SUMMARY
There are no US FDA-approved therapies for the management of frontotemporal dementia (FTD). Evidence-based medicine that would support a FDA indication for the treatment of FTD requires large-scale, randomized, double-blind, placebo-controlled trials that do not currently exist. Progress in obtaining approval and therapeutic indications for FTD has been severely hampered by the heterogeneity of clinical and pathological phenotypes seen in various FTD disease states. These issues are often misinterpreted by clinicians, caregivers and patients suggesting that potential treatment options are nonexistent for this devastating disease. This article discusses these issues in the context of recent studies and publications investigating therapeutic options in FTD, and further suggests a rationale for individualized therapy in FTD. Targeting the myriad of symptoms seen in FTD requires recognition of such symptoms that may play primary or secondary roles in the spectrum of deficits that lead to functional disability in FTD, and the availability of a wide range of therapeutic options that may be helpful in alleviating such symptomatology. Fortunately, agents targeting the many cognitive, behavioral, psychiatric and motor symptoms that can be seen in FTD are readily available, having been previously developed and approved for symptomatic benefit in other disease states. In contrast to the widespread belief that beneficial treatments are not available for FTD today, our therapeutic armament is stocked with pharmacological tools that may improve quality of life for those suffering from this devastating and incurable class of degenerative diseases.
Rational selection of treatments for fronto-temporal dementia (FTD) is a major challenge for clinicians [1–17]. No US FDA-approved therapies for the management of FTD exist. Evidence-based medicine that would support a FDA indication for the treatment of FTD requires large-scale, randomized, double-blind, placebo-controlled trials that are currently lacking. Progress in obtaining approval and therapeutic indications for FTD has been severely hampered by the heterogeneity of clinical and pathological phenotypes seen in various FTD disease states [1–17]. Clinical heterogeneity confounds the selection of appropriate outcome measures currently, while underlying pathological heterogeneity may prove to be a major confound as we move into an era of disease-modifying therapies that target biological mechanisms for neuronal degeneration [18–21]. These issues may be misinterpreted by clinicians, caregivers and patients, suggesting that potential treatment options are nonexistent for this devastating disease today. This article discusses these issues in the context of recent studies and publications investigating therapeutic options in FTD, and further suggests a rationale for individualized therapy in FTD using readily available agents that have been developed for symptomatic benefit in other disease states [1–17]. While the therapeutic armament for the treatment of FTD is not ideal at present, a wealth of potential strategies exist that may alleviate symptoms, improve quality of life and make this devastating disease more tolerable for the countless afflicted and their caregivers.
Issues of clinical heterogeneity in FTD
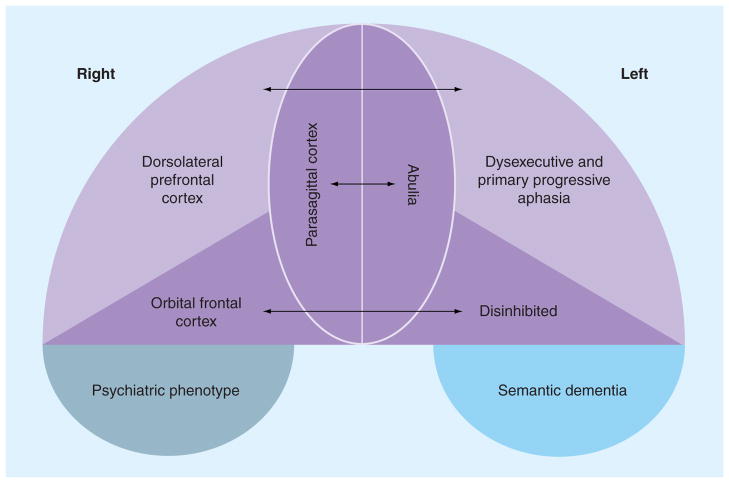
Clinical (symptomatic) heterogeneity stems partly from the complex functions of the frontal and temporal lobes that can be preferentially affected in various forms of FTD (Figure 1) [2,6–9,11,14–16,22–26]. In addition, brainstem, spinal cord and striatal involvement can produce a myriad of coexistent symptoms including bulbar, corticospinal and extrapyramidal dysfunction that further add to the heterogeneity of clinical phenotypes in FTD and complicate the treatment of FTD [2,6–9,11,14–16,24,25,27–29]. The focal predilection of FTD pathology for discrete brain areas remains a mystery. A total of eight different major clinical phenotypes of FTD exist and are highlighted in Table 1. Such clinical heterogeneity is further confounded by the fact that each distinct clinical presentation may be either sporadic or caused by genetic mutations in at least five distinct genes, and are associated with multiple different pathological features [30–32]. Given the many disparate clinical phenotypes and diagnostic classifications for FTD that exist today and the lack of evidence-based or FDA-approved therapies for FTD, clinicians may feel as though therapeutic options are limited or not available. Redefining the disease in an individual on the basis of signs and symptoms that may be amenable to targeted intervention provides an alternative treatment strategy based on empirical trial and error in a given person suffering from FTD. Symptomatic involvement in FTD often encompasses multiple domains including cognitive, behavioral, psychiatric, motor and sensory systems (Table 2). This wide range of clinical manifestations is fertile ground for the empiric evaluation of therapies that may control signs and symptoms, as well as promote quality of life, for persons suffering from FTD. An exploration of therapeutic options focused on symptom reduction is the focus of this article.
Figure 1.
Coronal view of frontal and temporal lobes depicting neuroanatomic subdivisions and clinical phenotypes associated with select involvement of these areas.
Table 1.
Diagnostic clinical categories of frontotemporal dementia.
| Diagnostic category | Cardinal features |
|---|---|
| bvFTD | Early behavioral changes characterized by impaired social and interpersonal interactions |
| PPA | Early expressive language difficulties with relatively intact comprehension |
| SD | Early loss of semantic knowledge with preserved language fluency |
| FTDP | Early parkinsonism in association with any FTD clinical phenotype, often associated with tau mutations on chromosome 17 |
| FTD-MND | Early signs of MND in the setting of any clinical FTD phenotype |
| FTD with inclusion body myopathy and Paget’s disease of the bone | Characteristic inclusion body myopathy weakness (knee extensor and finger flexor involvement predominates) and Paget’s disease associated with bvFTD phenotype and abnormalities in VCP |
| PSP | Axial rigidity, vertical (downward) gaze paralysis |
| CBD | Asymmetric rigidity, cortical sensory loss and apraxia |
bvFTD: Behavioral variant of frontotemporal dementia; CBD: Corticobasal degeneration; FTD: Frontotemporal dementia; FTDP: Frontotemporal dementia with parkinsonism; MND: Motor neuron disease; PPA: Primary progressive aphasia; PSP: Progressive supranuclear palsy; SD: Semantic dementia; VCP: Valosin-containing protein.
Table 2.
Clinical symptoms that can be associated with frontotemporal dementia phenotypes.
| Clinical category of signs and symptoms | Specific clinical features |
|---|---|
| Cognitive | Executive, attention, memory, language, praxis impairments |
| Behavioral | Socially inappropriate behavior, loss of interpersonal skills, personality change |
| Psychiatric | Abulia, apathy, obsessive–compulsive tendencies, depression, anxiety, motor restlessness, irritability, agitation, lability of mood, pseudobulbar affect |
| Motor | Rigidity, bradykinesia, muscle atrophy, fasciculations, bulbar motor involvement including swallowing difficulties, extraocular movement abnormalities, falls |
| Sensory (common in corticobasal dementia, and rare in other forms of frontotemporal dementia) | Extinction to double simultaneous stimulation, agraphesthesia, astereoagnosis |
Issues of genetic heterogeneity
Over the last 15 years, tremendous advances have been made in understanding the genetics of FTD [30–32]. For years, researchers and clinicians had understood that FTD may run in families, exhibiting an autosomal dominant pattern of inheritance. Over the last 15 years, mutations in tau [30,32–37], progranulin [31,32,38,39], valosin-containing protein [40–42], CHMP2B [43–45] and tar DNA-binding protein (TDP)-43 [46–48] have been linked to FTD. Critical to our understanding of the therapeutics of FTD is an appreciation that all known mutations leading to autosomal dominant FTD can give rise to a myriad of clinical phenotypes even within the same family pedigree [30–32,38,49–52]. Despite the wealth of discovery in the genetics of FTD, no evidence exists to support any contentions that specific neurotransmitter systems and/or neuroanatomic circuits are preferentially involved in any distinct genetic variants identified to date. Current treatment strategies should focus on symptom identification and alleviation irrespective of genetic status.
Issues of pathologic heterogeneity
The clinical features of FTD reflect pathological brain changes that can vary widely in both appearance and etiology, and these changes are referred to in the pathological literature as variants of frontotemporal lobar dementia (FTLD; see Figure 2 for overview of common pathological phenotypes of FTLD) [30,31,34,51,53,54]. Classic Pick’s disease, FTLD-17 linked to tau mutations, progressive supranuclear palsy and corticobasal degeneration are characterized pathologically by the presence of tau inclusions [33,35,37,54–57]. FTD cases associated with progranulin mutations can be characterized pathologically by the absence of progranulin reactivity in affected regions of the cortex [38,49,50]. Rare cases in familial forms of FTD associated with mutations in the valosin-containing protein gene can be identified with antibodies to valosin [40–42]. Recent discoveries include the purification and identification of TDP-43 as a specific constituent of FTLD pathology in most cases of FTD related to GRN mutations and to the FTLD cases previously classified as FTLD with ubiquitin-positive, tau-negative inclusions [31,32,46,47,49,53,54,58]. While the role of TDP-43 in the development of FTD remains unclear, the presence of aberrant TDP-43 pathology is seen in some cases with signs and symptoms of motor neuron disease (MND) in association with FTLD (FTLD-MND) [31,32,47–49,53,54,58]. Among the most recent advances in the neuropathologic evaluation of FTLD is the discovery of variants of FTLD that develop pathologic inclusions related to the fused-in-sarcoma protein (coded by the fused-in-sarcoma protein [47]). Complicating this scenario is the presence of frontal or temporal variants of more common diseases such as Alzheimer’s disease (AD) [59–67], dementia with Lewy bodies [68,69] and vascular dementia [67,70]. Clinical FTD phenotypes are well represented in this cluster of common neurodegenerative diseases [67].
Figure 2. Gross pathology and histopathological findings in frontotemporal dementia.
