Abstract
In this review, we discuss the pharmacological and clinical properties of irbesartan, a noncompetitive angiotensin II receptor type 1 antagonist, successfully used for more than a decade in the treatment of essential hypertension. Irbesartan exerts its antihypertensive effect through an inhibitory effect on the pressure response to angiotensin II. Irbesartan 150–300 mg once daily confers a lasting effect over 24 hours, and its antihypertensive efficacy is further enhanced by the coadministration of hydrochlorothiazide. Additionally and partially beyond its blood pressure-lowering effect, irbesartan reduces left ventricular hypertrophy, favors right atrial remodeling in atrial fibrillation, and increases the likelihood of maintenance of sinus rhythm after cardioversion in atrial fibrillation. In addition, the renoprotective effects of irbesartan are well documented in the early and later stages of renal disease in type 2 diabetics. Furthermore, both the therapeutic effectiveness and the placebo-like side effect profile contribute to a high adherence rate to the drug. Currently, irbesartan in monotherapy or combination therapy with hydrochlorothiazide represent a rationale pharmacologic approach for arterial hypertension and early-stage and late-stage diabetic nephropathy in hypertensive type II diabetics.
Keywords: AT1 receptor blockers, renin-angiotensin-aldosterone system, heart, renal, arterial, hypertension
Introduction
The renin-angiotensin-aldosterone system plays a pivotal role in the regulation of blood pressure and body sodium and water homeostasis. The renin-angiotensin-aldosterone system is implicated in the pathogenesis and progression of numerous cardiovascular and renal pathologies, including hypertension, structural cardiac remodeling, myo-cardial infarction, heart failure, and chronic kidney disease.1,2 The inhibition of the renin-angiotensin-aldosterone system is therefore a therapeutic target.
In our pharmacological arsenal, we currently have four weapons that inhibit the renin-angiotensin-aldosterone system through either direct or complementary mechanisms. Angiotensin-converting enzyme inhibitors block the conversion of angiotensin I to angiotensin II; angiotensin receptor blockers selectively antagonize angiotensin II at the AT1 receptors; aldosterone receptor blockers reduce the effects of aldosterone; and renin inhibitors, the newest drug group, directly inhibit human renin.
Angiotensin receptor blockers have been available for management of hypertension for almost 20 years. So far, seven angiotensin receptor blockers have been approved by the US Food and Drug Administration, with slightly different therapeutic indications (Table 1). In comparison with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers have very similar antihypertensive efficacy, but a better side effect profile, mainly because they are not associated with cough, a major side effect of ACE inhibitors.3,4 Several angiotensin receptor blockers have a long plasma half-life and binding time to the AT1 receptor, enabling a once a day administration. Because the side effect profile of drugs and the complexity of dosage regimens are known to have the greatest impact on patient adherence, angiotensin receptor blockers have an ideal profile, with a placebo-like tolerability and pharmacokinetic properties allowing a once-daily dosing regimen.5
Table 1.
Indications of the seven approved angiotensin receptor blocker (listed in order of date of appearance on the market)89,90
| Essential hypertension | Heart failure | Cardiovascular prevention | Nephropathy | |
|---|---|---|---|---|
| Losartan | X | X | X | X |
| Symptomatic NYHA II–IIIa | ↓ stroke risk in LVH | Hypertensive, type II diabetics (↑ creatinine and/or albuminuria ≥300 mg/day) | ||
| Valsartan | X | X | ||
| Symptomatic NYHA II–IIIb | ||||
| X | ||||
| Asymptomatic if recent MI and LVEF ≤ 40% | ||||
| Candesartan | X | X | ||
| If LVEF ≤ 40%a | ||||
| Irbesartan | X | X | ||
| Hypertensive, type II diabetics, (↑ creatinine and/or albuminuria ≥30 mg/day) | ||||
| Olmesartan | X | |||
| Telmisartan | X | X | ||
| ↓ MI and stroke risk in high CV risk and/or diabetic patients with target organ damage | ||||
| Eprosartan | X |
Notes:
As add-on therapy to angiotensin-converting enzyme inhibitors or as an alternative to angiotensin-converting enzyme inhibitors in patients unable to tolerate angiotensin-converting enzyme inhibitors;
As add-on therapy to angiotensin-converting enzyme inhibitors when beta-blockers cannot be used or as an alternative to angiotensin-converting enzyme inhibitors in patients unable to tolerate angiotensin-converting enzyme inhibitors;
↓ indicates decrease.
Abbreviations: ARB, angiotensin receptor blocker; ACE-I, ACE inhibitor; LVEF, left ventricular ejection fraction; CV, cardiovascular; MI, myocardial infarction; LVH, left ventricular hypertrophy.
Irbesartan (Aprovel®, Avapro®, Irbetan®, Karvea®) is a well established angiotensin receptor blocker, approved worldwide for the treatment of hypertension. In essential hypertension, irbesartan lowers blood pressure over 24 hours. The usual starting dosage is 150 mg once daily, but the dose can be uptitrated to 300 mg once daily if necessary.6 In many countries (US, Canada, Europe), irbesartan is also approved for the treatment of nephropathy in patients with hypertension and type 2 diabetes mellitus. In the latter indication, 300 mg once daily is the recommended maintenance dosage.6
This review summarizes the pharmacokinetic and phar-macodynamic characteristics of irbesartan, with a particular focus on recent clinical evidence about the therapeutic effi-cacy and tolerability of irbesartan when used as oral mono-therapy or combination therapy in essential hypertension, diabetic nephropathy, and cardiac disease.
