubidomycin (daunorubicin) and FAMP-Ida is fludarabine monophosphate. Figure is adapted from Reference 22.
Acknowledgments
The authors acknowledge the assistance of the COG Publications and Operations offices. The work was supported by grants CA 13539, CA 98543, CA 032685 and CA 087025 from the National Institutes of Health. A complete listing of grant support for research conducted by CCG before 2003 is available online at http://www.childrensoncologygroup.org/admin/grantinfo.htm. NCI provided Chiron Interleukin-2 to the CCG institutions. The National Childhood Cancer Foundation, the MACC Fund and the Crawdaddy Foundation also supported the laboratory work of Dr. Sondel. The Yetta Dietch Novotny Chair in Clinical Oncology supported Dr. Lange’s contributions.
Footnotes
Author contributions: Beverly Lange, designed and oversaw CCG-2961 and wrote first draft of manuscript; Richard Yang, Jacek Gan, and Jaquelyn A. Hank performed and analyzed laboratory studies on IL-2 receptor and wrote methods section; Eric L. Sievers designed IL-2 clinical trial; Todd A. Alonzo PhD and Robert B. Gerbing MA performed and distributed data analyses and wrote statistical section; Paul M. Sondel designed B-972 correlative trial, oversaw specimen processing and reporting, and wrote background. All authors reviewed data and edited the manuscript.
Conflicts of Interest: Dr. Sievers’ is employed by Seattle Genetics, which has a product that could compete with IL-2. There are not other conflicts.
References
- 1.Mackall CL, Gress RE. Pathways of T-cell regeneration in mice and humans: implications for bone marrow transplantation and immunotherapy. Immunol Rev. 1997;157:61–721. doi: 10.1111/j.1600-065x.1997.tb00974.x. [DOI] [PubMed] [Google Scholar]
- 2.Ruggeri L, Capanni M, Mancusi A, Martelli MF, Velardi A, et al. The impact of donor natural killer cell alloreactivity on allogeneic hematopoietic transplantation. Transpl Immunol. 2005:203–6. doi: 10.1016/j.trim.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;11:24371–83. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 4.Waldman TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 5.McDermott DF. Immunotherapy of metastatic renal cell carcinoma. Cancer. 2009;115:2298–305. doi: 10.1002/cncr.24236. [DOI] [PubMed] [Google Scholar]
- 6.Bhatia S, Tykodi SS, Thompson JA. Treatment of metastatic melanoma: an overview. Oncology. 2009;23:488–96. [PMC free article] [PubMed] [Google Scholar]
- 7.Ringdén O, Karlsson H, Olsson R, Omazic B, Uhlin M. The allogeneic graft-versus-cancer effect. Br J Haematol. 2009;147:614–33. doi: 10.1111/j.1365-2141.2009.07886.x. [DOI] [PubMed] [Google Scholar]
- 8.Toren A, Ackerstein A, Slavin S, Nagler A. Role of interleukin-2 in human hematological malignancies. Med Oncol. 1995;12:177–86. doi: 10.1007/BF01571195. [DOI] [PubMed] [Google Scholar]
- 9.Kuhn DJ, Dou QP. The role of interleukin-2 receptor alpha in cancer. Front Biosci. 2005;10:1462–74. doi: 10.2741/1631. [DOI] [PubMed] [Google Scholar]
- 10.Murakami S. Soluble interleukin-2 receptor in cancer. Front Biosci. 2004;9:3085–90. doi: 10.2741/1461. [DOI] [PubMed] [Google Scholar]
- 11.Bogner MP, Voss SD, Bechhofer R, Hank JA, Roper M, Poplack D, et al. Serum CD25 levels during interleukin-2 therapy: dose dependence and correlations with clinical toxicity and lymphocyte surface sCD25 expression. J Immunother. 1992;11:111–8. [PubMed] [Google Scholar]
- 12.Bien E, Balcerska A. Serum soluble interleukin 2 receptor alpha in human cancer of adults and children: a review. Biomarkers. 2008;13:1–26. doi: 10.1080/13547500701674063. [DOI] [PubMed] [Google Scholar]
- 13.Higuchi CM, Thompson JA, Petersen FB, Buckner CD, Fefer A. Toxicity and immunomodulatory effects of interleukin-2 after autologous bone marrow transplantation for hematologic malignancies. Blood. 1991;71:2561–8. [PubMed] [Google Scholar]
- 14.Foa R, Meloni G, Guarini A, Vignetti M, Marchis D, Tosti S, Tos AG, Vischia F, Mandelli F, Gavosto F. Interleukin 2 (IL-2) in the management of acute myeloid leukemia: clinical and biological findings. Leukemia. 1992;6(Suppl 3):115S–116S. [PubMed] [Google Scholar]
- 15.Meloni G, Foà R, Tosti S, Vignetti M, Mancini F, Guarini A, et al. Autologous bone marrow transplantation followed by interleukin-2 in children with advanced leukemia: a pilot study. Leukemia. 1993;6:780–5. [PubMed] [Google Scholar]
- 16.Benyunes MC, Massumoto C, York A, Higuchi CM, Buckner CD, Thompson JA, Petersen FB, Fefer A. Interleukin-2 with or without lymphokine-activated killer cells as consolidative immunotherapy after autologous bone marrow transplantation for acute myelogenous leukemia. Bone Marrow Transplant. 1993;12:159–63. [PubMed] [Google Scholar]
- 17.Fefer A, Benyunes MC, Massumoto C, Higuchi C, York A, Buckner CD, Thompson JA. Interleukin-2 therapy after autologous bone marrow transplantation for hematologic malignancies. Semin Oncol. 1993;20:41–5. [PubMed] [Google Scholar]