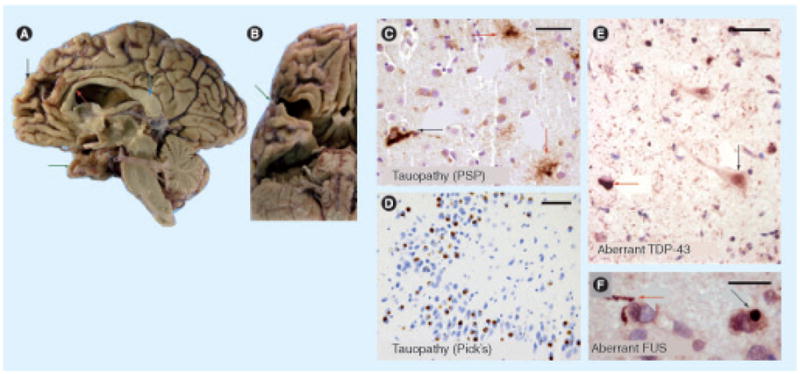
Frontotemporal dementias (FTDs) are devastating neurological diseases with characteristic gross and microscopic pathological features. Gross photographs from FTD cases (medial aspect (A) and inferior aspect (B)) demonstrate the dramatic atrophy of the anterior portions of the cerebral neocortex – the frontal and anterior temporal cortices. A hemisected brain viewed from the midline (A) demonstrates atrophy in the frontal gyri (black arrow), along with atrophy of the genu of the corpus callosum (red arrow) in contrast to the relatively spared posterior cortex and splenium of the corpus collusum (blue arrow). Green arrows (A & B) show the markedly atrophic anterior portion of the temporal lobes. Immunohistochemical stains ((C–F), with nuclei counterstained with hematoxylin) can show diagnostic features that help discriminate between FTD subtypes. Tauopathies (C & D) can show divergent features. In progressive supranuclear palsy (C), anti-phospho-tau antibody labels both neurons (black arrow) and fine ‘tufted astrocytes’ (red arrows) in the striatum. In Pick’s disease (D), anti-tau antibody highlights roughly spherical ‘Pick body’ inclusions within the cytoplasm of hippocampal dentate granule cells. Relatively newly defined histopathological features associated with FTDs include aberrant immunohistochemical staining with TDP-43 (e) and FUS (F). TDP-43 protein (e) is normally present in the cell nuclei (relatively normally stained cell indicated with black arrow). However, in FTD, amyotrophic lateral sclerosis and hippocampal sclerosis dementia, cells display aberrant intracytoplasmic TDP-43 inclusions (red arrow) and aberrant background staining of neurites. Similarly, aberrant anti-FUS (F) staining labels neurites (red arrow) and intracytoplasmic inclusions (black arrow) in some FTD brains. Scale bars = (C) 50 μm, (D) 100 μm, (e) 50 μm and (F) 30 μm.
FUS: Fused in sarcoma; PSP: Progressive supranuclear palsy; TDP: Tar DNA-binding protein.
While prediction of underlying pathology and its implications for treatment of persons with FTD is in its infancy, future advances will require therapies tailored to individual disease subtypes. In the meantime, therapeutic treatment options should remain focused on symptomatic benefit. Exceptions to this rule may include cases with suspected underlying AD or cerebrovascular pathology, in which therapeutic regimens may include a weighted use of AD-approved therapies or a focus on vascular risk factor modification.
Neuroanatomic & neurotransmitter system involvement in FTD
An understanding of particular neuroanatomic pathway and neurotransmitter system involvement is important for defining therapeutic strategies that may target the clinical features of FTD. Much evidence exists to support the contention that not only frontal and temporal lobe systems are defunct in FTD, but also that specific neurotransmitter systems are dysregulated in these conditions (Table 3). Cholinergic [71–76], dopaminergic [77–81], GABAergic [72,82], glutamatergic [72,82], noradrenergic [72,80,81] and serotonergic [73,83–85] system involvement have all been implicated in the development of the signs and symptoms of FTD. As such, modulation of such key pathways and neurotransmitter systems may play a central role in our understanding of currently available therapeutic options for FTD. Identifying target symptoms, localizing such symptoms to specific neuroanatomic and neurotransmitter pathways, and identifying potential strategies for therapeutic intervention are critical steps in the development of a personalized therapeutic regimen for individuals suffering from FTD.
Table 3.
Studies of neuroanatomic and neurotransmitter system involvement in frontotemporal dementia.
| Author | System | Evidence for involvement | Clinical features | Ref. |
|---|---|---|---|---|
| Odawara et al. | Cholinergic system | Total mAchR in the temporal cortex was lower in FTD than AD or controls. M1 receptors were specifically reduced and M2 receptors were increased in FTD over controls, AD and dementia with Lewy bodies cases | Cognition and language | [71] |
| Uhl et al. | Cholinergic system | Decreased cell number in nbM | Cognition | [74] |
| Weinberger et al. | Cholinergic system | 123IQNB PET imaging of mAchRs demonstrated reductions in frontal and anterior temporal cortices | Cognition | [75] |
| Wood et al. | Cholinergic system | Choline acetyltransferase activity in the cortex from the basal forebrain is intact in Pick’s disease | Cognition | [76] |
| Hansen et al. | Cholinergic system | Levels of choline acetyltransferase were normal, whereas muscarinic receptor binding was decreased in bvFTD | Cognition | [111] |
| Engelborghs et al. | Dopaminergic system | CSF norepinephrine levels were positively correlated with dementia severity. CSF HVA:5-HIAA ratios (reflecting serotonergic modulation of dopaminergic neurotransmission) were correlated with aggressive behavior | Aggression and agitation | [138] |
| Frisoni et al. | Dopaminergic system | [123I]-IBZM SPECT showed reduced ligand uptake in superior frontal regions suggesting marked involvement of the frontal cortical dopaminergic system in bvFTD | Executive function and motor planning | [77] |
| Rinne et al. | Dopaminergic system | [11C]CFT (cocaine analog) uptake in the caudate and putamen was reduced to 86 and 82% of the control value, respectively. UPDRS scores showed a negative correlation with [11C]CFT uptake in both the putamen and caudate | Motor function and extrapyramidal signs | [79] |
| Kanazawa et al. | Dopaminergic system | Dopamine reduction in the striatum of Pick’s disease was disproportionately greater than expected for various degrees of nigral cell loss | Motor function and extrapyramidal signs | [78] |
| Sedaghat et al. | Dopaminergic system | SPECT using 123I-FP-CIT demonstrated a mean 65% reduction of tracer uptake in FTD compared with control cases | Extrapyramidal signs assessed by the UPDRS | [139] |
| Ferrer | Glutamatergic and GABAergic systems | Histopathological assessment demonstrates loss of glutamatergic pyramidal cells and calbindin- D28k-immunoreactive GABAergic local-circuit neurons in the upper cortical layers | Cortical connectivity involved in cognition, language and behavior | [82] |
| Bowen et al. | Serotonergic system | 5HT1A and 5HT2A receptors are reduced in FTD | Depression, anxiety and behavior | [83] |
| Franceschi et al. | Serotonergic system | In vivo measurements of [11C]MDL indicated a significant reduction of 5HT2A receptors in orbitofrontal, frontal medial and cingulate cortices | Behavior, depression and anxiety | [84] |
| Procter et al. | Cholinergic, serotonergic and glutamatergic systems | No evidence of any cholinergic abnormality in FTD. Serotonin receptors were decreased in frontal and temporal cortex. No loss of kainate receptors but loss of AMPA receptors from both temporal and frontal lobes | Depression, anxiety and cortical connectivity involved in cognition, language and behavior | [72] |
| Sjogren et al. | Dopaminergic, noradrenergic and serotonergic systems | HVA was significantly reduced in FTD and AD. 5-HIAA and HMPG were not significantly different from controls. No significant effect of neuroleptic or antidepressant medication, extrapyramidal signs or FTD subtype on monoamine metabolites was seen | Motor function and extrapyramidal signs | [80] |
| Sparks et al. | Cholinergic and serotonergic systems | Choline acetyltransferase activity was decreased in the hypothalamus and nbM. Acetylcholinesterase activity was decreased in the nbM. Serotonin binding was decreased in frontal and temporal cortices | Cognition, depression, anxiety and behavior | [73] |
| Sparks et al. | Monoaminergic systems | MAO-B was decreased in the nbM and increased in the hypothalamus, while MAO-A was increased in the hypothalamus and decreased in the nbM and temporal pole | Cognition, depression, anxiety and behavior | [81] |
| Yang et al. | Noradrenergic and serotonergic systems | Reduced cell numbers in the serotoninergic dorsal raphe, but not the locus coeruleus, are seen in FTD irrespective of the presence or absence of motor neuron disease | Depression, anxiety and behavior | [85] |
5HT: 5-hydroxytryptamine; AD: Alzheimer’s disease; AMPA: 2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl)propanoic acid; bvFTD: Behavioral variant of frontotemporal dementia; CFT: (−)-2-β-Carbomethoxy-3-β-(4-fluorophenyl) tropane; CIT: β-carbomethoxy-3 β-(4-iodophenyltropane); CSF: Cerebrospinal fluid; FP: Fluoropropyl; FTD: Frontotemporal dementia; HIAA: Hydroxyindolacetic acid; HMPG: 4-hydroxy-3-methoxyphenyl glycol; HVA: Homovanillic acid; IBZM: Iodobenzamide; 123IQNB: 3-quinuclidinyl-4-iodobenzilate labeled with iodine 123; mAchR: Muscarinic acetylcholine receptors; MAO: Monoamine oxidase; MDL: [(R)-(+)-4-(1- hydroxy-1-(2, 3-dimethoxyphenyl) methy1)-N-2-(4-fluorophenylethyl) piperidine]; nbM: Nucleus basalis of Meynert; SPECT: Single photon emission computed tomography; UPDRS: Unified Parkinson’s Disease Rating Scale.
