Abstract
Pancreatic ductal adenocarcinoma (PDAC) is associated with a pronounced collagen-rich fibrosis known as desmoplastic reaction; however the role of fibrosis in PDAC is poorly understood. In this report we show that collagen can regulate the tumor suppressive let-7 family of microRNAs in pancreatic cancer cells. PDAC cells growing in 3D collagen gels repress mature let-7 without affecting the precursor form of let-7 in part via increased expression of membrane type 1-matrix metalloproteinase (MT1-MMP, MMP-14) and ERK1/2 activation. PDAC cells in collagen also demonstrate increased TGF-β1 signaling, and blocking TGF-β1 signaling attenuated collagen-induced MT1-MMP expression, ERK1/2 activation, and repression of let-7 levels. Although MT1-MMP overexpression was not sufficient to inhibit let-7 on 2D tissue culture plastic, overexpression of MT1-MMP in PDAC cells embedded in 3D collagen gels or grown in vivo repressed let-7 levels. Importantly, MT1-MMP expression significantly correlated with decreased levels of let-7 in human PDAC tumor specimens. Overall, our study emphasizes the interplay between the key proteinase MT1-MMP and its substrate type I collagen in modulating microRNA expression, and identifies an additional mechanism by which fibrosis may contribute to PDAC progression.
Keywords: TGF-β1, collagen, ERK1/2, MT1-MMP, let-7, fibrosis
Human pancreatic ductal adenocarcinoma (PDAC) is frequently associated with an intense area of fibrosis known as the desmoplastic reaction (Jemal and Siegel et al., 2007; Mahadevan and Von Hoff et al., 2007). Desmoplastic reaction is composed of bands of fibrous stroma surrounding the malignant cells, while areas of relatively normal pancreas exhibit minimal fibrosis (Hezel and Kimmelman et al., 2006; Ottaviano and Sun et al., 2006). Human PDAC tumors exhibit increased amounts of type I collagen compared to normal pancreas and, with loss of basement membrane, the malignant cells are directly exposed to type I collagen (Imamura and Iguchi et al., 1995). In human pancreatic tumor tissue, there is a strong correlation between the expression of TGF-β1 and type I collagen (Aoyagi and Oda et al., 2004), and TGF-β1 overexpression in pancreatic cancer cells can induce desmoplatic reaction in an experimental model of human pancreatic carcinoma (Lohr and Schmidt et al., 2001).
Previously, we had shown that type I collagen modulated the behavior of PDAC cells by enhancing cell motility and increasing expression of membrane type 1-matrix metalloproteinase (MT1-MMP, MMP-14), a key collagenase that allows cells to invade through the collagen-rich extracellular matrix (Ottaviano and Sun et al., 2006). MT1-MMP is overexpressed in pancreatic tumors mainly in areas of fibrosis, and expression of MT1-MMP is increased in metastatic PDAC lesions compared to the primary tumors. Animal studies support MT1-MMP as a primary regulator of interstitial collagenolysis, as mice genetically deficient in MT1-MMP have severe growth defects as a result of failure to process interstitial collagens during bone and soft tissue formation (Holmbeck and Bianco et al., 1999; Zhou and Apte et al., 2000). This conclusion is supported by in vitro studies using organotypic and 3D culture systems which demonstrate that only MT1-MMP confers a 3D growth advantage by removing matrix confines and allowing changes in cellular geometry necessary for proliferation (Hotary and Allen et al., 2003; Sabeh and Ota et al., 2004).
MicroRNAs are small single-stranded, non-coding RNAs that bind to target mRNA and inhibit translation or promote degradation of the transcript (Lewis and Burge et al., 2005; Lim and Lau et al., 2005). A recent study showed that members of the let-7 family of microRNAs, initially identified as key regulators of embryonic development in C.elegans (Reinhart and Slack et al., 2000), were decreased in human PDACs (Torrisani and Bournet et al., 2009). Let-7 expression increases with differentiation and is present in high levels in mature tissue (Erkan and Kleeff et al., 2007), where it inhibits cell proliferation by monitoring DNA replication, mitosis and cytokinesis (Johnson and Esquela-Kerscher et al., 2007). Overexpression of let-7 in mutant K-Ras-positive lung cancer cells inhibits tumor growth in vivo, while overexpression of let-7 in breast tumor-initiating cells inhibits tumor formation and metastasis in vivo (Yu and Yao et al., 2007). Interestingly, K-Ras, which is mutated in > 90% of PDAC, is also a target of let-7 microRNA (Johnson and Grosshans et al., 2005; Kumar and Erkeland et al., 2008; Morris and McManus et al., 2005).