- 18.Bergmann L, Heil G, Kolbe K, Lengfelder E, Puzicha E, Martin H, Lohmeyer J, Mitrou PS, Hoelzer D. Interleukin-2 bolus infusion as late consolidation therapy in 2nd remission of acute myeloblastic leukemia. Leuk Lymphoma. 1995;16:271–9. doi: 10.3109/10428199509049766. [DOI] [PubMed] [Google Scholar]
- 19.Meloni G, Trisolini SM, Capria S, Torelli GF, Baldacci E, Torromeo C. How long can we give interleukin-2? Clinical and immunological evaluation of AML patients after 10 or more years of IL2 administration. Leukemia. 2002;16:2016–8. doi: 10.1038/sj.leu.2402566. [DOI] [PubMed] [Google Scholar]
- 20.Stein AS, O’Donnell MR, Slovak ML, Snyder DS, Nademanee AP, Parker P. Interleukin-2 after autologous stem-cell transplantation for adult patients with acute myeloid leukemia in first complete remission. J Clin Oncol. 2003;21:615–23. doi: 10.1200/JCO.2003.12.125. [DOI] [PubMed] [Google Scholar]
- 21.Stone RM, DeAngelo DJ, Janosova A, Galinsky I, Canning C, Ritz J, Soiffer RJ. Low dose interleukin-2 following intensification therapy with high dose cytarabine for acute myelogenous leukemia in first complete remission. Am J Hematol. 2008;83:77, 1–7. doi: 10.1002/ajh.21253. [DOI] [PubMed] [Google Scholar]
- 22.Baer MR, George SL, Caligiuri MA, Sanford BL, Bothun SM, Mrózek K, et al. Low-dose interleukin-2 immunotherapy does not improve outcome of patients age 60 years and older with acute myeloid leukemia in first complete remission: Cancer and Leukemia Group B Study 9720. J Clin Oncol. 2008;26:4934–9. doi: 10.1200/JCO.2008.17.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pautas C, Merabet F, Thomas X, Raffoux E, Gardin C, Corm S, et al. Randomized study of intensified anthracycline doses for induction and recombinant interleukin-2 for maintenance in patients with acute myeloid leukemia age 50 to 70 years: results of the ALFA-9801 study. J Clin Oncol. 2010;28:808–14. doi: 10.1200/JCO.2009.23.2652. [DOI] [PubMed] [Google Scholar]
- 24.Sievers EL, Lange BJ, Sondel PM, Krailo MD, Gan J, Liu-Mares W, Feig SA. Feasibility, toxicity, and biologic response of interleukin-2 after consolidation chemotherapy for acute myelogenous leukemia: a report from the Children’s Cancer Group. J Clin Oncol. 1998;16:914–9. doi: 10.1200/JCO.1998.16.3.914. [DOI] [PubMed] [Google Scholar]
- 25.Lange BJ, Smith FO, Feusner J, Barnard DR, Dinndorf P, Feig S, et al. Outcomes in CCG-2961, a children’s oncology group phase 3 trial for untreated pediatric acute myeloid leukemia: a report from the children’s oncology group. Blood. 2008;111:1044–53. doi: 10.1182/blood-2007-04-084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French-American-British Cooperative Group. Ann Intern Med. 1985;103:620–5. doi: 10.7326/0003-4819-103-4-620. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J AM Stat Assoc. 1958;53:457. [Google Scholar]
- 28.Peto R, Peto J. Asymptotically efficient rank in variant test procedures. J R Stat Soc A. 1972;2:185–206. [Google Scholar]
- 29.Mann HB, Whitney DR. On a test of whether one or two random variables is stochastically larger than the other. Ann Math Stat. 1947;18:50–60. [Google Scholar]
- 30.Robinson N, Sanders JE, Benyunes MC, Beach K, Lindgren C, Thompson JA, et al. Phase I trial of interleukin-2 after unmodified HLA-matched sibling bone marrow transplantation for children with acute leukemia. Blood. 1996;87:1249–54. [PubMed] [Google Scholar]
- 31.Hank JA, Surfus J, Gan J, Albertini M, Lindstrom M, Schiller JH, et al. Distinct clinical and laboratory activity of two recombinant interleukin-2 preparations. Clin Cancer Res. 1999;5:281–9. [PubMed] [Google Scholar]
- 32.Wells RJ, Woods WG, Buckley JD, Odom LF, Benjamin D, Bernstein I, et al. Treatment of newly diagnosed children and adolescents with acute myeloid leukemia: a Children’s Cancer Group study. J Clin Oncol. 1994;12(11):2367–77. doi: 10.1200/JCO.1994.12.11.2367. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H, Chua KS, Guimond M, Kapoor V, Brown MV, et al. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nat Med. 2005;11:1238–43. doi: 10.1038/nm1312. [DOI] [PubMed] [Google Scholar]
- 34.Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28:955–9. doi: 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman S, Chen H, Smith M, et al. Chimeric Anti-GD2 Antibody with GM-CSF, IL2 and 13-cis Retinoic Acid for High-risk Neuroblastoma: A Children’s Oncology Group (COG) Phase 3 Study. New England J Med. 2010;363:1324–34. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Consort Diagram for CCG-2961: IDACTER is idarubicin, cytarabine, thioguanaine, etoposide, and rubidomycin (daunorubicin) and FAMP-Ida is fludarabine monophosphate. Figure is adapted from Reference 22.