Overview of pharmacology
Irbesartan is an imidazole derivative with a bipentyl-tetrazole side chain. It does not require biotransformation to exert its pharmacological action. The molecule has a high affinity for the AT1 receptor in human vascular smooth muscle cells, inducing in vitro a rightward shift of the angiotensin II concentration-response curve and a depression of the maximal response to angiotensin II characteristic of insurmountable blockade of AT1 receptors.7 Following oral administration, the absolute average bioavailability is high (60%–80%), the highest in its class, and is not affected by concomitant food intake.8 In healthy subjects, peak plasma concentration and the area under the curve are dose-dependent, whereas the time to peak plasma concentration is dose-independent (1.5–2.0 hours). Steady-state plasma concentrations are reached after three days of once-daily administration, with an elimination half-life of about 11–15 hours, and no evidence of accumulation over one-week multiple dosing.9 The degree of plasma protein binding is ≥90%.10 Irbesartan is strongly metabolized via hepatic glucuronidation and oxidation (mainly by the cytochrome P450 2C9 isoenzyme) and excreted by both biliary (80%) and renal (20%) routes. No active metabolites have been identified. No gender-related or age-related dosage adjustment is necessary, not even for patients with mild-to-moderate hepatic insufficiency, heart failure, or renal insufficiency.11–14 Irbesartan is strictly con-traindicated in the second and third trimesters of pregnancy and during lactation.15
Drug interactions
Potential drug interactions with cytochrome P450 2C9 have been extensively analyzed.16 Fluconazole, a cytochrome P450 2C9 inhibitor, increases steady-state peak plasma concentration (19%) and area under the curve (63%), without altering the time to peak plasma concentration.17 No data are available regarding the impact of this interaction on antihypertensive efficacy. The pharmacokinetic profile of irbesartan is not affected by nifedipine, warfarin, simvastatin, tolbutamide, hydrochlorothiazide, or magnesium-aluminum hydroxide antacids. Irbesartan does not alter the steady-state phar-macokinetics of digoxin.6,16 When combined with a cyclo-oxygenase 2 inhibitor in slightly volume-depleted subjects with normal renal function, irbesartan does not affect renal hemodynamics and renal salt handling.18 A pharmacogenetic study has confirmed the role of the cytochrome P450 2C9 enzyme in the metabolism of irbesartan. In a Chinese population, carriers of the cytochrome P450 2C9*3 allele had higher levels of irbesartan at 6 and 14 hours.19
Therapeutic efficacy in hypertension
The antihypertensive efficacy of irbesartan has been established in numerous, large, randomized active-controlled or placebo-controlled trials of up to three months’ duration.20–34 As expected, irbesartan in monotherapy is superior to placebo in lowering both systolic and diastolic blood pressure.15,30,31 The blood pressure effect was manifest within two weeks of starting treatment, and achieved maximum reduction after 2–6 weeks.30 A dose-response relationship over a dose range of 1–900 mg once daily was observed, reaching a plateau with doses ≥300 mg once daily.31 The placebo-subtracted reduction in office blood pressure was approximately 8–10/5–6 mmHg.31 In studies involving ambulatory blood pressure assessment, irbesartan was effective in producing sustained 24-hour blood pressure control.22,23,29,35 A trough-to-peak ratio ≥0.6 was achieved with doses ≥150 mg once daily.31
Comparative clinical trials performed in mild-to-moderate hypertension showed equal efficacy, but better tolerability, compared with the other major antihypertensive classes, ie, beta-blockers (atenolol), calcium antagonists (amlo-dipine), angiotensin-converting enzyme inhibitors (enalapril), and renin inhibitors (aliskiren), and superior efficacy as compared with doxazosin.20–22,26–28,36–38 In intraclass comparative clinical trials (Table 2), irbesartan was at least as effective as losartan, significantly more effective than valsartan, but less effective than olmesartan at reducing office diastolic blood pressure.24,25,35,39 However, in an additional analysis considering 24-hour ambulatory blood pressure values as a secondary variable, no significant difference was found between olmesartan and irbesartan in terms of blood pressure (13/9 mmHg for olmesartan 20 mg once daily versus 11/7 mmHg for irbesartan 150 mg once daily (not statistically significant), nor in terms of the percentage of patients reaching a mean 24-hour blood pressure <130/80 mmHg (21% versus 14%, not statistically significant).40
Table 2.
Antihypertensive efficacy of irbesartan, comparing oral irbesartan monotherapy with other classes of antihypertensive drugs in patients with mild-to-moderate hypertension
| Comparison class (agent) | Dosage (mg/day) | IRB noninferior | IRB superior | IRB inferior |
|---|---|---|---|---|
| ACE inhibitor | ||||
| ENA | IRB 150–300, ENA 10–20 | Chiou et al27 | ||
| IRB 150–300, ENA 10–20 | Coca et al22 | |||
| IRB 150–300, ENA 10–20 | Lacourciere20 | |||
| IRB 75–300, ENA 5–10 | Mimran et al21 | |||
| Renin inhibitor | ||||
| ALI | IRB 150, ALI 150 | Gradman et al36 | ||
| IRB 150, ALI 300 | Gradman et al36 | |||
| Calcium channel blocker | ||||
| AML | IRB 150, AML 5 | Gaudio et al61 | ||
| Beta-blocker | ||||
| ATE | IRB 75–150, ATE 50–100 | Stumpe et al28 | ||
| Alpha-1 antagonist | ||||
| DOX | IRB 300, DOX 4 | Derosa et al38 | ||
| Angiotensin II receptor blockers | ||||
| LOS | IRB 150, LOS 100 | Kassler-Taub et al24 | ||
| IRB 300, LOS 100 | Kassler-Taub et al24 | |||
| IRB 200, LOS 100 | Yoshinaga91 | |||
| IRB 150–300, LOS 50–100 | Oparil et al25 | |||
| VAL | IRB 150, VAL 80 | Mancia et al23 | ||
| OLM | IRB 150, OLM 20 | Oparil et al39 |
Abbreviations: ACE, angiotensin-converting enzyme inhibitor; CCB, calcium channel blocker; BB, beta blocker; ATE, atenolol; IRB, irbesartan; ENA, enalapril; ALI, aliskiren; AML, amlodipine; LOS, losartan; OLM, olmesartan; VAL, valsartan; DOX, doxazosin.
In a recent comparative study, we demonstrated significant differences between angiotensin receptor blockers in their capacity to induce sustained vascular blockade of angiotensin II receptors when the renin-angiotensin-aldosterone system is activated by a thiazide diuretic.41 The blood pressure response to angiotensin II infusion was reduced by more than 90% with irbesartan 300 mg, irbesartan-hydrochlorothiazide 300/25 mg, and/or olmesartan-hydrochlorothiazide 20/25 mg, and by nearly 60% with valsartan-hydrochlorothiazide 160/25 mg and losartan-hydrochlorothiazide 100/25 mg (P < 0.05). In the kidney, angiotensin II infusion reduced renal plasma flow by 36% at baseline (P < 0.001). Irbesartan ±hydrochlorothiazide and olmesartan-hydrochlorothiazide blocked the renal hemodynamic response to angiotensin II almost completely, whereas valsartan-hydrochlorothiazide and losartan-hydrochlorothiazide only blunted this effect, by 34% and 45%, respectively.
Finally, a Portuguese group evaluated the effect of irbesar-tan on the circadian rhythm of blood pressure. In salt-sensitive hypertensive patients with a nondipper profile on a high-salt diet (n =12), irbesartan restored the nocturnal blood pressure decline in a dose-dependent manner.42
Efficacy in hypertension when combined with other drugs
Combination of an angiotensin receptor blocker with hydro-chlorothiazide provides additive blood pressure reduction. There is a strong pathophysiological rationale supporting this association. Diuretic-induced reduction in total body sodium provokes a secondary rise of renin, which may counterbalance its diuretic and antihypertensive effect. Simultaneously blocking the renin-angiotensin-aldosterone system prevents the action of a reactive hyperreninemia and maintains the blood pressure-lowering effect of salt depletion. The synergy between the two drugs has been shown in several clinical trials.
In a 4 × 4 placebo-controlled study involving 683 patients with mild-to-moderate hypertension, patients were randomized to one of 16 different double-blind combinations of irbesartan (0–300 mg once daily) and hydrochlorothiazide (0–25 mg once daily).34 At week 8, mean changes from baseline in trough blood pressure and total responder rates increased in a dose-dependent manner in both the single therapy and combination therapy groups. Combination therapy was more effective than either drug alone.
Two placebo-controlled studies performed in patients with mild-to-moderate hypertension showed that irbesartan 75 mg or 150 mg + hydrochlorothiazide 12.5 mg reduced blood pressure more effectively than placebo or either drug alone in both seated trough blood pressure and 24-hour ambulatory blood pressure.32,43 Irbesartan 150 mg + hydro-chlorothiazide 12.5 mg once daily resulted in reductions of 4–7/2–4 mmHg, additional to those with the individual components.15 Further, the combination therapy reduced blood pressure in patients inadequately controlled by mono-therapy with irbesartan or hydrochlorothiazide.33,44
The largest trial demonstrating the efficacy of irbesartan-hydrochlorothiazide combination therapy was INCLUSIVE (Irbesartan-Hydrochlorothiazide Blood Pressure Reductions in Diverse Patient Populations), a prospective, open-label, single-arm study (n = 844). INCLUSIVE extended the previous reported findings by evaluating the efficacy and safety of a fixed combination in patients with uncontrolled systolic blood pressure after four weeks’ monotherapy. Progressive uptitra-tion to high-dose irbesartan-hydrochlorothiazide 300/25 mg once daily, if necessary, lead to substantial reductions in systolic blood pressure (–23.0 ± 13.3 mmHg, P < 0.001) between baseline and week 18, and allowed systolic blood pressure goals to be attained in 75% of patients.