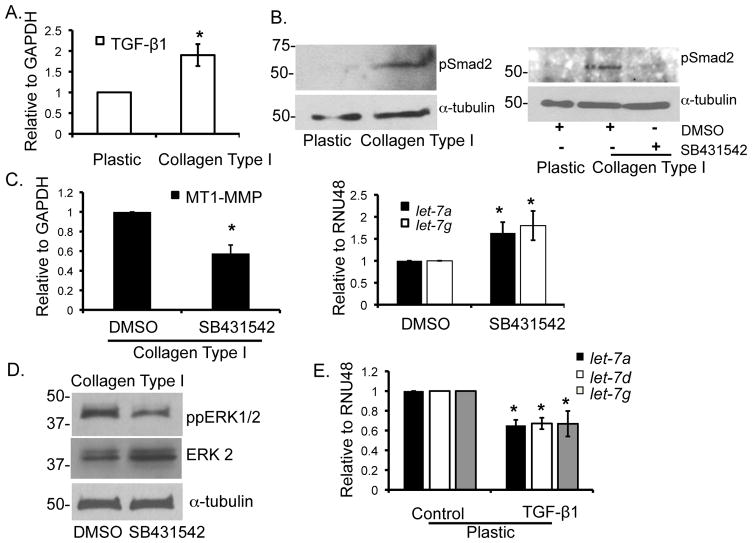
Although the collagen microenvironment has been shown to regulate gene expression, the extent to which the effect is mediated through modulation of microRNAs has not been previously examined. Therefore, in this study we examined the effect of collagen on let-7 family microRNAs. Initially, we examined the relative expression of the various members of the let-7 family of microRNAs in pancreatic cancer cells (Panc1) grown on tissue culture plastic. As shown in Supplemental Fig. 1, there was differing expression of the various members of the let-7 family. Expression of let-7a and let-7e was most pronounced, let-7g and let-7i were in the intermediate range, while the relative levels of let-7c, let-7d, let-7f and miR-98 were significantly reduced. We next examined the effect of collagen on 3 members of let-7 family based on varying expression levels (let-7a, let-7d and let-7g). Panc1 cells were grown on 2D tissue culture plastic or embedded in 3D type I collagen gels for 24 hours, and then processed for microRNA and mRNA expression. Compared to Panc1 cells grown on tissue culture plastic, Panc1 cells growing in 3D type I collagen gels show 80% reduction in the mature let-7 levels (Fig. 1A) without affecting the expression of the precursor primary form of let-7 (Supplemental Fig. 2), indicating that collagen regulates the post-transcriptional processing of let-7 from the precursor form to the mature form. Since type I collagen induces MT1-MMP expression in PDAC cells [(Ottaviano and Sun et al., 2006) and Fig. 1B], we examined whether MT1-MMP may be involved in collagen-repression of let-7. Initially, we examined the effect of the broad-spectrum MMP inhibitor GM6001 on let-7 levels. As shown in Fig. 1C, GM6001 partially rescued the effect of collagen on let-7 levels. Moreover, siRNA knockdown of MT1-MMP also partially rescued let-7 in 3D collagen (Fig. 1D), suggesting that collagen repression of let-7 is mediated in part via increased MT1-MMP expression.
Figure 1. Collagen repression of let-7 microRNA involves MT1-MMP.