Existing clinical data regarding therapeutic strategies for FTD
Only a handful of randomized, double-blind, placebo-controlled trials of treatments for FTD exist in the literature today (Table 4). These studies include small numbers of patients, with limited descriptions of the clinical or pathological phenotypes and genotypes on subjects enrolled. As FTD is a relatively rare entity, inclusion of heterogeneous populations of subjects has been necessary to accomplish even these small-scale forays into clinical trial development in FTD. Unfortunately, the issue of clinical and pathologic heterogeneity introduces such tremendous variability into the samples studied that the likelihood of achieving a significant outcome is practically nonexistent [18,25,86–88]. Selecting the appropriate outcome measures for such a study is also a major confound [18,25,86–88]. Cognitive outcomes in FTD trials are also confounded by preserved performance on standard measures used in the design of clinical trials for AD and the inability to accurately evaluate cognition in subjects with language impairments using standardized testing instruments [18,87]. As such, the field has focused on behavioral and psychiatric outcome measures, without considering inclusion of related symptomatology in the selection of subjects for such trials [18,87]. For example, evaluating the effects of a selective serotonin reuptake inhibitor on FTD symptoms requires a high rate of incident appearance of symptoms such as depression, anxiety or compulsivity that may be alleviated by these agents. Advances in the development of therapies for the symptoms of FTD require the following four requirements that currently do not exist:
Table 4.
Clinical trials by class of agent in frontotemporal dementia.
| Author | Study | Design | Subjects | Major findings | Ref. |
|---|---|---|---|---|---|
| Randomized, double-blind, placebo-controlled studies | |||||
| Lebert et al. | Trazodone in bvFTD | Randomized, double-blind, placebo-controlled, crossover study | 26 subjects with bvFTD | Significant decrease in NPI scores, mediated by improvements in irritability, agitation, depression and eating disorders was seen for the treated group | [97] |
| Deakin et al. | Paroxetine in FTD | Double-blind, placebo-controlled, crossover trial, 6 weeks duration, paroxetine 40 mg/day | Ten subjects with bvFTD | No differences in NPI or Cambridge Behavioral Inventory. Worse performance on paired associates learning task, reversal learning and a delayed pattern recognition task noted in treated subjects | [96] |
| Moretti et al. | Paroxetine vs piracetam FTD | Randomized 14-month study of paroxetine 20 mg/day vs piracetam 1200 mg/day | Eight patients in each group, aged 64–68 years, with a diagnosis of bvFTD | Patients treated with paroxetine showed significant improvements in behavioral symptoms, reflected by a reduction of caregiver stress | [99] |
| Rahman et al. | Methylphenidate in FTD | Within-subjects, double-blind, placebo-controlled study, crossover design of a single dose of methylphenidate 40 mg | Eight subjects with bvFTD | Reduced risk-betting was seen on the Cambridge Gamble Task, but no effects on other measures of cognitive function were observed | [106] |
| Kertesz et al. | Galantamine in bvFTD and PPA | 18-week open-label and 8-week randomized placebo-controlled trial | 36 bvFTD and PPA subjects | Improvement in global severity score in PPA was seen, but was not significant after correction for multiple comparisons. Language scores for PPA remained stable for treated subjects compared with a decline seen in placebo subjects | [92] |
| Reed et al. | Bromocriptine in PPA | 8-week escalating dose followed by 1-month washout and crossover, randomized, double-blind, placebo-controlled trial | Six subjects with PPA | Increased mean utterance length was observed in the treated subjects, although decline was still noted irrespective of treatment arm | [112] |
| Open-label studies | |||||
| Boxer et al. | Memantine in bvFTD, PPA and SD | Open-label, 26-week duration, memantine 20 mg/day | 21 subjects with bvFTD, 13 with SD and nine with PPA | Transient improvement in NPI scores seen predominantly in bvFTD group | [89] |
| Diehl-Schmid et al. | Memantine in bvFTD | Open-label, 6-month duration, memantine 20 mg/day | 16 subjects with bvFTD | No changes in NPI or Frontal Behavioral Inventory were seen. ADAS-Cog scores increased, reflecting cognitive decline | [90] |
| Moretti et al. | Rivastigmine in FTD | Open-label, 12-month study of rivastigmine 3–9 mg/day | 20 subjects aged 60–75 years with bvFTD | Reductions in NPI, Behavioral Pathology in Alzheimer’s Disease Rating Scale and Cornell Scale for Depression in Dementia scores were seen in the rivastigmine group. Caregiver burden was reduced. Executive function stabilized in the rivastigmine group | [94] |
| Swartz et al. | Serotonin reuptake inhibitors in FTD | Open-label, 3-month trial of fluoxetine, sertraline or paroxetine | 11 subjects with bvFTD | After treatment, disinhibition, depressive symptoms, carbohydrate craving and compulsions all showed improvement in at least half the subjects in which they had been present | [100] |
| Case series | |||||
| Mendez et al. | Donepezil in bvFTD | Case–control study of donepezil over 6 months | 12 subjects in each group of treated or control bvFTD subjects | Treated subjects worsened on FTD Inventory. Four out of 12 treated subjects had increased disinhibition and compulsivity | [93] |
| Swanberg | Memantine in FTD | Case series, open-label | Three subjects with bvFTD | All subjects had improvement in total NPI score with specific improvements in scores of apathy, agitation and anxiety | [113] |
| Prodan et al. | Sertraline in SD | Case series treated with sertraline 25–50 mg/day with 6–24-month follow-up period | Two men and two women with SD and agitation and hostile behavior | Marked reductions in aggressive behavior and total NPI-Q scores were seen for all subjects | [116] |
| Moretti et al. | Selegiline in FTD | Case series treated with selegiline 1.25 mg/day | Three subjects with bvFTD | Improvements seen on NPI, and suggestion of cognitive benefit on Stroop errors and Paced Auditory Serial Addition Task performance | [98] |
| Adler et al. | Moclobemide in FTD | Case series treated with moclobemide 300–600 mg/day for 14 days | Six subjects with bvFTD | Depressive symptoms, aggressive behavior, irritability, distractibility, mental rigidity, stereotypy of speech and perseverations, pacing and motor stereotypy and problem-solving improved variably | [95] |
| Czarnecki et al. | Sensitivity to atypical antipsychotics | Case series of subjects treated with risperidone, olanzapine and quetiapine | Three subjects with bvFTD | Subjects developed parkinsonism and tardive antecollis | [102] |
| Case reports | |||||
| Curtis et al. | Risperidone in bvFTD | Case report | 42-year-old woman with Pick’s disease | General improvement in psychotic symptoms of agitation, delusions and hallucinations | [101] |
| Fellgiebel et al. | Aripiprazole partially restores frontal glucose metabolism | Case report using longitudinal FDG-PET | 73-year-old male with bvFTD | Frontal glucose metabolism declined over 12 months with conventional treatment, but improved after 1 month of treatment | [103] |
| Goforth et al. | Methylphenidate in FTD | Case report of methylphenidate using quantitative EEG | Single subject with bvFTD | Left greater than right bifrontotemporal slowing normalized partially | [105] |
| Cruz et al. | Topiramate for alcohol abuse in FTD | Case report | 53-year-old male with bvFTD | Topiramate reduced alcohol abuse but not other obsessive tendencies | [109] |
ADAS-Cog: Alzheimer’s Disease Assessment Scale cognitive component; bvFTD: Behavioral variant of frontotemporal dementia; FTD: Frontotemporal dementia; NPI: Neuropsychiatric inventory; PPA: Primary progressive aphasia; SD: Semantic dementia.
Development of widespread clinical networks to recruit homogenous patient populations;
Development and validation of outcome measures designed specifically for subjects with FTD;
Rational design of FTD-specific clinical inclusion/exclusion criteria;
Selection of appropriate outcome measures that accurately reflect plausible changes in symptoms targeted by the proposed intervention [18,25,86–88].
Other available clinical data have focused on the results of small-scale, open-label studies, retrospective case series or single case reports in the literature (Table 4). Although they represent the current state-of-the-art, these studies are plagued by confounds and have failed to adequately define appropriate treatment options for FTD. To date, studies have investigated a wide range of potential therapeutic options including the NMDA receptor antagonist memantine [89,90], cholinesterase inhibitors [91–94], serotonin reuptake inhibitors and other antidepressants [95–100], antipsychotics [101–104], psychostimulants [105,106], inflammatory mediators [107,108] and antiepileptics [109]. Despite the limitations of these studies, they clearly suggest that the range of potential therapeutic options in FTD is broad; however, considering which agent(s) to use and in what order, as well as which patients might respond to which therapies cannot be derived confidently from the existing literature.
A clinically plausible approach to the treatment of FTD symptomatology
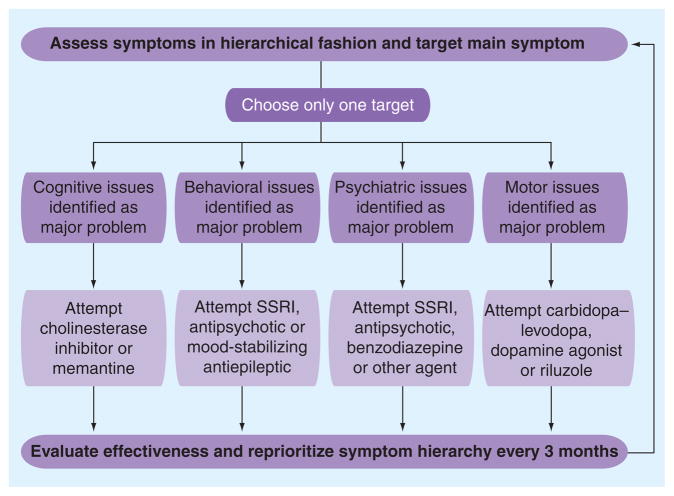
Major considerations in selecting appropriate treatment for FTD include identification and hierarchical categorization of discrete clinical symptoms on the basis of contributions to functional decline and safety considerations. Therapeutic interventions should include successive empiric trials of symptomatic therapies targeting the individualized hierarchical symptom list from the top down. This strategy relies on: communicating frequently with caregivers and evaluating the target patient often; recognizing that some clinically plausible strategies may fail in one patient and succeed in another and that the failure of an agent does not mean other agents in the same class will fail; and ensuring that the dosage and therapeutic trial length are adequate to understand the effects in the target patient. Current clinical strategies to maximize symptom control in FTD may benefit from the use of an algorithmic approach defined in Figure 3.
Figure 3. A clinically plausible algorithm for empiric evaluation of potential symptomatic therapies for the treatment of frontotemporal dementia.
SSRI: Selective serotonin reuptake inhibitor.