Endothelial effects
Angiotensin II has emerged as a key mediator of arteriosclerosis, by various pathogenic pathways. It induces vasocon-striction, triggers oxidative stress, stimulates the release of proinflammatory cytokines and growth factors, and induces a procoagulant state through activation of platelets and of the plasminogen-activator inhibitor. These pathophysiological effects are mediated by the AT1 receptor, whereas the AT2 receptor might have protective functions.45 The vascular protective properties of irbesartan on vascular endothelium, proven in a number of in vitro and in vivo studies, have recently been reviewed in detail by Derosa.46,47
Efficacy in diabetic nephropathy
The efficacy of irbesartan at slowing the progression of renal damage in hypertensive type 2 diabetic patients was clearly demonstrated in PRIME (Program for Irbesartan Mortality and Morbidity Evaluation).48 PRIME consisted of two large (n > 500), at least two years in duration, randomized, double-blind, placebo-controlled trials, ie, IRMA 2 (the Irbesartan Microalbuminuria Type 2 Diabetes on Hypertensive Patients trial)49 and IDNT (the Irbesartan in Diabetic Nephropathy trial).50
The IRMA 2 trial was performed in patients (n = 590) with early-stage renal damage, as indicated by microalbuminuria (30–300 mg/day) but normal creatinine levels.49 The aim of the study was to compare the effects of irbesartan 150 and 300 mg once daily and placebo on the progression to overt nephropathy, defined as the conversion of microalbuminuria to albuminuria. In the two years of follow-up, the primary endpoint of overt nephropathy was reached by significantly fewer recipients of irbesartan 300 mg once daily compared with placebo (unadjusted hazards ratio for diabetic nephropa-thy 0.3, 95% confidence interval [CI] 0.14–0.61, P < 0.001). Irbesartan 150 mg once daily significantly reduced urinary protein (albumin excretion rate) compared with placebo, but without attaining the primary endpoint. The effect of irbesartan on microalbuminuria was partly independent of its blood pressure-lowering effect.
IDNT evaluated the efficacy of irbesartan in 1715 hypertensive, type 2 diabetic patients with established nephropathy, as indicated by overt proteinuria (>900 mg/day) and elevated serum creatinine.50 The relative risk of reaching the composite primary endpoint (doubling baseline serum creatinine level, onset of end-stage renal disease, or death from any cause) was significantly lower with irbesartan 300 mg once daily than with placebo (unadjusted relative risk 0.80, 95% CI 0.66–0.97; P = 0.02) or with amlodipine 10 mg once daily (unadjusted relative risk 0.77, 95% CI 0.63–0.93; P =0.006). Again, the difference remained significant after adjustment for mean arterial pressure, suggesting a blood pressure-independent effect.
In a post hoc analysis of IDNT, the systolic blood pressure achieved strongly predicted renal outcome, and progressive lowering to 120 mmHg was associated with improved renal and patient survival, independently of baseline renal function.51 Below this threshold, all-cause mortality increased. Additional evidence of a beneficial antiproteinuric effect of irbesartan beyond blood pressure control emerged from a small placebo-controlled crossover trial, where irbesartan improved microalbuminuria also in a normotensive subgroup of diabetic patients with early-stage microalbuminuric nephropathy.52
There are some arguments to explain this renoprotective effect. Irbesartan has been found to induce renal vasodilata-tion without altering glomerular filtration rate, to improve endothelial function, and to reduce oxidative stress and inflammation in the kidney.49,53–58 In an animal model, irbesartan normalized the deficiency in podocytary nephrin expression, a protein involved in glomerular filtration barrier function.59 All these data suggest that blockade of the AT1 receptor confers renal protection beyond its purely hemodynamic effect.
The recently published IMPROVE (Irbesartan in the Management of Proteinuric Patients at High Risk for Vascular Events) study analyzed the potential benefit of a dual blockade of the renin-angiotensin-aldosterone system on albuminuria, in a population of hypertensive, mainly diabetic (87% with type 2 diabetes, 2% with type 1 diabetes patients at high cardiovascular risk, with albuminuric kidney disease.60 Patients were randomized to 20 weeks’ treatment with ramipril 10 mg + irbesartan 150–300 mg (both once daily) or ramipril 10 mg + placebo. The study showed no further benefit on albuminuria reduction in patients treated with combination therapy, despite better blood pressure control. Subgroup analyses showed that the largest reduction in albuminuria occurred in patients with overt nephropathy, without reaching statistical significance.
Efficacy in cardiac disease
The evidence of a positive impact of irbesartan on left ventricular mass in patients with mild-to-moderate hypertension is based on two comparative trials, ie, an open-label/blinded-endpoint study (n = 60) and a randomized double-blind study (n = 115).61,62 Over a six-month period, irbesartan 150–300 mg once daily was found to induce significantly greater left ventricular mass index regression than amlo-dipine 5–10 mg once daily and atenolol 50–100 mg once daily, despite similar blood pressure control.61,62 Moreover, compared with atenolol, irbesartan significantly reduced QT and corrected QT interval dispersion (the difference between maximal and minimal QT intervals within a 12-lead surface electrocardiogram), with a theoretical reduction in the risk of arrhythmias.63 There was a similar improvement of diastolic function in both groups, related to changes in ventricular geometry and blood pressure control for irbesartan, and only to blood pressure reduction for atenolol.64 Of note, similar beneficial cardiac effects have been demonstrated with other angiotensin receptor blockers, indicating that these effects are not unique to irbesartan.
In the IDNT trial, irbesartan reduced the incidence of congestive heart failure episodes (most, but not all, requiring hospitalization) compared with placebo (hazards ratio 0.72, 95% CI 0.52–1.0; P = 0.048) or amlodipine (hazards ratio 0.65, 96% CI 0.48–0.87; P = 0.004).50,65
More recently, the data of the I-PRESERVE trial have been published.66 This was a large, multicenter, placebo-controlled trial performed in a population of 4128 patients ≥60 years, with New York Heart Association Class II–IV heart failure and an ejection fraction ≥45%. Despite a reduction in blood pressure of 3.6/1.9 mmHg over four years of follow-up, irbesartan 300 mg once daily did not yield cardiovascular benefits over placebo on the primary composite outcome of all-cause mortality or hospitalization for a cardiovascular cause. These data tend to confirm the absence of benefits of renin-angiotensin-aldosterone system inhibition in patients with diastolic dysfunction.
Data from studies with angiotensin-converting enzyme inhibitors provided evidence that the renin-angiotensin-aldosterone system is involved in atrial remodeling in atrial fibrillation. Some trials have evaluated the effect of irbesar-tan in atrial fibrillation. A Spanish prospective trial showed that adding irbesartan to amiodarone was more effective in the maintenance of sinus rhythm than amiodarone alone in patients with persistent atrial fibrillation after cardiover-sion to sinus rhythm.67 Patients treated with amiodarone-irbesartan had a greater probability of remaining free of atrial fibrillation than patients treated with amiodarone alone over a median follow-up time of 254 days (79.5% versus 55.9%, P = 0.007).