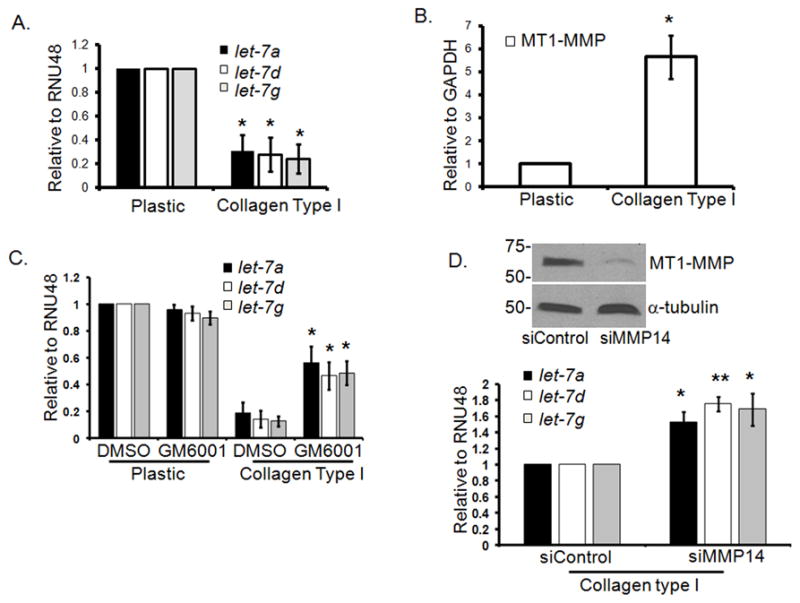
Panc1 cells were grown on tissue culture plastic or in 3D type I collagen gels (5 mg/ml) in serum containing medium for 24 hours. A. MicroRNA were isolated using mirVana kit (Applied Biosystems, Foster City, CA) and analyzed for expression of the mature let-7a, let-7d and let-7g by qRT-PCR, with RNU48 as the endogenous control, using gene specific Taqman probes (Applied Biosystems, Foster City, CA). The data were quantified by the comparative CT method for relative gene expression from 3 experiments. *, p < 0.05 relative to cells grown on plastic. B. Similarly, MT1-MMP and GAPDH (endogenous control) mRNA were analyzed by qRT-PCR. *, p < 0.05 relative to cells grown on plastic. Data are an average from 3 separate experiments. C. Panc1 cells were grown on plastic or in 3D type I collagen gels (5 mg/ml) and treated with either DMSO or GM6001 (10 μM; EMD, Gibbstown, NJ) for 24 hours. MicroRNA were isolated and analyzed for let-7 and RNU48 (endogenous control) expression by qRT-PCR. *, p <0.05 relative to DMSO-treated cells grown in collagen. Data are an average of 3 separate experiments. D. Panc1 cells were transiently transfected with siRNA against MT1-MMP or control siRNA (50 nmoles) using Nucleofector Kit R (Lonza, Walkersville, MD). Cells were allowed to recover overnight and then plated on plastic or in collagen gels for 24 hours. MT1-MMP knockdown was analyzed by immunoblotting for cells grown on plastic (upper panel), while microRNA were analyzed for let-7 and RNU48 (endogenous control) expression by qRT-PCR. * p< 0.05; **, p < 0.01 relative to siControl transfected cells grown in collagen. Data are an average of 3 separate experiments.
Since MT1-MMP has previously been shown to promote MEK/ERK1/2 activation (Sounni and Rozanov et al., 2009), we examined the effect of MT1-MMP siRNA on collagen-induced ERK1/2 phosphorylation. As shown in Fig. 2A, decreasing MT1-MMP levels in Panc1 cells grown in 3D type I collagen attenuated ERK1/2 phosphorylation (Fig. 2A). Moreover, collagen-induced ERK1/2 phosphorylation was mediated by MEK1/2 since U0126 completely blocked ERK1/2 activation (Fig. 2B). However, the activation of ERK1/2 was independent of the EGF receptor (EGFR). Treatment with EGFR inhibitors AG1478 or PD153035 did not inhibit collagen mediated ERK1/2 activation (Fig. 2C). As ERK1/2 can repress let-7 levels (Dangi-Garimella and Yun et al., 2009) we examined the effect of ERK1/2 on collagen regulation of let-7. Panc1 cells growing on plastic or in 3D type I collagen were treated with vehicle control (DMSO) or U0126 for 24 hours. Treatment with U0126 significantly rescued the levels of mature let-7 in 3D collagen (Fig. 2D) without any effect on the precursor form of let-7 (Supplemental Fig. 3), demonstrating that collagen activates the MEK1/2-ERK1/2 signaling pathway to inhibit processing of let-7 to the mature form in pancreatic cancer cells. Our findings are consistent with a recently published study that showed a role for ERK in stabilizing the protein complex that regulates processing of precursor microRNAs (Paroo and Ye et al., 2009). Specifically, mitogenic signaling mediated ERK activation was shown to increase levels of growth-promoting microRNAs while decreasing expression of the tumor suppressor microRNAs let-7.