Cognitive symptom management
Frontal and temporal dysfunction in FTD can give rise to cognitive symptoms that can be classified as dysexecutive, attentional or language based, involving either fluency (language production) or semantic knowledge (dysnomia) [2,6–9,11,14–16,22–26]. Cholinergic [71–76] and, to a lesser degree, glutamatergic [72,82] dysfunction have been documented in FTD, suggesting that agents targeting these neurotransmitter systems may prove beneficial in FTD (Table 3). Approved therapies for cognitive dysfunction in degenerative disease states (AD and Parkinson’s dementia) include cholinesterase inhibitors as well as memantine, an NMDA receptor partial antagonist [1,3–6,10,13]. Available evidence suggests that cholinesterase inhibitors may stabilize loss of language and executive functions in primary progressive aphasia and bvFTD phenotypes [91–94]. Memantine may actually worsen global cognitive function, as evidenced by increases in Alzheimer’s Disease Assessment Scale cognitive component scores in a single open-label treatment study of 16 subjects with FTD. The reasons for this are unknown [89]. Still, undisputed efficacy in AD suggests that memantine may still have a role in the therapeutic armament for FTD if underlying AD pathology is suspected. While further studies are needed to clarify direction and magnitude of effects of these agents, a wealth of clinical and biological data support the use of such agents in empiric attempts to alleviate symptoms in FTD [3,71–77,89–92,94,110,111]. Other small-scale studies and case reports have suggested that such agents may have significant psychiatric and behavioral effects that may negate their utility in some patients with FTD because symptoms worsen rather than abate with their use [89,93]. Another small trial of bromocriptine demonstrated significant improvement in mean length of utterance scores, suggesting improved language fluency, after treatment compared with placebo in both the initial and crossover arms of the study [112]. Progressive decline was seen in both the treatment and placebo groups, however, suggesting that the beneficial effects of bromocriptine treatment are limited.
Behavioral & psychiatric symptom management
Behavioral symptoms that may be amenable to pharmacological management include obsessive– compulsive tendencies and motor restlessness. Socially inappropriate behaviors, loss of interpersonal skills and personality change are symptoms that appear to defy many current therapeutic strategies. While one study has suggested that cholinesterase inhibitors may reduce the ‘behavioral pathology’ in the Alzheimer’s Disease Rating Scale [94], another has demonstrated that subjects treated with donepezil exhibited increased compulsivity and disinhibition [93]. Memantine, on the other hand, while not demonstrating efficacy on cognitive outcomes, has shown benefit in reducing Neuropsychiatric Inventory scores (including many behavioral components) in two studies [89,113], but failed to show benefit in a third open-label study [90]. Studies in AD have largely demonstrated benefits on behavioral outcomes using these agents, suggesting that they may have some utility in alleviating the behavioral symptoms of FTD especially in cases where an underlying AD pathology is suspect.
Other strategies for targeting obsessive– compulsive tendencies and motor restlessness rely on the use of antidepressant and antipsychotic therapies. Again, available data are less than optimal and provide some conflicting evidence. Trazodone [5,13,18,97], paroxetine [5,13,18,96,99,100], sertraline [13,100,114–116], selegiline [10,94,98] and moclobemide [18,95] have all shown beneficial effects in the treatment of behavioral symptoms of FTD, and yet another study of paroxetine showed no benefit in treating the behavioral symptoms of FTD as measured by the Neuropsychiatric Inventory or Cambridge Behavioral Inventory [96].
Atypical antipsychotics need to be used with caution and require open disclosure regarding the FDA ‘black-box’ warning for use in the elderly with dementia [117–122]. Increased risk of stroke or myocardial infarction from 2.6 to 4.5% per year has been noted [118]. The applicability of such a warning to the generally younger patients with atypical dementia characterized by FTD is unclear at present. Until such issues are clarified, discretion in the use of such agents must be exercised [118–122]. With this caveat in mind, use of antipsychotics in FTD may represent the only choice available to clinicians and family members that need to control behavioral issues that pose an immediate threat to safety. Available evidence in FTD suggests that psychotic symptoms of agitation, delusions and hallucinations may improve with the use of such agents [4,13,16,94,101,103,104,115,123]. Aripiprazole increased frontal glucose metabolism in a case report, suggesting that the use of such agents may help to restore frontal dysfunction [103]. In addition to the caution invoked by the black box warning, a small retrospective case series of subjects treated with risperidone, olanzapine and quetiapine showed increased risk of parkinsonism and tardive antecollis, suggesting extreme sensitivity to such agents in some patients with FTD [102].
Methylphenidate, a psychostimulant, reduced risk-betting behavior on the Cambridge Gamble Task in a randomized, placebo-controlled, double-blind study [106] and partially normalized quantitative EEG patterns in a single case report [105], suggesting it may prove beneficial in the modulation of behavior in FTD. Topiramate therapy alleviated obsessive alcohol consumption in a single case report, but did not have an effect on other obsessive tendencies [109]. The practical use of such agents across the FTD spectrum remains to be elucidated.
Abulia, apathy, depression, anxiety, irritability, agitation, lability of mood and pseudo-bulbar affect can all represent major challenges in the management of FTD. These symptoms are presumably attributable to the dysregulation of central serotonergic, dopaminergic and nor-adrenergic pathways that are well documented in FTD (Table 3). Since antidepressants such as trazodone [5,13,18,97], paroxetine [5,13,18,96,99,100], sertraline [13,100,114–116], selegiline [10,94,98] and moclobemide [18,95] have all shown beneficial effects in the alleviation of neuropsychiatric symptoms in FTD, other agents of this class should also prove equally effective.
Potential negative effects of antidepressants seem to be minimal, although one randomized, placebo-controlled, double-blind study demonstrated reductions in cognitive abilities on paired associates learning task, reversal learning and a delayed pattern recognition task [96]. Thus, while antidepressant therapies appear safe in most published studies, the clinician and caregiver must be alert for any deleterious effects even in the safest of classes of drugs used for symptomatic control in FTD.
The use of antipsychotics for the psychiatric symptoms of FTD should take into account the same caveats and considerations mentioned earlier for the treatment of the behavioral symptoms of FTD [118–122]. As with treatment of refractory depression, bipolar disorder and schizoaffective disorder, the use of antipsychotics can prove to be a mainstay in the treatment of psychiatric symptoms refractory to far safer antidepressant regimens that are typically used as first-line agents in FTD and other psychiatric disorders.
Pseudobulbar symptoms (pathological laughter and crying) are frequently seen in FTD-MND and progressive supranuclear palsy, mediated presumably through pathological brainstem involvement in these FTD variants. Previous data suggest that tricyclic antidepressants and serotonin reuptake inhibitors may be effective in the treatment of pseudobulbar symptoms [124,125]. Recent evidence suggests that the combination of dextromethorphan and quinidine can alleviate such symptoms [124–128]. This combination includes dextromethorphan, as the presumed active agent, activating σ-1 opiod receptors in the brainstem, while quinidine enhances CNS penetration by reducing peripheral metabolism via the cytochrome P450 system [124–128]. Pseudobulbar symptoms were significantly ameliorated in patients with amyotrophic lateral sclerosis (ALS) and multiple sclerosis. This agent has not been tested in FTD variants lacking an ALS/MND diagnosis that may also exhibit pseudobulbar symptoms.
Motor symptom management
Rigidity, bradykinesia, muscle atrophy, fasciculations, bulbar motor involvement including swallowing difficulties, extraocular movement abnormalities and falls can all be signs of extra-pyramidal, brainstem or MND involvement in FTD [15,51,55,129–132]. Treatment of parkinsonism should follow the general guidelines for the treatment of idiopathic Parkinson’s disease by using the routine carbidopa–levodopa and dopaminergic agonist therapy. While such treatments can exacerbate behavioral and psychiatric symptoms of FTD, this should not be a consideration if motor deficits rise to become the most problematic symptoms in the hierarchical categorization of symptoms. Signs and symptoms of MND (e.g., muscle atrophy, fasciculations and bulbar motor involvement including swallowing difficulties) are largely dealt with utilizing physical and occupational strategies. Pharmacological management remains poor, but the use of riluzole is an option. This agent has been shown to slow progression of disability in ALS/MND [133–136]. While no current evidence exists for the efficacy of riluzole in FTD-MND, the pathological and genetic linkages between FTD and ALS/MND suggest that the same disease process is at work [47,48,58,137]. Therefore, therapies designed to extend disease duration by maximizing motor function may be viable options in cases where cognitive and behavioral deterioration does not preclude quality of life.
Caveats & considerations in interpretation of therapeutic strategies in FTD
All of the studies reviewed and commented on earlier suffer from a myriad of inadequacies including subject heterogeneity, small sample size, suboptimal outcome measures and a lack of consistency across studies. In addition, much of what we have learned regarding therapeutic options in FTD is based on small case series or even single case reports in the literature. A primary consideration for physicians that treat subjects with FTD is the caveat that the efficacy of any therapeutic option in an individual case of FTD cannot be reliably anticipated on the basis of the results of published studies in FTD to date. Despite the many therapeutic options available today, no proven therapies for FTD have been identified. Specific recommendations for treatment strategies in persons suffering from FTD cannot be made at present, and the earlier discussion is in no way meant to promote any specific therapeutic option. Until such a time when reliable data are available, treating clinicians will have to rely on individualized, symptom-targeted strategies based on empiric trial and error in any given patient.
Future perspective
Rapidly advancing discoveries in the pathobiology and genetics of FTD over the last decade has led to increased recognition of FTD as the major degenerative cause for dementia in persons under the age of 65 years. These discoveries have led to the current recognition that proven therapies for FTD are lacking. Future developments in the area of therapeutics over the next decade will focus on developing widespread clinical networks to recruit homogenous patient populations. Indeed, such multicenter collaborative initiatives are already underway and will propel research in the therapeutics of FTD forward. Further development and validation of outcome measures designed specifically for subjects with FTD is an active area of research focus and again will provide the tools needed to rationally test currently available and future developmental therapeutics.
Unfortunately, as many of the existing therapeutics discussed herein are coming off-patent and being developed as generics, pharmaceutical backing for definitive clinical trials with these compounds will become essentially nonexistent. As Federal and other extramural sources of funding are further limited by the current and anticipated economic situation, Phase II and III trials for the agents discussed herein will largely come to halt. Unless priorities for Federal funding or Pharma interests change significantly, we will not see definitive research or therapeutic approvals for existing medications that we rely on currently for symptom management in FTD. Over the next 5–10 years the clinician will be left practicing empiric rather than evidence-based medicine in FTD using off-label indications to battle this devastating disease.
