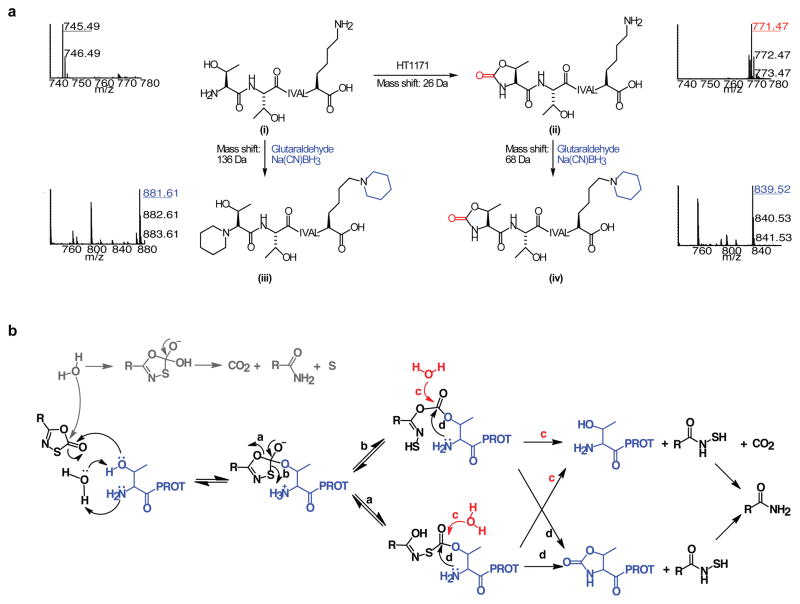
Figure 3. LC-MS/MS identification of the modified N-terminus of the Mtb proteasome treated with oxathiazol-2-ones.
(a) Mass spectra of tryptic N-terminal heptapeptides from Mtb proteasomes that were untreated (i), HT1171-treated (ii), treated with glutaraldehyde/Na(CN)BH3 after trypsin digestion (iii), or treated with glutaraldehyde/Na(CN)BH3 after HT1171 treatment and trypsin digestion (iv). All ions were confirmed by MS/MS analysis (Fig. S7a–d). Reaction equations illustrate proposed modification of active site Thr1 by oxathiazol-2-one (i
 ii), and modification of primary amino groups at Thr1 and Lys7 with glutaraldehyde and Na(CN)BH3 (i
ii), and modification of primary amino groups at Thr1 and Lys7 with glutaraldehyde and Na(CN)BH3 (i
 iii and ii
iii and ii
 iv). (b) Proposed mechanism of proteasome inactivation by oxathiazol-2-one. Paths marked by a, b, d lead to irreversible inhibition. In paths marked by c, hydrolysis of the inhibitor-enzyme intermediate allows the proteasome to degrade the oxathiazol-2-one without losing activity.
iv). (b) Proposed mechanism of proteasome inactivation by oxathiazol-2-one. Paths marked by a, b, d lead to irreversible inhibition. In paths marked by c, hydrolysis of the inhibitor-enzyme intermediate allows the proteasome to degrade the oxathiazol-2-one without losing activity.