This finding has recently been reconfirmed in a randomized, controlled Chinese trial performed in patients with atrial fibrillation following rheumatic valve replacement and cardioversion. The combination of amiodarone and irbesartan demonstrated a higher rate of maintenance of sinus rhythm (69.8% versus 40.5%, P = 0.01) and better atrial fibrillation-free survival (P = 0.006) than amiodarone alone during the one-year follow-up period.68
The ACTIVE-I study is part of a larger research program in atrial fibrillation, which 9016 patients enrolled in 41 countries were randomly assigned to receive irbesartan or placebo for a mean of 4.1 years.69 The study was completed in June 2009. The difference in systolic blood pressure between the groups was approximately 3 mmHg. According to the study results, irbesartan was not associated with a reduction in the first coprimary endpoint of major vascular events, the composite of cardiovascular death, heart attack, or stroke (5.4% per year in each group, P = 0.846). There was a slight but nonsignificant reduction in the second coprimary endpoint of major vascular events plus hospitalization for heart failure (7.3% in the irbesartan group versus 7.7% in the placebo group, P = 0.122) due to a 14% reduction in the risk for heart failure hospitalization in the irbesartan group versus the placebo group (2.7% versus 3.2%, P = 0.018). A post hoc analysis revealed a 13% reduction in the composite endpoint of stroke, non-central nervous system embolism, and transient ischemic attack in patients taking irbesartan versus placebo (2.9% versus 3.4%, P = 0.02).
Safety and tolerability
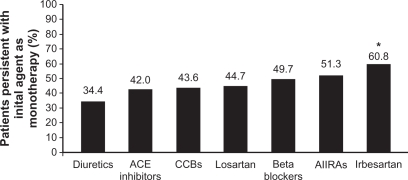
Poor adherence with therapy has been recognized as a causal factor of failure of blood pressure control.70 Persistence with a drug, defined as the time a patient remains on the prescribed medication, can be regarded as a good general indicator of the satisfaction of both patients and physicians with the efficacy and tolerability of the treatment. It is therefore not surprising that the persistence rate varies between drug classes. A British comparative study and a large Canadian cohort study showed angiotensin-converting enzyme inhibitors as the best and diuretics as the poorest persistence builders.70,71 Data regarding persistence with angiotensin receptor blockers, not yet included in the previous studies, are now emerging. Two population-based trials including angiotensin receptor blockers revealed that patients have a better persistence with angiotensin receptor blockers than with all other antihypertensive drug classes up to three years.72,73 In a European cohort study of 2416 newly diagnosed hypertensive patients treated with monotherapy by general practitioners, irbesartan scored highest, with a persistence rate at one year of 60.8%, compared with other angio-tensin receptor blockers and other drug classes (Figure 1).74 This improved persistence has been attributed in part to the efficacy of the compounds and mainly to the placebo-like side effect profile, verified for all clinically relevant dosages of irbesartan.23,24,37,75,76 In a pooled analysis of nine 4–12-week, placebo-controlled studies involving 2606 mild-to-moderate hypertensive patients, the overall incidence of adverse events was similar in the irbesartan group (<900 mg once daily) and placebo-treated group (21% versus 20%, respectively), without clinical relevant differences in type of adverse events.75 Adverse events reported in ≥1% of irbesartan recipients and with a numerically higher incidence than with placebo, included diarrhea, dyspepsia/heartburn, and fatigue. None of these adverse events occurred at an incidence ≥5%. The tolerability profile of irbesartan is, in many aspects, comparable with that of other angiotensin receptor blockers.
Figure 1.
Persistence at one year by antihypertensives.74
Copyright © 2002, Nature Publishing Group. Reproduced with permission from Hasford et al. http://www.nature.com/jhh/index.html;
Notes: *P = 0.001 versus all other antihypertensive classes and versus losartan; P = 0.009 versus all other angiotensin II receptor antagonists.
Abbreviations: ACE, angiotensin-converting enzyme; CCBs, calcium channel blockers; AIIRAs, angiotensin II receptor antagonists.
The results of a post-marketing survey in Switzerland including 4769 hypertensive patients treated with irbesartan further emphasized the value of the good tolerability profile in enhancing treatment adherence.77 Of the 4639 patients with complete follow-up data, 82.5% were persistent with irbesartan for more than four months. The tolerability profile emerged as the most important predictive factor of long-term persistence with therapy. The favorable safety profile was also confirmed in long-term treatment. In two-year extension studies, irbesartan as monotherapy or as combination therapy with hydrochlorothiazide was associated with low discontinuation rates for adverse events (5.3%–9.1%) and low incidences of serious adverse events (5.3%–8.6%).78,79 In previously reported comparison studies with other anti-hypertensive major classes, the overall incidence of adverse events with irbesartan was similar to that of the comparator agent, including atenolol,28 enalapril,20–22,37 amlodipine,26 doxazosin,38 aliskiren,36 and other angiotensin receptor blockers. The incidence of cough was significantly lower with irbesartan than with the angiotensin-converting enzyme inhibitor, enalapril (overall range 0%–10% versus 8%–21%, respectively).20–22,27 However, the incidence of cough was comparable with other angiotensin receptor blockers.
The good tolerability profile is conserved when irbesartan is administered in combination with hydrochlorothiazide, with an overall incidence rate of adverse events comparable with that of placebo. Adverse events, transient and mild, are similar to those found in irbesartan and/or hydrochlorothiazide monotherapy.32–34 Safety and tolerability of fixed-dose irbesar-tan-hydrochlorothiazide for rapid control of severe hypertension has recently been confirmed in a randomized, controlled trial.80 Despite more rapid and aggressive blood pressure-lowering, initial fixed-dose irbesartan-hydrochlorothiazide demonstrated a comparable adverse event profile to irbesartan monotherapy in patients with severe hypertension.
When considering hypertensive patients with type 2 diabetes and nephropathy, the adverse event experiences were generally similar to those reported by the hypertensive population. However, in the population with overt nephropa-thy in the IDNT trial, the incidence rates of dizziness, ortho-static dizziness, orthostatic hypotension, and hyperkalemia (≥6.0 mmol/L) were significantly higher with irbesartan 300 mg once daily than with placebo.50 The occurrence of hyperkalemia led to significantly higher discontinuation rates in the irbesartan treated-group (1.9%) than in the placebo (0.5%) or amlodipine group (0.4%, P = 0.01 for both between-group comparisons). Of note, hyperkalemia is a relative contraindication to the prescription of blockers of the renin-angiotensin system, and the addition of an angio-tensin receptor blocker, on top of an angiotensin-converting enzyme inhibitor or a direct renin inhibitor, may favor the development of life-threatening hyperkalemia, particularly in patients with reduced renal function.