Fortunately, the future of FTD therapeutics has been engaged in the search for new therapies that may again invigorate extramural funding and Pharma initiatives. It is clear that effective, targeted disease-modifying therapies are needed for FTD. Symptomatic treatment is merely a ‘way station’ on the way to our search for treatments that will slow or stop this devastating cluster of diseases grouped under the rubric of FTD. Agents targeting tau, progranulin, TDP-43, valosin-containing protein, CHMP2B and other potential molecular players in the development of FTD are under development [2,4,18–21]. Progress in this area is critically dependent on developing a widespread infrastructure to obtain more homogeneous patient populations, moving potential compounds into definitive clinical trials and developing appropriate assessment measures to operationalize outcomes for fully validated efficacy of future therapeutic agents [18,20].
Conclusion
In summary, targeting the many symptoms seen in FTD requires the recognition of such symptoms that play primary or secondary roles in the spectrum of deficits leading to functional disability in FTD. Agents targeting the cognitive, behavioral, psychiatric and motor symptoms of FTD are readily available, having been previously developed and approved for symptomatic benefit in other disease states. In contrast to the widespread belief that beneficial treatments are not available for FTD, our therapeutic armament is well-stocked with pharmacological tools that may improve quality of life for those suffering from this devastating and incurable class of degenerative diseases.
Practice Points.
No US FDA-approved therapies for frontotemporal dementia (FTD) exist.
Available clinical trial data to date are inconclusive regarding off-label recommendations for any treatment option.
The lack of clinical data or FDA approval does not mean that treatments targeting the symptoms of FTD will not show benefit in some individuals with FTD.
FTD is clinically, pathogically and genetically heterogenous, and this heterogeneity limits global recommendations for therapeutic treatment in FTD.
Treatment of FTD should begin by defining relevant signs and symptoms that are impairing quality of life in each person suffering from FTD in order to develop an individualized therapeutic strategy with maximal chances for success.
Each treatment option must be tested empirically in each patient individually, employing only one therapeutic strategy at a time, monitoring for symptomatic improvement and/or side effects (in essence, a clinical trial with n = 1).
Hierarchical prioritization of symptoms should be repeated after each successive therapeutic trial to identify the primary target sign or symptom before testing additional medications.
Acknowledgments
The authors gratefully acknowledge the many subjects, caregivers, friends and families of persons with Alzheimer’s disease that have engaged in clinical research activities that allow discoveries to be made. We also thank P Thomason for critical review and editing of the manuscript.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
GA Jicha is supported by funding from the NIH/NIA 1 P30 AG028383 & 2R01AG019241–06A2, NIH LRP 1 L30 AG032934, and the Sanders-Brown Foundation. GA Jicha has also received research support for clinical trial activities from NIH/NIA ADCS U01AG010483, Pfizer, Elan, Janssen, Medivation and Baxter. PT Nelson is supported by funding from the NIH/NIA 1 P30 AG028383, R21AG036875, R01NS061933. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Allain H, Bentue-Ferrer D, Tribut O, Merienne M, Belliard S. Drug therapy of frontotemporal dementia. Hum Psychopharmacol. 2003;18:221–225. doi: 10.1002/hup.472. [DOI] [PubMed] [Google Scholar]
- 2.Arvanitakis Z. Update on frontotemporal dementia. Neurologist. 2010;16:16–22. doi: 10.1097/NRL.0b013e3181b1d5c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3▪▪.Bei H, Ross L, Neuhaus J. Off-label medication use in frontotemporal dementia. Am J Alzheimers Dis Other Demen. 2010;25:128–133. doi: 10.1177/1533317509356692. Novel exploration of off-label medication use by expert clinicians in the area of frontotemporal dementia (FTD) demonstrates that therapeutic options do exist, and are being used currently for symptomatic treatment of this devastating disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boxer AL, Boeve BF. Frontotemporal dementia treatment: current symptomatic therapies and implications of recent genetic, biochemical, and neuroimaging studies. Alzheimer Dis Assoc Disord. 2007;21:S79–S87. doi: 10.1097/WAD.0b013e31815c345e. [DOI] [PubMed] [Google Scholar]
- 5.Chow TW. Treatment approaches to symptoms associated with frontotemporal degeneration. Curr Psychiatry Rep. 2005;7:376–380. doi: 10.1007/s11920-005-0040-5. [DOI] [PubMed] [Google Scholar]
- 6.Galariotis V, Bodi N, Janka Z, Kalman J. Frontotemporal dementia – part III. Clinical diagnosis and treatment. Ideggyogy Sz. 2005;58:292–297. [PubMed] [Google Scholar]
- 7.Galariotis V, Bodi N, Janka Z, Kalman J. Frontotemporal dementia – part I. History, prevalence, clinical forms. Ideggyogy Sz. 2005;58:164–171. [PubMed] [Google Scholar]
- 8.Graff-Radford NR, Woodruff BK. Frontotemporal dementia. Semin Neurol. 2007;27:48–57. doi: 10.1055/s-2006-956755. [DOI] [PubMed] [Google Scholar]
- 9.Honig LS, Bell K, Chin SS. Frontotemporal dementia. Sci Aging Knowledge Environ. 2003;2003(13):DN1. doi: 10.1126/sageke.2003.13.dn1. [DOI] [PubMed] [Google Scholar]
- 10.Kaye ED, Petrovic-Poljak A, Verhoeff NP, Freedman M. Frontotemporal dementia and pharmacologic interventions. J Neuropsychiatry Clin Neurosci. 2010;22:19–29. doi: 10.1176/jnp.2010.22.1.19. [DOI] [PubMed] [Google Scholar]
- 11.Kirshner HS. Frontotemporal dementia and primary progressive aphasia: an update. Curr Neurol Neurosci Rep. 2010;10:504–511. doi: 10.1007/s11910-010-0145-z. [DOI] [PubMed] [Google Scholar]
- 12.Leger GC, Johnson N. A review on primary progressive aphasia. Neuropsychiatr Dis Treat. 2007;3:745–752. doi: 10.2147/ndt.s1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendez MF. Frontotemporal dementia: therapeutic interventions. Front Neurol Neurosci. 2009;24:168–178. doi: 10.1159/000197896. [DOI] [PubMed] [Google Scholar]
- 14.Roberson ED. Frontotemporal dementia. Curr Neurol Neurosci Rep. 2006;6:481–489. doi: 10.1007/s11910-006-0050-7. [DOI] [PubMed] [Google Scholar]
- 15.Sjogren M, Andersen C. Frontotemporal dementia – a brief review. Mech Ageing Dev. 2006;127:180–187. doi: 10.1016/j.mad.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Weder ND, Aziz R, Wilkins K, Tampi RR. Frontotemporal dementias: a review. Ann Gen Psychiatry. 2007;6:15. doi: 10.1186/1744-859X-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeaworth RC, Burke WJ. Frontotemporal dementia: a different kind of dementia. Arch Psychiatr Nurs. 2000;14:249–253. doi: 10.1053/apnu.2000.9816. [DOI] [PubMed] [Google Scholar]
- 18▪▪.Freedman M. Frontotemporal dementia: recommendations for therapeutic studies, designs, and approaches. Can J Neurol Sci. 2007;34(Suppl 1):S118–S124. doi: 10.1017/s0317167100005680. This represents an in-depth review and opinion on shortcomings of prior therapeutic studies in FTD and further presents rational suggestions for improving clinical trials of therapeutic agents in this heterogeneous disease state. [DOI] [PubMed] [Google Scholar]
- 19.Gozes I. Tau pathology and future therapeutics. Curr Alzheimer Res. 2010;7(8):685–696. doi: 10.2174/156720510793611628. [DOI] [PubMed] [Google Scholar]
- 20▪.Trojanowski JQ, Duff K, Fillit H, et al. New directions for frontotemporal dementia drug discovery. Alzheimers Dement. 2008;4:89–93. doi: 10.1016/j.jalz.2007.06.001. Review of emerging therapeutic options in FTD based on recent discoveries in the area of pathobiology and genetics provides a unique look forward at the future of FTD therapeutics undergoing preclinical exploration currently. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21▪.Vossel KA, Miller BL. New approaches to the treatment of frontotemporal lobar degeneration. Curr Opin Neurol. 2008;21:708–716. doi: 10.1097/WCO.0b013e328318444d. Review of emerging therapeutic options in FTD based on recent discoveries in the area of pathobiology and genetics provides a unique look forward at the future of FTD therapeutics undergoing preclinical exploration currently. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kertesz A. Frontotemporal dementia, Pick’s disease. Ideggyogy Sz. 2010;63:4–12. [PubMed] [Google Scholar]
- 23.Kertesz A. Pick complex: an integrative approach to frontotemporal dementia: primary progressive aphasia, corticobasal degeneration, and progressive supranuclear palsy. Neurologist. 2003;9:311–317. doi: 10.1097/01.nrl.0000094943.84390.cf. [DOI] [PubMed] [Google Scholar]
- 24.Pasquier F, Lebert F, Lavenu I, Guillaume B. The clinical picture of frontotemporal dementia: diagnosis and follow-up. Dement Geriatr Cogn Disord. 1999;10(Suppl 1):10–14. doi: 10.1159/000051206. [DOI] [PubMed] [Google Scholar]
- 25.Pijnenburg YA, Mulder JL, van Swieten JC, et al. Diagnostic accuracy of consensus diagnostic criteria for frontotemporal dementia in a memory clinic population. Dement Geriatr Cogn Disord. 2008;25:157–164. doi: 10.1159/000112852. [DOI] [PubMed] [Google Scholar]