Figure 2. ERK1/2 mediates collagen repression of let-7 in PDAC cells.
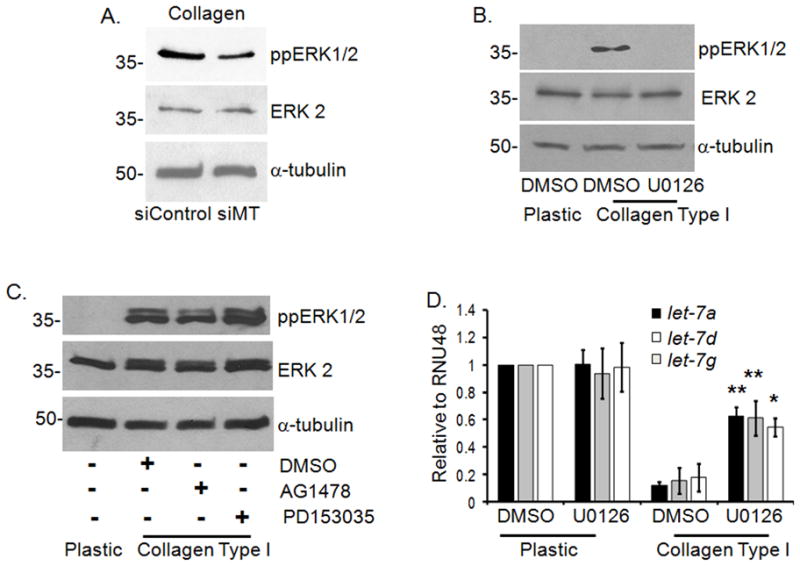
A. Panc1 cells were transiently transfected with siRNA against MT1-MMP or control siRNA (50 nmoles) as detailed in Fig. 1D. Cells were allowed to recover overnight and then plated in 3D type I collagen gels (5 mg/ml) for 24 hours, the matrix was dissolved in collagenase (Worthington Biologicals, Lakewood, NJ) and then lysed in m-RIPA buffer. Lysates were immunoblotted for ppERK1/2, ERK/12 and α-tubulin (loading control). Data is representative of 3 separate experiments. B. Panc1 cells were grown on tissue culture plastic or in 3D type I collagen gels (5 mg/ml) for 24 hours and treated with vehicle control (DMSO) or U0126 (10 μM; Cell Signaling, Danvers, MA) for 24 hours. Cells grown in collagen were recovered with collagenase treatment and then lysed in m-RIPA buffer, prior to immunoblotting for ppERK1/2 and α-tubulin (loading control). Data is representative of 4 separate experiments. C. Panc1 cells were grown on plastic or in 3D type I collagen gels (5 mg/ml) for 24 hours and then treated with DMSO, AG1478 (10 μM; Calbiochem, Gibbstown, NJ) or PD153035 (10 μM; Calbiochem, Gibbstown, NJ) for an additional 24 hours. Cells were lysed as before and then immunoblotted for ppERK1/2, ERK2 and α-tubulin (loading control). Data is representative of 3 separate experiments. D. Panc1 cells grown on plastic or in collagen gels were treated with DMSO or U0126 for 24 hours. MicroRNA were isolated using mirVana kit and analyzed for expression of let-7a, let-7d and let-7g by qRT-PCR, with RNU48 as the endogenous control, using gene specific Taqman probes. The data were quantified by the comparative CT method for relative gene expression from 4 separate experiments. *, p < 0.05; **, p < 0.01 relative to DMSO-treated cells grown in collagen.