Recent research has focused on the impact of irbesartan on quality of life and exercise performance in cardiology patients. In a randomized, controlled trial focused on cardiac insufficiency symptoms, irbesartan added to angiotensin-converting enzyme inhibitors produced significant improvement in physical capacity (six-minute hall-walk distance, P < 0.01), exercise time (P = 0.01), New York Heart Association class (P < 0.005), and quality of life score (P < 0.005) compared with placebo.81
Based on the results of IDNT, a number of modeled (Markov modeling) pharmacoeconomic analyses were published.50 Treatment with irbesartan in hypertensive patients with type 2 diabetes and nephropathy resulted in improved life expectancy and appeared to be cost-saving compared with amlodipine or control therapy over a prospective period of 10 years and/or 25 years for the US, Canada, and some European countries (Belgium, France, Italy, Spain, UK).82–87 The early initiation of irbesartan (at the microalbuminuric stage) improved life expectancy and saved costs compared with later initiation (in the presence of overt nephropathy).88
Conclusion
Used as monotherapy or in association with hydrochlorothiazide, irbesartan is an effective antihypertensive drug in a variety of mild-to-moderate hypertensive populations, including patients with diabetes, obesity, renal insufficiency, and cardiovascular disease. In comparative trials, irbesartan is at least as effective and sometimes superior to comparator agents of the major antihypertensive classes. There is some evidence that irbesartan provides protective cardiovascular effects beyond its antihypertensive action. This is particularly true for its beneficial effects on slowing the progression of early-stage and late-stage renal disease in hypertensive patients with type 2 diabetes and on promoting regression of left ventricular mass in patients with hypertension and left ventricular hypertrophy. Recent research has further highlighted the positive role of irbesartan in preventing recurrence of arrhythmia in patients with persistent atrial fibrillation, when added to classical antiarrhythmic therapy. Finally, some data suggest an additional benefit in cardiac disease, through a reduction in the risk of heart failure episodes, as observed with other angiotensin receptor blockers.
In addition to its therapeutic efficacy, irbesartan can claim, like other angiotensin receptor blockers, an extremely favorable, placebo-like side effect profile, as has been shown in numerous real-life trials, even in the long term. It is therefore not surprising that irbesartan scores well for patient acceptation and adherence rates.
Footnotes
Disclosure
The preparation of this review was not supported by any external funding.
References
- 1.Probstfield JL, O’Brien KD. Progression of cardiovascular damage: The role of renin-angiotensin system blockade. Am J Cardiol. 2010;105:10A–20A. doi: 10.1016/j.amjcard.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Ribeiro AB. Angiotensin II antagonists – therapeutic benefits spanning the cardiovascular disease continuum from hypertension to heart failure and diabetic nephropathy. Curr Med Res Opin. 2006;22:1–16. doi: 10.1185/030079905X75041. [DOI] [PubMed] [Google Scholar]
- 3.Grossman E, Messerli FH, Neutel JM. Angiotensin II receptor blockers: Equal or preferred substitutes for ACE inhibitors. Arch Intern Med. 2000;160:1905–1911. doi: 10.1001/archinte.160.13.1905. [DOI] [PubMed] [Google Scholar]
- 4.Chan P, Tomlinson B, Huang TY, Ko JT, Lin TS, Lee YS. Double-blind comparison of losartan, lisinopril, and metolazone in elderly hypertensive patients with previous angiotensin-converting enzyme inhibitor-induced cough. J Clin Pharmacol. 1997;37:253–257. doi: 10.1002/j.1552-4604.1997.tb04788.x. [DOI] [PubMed] [Google Scholar]
- 5.Burnier M. Medication adherence and persistence as the cornerstone of effective antihypertensive therapy. Am J Hypertens. 2006;19:1190–1196. doi: 10.1016/j.amjhyper.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Croom KF, Plosker GL. Irbesartan: A review of its use in hypertension and diabetic nephropathy. Drugs. 2008;68:1543–1569. doi: 10.2165/00003495-200868110-00008. [DOI] [PubMed] [Google Scholar]
- 7.Herbert JM, Delisee C, Dol F, et al. Effect of SR47436, a novel angio-tensin II AT1 receptor antagonist, on human vascular smooth muscle cells in vitro. Eur J Pharmacol. 1994;251:143–150. doi: 10.1016/0014-2999(94)90394-8. [DOI] [PubMed] [Google Scholar]
- 8.Brunner HR. The new angiotensin II receptor antagonist, irbesartan: Pharmacokinetic and pharmacodynamic considerations. Am J Hypertens. 1997;10:311S–317S. doi: 10.1016/s0895-7061(97)00391-9. [DOI] [PubMed] [Google Scholar]
- 9.Marino MR, Langenbacher K, Ford NF, Uderman HD. Pharmacokinetics and pharmacodynamics of irbesartan in healthy subjects. J Clin Pharmacol. 1998;38:246–255. doi: 10.1002/j.1552-4604.1998.tb04422.x. [DOI] [PubMed] [Google Scholar]
- 10.Ruilope L. Human pharmacokinetic/pharmacodynamic profile of irbesartan: A new potent angiotensin II receptor antagonist. J Hypertens Suppl. 1997;15:S15–S20. [PubMed] [Google Scholar]
- 11.Vachharajani NN, Shyu WC, Smith RA, Greene DS. The effects of age and gender on the pharmacokinetics of irbesartan. Br J Clin Pharmacol. 1998;46:611–613. doi: 10.1046/j.1365-2125.1998.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marino MR, Langenbacher KM, Raymond RH, Ford NF, Lasseter KC. Pharmacokinetics and pharmacodynamics of irbesartan in patients with hepatic cirrhosis. J Clin Pharmacol. 1998;38:347–356. doi: 10.1002/j.1552-4604.1998.tb04434.x. [DOI] [PubMed] [Google Scholar]
- 13.Kostis JB, Vachharajani NN, Hadjilambris OW, Kollia GD, Palmisano M, Marino MR. The pharmacokinetics and pharmacodynamics of irbesartan in heart failure. J Clin Pharmacol. 2001;41:935–942. doi: 10.1177/00912700122010906. [DOI] [PubMed] [Google Scholar]
- 14.Sica DA, Marino MR, Hammett JL, Ferreira I, Gehr TW, Ford NF. The pharmacokinetics of irbesartan in renal failure and maintenance hemodialysis. Clin Pharmacol Ther. 1997;62:610–618. doi: 10.1016/S0009-9236(97)90080-1. [DOI] [PubMed] [Google Scholar]
- 15.Croom KF, Curran MP, Goa KL, Perry CM. Irbesartan: A review of its use in hypertension and in the management of diabetic nephropathy. Drugs. 2004;64:999–1028. doi: 10.2165/00003495-200464090-00011. [DOI] [PubMed] [Google Scholar]
- 16.Marino MR, Vachharajani NN. Drug interactions with irbesartan. Clin Pharmacokinet. 2001;40:605–614. doi: 10.2165/00003088-200140080-00004. [DOI] [PubMed] [Google Scholar]