- 26.Rose DZ. Primary progressive aphasia. Cleve Clin J Med. 2007;74:9. doi: 10.3949/ccjm.74.1.9. [DOI] [PubMed] [Google Scholar]
- 27.Froelich-Fabre S, Skoglund L, Ostojic J, et al. Clinical and molecular aspects of frontotemporal dementia. Neurodegener Dis. 2004;1:218–224. doi: 10.1159/000080989. [DOI] [PubMed] [Google Scholar]
- 28.Padovani A, Agosti C, Premi E, Bellelli G, Borroni B. Extrapyramidal symptoms in frontotemporal dementia: prevalence and clinical correlations. Neurosci Lett. 2007;422:39–42. doi: 10.1016/j.neulet.2007.05.049. [DOI] [PubMed] [Google Scholar]
- 29.Reich SG, Grill SE. Corticobasal degeneration. Curr Treat Options Neurol. 2009;11:179–185. doi: 10.1007/s11940-009-0021-9. [DOI] [PubMed] [Google Scholar]
- 30.Galariotis V, Bodi N, Janka Z, Kalman J. Frontotemporal dementia – Part II. Differential diagnosis, genetics, molecular pathomechanism and pathology. Ideggyogy Sz. 2005;58:220–224. [PubMed] [Google Scholar]
- 31.Liscic RM. Frontotemporal dementias: update on recent developments in molecular genetics and neuropathology. Arch Hig Rada Toksikol. 2009;60:117–122. doi: 10.2478/10004-1254-60-2009-1921. [DOI] [PubMed] [Google Scholar]
- 32.Neumann M, Tolnay M, Mackenzie IR. The molecular basis of frontotemporal dementia. Expert Rev Mol Med. 2009;11:e23. doi: 10.1017/S1462399409001136. [DOI] [PubMed] [Google Scholar]
- 33.D’Souza I, Schellenberg GD. Regulation of tau isoform expression and dementia. Biochim Biophys Acta. 2005;1739:104–115. doi: 10.1016/j.bbadis.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Hodges JR, Miller B. The classification, genetics and neuropathology of frontotemporal dementia. Introduction to the special topic papers: part I. Neurocase. 2001;7:31–35. doi: 10.1093/neucas/7.1.31. [DOI] [PubMed] [Google Scholar]
- 35.Schraen-Maschke S, Dhaenens CM, Delacourte A, Sablonniere B. Microtubule-associated protein tau gene: a risk factor in human neurodegenerative diseases. Neurobiol Dis. 2004;15:449–460. doi: 10.1016/j.nbd.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 36.Wilhelmsen KC, Clark LN, Miller BL, Geschwind DH. Tau mutations in frontotemporal dementia. Dement Geriatr Cogn Disord. 1999;10(Suppl 1):88–92. doi: 10.1159/000051221. [DOI] [PubMed] [Google Scholar]
- 37.Yoshiyama Y, Lee VM, Trojanowski JQ. Frontotemporal dementia and tauopathy. Curr Neurol Neurosci Rep. 2001;1:413–421. doi: 10.1007/s11910-001-0100-0. [DOI] [PubMed] [Google Scholar]
- 38.van Swieten JC, Heutink P. Mutations in progranulin (GRN) within the spectrum of clinical and pathological phenotypes of frontotemporal dementia. Lancet Neurol. 2008;7:965–974. doi: 10.1016/S1474-4422(08)70194-7. [DOI] [PubMed] [Google Scholar]
- 39.Yu CE, Bird TD, Bekris LM, et al. The spectrum of mutations in progranulin: a collaborative study screening 545 cases of neurodegeneration. Arch Neurol. 2010;67:161–170. doi: 10.1001/archneurol.2009.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guinto JB, Ritson GP, Taylor JP, Forman MS. Valosin-containing protein and the pathogenesis of frontotemporal dementia associated with inclusion body myopathy. Acta Neuropathol. 2007;114:55–61. doi: 10.1007/s00401-007-0224-7. [DOI] [PubMed] [Google Scholar]
- 41.Kimonis VE, Fulchiero E, Vesa J, Watts G. VCP disease associated with myopathy, Paget disease of bone and frontotemporal dementia: review of a unique disorder. Biochim Biophys Acta. 2008;1782:744–748. doi: 10.1016/j.bbadis.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Weihl CC, Pestronk A, Kimonis VE. Valosin-containing protein disease: inclusion body myopathy with Paget’s disease of the bone and fronto-temporal dementia. Neuromuscul Disord. 2009;19:308–315. doi: 10.1016/j.nmd.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Momeni P, Rogaeva E, Van Deerlin V, et al. Genetic variability in CHMP2B and frontotemporal dementia. Neurodegener Dis. 2006;3:129–133. doi: 10.1159/000094771. [DOI] [PubMed] [Google Scholar]
- 44.Skibinski G, Parkinson NJ, Brown JM, et al. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat Genet. 2005;37:806–808. doi: 10.1038/ng1609. [DOI] [PubMed] [Google Scholar]
- 45.van der Zee J, Urwin H, Engelborghs S, et al. CHMP2B C-truncating mutations in frontotemporal lobar degeneration are associated with an aberrant endosomal phenotype in vitro. Hum Mol Genet. 2008;17:313–322. doi: 10.1093/hmg/ddm309. [DOI] [PubMed] [Google Scholar]
- 46.Borroni B, Bonvicini C, Alberici A, et al. Mutation within TARDBP leads to frontotemporal dementia without motor neuron disease. Hum Mutat. 2009;30:E974–E983. doi: 10.1002/humu.21100. [DOI] [PubMed] [Google Scholar]
- 47.Mackenzie IR, Rademakers R, Neumann M. TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol. 2010;9:995–1007. doi: 10.1016/S1474-4422(10)70195-2. [DOI] [PubMed] [Google Scholar]
- 48.Origone P, Caponnetto C, Bandettini Di Poggio M, et al. Enlarging clinical spectrum of FALS with TARDBP gene mutations: S393L variant in an Italian family showing phenotypic variability and relevance for genetic counselling. Amyotroph Lateral Scler. 2010;11:223–227. doi: 10.3109/17482960903165039. [DOI] [PubMed] [Google Scholar]
- 49.Gabryelewicz T, Masellis M, Berdynski M, et al. Intra-familial clinical heterogeneity due to FTLD-U with TDP-43 proteinopathy caused by a novel deletion in progranulin gene (PGRN) J Alzheimers Dis. 2010;22(4):1123–1133. doi: 10.3233/JAD-2010-101413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mackenzie IR. The neuropathology and clinical phenotype of FTD with progranulin mutations. Acta Neuropathol. 2007;114:49–54. doi: 10.1007/s00401-007-0223-8. [DOI] [PubMed] [Google Scholar]
- 51▪▪.Seelaar H, Rohrer JD, Pijnenburg YA, Fox NC, van Swieten JC. Clinical, genetic and pathological heterogeneity of frontotemporal dementia: a review. J Neurol Neurosurg Psychiatry. 2010 doi: 10.1136/jnnp.2010.212225. (Epub ahead of print). Focuses on recent clinical, neuropsychological, imaging, genetic and pathological developments that have influenced our understanding of FTD as a complex and heterogeneous disorder. [DOI] [PubMed] [Google Scholar]
- 52.van Swieten JC, Rosso SM, van Herpen E, Kamphorst W, Ravid R, Heutink P. Phenotypic variation in frontotemporal dementia and parkinsonism linked to chromosome 17. Dement Geriatr Cogn Disord. 2004;17:261–264. doi: 10.1159/000077150. [DOI] [PubMed] [Google Scholar]
- 53▪.Cairns NJ, Bigio EH, Mackenzie IR, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. The molecular and pathological heterogeneity of frontotemporal lobar dementia is explored with an emphasis on clarifying nomenclature and establishing a uniform nosology in the field. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kovari E. Neuropathological spectrum of frontal lobe dementias. Front Neurol Neurosci. 2009;24:149–159. doi: 10.1159/000197894. [DOI] [PubMed] [Google Scholar]
- 55.Kertesz A, Munoz D. Relationship between frontotemporal dementia and corticobasal degeneration/progressive supranuclear palsy. Dement Geriatr Cogn Disord. 2004;17:282–286. doi: 10.1159/000077155. [DOI] [PubMed] [Google Scholar]
- 56.Rosso SM, van Swieten JC. New developments in frontotemporal dementia and parkinsonism linked to chromosome 17. Curr Opin Neurol. 2002;15:423–428. doi: 10.1097/00019052-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 57.Zhukareva V, Trojanowski JQ, Lee VM. Assessment of pathological tau proteins in frontotemporal dementias: qualitative and quantitative approaches. Am J Geriatr Psychiatry. 2004;12:136–145. [PubMed] [Google Scholar]
- 58.Mackenzie IR. The neuropathology of FTD associated With ALS. Alzheimer Dis Assoc Disord. 2007;21:S44–S49. doi: 10.1097/WAD.0b013e31815c3486. [DOI] [PubMed] [Google Scholar]
- 59.Back-Madruga C, Boone KB, Briere J, et al. Functional ability in executive variant Alzheimer’s disease and typical Alzheimer’s disease. Clin Neuropsychol. 2002;16:331–340. doi: 10.1076/clin.16.3.331.13846. [DOI] [PubMed] [Google Scholar]
- 60.Habek M, Hajnsek S, Zarkovic K, Chudy D, Mubrin Z. Frontal variant of Alzheimer’s disease: clinico–CSF–pathological correlation. Can J Neurol Sci. 2010;37:118–120. [PubMed] [Google Scholar]
- 61.Johnson JK, Head E, Kim R, Starr A, Cotman CW. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch Neurol. 1999;56:1233–1239. doi: 10.1001/archneur.56.10.1233. [DOI] [PubMed] [Google Scholar]
- 62.Kosunen O, Soininen H, Paljarvi L, Heinonen O, Talasniemi S, Riekkinen PJ., Sr Diagnostic accuracy of Alzheimer’s disease: a neuropathological study. Acta Neuropathol. 1996;91:185–193. doi: 10.1007/s004010050412. [DOI] [PubMed] [Google Scholar]
- 63.Larner AJ. “Frontal variant Alzheimer’s disease”: a reappraisal. Clin Neurol Neurosurg. 2006;108:705–708. doi: 10.1016/j.clineuro.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 64.Perry RJ, Hodges JR. Differentiating frontal and temporal variant frontotemporal dementia from Alzheimer’s disease. Neurology. 2000;54:2277–2284. doi: 10.1212/wnl.54.12.2277. [DOI] [PubMed] [Google Scholar]
- 65.Talbot K, Young RA, Jolly-Tornetta C, Lee VM, Trojanowski JQ, Wolf BA. A frontal variant of Alzheimer’s disease exhibits decreased calcium-independent phospholipase A2 activity in the prefrontal cortex. Neurochem Int. 2000;37:17–31. doi: 10.1016/s0197-0186(00)00006-1. [DOI] [PubMed] [Google Scholar]