As we had previously shown that MT1-MMP expression involves TGF-β1 signaling in pancreatic cancer cells (Ottaviano and Sun et al., 2006), we also examined whether TGF-β1 may play a role in collagen repression of let-7. Initially, we evaluated the effect of collagen on TGF-β1 signaling. As shown in Figs. 3A and 3B, Panc1 cells growing in 3D type I collagen demonstrate a 2-fold increase in TGF-β1 mRNA and increased Smad2 phosphorylation, which was blocked using a highly specific TGF-β type I receptor (TβRI) inhibitor SB431542. Significantly, treatment with SB431542 attenuated the effect of collagen on MT1-MMP, let-7, and ERK1/2 phosphorylation (Figs. 3C and 3D). To directly demonstrate that TGF-β1 regulates let-7 expression, Panc1 cells grown on plastic were treated with recombinant human TGF-β1 for 24 hours and let-7 expression was analyzed. As seen in Fig. 3E, TGF-β1 treatment resulted in approximately 40% inhibition of let-7 expression. These results demonstrate that collagen, in part by enhancing TβRI activity, promotes MT1-MMP expression and ERK1/2 phosphorylation to repress let-7 in PDAC cells.
Figure 3. TGF-β receptor type I (TβRI) mediates collagen repression of let-7.
A. The mRNA isolated from Panc1 cells grown on plastic or in 3D type I collagen gels for 24 hours was analyzed by qRT-PCR for TGF-β1 expression with GAPDH as the endogenous control. *, p < 0.05 relative to cells grown on plastic. Data are an average of 3 separate experiments. B. The lysates were analyzed for pSmad2 and α-tubulin (loading control) by immunoblotting (left panel). Panc1 cells grown in 3D type I collagen gels were treated with DMSO (control) or TGF-β type I receptor (TβRI) inhibitor SB431542 (10 μM, TOCRIS, Ellisville, MO) for 24 hours and cell lysates immunoblotted for pSmad2 and α-tubulin (right panel). Data is representative of at least 2 separate experiments. C. Panc1 cells growing in 3D type I collagen gels were treated with DMSO or SB431542 and changes in MT1-MMP and let-7 monitored by qRT-PCR. *, p < 0.05 relative to DMSO-treated cells grown in collagen. Data are an average of 4 separate experiments. D. The protein lysates were immunoblotted for phospho-ERK1/2, ERK2 and α-tubulin (loading control). Data are representative of at least 3 separate experiments. E. Panc1 cells grown on plastic were induced with recombinant human TGF-β1 (10 ng/ml) for 24 hours; microRNA isolated from the cells was analyzed for let-7 by qRT-PCR from 3 separate experiments.
We further evaluated the role of MT1-MMP in the regulation of let-7 expression by generating Panc1 cells expressing full-length or tail-less (ΔC) MT1-MMP protein. Since the MT1-MMP protein without the tail is retained on the surface longer, there was increased MMP-2 activation by cells expressing the tail-less MT1-MMP protein compared to the wild-type MT1-MMP protein (Fig. 4A). Although overexpression of MT1-MMP was not sufficient to repress let-7 levels on plastic (data not shown), overexpression of MT1-MMP protein in PDAC cells grown in collagen further repressed let-7 levels compared to control PDAC cells (Fig. 4B). These results indicate that MT1-MMP repression of let-7 requires the collagen-milieu.
Figure 4. MT1-MMP represses let-7 microRNA in vivo.