- 17.Kovacs SJ, Wilton J, Blum R. Steady state (SS) pharmacokinetics (PK) of irbesartan alone and in combination with fluconazole (F) Clin Pharmacol Ther. 1999;65 Abstr 132. [Google Scholar]
- 18.Kistler T, Ambuhl PM. Renal safety of combined cyclooxygenase 2 (COX-2) inhibitor and angiotensin II receptor blocker administration in mild volume depletion. Swiss Med Wkly. 2001;131:193–198. doi: 10.4414/smw.2001.09680. [DOI] [PubMed] [Google Scholar]
- 19.Hong X, Zhang S, Mao G, et al. CYP2C9*3 allelic variant is associated with metabolism of irbesartan in Chinese population. Eur J Clin Pharmacol. 2005;61:627–634. doi: 10.1007/s00228-005-0976-8. [DOI] [PubMed] [Google Scholar]
- 20.Lacourciere Y. A multicenter, randomized, double-blind study of the antihypertensive efficacy and tolerability of irbesartan in patients aged > or = 65 years with mild to moderate hypertension. Clin Ther. 2000;22:1213–1224. doi: 10.1016/s0149-2918(00)83064-7. [DOI] [PubMed] [Google Scholar]
- 21.Mimran A, Ruilope L, Kerwin L, et al. A randomised, double-blind comparison of the angiotensin II receptor antagonist, irbesartan, with the full dose range of enalapril for the treatment of mild-to-moderate hypertension. J Hum Hypertens. 1998;12:203–208. doi: 10.1038/sj.jhh.1000591. [DOI] [PubMed] [Google Scholar]
- 22.Coca A, Calvo C, Garcia-Puig J, et al. A multicenter, randomized, double-blind comparison of the efficacy and safety of irbesartan and enalapril in adults with mild to moderate essential hypertension, as assessed by ambulatory blood pressure monitoring: The MAPAVEL Study (Monitorizacion Ambulatoria Presion Arterial APROVEL) Clin Ther. 2002;24:126–138. doi: 10.1016/s0149-2918(02)85010-x. [DOI] [PubMed] [Google Scholar]
- 23.Mancia G, Korlipara K, van RP, Villa G, Silvert B. An ambulatory blood pressure monitoring study of the comparative antihypertensive efficacy of two angiotensin II receptor antagonists, irbesartan and valsartan. Blood Press Monit. 2002;7:135–142. doi: 10.1097/00126097-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Kassler-Taub K, Littlejohn T, Elliott W, Ruddy T, Adler E. Comparative efficacy of two angiotensin II receptor antagonists, irbesartan and losartan in mild-to-moderate hypertension. Irbesartan/Losartan Study Investigators. Am J Hypertens. 1998;11:445–453. doi: 10.1016/s0895-7061(97)00491-3. [DOI] [PubMed] [Google Scholar]
- 25.Oparil S, Guthrie R, Lewin AJ, et al. An elective-titration study of the comparative effectiveness of two angiotensin II-receptor blockers, irbesartan and losartan. Irbesartan/Losartan Study Investigators. Clin Ther. 1998;20:398–409. doi: 10.1016/s0149-2918(98)80051-9. [DOI] [PubMed] [Google Scholar]
- 26.Neutel JM, Germino FW, Smith D. Comparison of monotherapy with irbesartan 150 mg or amlodipine 5 mg for treatment of mild-to-moderate hypertension. J Renin Angiotensin Aldosterone Syst. 2005;6:84–89. doi: 10.3317/jraas.2005.014. [DOI] [PubMed] [Google Scholar]
- 27.Chiou KR, Chen CH, Ding PY, et al. Randomized, double-blind comparison of irbesartan and enalapril for treatment of mild to moderate hypertension. Zhonghua Yi Xue Za Zhi (Taipei) 2000;63:368–376. [PubMed] [Google Scholar]
- 28.Stumpe KO, Haworth D, Hoglund C, et al. Comparison of the angiotensin II receptor antagonist irbesartan with atenolol for treatment of hypertension. Blood Press. 1998;7:31–37. doi: 10.1080/080370598437547. [DOI] [PubMed] [Google Scholar]
- 29.Fogari R, Ambrosoli S, Corradi L, et al. 24-hour blood pressure control by once-daily administration of irbesartan assessed by ambulatory blood pressure monitoring. Irbesartan Multicenter Investigators’ Group. J Hypertens. 1997;15:1511–1518. doi: 10.1097/00004872-199715120-00020. [DOI] [PubMed] [Google Scholar]
- 30.Pool JL, Guthrie RM, Littlejohn TW, III, et al. Dose-related antihyperten-sive effects of irbesartan in patients with mild-to-moderate hypertension. Am J Hypertens. 1998;11:462–470. doi: 10.1016/s0895-7061(97)00501-3. [DOI] [PubMed] [Google Scholar]
- 31.Reeves RA, Lin CS, Kassler-Taub K, Pouleur H. Dose-related efficacy of irbesartan for hypertension: An integrated analysis. Hypertension. 1998;31:1311–1316. doi: 10.1161/01.hyp.31.6.1311. [DOI] [PubMed] [Google Scholar]
- 32.Howe P, Phillips P, Saini R, Kassler-Taub K. The antihypertensive efficacy of the combination of irbesartan and hydrochlorothiazide assessed by 24-hour ambulatory blood pressure monitoring. Irbesartan Multicenter Study Group. Clin Exp Hypertens. 1999;21:1373–1396. doi: 10.3109/10641969909070855. [DOI] [PubMed] [Google Scholar]
- 33.Rosenstock J, Rossi L, Lin CS, MacNeil D, Osbakken M. The effects of irbesartan added to hydrochlorothiazide for the treatment of hypertension in patients non-responsive to hydrochlorothiazide alone. J Clin Pharm Ther. 1998;23:433–440. doi: 10.1046/j.1365-2710.1998.00184.x. [DOI] [PubMed] [Google Scholar]
- 34.Kochar M, Guthrie R, Triscari J, Kassler-Taub K, Reeves RA. Matrix study of irbesartan with hydrochlorothiazide in mild-to-moderate hypertension. Am J Hypertens. 1999;12:797–805. doi: 10.1016/s0895-7061(99)00053-9. [DOI] [PubMed] [Google Scholar]
- 35.Kawano Y, Sato Y, Yoshinaga K. A randomized trial of the effect of an angiotensin II receptor blocker SR47436 (irbesartan) on 24-hour blood pressure in patients with essential hypertension. Hypertens Res. 2008;31:1753–1763. doi: 10.1291/hypres.31.1753. [DOI] [PubMed] [Google Scholar]
- 36.Gradman AH, Schmieder RE, Lins RL, Nussberger J, Chiang Y, Bedigian MP. Aliskiren, a novel orally effective renin inhibitor, provides dose-dependent antihypertensive efficacy and placebo-like tolerability in hypertensive patients. Circulation. 2005;111:1012–1018. doi: 10.1161/01.CIR.0000156466.02908.ED. [DOI] [PubMed] [Google Scholar]
- 37.Larochelle P, Flack JM, Marbury TC, Sareli P, Krieger EM, Reeves RA. Effects and tolerability of irbesartan versus enalapril in patients with severe hypertension. Irbesartan Multicenter Investigators. Am J Cardiol. 1997;80:1613–1615. doi: 10.1016/s0002-9149(97)00784-4. [DOI] [PubMed] [Google Scholar]
- 38.Derosa G, Cicero AF, Gaddi A, Mugellini A, Ciccarelli L, Fogari R. Effects of doxazosin and irbesartan on blood pressure and metabolic control in patients with type 2 diabetes and hypertension. J Cardiovasc Pharmacol. 2005;45:599–604. doi: 10.1097/01.fjc.0000161403.91456.39. [DOI] [PubMed] [Google Scholar]