- 66.Taylor KI, Probst A, Miserez AR, Monsch AU, Tolnay M. Clinical course of neuropathologically confirmed frontal-variant Alzheimer’s disease. Nat Clin Pract Neurol. 2008;4:226–232. doi: 10.1038/ncpneuro0746. [DOI] [PubMed] [Google Scholar]
- 67.Gislason TB, Sjogren M, Larsson L, Skoog I. The prevalence of frontal variant frontotemporal dementia and the frontal lobe syndrome in a population based sample of 85 year olds. J Neurol Neurosurg Psychiatry. 2003;74:867–871. doi: 10.1136/jnnp.74.7.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Downes JJ, Priestley NM, Doran M, Ferran J, Ghadiali E, Cooper P. Intellectual, mnemonic, and frontal functions in dementia with Lewy bodies: a comparison with early and advanced Parkinson’s disease. Behav Neurol. 1998;11:173–183. [PubMed] [Google Scholar]
- 69.Engelborghs S, Maertens K, Marien P, et al. Behavioural and neuropsychological correlates of frontal lobe features in dementia. Psychol Med. 2006;36:1173–1182. doi: 10.1017/S003329170600777X. [DOI] [PubMed] [Google Scholar]
- 70.Stewart JT. Psychiatric and behavioral manifestations of vascular dementia. Am J Geriatr Cardiol. 2007;16:165–170. doi: 10.1111/j.1076-7460.2007.06038.x. [DOI] [PubMed] [Google Scholar]
- 71.Odawara T, Shiozaki K, Iseki E, Hino H, Kosaka K. Alterations of muscarinic acetylcholine receptors in atypical Pick’s disease without Pick bodies. J Neurol Neurosurg Psychiatry. 2003;74:965–967. doi: 10.1136/jnnp.74.7.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Procter AW, Qurne M, Francis PT. Neurochemical features of frontotemporal dementia. Dement Geriatr Cogn Disord. 1999;10(Suppl 1):80–84. doi: 10.1159/000051219. [DOI] [PubMed] [Google Scholar]
- 73.Sparks DL, Markesbery WR. Altered serotonergic and cholinergic synaptic markers in Pick’s disease. Arch Neurol. 1991;48:796–799. doi: 10.1001/archneur.1991.00530200032014. [DOI] [PubMed] [Google Scholar]
- 74.Uhl GR, Hilt DC, Hedreen JC, Whitehouse PJ, Price DL. Pick’s disease (lobar sclerosis): depletion of neurons in the nucleus basalis of Meynert. Neurology. 1983;33:1470–1473. doi: 10.1212/wnl.33.11.1470. [DOI] [PubMed] [Google Scholar]
- 75.Weinberger DR, Gibson R, Coppola R, et al. The distribution of cerebral muscarinic acetylcholine receptors in vivo in patients with dementia. A controlled study with 123IQNB and single photon emission computed tomography. Arch Neurol. 1991;48:169–176. doi: 10.1001/archneur.1991.00530140061018. [DOI] [PubMed] [Google Scholar]
- 76.Wood PL, Etienne P, Lal S, et al. A postmortem comparison of the cortical cholinergic system in Alzheimer’s disease and Pick’s disease. J Neurol Sci. 1983;62:211–217. doi: 10.1016/0022-510x(83)90200-9. [DOI] [PubMed] [Google Scholar]
- 77.Frisoni GB, Pizzolato G, Bianchetti A, et al. Single photon emission computed tomography with [99Tc]-HM-PAO and [123I]-IBZM in Alzheimer’s disease and dementia of frontal type: preliminary results. Acta Neurol Scand. 1994;89:199–203. doi: 10.1111/j.1600-0404.1994.tb01661.x. [DOI] [PubMed] [Google Scholar]
- 78.Kanazawa I, Kwak S, Sasaki H, et al. Studies on neurotransmitter markers of the basal ganglia in Pick’s disease, with special reference to dopamine reduction. J Neurol Sci. 1988;83:63–74. doi: 10.1016/0022-510x(88)90020-2. [DOI] [PubMed] [Google Scholar]
- 79.Rinne JO, Laine M, Kaasinen V, Norvasuo-Heila MK, Nagren K, Helenius H. Striatal dopamine transporter and extrapyramidal symptoms in frontotemporal dementia. Neurology. 2002;58:1489–1493. doi: 10.1212/wnl.58.10.1489. [DOI] [PubMed] [Google Scholar]
- 80.Sjogren M, Minthon L, Passant U, Blennow K, Wallin A. Decreased monoamine metabolites in frontotemporal dementia and Alzheimer’s disease. Neurobiol Aging. 1998;19:379–384. doi: 10.1016/s0197-4580(98)00086-4. [DOI] [PubMed] [Google Scholar]
- 81.Sparks DL, Woeltz VM, Markesbery WR. Alterations in brain monoamine oxidase activity in aging, Alzheimer’s disease, and Pick’s disease. Arch Neurol. 1991;48:718–721. doi: 10.1001/archneur.1991.00530190064017. [DOI] [PubMed] [Google Scholar]
- 82.Ferrer I. Neurons and their dendrites in frontotemporal dementia. Dement Geriatr Cogn Disord. 1999;10(Suppl 1):55–60. doi: 10.1159/000051214. [DOI] [PubMed] [Google Scholar]
- 83.Bowen DM, Procter AW, Mann DM, et al. Imbalance of a serotonergic system in frontotemporal dementia: implication for pharmacotherapy. Psychopharmacology (Berl) 2008;196:603–610. doi: 10.1007/s00213-007-0992-8. [DOI] [PubMed] [Google Scholar]
- 84.Franceschi M, Anchisi D, Pelati O, et al. Glucose metabolism and serotonin receptors in the frontotemporal lobe degeneration. Ann Neurol. 2005;57:216–225. doi: 10.1002/ana.20365. [DOI] [PubMed] [Google Scholar]
- 85.Yang Y, Schmitt HP. Frontotemporal dementia: evidence for impairment of ascending serotoninergic but not noradrenergic innervation. Immunocytochemical and quantitative study using a graph method. Acta Neuropathol. 2001;101:256–270. doi: 10.1007/s004010000293. [DOI] [PubMed] [Google Scholar]
- 86.Gordon E, Rohrer JD, Kim LG, et al. Measuring disease progression in frontotemporal lobar degeneration: a clinical and MRI study. Neurology. 2010;74:666–673. doi: 10.1212/WNL.0b013e3181d1a879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kipps CM, Nestor PJ, Dawson CE, Mitchell J, Hodges JR. Measuring progression in frontotemporal dementia: implications for therapeutic interventions. Neurology. 2008;70:2046–2052. doi: 10.1212/01.wnl.0000313366.76973.8a. [DOI] [PubMed] [Google Scholar]
- 88.Mioshi E, Hsieh S, Savage S, Hornberger M, Hodges JR. Clinical staging and disease progression in frontotemporal dementia. Neurology. 2010;74:1591–1597. doi: 10.1212/WNL.0b013e3181e04070. [DOI] [PubMed] [Google Scholar]
- 89.Boxer AL, Lipton AM, Womack K, et al. An open-label study of memantine treatment in 3 subtypes of frontotemporal lobar degeneration. Alzheimer Dis Assoc Disord. 2009;23:211–217. doi: 10.1097/WAD.0b013e318197852f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Diehl-Schmid J, Forstl H, Perneczky R, Pohl C, Kurz A. A 6-month, open-label study of memantine in patients with frontotemporal dementia. Int J Geriatr Psychiatry. 2008;23:754–759. doi: 10.1002/gps.1973. [DOI] [PubMed] [Google Scholar]
- 91.FitzGerald DB, Crucian GP, Mielke JB, et al. Effects of donepezil on verbal memory after semantic processing in healthy older adults. Cogn Behav Neurol. 2008;21:57–64. doi: 10.1097/WNN.0b013e3181799df1. [DOI] [PubMed] [Google Scholar]
- 92.Kertesz A, Morlog D, Light M, et al. Galantamine in frontotemporal dementia and primary progressive aphasia. Dement Geriatr Cogn Disord. 2008;25:178–185. doi: 10.1159/000113034. [DOI] [PubMed] [Google Scholar]
- 93.Mendez MF, Shapira JS, McMurtray A, Licht E. Preliminary findings: behavioral worsening on donepezil in patients with frontotemporal dementia. Am J Geriatr Psychiatry. 2007;15:84–87. doi: 10.1097/01.JGP.0000231744.69631.33. [DOI] [PubMed] [Google Scholar]
- 94.Moretti R, Torre P, Antonello RM, Cattaruzza T, Cazzato G, Bava A. Rivastigmine in frontotemporal dementia: an open-label study. Drugs Aging. 2004;21:931–937. doi: 10.2165/00002512-200421140-00003. [DOI] [PubMed] [Google Scholar]
- 95.Adler G, Teufel M, Drach LM. Pharmacological treatment of frontotemporal dementia: treatment response to the MAO-A inhibitor moclobemide. Int J Geriatr Psychiatry. 2003;18:653–655. doi: 10.1002/gps.894. [DOI] [PubMed] [Google Scholar]
- 96.Deakin JB, Rahman S, Nestor PJ, Hodges JR, Sahakian BJ. Paroxetine does not improve symptoms and impairs cognition in frontotemporal dementia: a double-blind randomized controlled trial. Psychopharmacology (Berl) 2004;172:400–408. doi: 10.1007/s00213-003-1686-5. [DOI] [PubMed] [Google Scholar]
- 97.Lebert F, Stekke W, Hasenbroekx C, Pasquier F. Frontotemporal dementia: a randomised, controlled trial with trazodone. Dement Geriatr Cogn Disord. 2004;17:355–359. doi: 10.1159/000077171. [DOI] [PubMed] [Google Scholar]
- 98.Moretti R, Torre P, Antonello RM, Cazzato G, Bava A. Effects of selegiline on fronto-temporal dementia: a neuropsychological evaluation. Int J Geriatr Psychiatry. 2002;17:391–392. doi: 10.1002/gps.602. [DOI] [PubMed] [Google Scholar]
- 99.Moretti R, Torre P, Antonello RM, Cazzato G, Bava A. Frontotemporal dementia: paroxetine as a possible treatment of behavior symptoms. A randomized, controlled, open 14-month study. Eur Neurol. 2003;49:13–19. doi: 10.1159/000067021. [DOI] [PubMed] [Google Scholar]
- 100.Swartz JR, Miller BL, Lesser IM, Darby AL. Frontotemporal dementia: treatment response to serotonin selective reuptake inhibitors. J Clin Psychiatry. 1997;58:212–216. [PubMed] [Google Scholar]
- 101.Curtis RC, Resch DS. Case of pick’s central lobar atrophy with apparent stabilization of cognitive decline after treatment with risperidone. J Clin Psychopharmacol. 2000;20:384–385. doi: 10.1097/00004714-200006000-00018. [DOI] [PubMed] [Google Scholar]
- 102.Czarnecki K, Kumar N, Josephs KA. Parkinsonism and tardive antecollis in frontotemporal dementia – increased sensitivity to newer antipsychotics? Eur J Neurol. 2008;15:199–201. doi: 10.1111/j.1468-1331.2007.02032.x. [DOI] [PubMed] [Google Scholar]