A. Full-length MT1-MMP and ΔC mutant were subcloned into pRetroX-Tight-Pur vector (Clontech, Mountainview, CA). Panc1 cells inducibly expressing MT1-MMP were then generated as previously described (Dangi-Garimella and Redig et al., 2010) to create Panc1-tet-V, Panc1-tet-MT and Panc1-tet-ΔC. These cells were grown on plastic, induced with doxycycline (2 μg/ml) for 24 hours, and then immunoblotted for MT1-MMP and α-tubulin (loading control) (upper panel). To examine MT1-MMP activity, the conditioned medium was analyzed for MMP-2 activation using gelatin zymography (lower panel). Data are representative of at least 4 separate experiments. B. Panc1-tet-V, Panc1-tet-MT and Panc1-tet-ΔC cells were grown on tissue culture plastic or in 3D collagen gels (5 mg/ml), induced with doxycycline for 64 hours and changes in let-7 and RNU48 (endogenous control) expression were analyzed by qRT-PCR from 3 separate experiments. *, p < 0.05 relative to control Panc1-tet-V cells. C. Mice were housed, fed and treated in accordance with the guidelines approved by the Northwestern University IACUC. Eight-week old athymic nu/nu animals (n=8) were subcutaneously injected with 5 × 106 Panc1-tet-V cells in the left flank and Panc1-tet-ΔC cells in the right flank of the same animal. Twenty-four hours later, doxycycline was added to the drinking water to induce MT1-MMP expression, and monitored twice a week for the development of skin tumors. The mice were euthanized, following which the tumors were dissected and placed in RNAlater (Qiagen, Valencia, CA). The RNAlater-preserved tumors were processed and analyzed for human MT1-MMP and human GAPDH mRNA or human let-7 and human sno135 microRNA expression by quantitative real-time PCR. For each animal the relative expression of let-7a, let-7d and let-7g was calculated in Panc1-tet-ΔC tumors and normalized to the levels present in the Panc1-tet-V tumors, with the let-7 expression in the control animals arbitrarily set at 1.0. Relative let-7 values < 1.0 indicate repression by MT1-MMP. D. Pancreatic tissue was obtained from patients with pancreatic adenocarcinoma and de-identified on an IRB-approved protocol. The cancerous and adjacent normal tissue samples were dissected and processed for RNA extraction using Trizol. The RNA quality was checked using Bioanalyzer-309 and samples with RNA Integrity Number (RIN) greater than 6 were used for RNA and microRNA studies. The let-7 levels relative to RNU48 and MT1-MMP expression relative to GAPDH in PDAC tumors (n=11) were normalized to the levels present in the matched adjacent normal pancreas, which was arbitrarily set at 1.0. Spearman’s correlation (GraphPad Instat) was used to determine significance between MT1-MMP and let-7 levels in these tumors.
We next injected Panc1 cells into nude mice to examine whether MT1-MMP can modulate let-7 in vivo. Panc1-tet-V and Panc1-tet-ΔC cells were subcutaneously injected into the left and right flanks of nude mice respectively, and the mice were maintained on doxycycline-containing water for 3 weeks to induce gene expression. The tumors were then collected and processed for mRNA and microRNA expression. For each mouse, the let-7 levels in the Panc1-tet-ΔC tumors were normalized to the matched Panc1-tet-V tumors, arbitrarily setting the let-7 in the Panc1-tet-V tumors to 1.0. As seen in Fig. 4C, each ΔC-expressing tumor had lower levels of let-7 relative to the corresponding control tumor, indicating that MT1-MMP is also able to repress let-7 in vivo. Additionally, even though the effect was less pronounced compared to the ΔC-expressing tumors, Panc1 tumors expressing the wild-type MT1-MMP showed similar results, with lower levels of at least one of the let-7 family members in the MT1-MMP expressing tumors relative to control tumors (Supplemental Fig. 4). To provide additional support for our hypothesis that MT1-MMP can repress let-7 levels in the in vivo microenvironment, we examined the relationship between MT1-MMP and let-7 in human PDAC tumors (Fig. 4D). RNA and microRNA isolated from human PDAC tumor samples were analyzed for expression of MT1-MMP and let-7 and normalized to the adjacent matched normal pancreas. We found a statistically significant inverse relationship between MT1-MMP and let-7 (r= −0.7091, p= 0.0182).
Overall, in this report we show that PDAC cells growing in 3D type I collagen gels repress let-7 microRNA, in part via increased TGF-β1-mediated expression of MT1-MMP and ERK1/2 activation. It was recently shown that TGF-β1 can downregulate expression of miR-200 family members, and upregulate the oncogenic microRNA miR-21 (Qian and Katsaros et al., 2009). We now provide evidence that TGF-β1 signaling can also regulate let-7 family members. Let-7 expression can inhibit pancreatic cancer cell proliferation in vitro; however, let-7 did not affect progression of the primary tumor in vivo (Torrisani and Bournet et al., 2009). Interestingly, work done by Oh et al suggests that let-7 could have therapeutic potential in the treatment of pancreatic cancer (Oh and Kim et al.). Expression of let-7 in A549 (lung cancer) and AsPC1 (pancreatic cancer) cells decreased K-Ras levels and potentiated the effect of radiation.