- 39.Oparil S, Williams D, Chrysant SG, Marbury TC, Neutel J. Comparative efficacy of olmesartan, losartan, valsartan, and irbesartan in the control of essential hypertension. J Clin Hypertens (Greenwich) 2001;3:283–291. 318. doi: 10.1111/j.1524-6175.2001.01136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith DH, Dubiel R, Jones M. Use of 24-hour ambulatory blood pressure monitoring to assess antihypertensive efficacy: A comparison of olmesartan medoxomil, losartan potassium, valsartan, and irbesartan. Am J Cardiovasc Drugs. 2005;5:41–50. doi: 10.2165/00129784-200505010-00006. [DOI] [PubMed] [Google Scholar]
- 41.Coltamai L, Maillard M, Simon A, Vogt B, Burnier M. Comparative vascular and renal tubular effects of angiotensin II receptor blockers combined with a thiazide diuretic in humans. J Hypertens. 2010;28:520–526. doi: 10.1097/HJH.0b013e3283346be1. [DOI] [PubMed] [Google Scholar]
- 42.Polonia J, Diogo D, Caupers P, Damasceno A. Influence of two doses of irbesartan on non-dipper circadian blood pressure rhythm in salt-sensitive black hypertensives under high salt diet. J Cardiovasc Pharmacol. 2003;42:98–104. doi: 10.1097/00005344-200307000-00015. [DOI] [PubMed] [Google Scholar]
- 43.Weber M, Saini R, Kassler-Taub K. Irbesartan combined with low-dose hydrochlorothiazide for mild-to-moderate hypertension. J Hypertens. 1998;(Suppl 2) Abstr 129. [Google Scholar]
- 44.Weir MR, Tolchin N, Toth P. Addition of hydrochlorothiazide to irbesartan produces further dose-related reduction in blood pressure within two weeks. Hypertension. 1998;(Suppl 2):130. [Google Scholar]
- 45.Hunyady L, Catt KJ. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol. 2006;20:953–970. doi: 10.1210/me.2004-0536. [DOI] [PubMed] [Google Scholar]
- 46.Hirata Y, Nagata D, Suzuki E, Nishimatsu H, Suzuki J, Nagai R. Diagnosis and treatment of endothelial dysfunction in cardiovascular disease. Int Heart J. 2010;51:1–6. doi: 10.1536/ihj.51.1. [DOI] [PubMed] [Google Scholar]
- 47.Derosa G, AT Salvadeo S. Endothelial function, blood pressure control, and risk modification: Impact of irbesartan alone or in combination. IBPC. 2010 May 18; doi: 10.2147/ibpc.s6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ravera M, Ratto E, Vettoretti S, Parodi D, Deferrari G. Prevention and treatment of diabetic nephropathy: The program for irbesar-tan mortality and morbidity evaluation. J Am Soc Nephrol. 2005;16(Suppl 1):S48–S52. doi: 10.1681/asn.2004110957. [DOI] [PubMed] [Google Scholar]
- 49.Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 50.Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 51.Pohl MA, Blumenthal S, Cordonnier DJ, et al. Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: Clinical implications and limitations. J Am Soc Nephrol. 2005;16:3027–3037. doi: 10.1681/ASN.2004110919. [DOI] [PubMed] [Google Scholar]
- 52.Sasso FC, Carbonara O, Persico M, et al. Irbesartan reduces the albumin excretion rate in microalbuminuric type 2 diabetic patients independently of hypertension: A randomized double-blind placebo-controlled crossover study. Diabetes Care. 2002;25:1909–1913. doi: 10.2337/diacare.25.11.1909. [DOI] [PubMed] [Google Scholar]
- 53.Burnier M, Hagman M, Nussberger J, et al. Short-term and sustained renal effects of angiotensin II receptor blockade in healthy subjects. Hypertension. 1995;25:602–609. doi: 10.1161/01.hyp.25.4.602. [DOI] [PubMed] [Google Scholar]
- 54.Schmitt F, Martinez F, Brillet G, et al. Acute renal effects of AT1-receptor blockade after exogenous angiotensin II infusion in healthy subjects. J Cardiovasc Pharmacol. 1998;31:314–321. doi: 10.1097/00005344-199802000-00019. [DOI] [PubMed] [Google Scholar]
- 55.Pechere-Bertschi A, Nussberger J, Decosterd L, et al. Renal response to the angiotensin II receptor subtype 1 antagonist irbesartan versus enalapril in hypertensive patients. J Hypertens. 1998;16:385–393. doi: 10.1097/00004872-199816030-00016. [DOI] [PubMed] [Google Scholar]
- 56.Persson F, Rossing P, Hovind P, et al. Irbesartan treatment reduces biomarkers of inflammatory activity in patients with type 2 diabetes and microalbuminuria: An IRMA 2 substudy. Diabetes. 2006;55:3550–3555. doi: 10.2337/db06-0827. [DOI] [PubMed] [Google Scholar]
- 57.Ceriello A, Assaloni R, Da RR, et al. Effect of atorvastatin and irbe-sartan, alone and in combination, on postprandial endothelial dysfunction, oxidative stress, and inflammation in type 2 diabetic patients. Circulation. 2005;111:2518–2524. doi: 10.1161/01.CIR.0000165070.46111.9F. [DOI] [PubMed] [Google Scholar]
- 58.Sola S, Mir MQ, Cheema FA, et al. Irbesartan and lipoic acid improve endothelial function and reduce markers of inflammation in the metabolic syndrome: Results of the Irbesartan and Lipoic Acid in Endothelial Dysfunction (ISLAND) study. Circulation. 2005;111:343–348. doi: 10.1161/01.CIR.0000153272.48711.B9. [DOI] [PubMed] [Google Scholar]
- 59.Bonnet F, Cooper ME, Kawachi H, Allen TJ, Boner G, Cao Z. Irbesartan normalises the deficiency in glomerular nephrin expression in a model of diabetes and hypertension. Diabetologia. 2001;44:874–877. doi: 10.1007/s001250100546. [DOI] [PubMed] [Google Scholar]
- 60.Bakris GL, Ruilope L, Locatelli F, et al. Treatment of microalbuminuria in hypertensive subjects with elevated cardiovascular risk: Results of the IMPROVE trial. Kidney Int. 2007;72:879–885. doi: 10.1038/sj.ki.5002455. [DOI] [PubMed] [Google Scholar]
- 61.Gaudio C, Ferri FM, Giovannini M, et al. Comparative effects of irbe-sartan versus amlodipine on left ventricular mass index in hypertensive patients with left ventricular hypertrophy. J Cardiovasc Pharmacol. 2003;42:622–628. doi: 10.1097/00005344-200311000-00007. [DOI] [PubMed] [Google Scholar]
- 62.Malmqvist K, Kahan T, Edner M, et al. Regression of left ventricular hypertrophy in human hypertension with irbesartan. J Hypertens. 2001;19:1167–1176. doi: 10.1097/00004872-200106000-00023. [DOI] [PubMed] [Google Scholar]
- 63.Malmqvist K, Kahan T, Edner M, Bergfeldt L. Comparison of actions of irbesartan versus atenolol on cardiac repolarization in hypertensive left ventricular hypertrophy: Results from the Swedish Irbesartan Left Ventricular Hypertrophy Investigation Versus Atenolol (SILVHIA) Am J Cardiol. 2002;90:1107–1112. doi: 10.1016/s0002-9149(02)02777-7. [DOI] [PubMed] [Google Scholar]
- 64.Muller-Brunotte R, Edner M, Malmqvist K, Kahan T. Irbesartan and atenolol improve diastolic function in patients with hypertensive left ventricular hypertrophy. J Hypertens. 2005;23:633–640. doi: 10.1097/01.hjh.0000160222.17092.b8. [DOI] [PubMed] [Google Scholar]