- 103.Fellgiebel A, Muller MJ, Hiemke C, Bartenstein P, Schreckenberger M. Clinical improvement in a case of frontotemporal dementia under aripiprazole treatment corresponds to partial recovery of disturbed frontal glucose metabolism. World J Biol Psychiatry. 2007;8:123–126. doi: 10.1080/15622970601016538. [DOI] [PubMed] [Google Scholar]
- 104.Moretti R, Torre P, Antonello RM, Cazzato G, Griggio S, Bava A. Olanzapine as a treatment of neuropsychiatric disorders of Alzheimer’s disease and other dementias: a 24-month follow-up of 68 patients. Am J Alzheimers Dis Other Demen. 2003;18:205–214. doi: 10.1177/153331750301800410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Goforth HW, Konopka L, Primeau M, et al. Quantitative electroencephalography in frontotemporal dementia with methylphenidate response: a case study. Clin EEG Neurosci. 2004;35:108–111. doi: 10.1177/155005940403500212. [DOI] [PubMed] [Google Scholar]
- 106.Rahman S, Robbins TW, Hodges JR, et al. Methylphenidate (‘Ritalin’) can ameliorate abnormal risk-taking behavior in the frontal variant of frontotemporal dementia. Neuropsychopharmacology. 2006;31:651–658. doi: 10.1038/sj.npp.1300886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Decker DA, Heilman KM. Steroid treatment of primary progressive aphasia. Arch Neurol. 2008;65:1533–1535. doi: 10.1001/archneur.65.11.1533. [DOI] [PubMed] [Google Scholar]
- 108.Tobinick E. Perispinal etanercept produces rapid improvement in primary progressive aphasia: identification of a novel, rapidly reversible TNF-mediated pathophysiologic mechanism. Medscape J Med. 2008;10:135. [PMC free article] [PubMed] [Google Scholar]
- 109.Cruz M, Marinho V, Fontenelle LF, Engelhardt E, Laks J. Topiramate may modulate alcohol abuse but not other compulsive behaviors in frontotemporal dementia: case report. Cogn Behav Neurol. 2008;21:104–106. doi: 10.1097/WNN.0b013e31816bdf73. [DOI] [PubMed] [Google Scholar]
- 110.Huey ED, Putnam KT, Grafman J. A systematic review of neurotransmitter deficits and treatments in frontotemporal dementia. Neurology. 2006;66:17–22. doi: 10.1212/01.wnl.0000191304.55196.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hansen LA, Deteresa R, Tobias H, Alford M, Terry RD. Neocortical morphometry and cholinergic neurochemistry in Pick’s disease. Am J Pathol. 1988;131:507–518. [PMC free article] [PubMed] [Google Scholar]
- 112.Reed DA, Johnson NA, Thompson C, Weintraub S, Mesulam MM. A clinical trial of bromocriptine for treatment of primary progressive aphasia. Ann Neurol. 2004;56:750. doi: 10.1002/ana.20301. [DOI] [PubMed] [Google Scholar]
- 113.Swanberg MM. Memantine for behavioral disturbances in frontotemporal dementia: a case series. Alzheimer Dis Assoc Disord. 2007;21:164–166. doi: 10.1097/WAD.0b013e318047df5d. [DOI] [PubMed] [Google Scholar]
- 114.Anneser JM, Jox RJ, Borasio GD. Inappropriate sexual behaviour in a case of ALS and FTD: successful treatment with sertraline. Amyotroph Lateral Scler. 2007;8:189–190. doi: 10.1080/17482960601073543. [DOI] [PubMed] [Google Scholar]
- 115.Mendez MF, Shapira JS. The spectrum of recurrent thoughts and behaviors in frontotemporal dementia. CNS Spectr. 2008;13:202–208. doi: 10.1017/s1092852900028443. [DOI] [PubMed] [Google Scholar]
- 116.Prodan CI, Monnot M, Ross ED. Behavioural abnormalities associated with rapid deterioration of language functions in semantic dementia respond to sertraline. J Neurol Neurosurg Psychiatry. 2009;80:1416–1417. doi: 10.1136/jnnp.2009.173260. [DOI] [PubMed] [Google Scholar]
- 117.Dorsey ER, Rabbani A, Gallagher SA, Conti RM, Alexander GC. Impact of FDA black box advisory on antipsychotic medication use. Arch Intern Med. 2010;170:96–103. doi: 10.1001/archinternmed.2009.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118▪▪.Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33:957–970. doi: 10.1038/sj.npp.1301492. Thought leaders in the field of geriatric psychiatry wrestle with available data in light of the 2005 US FDA ‘black-box’ warning on the use of antipsychotic drugs in dementia. The authors keenly point out that behavioral and psychiatric symptoms are major problems that lack any approved therapies at present, suggest that antipsychotic therapy may still play an important role in the management of dementia, but also urge caution and the importance of shared decision-making when considering this therapeutic strategy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kirshner HS. Controversies in behavioral neurology: the use of atypical antipsychotic drugs to treat neurobehavioral symptoms in dementia. Curr Neurol Neurosci Rep. 2008;8:471–474. doi: 10.1007/s11910-008-0075-1. [DOI] [PubMed] [Google Scholar]
- 120.Recupero PR, Rainey SE. Managing risk when considering the use of atypical antipsychotics for elderly patients with dementia-related psychosis. J Psychiatr Pract. 2007;13:143–152. doi: 10.1097/01.pra.0000271655.02093.49. [DOI] [PubMed] [Google Scholar]
- 121.Salzman C, Jeste DV, Meyer RE, et al. Elderly patients with dementia-related symptoms of severe agitation and aggression: consensus statement on treatment options, clinical trials methodology, and policy. J Clin Psychiatry. 2008;69:889–898. doi: 10.4088/jcp.v69n0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang PS, Brookhart MA, Setoguchi S, Patrick AR, Schneeweiss S. Psychotropic medication use for behavioral symptoms of dementia. Curr Neurol Neurosci Rep. 2006;6:490–495. doi: 10.1007/s11910-006-0051-6. [DOI] [PubMed] [Google Scholar]
- 123.Chow TW, Binns MA, Cummings JL, et al. Apathy symptom profile and behavioral associations in frontotemporal dementia vs dementia of Alzheimer type. Arch Neurol. 2009;66:888–893. doi: 10.1001/archneurol.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rosen HJ, Cummings J. A real reason for patients with pseudobulbar affect to smile. Ann Neurol. 2007;61:92–96. doi: 10.1002/ana.21056. [DOI] [PubMed] [Google Scholar]
- 125.Schiffer R, Pope LE. Review of pseudobulbar affect including a novel and potential therapy. J Neuropsychiatry Clin Neurosci. 2005;17:447–454. doi: 10.1176/jnp.17.4.447. [DOI] [PubMed] [Google Scholar]
- 126.Brooks BR, Thisted RA, Appel SH, et al. Treatment of pseudobulbar affect in ALS with dextromethorphan/quinidine: a randomized trial. Neurology. 2004;63:1364–1370. doi: 10.1212/01.wnl.0000142042.50528.2f. [DOI] [PubMed] [Google Scholar]
- 127.Miller A. Pseudobulbar affect in multiple sclerosis: toward the development of innovative therapeutic strategies. J Neurol Sci. 2006;245:153–159. doi: 10.1016/j.jns.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 128.Panitch HS, Thisted RA, Smith RA, et al. Randomized, controlled trial of dextromethorphan/quinidine for pseudobulbar affect in multiple sclerosis. Ann Neurol. 2006;59:780–787. doi: 10.1002/ana.20828. [DOI] [PubMed] [Google Scholar]
- 129.Lillo P, Hodges JR. Frontotemporal dementia and motor neurone disease: overlapping clinic-pathological disorders. J Clin Neurosci. 2009;16:1131–1135. doi: 10.1016/j.jocn.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 130.Mathuranath PS, Xuereb JH, Bak T, Hodges JR. Corticobasal ganglionic degeneration and/or frontotemporal dementia? A report of two overlap cases and review of literature. J Neurol Neurosurg Psychiatry. 2000;68:304–312. doi: 10.1136/jnnp.68.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mitsuyama Y, Inoue T. Clinical entity of frontotemporal dementia with motor neuron disease. Neuropathology. 2009;29:649–654. doi: 10.1111/j.1440-1789.2009.01059.x. [DOI] [PubMed] [Google Scholar]
- 132.Snowden J, Neary D, Mann D. Frontotemporal lobar degeneration: clinical and pathological relationships. Acta Neuropathol. 2007;114:31–38. doi: 10.1007/s00401-007-0236-3. [DOI] [PubMed] [Google Scholar]
- 133.Bedlack RS. Amyotrophic lateral sclerosis: current practice and future treatments. Curr Opin Neurol. 2010;23:524–529. doi: 10.1097/WCO.0b013e32833c7ac2. [DOI] [PubMed] [Google Scholar]
- 134.Bellingham MC. A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: what have we learned in the last decade? CNS Neurosci Ther. 2011;17(1):4–31. doi: 10.1111/j.1755-5949.2009.00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cheah BC, Vucic S, Krishnan AV, Kiernan MC. Riluzole, neuroprotection and amyotrophic lateral sclerosis. Curr Med Chem. 2010;17:1942–1199. doi: 10.2174/092986710791163939. [DOI] [PubMed] [Google Scholar]
- 136.Miller RG, Jackson CE, Kasarskis EJ, et al. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2009;73:1218–1226. doi: 10.1212/WNL.0b013e3181bc0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Blair IP, Williams KL, Warraich ST, et al. FUS mutations in amyotrophic lateral sclerosis: clinical, pathological, neurophysiological and genetic analysis. J Neurol Neurosurg Psychiatry. 2010;81:639–645. doi: 10.1136/jnnp.2009.194399. [DOI] [PubMed] [Google Scholar]
- 138.Engelborghs S, Vloeberghs E, Le Bastard N, et al. The dopaminergic neurotransmitter system is associated with aggression and agitation in frontotemporal dementia. Neurochem Int. 2008;52:1052–1060. doi: 10.1016/j.neuint.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 139.Sedaghat F, Gotzamani-Psarrakou A, Dedousi E, et al. Evaluation of dopaminergic function in frontotemporal dementia using I-FP-CIT single photon emission computed tomography. Neurodegener Dis. 2007;4:382–385. doi: 10.1159/000105159. [DOI] [PubMed] [Google Scholar]