By gene signature analysis, additional microRNAs have also been shown to be differentially regulated between pancreatic cancer and normal pancreatic tissue or chronic pancreatitis (Bloomston and Frankel et al., 2007). Seven microRNAs (miR-21, miR-221, miR-222, miR-181a, b and d and miR-155) were confirmed to be overexpressed in the tumor samples when compared to benign pancreatic tissue. Significantly, miR-155 can inhibit the pro-apoptotic p53 target protein TP53INP1, thus promoting PDAC progression (Gironella and Seux et al., 2007). FISH analysis on tumor tissue microarrays also demonstrated that miR-21, which targets phosphatase and tensin homologue (PTEN), is overexpressed in pancreatic cancer tissue relative to benign pancreatic lesions or chronic pancreatitis and is associated with poor outcome (Dillhoff and Liu et al., 2008). Additionally, antisense-mediated inhibition of miR-21 along with miR-221 in pancreatic cancer cells reduced proliferation, increased apoptosis and sensitized cells to gemcitabine treatment (Park and Lee et al., 2009). Despite the importance of these microRNAs in pancreatic cancer progression, neither collagen nor MT1-MMP affected the levels of miR-21, miR-155, miR-221 or miR-375 in our model system.
Finally, even though microRNAs can regulate MMP expression and function (Gabriely and Wurdinger et al., 2008; Roy and Khanna et al., 2009), it was not heretofore shown that MMPs could in turn modulate microRNAs. Significantly, we show that MT1-MMP, which removes matrix barriers to allow cells to proliferate in the 3D microenvironment (Hotary and Allen et al., 2003; Sabeh and Ota et al., 2004), may also contribute to PDAC progression by modulating a microRNA program in the collagen-rich tumor microenvironment.
Supplementary Material
Acknowledgments
This research was supported by grant R01CA126888 (H.G.M.) from the NCI, and funding from the Elsa U. Pardee Foundation (H.G.M.) and the National Pancreas Foundation (H.G.M.).
References
- Aoyagi Y, Oda T, Kinoshita T, Nakahashi C, Hasebe T, Ohkohchi N, et al. Overexpression of TGF-beta by infiltrated granulocytes correlates with the expression of collagen mRNA in pancreatic cancer. Br J Cancer. 2004;91:1316–26. doi: 10.1038/sj.bjc.6602141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomston M, Frankel WL, Petrocca F, Volinia S, Alder H, Hagan JP, et al. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. Jama. 2007;297:1901–8. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- Dangi-Garimella S, Redig AJ, Shields MA, Siddiqui MA, Munshi HG. Rho-ROCK-myosin signaling meditates membrane type 1-matrix metalloproteinase-induced cellular aggregation of keratinocytes. J Biol Chem. 2010;285:28363–72. doi: 10.1074/jbc.M110.146019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangi-Garimella S, Yun J, Eves EM, Newman M, Erkeland SJ, Hammond SM, et al. Raf kinase inhibitory protein suppresses a metastasis signalling cascade involving LIN28 and let-7. EMBO J. 2009;28:347–58. doi: 10.1038/emboj.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillhoff M, Liu J, Frankel W, Croce C, Bloomston M. MicroRNA-21 is overexpressed in pancreatic cancer and a potential predictor of survival. J Gastrointest Surg. 2008;12:2171–6. doi: 10.1007/s11605-008-0584-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkan M, Kleeff J, Gorbachevski A, Reiser C, Mitkus T, Esposito I, et al. Periostin creates a tumor-supportive microenvironment in the pancreas by sustaining fibrogenic stellate cell activity. Gastroenterology. 2007;132:1447–64. doi: 10.1053/j.gastro.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Gabriely G, Wurdinger T, Kesari S, Esau CC, Burchard J, Linsley PS, et al. MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol Cell Biol. 2008;28:5369–80. doi: 10.1128/MCB.00479-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gironella M, Seux M, Xie MJ, Cano C, Tomasini R, Gommeaux J, et al. Tumor protein 53-induced nuclear protein 1 expression is repressed by miR-155, and its restoration inhibits pancreatic tumor development. Proc Natl Acad Sci U S A. 2007;104:16170–5. doi: 10.1073/pnas.0703942104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–49. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, Weiss SJ. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114:33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- Imamura T, Iguchi H, Manabe T, Ohshio G, Yoshimura T, Wang ZH, et al. Quantitative analysis of collagen and collagen subtypes I, III, and V in human pancreatic cancer, tumor-associated chronic pancreatitis, and alcoholic chronic pancreatitis. Pancreas. 1995;11:357–64. doi: 10.1097/00006676-199511000-00007. [DOI] [PubMed] [Google Scholar]
- Johnson CD, Esquela-Kerscher A, Stefani G, Byrom M, Kelnar K, Ovcharenko D, et al. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 2007;67:7713–22. doi: 10.1158/0008-5472.CAN-07-1083. [DOI] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–73. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- Lohr M, Schmidt C, Ringel J, Kluth M, Muller P, Nizze H, et al. Transforming growth factor-beta1 induces desmoplasia in an experimental model of human pancreatic carcinoma. Cancer Res. 2001;61:550–5. [PubMed] [Google Scholar]
- Oh JS, Kim JJ, Byun JY, Kim IA. Lin28-let7 modulates radiosensitivity of human cancer cells with activation of K-Ras. Int J Radiat Oncol Biol Phys. 76:5–8. doi: 10.1016/j.ijrobp.2009.08.028. [DOI] [PubMed] [Google Scholar]
- Ottaviano AJ, Sun L, Ananthanarayanan V, Munshi HG. Extracellular matrix-mediated membrane-type 1 matrix metalloproteinase expression in pancreatic ductal cells is regulated by transforming growth factor-beta1. Cancer Res. 2006;66:7032–40. doi: 10.1158/0008-5472.CAN-05-4421. [DOI] [PubMed] [Google Scholar]
- Park JK, Lee EJ, Esau C, Schmittgen TD. Antisense inhibition of microRNA-21 or -221 arrests cell cycle, induces apoptosis, and sensitizes the effects of gemcitabine in pancreatic adenocarcinoma. Pancreas. 2009;38:e190–9. doi: 10.1097/MPA.0b013e3181ba82e1. [DOI] [PubMed] [Google Scholar]
- Paroo Z, Ye X, Chen S, Liu Q. Phosphorylation of the human microRNA-generating complex mediates MAPK/Erk signaling. Cell. 2009;139:112–22. doi: 10.1016/j.cell.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian B, Katsaros D, Lu L, Preti M, Durando A, Arisio R, et al. High miR-21 expression in breast cancer associated with poor disease-free survival in early stage disease and high TGF-beta1. Breast Cancer Res Treat. 2009;117:131–40. doi: 10.1007/s10549-008-0219-7. [DOI] [PubMed] [Google Scholar]
- Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–6. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- Roy S, Khanna S, Hussain SR, Biswas S, Azad A, Rink C, et al. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res. 2009;82:21–9. doi: 10.1093/cvr/cvp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, et al. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167:769–81. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sounni NE, Rozanov DV, Remacle AG, Golubkov VS, Noel A, Strongin AY. TIMP-2 binding with cellular MT1-MMP stimulates invasion-promoting MEK/ERK signaling in cancer cells. Int J Cancer. 2009 doi: 10.1002/ijc.24690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisani J, Bournet B, du Rieu MC, Bouisson M, Souque A, Escourrou J, et al. let-7 MicroRNA transfer in pancreatic cancer-derived cells inhibits in vitro cell proliferation but fails to alter tumor progression. Hum Gene Ther. 2009;20:831–44. doi: 10.1089/hum.2008.134. [DOI] [PubMed] [Google Scholar]
- Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–23. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, et al. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci U S A. 2000;97:4052–7. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.