- 65.Berl T, Hunsicker LG, Lewis JB, et al. Cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial of patients with type 2 diabetes and overt nephropathy. Ann Intern Med. 2003;138:542–549. doi: 10.7326/0003-4819-138-7-200304010-00010. [DOI] [PubMed] [Google Scholar]
- 66.Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 67.Madrid AH, Bueno MG, Rebollo JM, et al. Use of irbesartan to maintain sinus rhythm in patients with long-lasting persistent atrial fibrillation: A prospective and randomized study. Circulation. 2002;106:331–336. doi: 10.1161/01.cir.0000022665.18619.83. [DOI] [PubMed] [Google Scholar]
- 68.Ji Q, Mei Y, Wang X, et al. Combination of irbesartan and amiodarone to maintain sinus rhythm in patients with persistent atrial fibrillation after rheumatic valve replacement. Circ J. 2010;74:1873–1879. doi: 10.1253/circj.cj-10-0254. [DOI] [PubMed] [Google Scholar]
- 69.Yusuf S. ACTIVE-I: Irbesartan linked with reduced HF complications, embolic events in patients with AF. Presented at the European Society of Cardiology Congress; Barcelona, Spain. August 29–September 29, 2009. [Google Scholar]
- 70.Jones JK, Gorkin L, Lian JF, Staffa JA, Fletcher AP. Discontinuation of and changes in treatment after start of new courses of antihypertensive drugs: A study of a United Kingdom population. BMJ. 1995;311:293–295. doi: 10.1136/bmj.311.7000.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Caro JJ, Speckman JL, Salas M, Raggio G, Jackson JD. Effect of initial drug choice on persistence with antihypertensive therapy: The importance of actual practice data. CMAJ. 1999;160:41–46. [PMC free article] [PubMed] [Google Scholar]
- 72.Marentette MA, Gerth WC, Billings DK, Zarnke KB. Antihypertensive persistence and drug class. Can J Cardiol. 2002;18:649–656. [PubMed] [Google Scholar]
- 73.Degli EE, Sturani A, Di MM, et al. Long-term persistence with antihyper-tensive drugs in new patients. J Hum Hypertens. 2002;16:439–444. doi: 10.1038/sj.jhh.1001418. [DOI] [PubMed] [Google Scholar]
- 74.Hasford J, Mimran A, Simons WR. A population-based European cohort study of persistence in newly diagnosed hypertensive patients. J Hum Hypertens. 2002;16:569–575. doi: 10.1038/sj.jhh.1001451. [DOI] [PubMed] [Google Scholar]
- 75.Simon TA, Gelarden RT, Freitag SA, Kassler-Taub KB, Davies R. Safety of irbesartan in the treatment of mild to moderate systemic hypertension. Am J Cardiol. 1998;82:179–182. doi: 10.1016/s0002-9149(98)00313-0. [DOI] [PubMed] [Google Scholar]
- 76.Man in’t Veld AJ. Clinical overview of irbesartan: Expanding the therapeutic window in hypertension. J Hypertens Suppl. 1997;15:S27–S33. [PubMed] [Google Scholar]
- 77.Burnier M, Hess B, Greminger P, Waeber B. Determinants of persistence in hypertensive patients treated with irbesartan: Results of a postmarketing survey. BMC Cardiovasc Disord. 2005;5:13. doi: 10.1186/1471-2261-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Littlejohn T, III, Saini R, Kassler-Taub K, Chrysant SG, Marbury T. Long-term safety and antihypertensive efficacy of irbesartan: Pooled results of five open-label studies. Clin Exp Hypertens. 1999;21:1273–1295. doi: 10.3109/10641969909070849. [DOI] [PubMed] [Google Scholar]
- 79.Raskin P, Guthrie R, Flack J, Reeves R, Saini R. The long-term antihyper-tensive activity and tolerability of irbesartan with hydrochlorothiazide. J Hum Hypertens. 1999;13:683–687. doi: 10.1038/sj.jhh.1000888. [DOI] [PubMed] [Google Scholar]
- 80.Franklin SS, Neutel JM. Efficacy and safety of irbesartan/HCTZ in severe hypertension according to cardiometabolic factors. J Clin Hypertens (Greenwich) 2010;12:487–494. doi: 10.1111/j.1751-7176.2010.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kum LC, Yip GW, Lee PW, et al. Comparison of angiotensin-converting enzyme inhibitor alone and in combination with irbesartan for the treatment of heart failure. Int J Cardiol. 2008;125:16–21. doi: 10.1016/j.ijcard.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 82.Rodby RA, Chiou CF, Borenstein J, et al. The cost-effectiveness of irbesartan in the treatment of hypertensive patients with type 2 diabetic nephropathy. Clin Ther. 2003;25:2102–2119. doi: 10.1016/s0149-2918(03)80208-4. [DOI] [PubMed] [Google Scholar]
- 83.Coyle D, Rodby RA. Economic evaluation of the use of irbesartan and amlodipine in the treatment of diabetic nephropathy in patients with hypertension in Canada. Can J Cardiol. 2004;20:71–79. [PubMed] [Google Scholar]
- 84.Palmer AJ, Annemans L, Roze S, Lamotte M, Rodby RA, Cordonnier DJ. An economic evaluation of irbesartan in the treatment of patients with type 2 diabetes, hypertension and nephropathy: Cost-effectiveness of Irbesartan in Diabetic Nephropathy Trial (IDNT) in the Belgian and French settings. Nephrol Dial Transplant. 2003;18:2059–2066. doi: 10.1093/ndt/gfg232. [DOI] [PubMed] [Google Scholar]
- 85.Palmer AJ, Annemans L, Roze S. Cost-effectiveness analysis of irbe-sartan in patients with type 2 diabetes, hypertension and nephropathy: The Italian perspective. Pharmaeconomics. 2005;7:43–57. [Google Scholar]
- 86.Palmer AJ, Annemans L, Roze S, et al. Irbesartan is projected to be cost and life saving in a Spanish setting for treatment of patients with type 2 diabetes, hypertension, and microalbuminuria. Kidney Int Suppl. 2005;93:S52–S54. doi: 10.1111/j.1523-1755.2005.09312.x. [DOI] [PubMed] [Google Scholar]
- 87.Palmer AJ, Annemans L, Roze S, Lamotte M, Rodby RA, Bilous RW. An economic evaluation of the Irbesartan in Diabetic Nephropathy Trial (IDNT) in a UK setting. J Hum Hypertens. 2004;18:733–738. doi: 10.1038/sj.jhh.1001729. [DOI] [PubMed] [Google Scholar]
- 88.Palmer AJ, Annemans L, Roze S, et al. Cost-effectiveness of early irbe-sartan treatment versus control (standard antihypertensive medications excluding ACE inhibitors, other angiotensin-2 receptor antagonists, and dihydropyridine calcium channel blockers) or late irbesartan treatment in patients with type 2 diabetes, hypertension, and renal disease. Diabetes Care. 2004;27:1897–1903. doi: 10.2337/diacare.27.8.1897. [DOI] [PubMed] [Google Scholar]
- 89.Drugs@FDA [homepage on the Internet]. US Food and Drug Administration 2011. [updated 2011 April 5]. Available from: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/. Accessed April 6, 2011.
- 90.Swiss Compendium. [on the Internet] 2010 http://www.kompendium.ch Accessed March 30 2011. [Google Scholar]
- 91.Yoshinaga K. A phase III clinical study of irbesartan, an angiotensin II receptor antagonist, in patients with essential hypertension: A double-blind, intergroup, comparative trial using losartan potassium as the comparator. Rinsholyaku. 2008;24:543–573. Japanese. [Google Scholar]