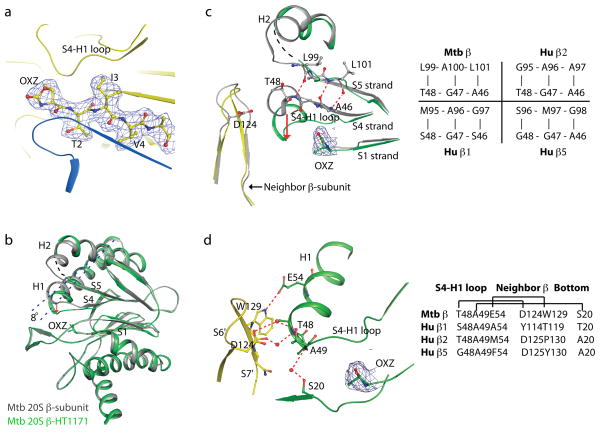
Figure 4. Crystal structure of the full-length Mtb 20S proteasome after exposure to HT1171 reveals cyclo-carbonylation of active site Thr1 and conformational changes in the β-subunit.
(a) 2Fo-Fc electron density map contoured at 1.2 σ and superimposed on the crystal structure of HT1171-treated proteasome at the active site in the β-subunit. Map was calculated by omitting oxazolidin-2-one and the N-terminal four amino acids from the crystal structure. (b) Superposition of HT1171-modifiedβ-subunit in green with native β-subunit in gray (PDB 2FHG). OXZ labels oxazolidin-2-one ring on Thr1. The conformational changes can be approximately described by an 8° tilt of H1 (dashed line) and a downward shift of the S4-H1 loop (red arrow). (c) Active site structure of HT1171-modified β-subunit in green, in comparison with native β-subunit structure in gray. In the native structure, A46G47T48 is part of the S4 strand forming a β-sheet with the S5 strand. In the HT1171-treated structure, A46G47T48 loses contacts with S5 and converts to the S4-H1 loop. Panel at right specifies amino acid pairs that form S4–S5 β-sheet interaction in this region in Mtb β chain prior to oxathiazol-2-one treatment and compares these with corresponding sequences in the proteolytically active human proteasome β chains. (d) Active site structure of HT1171-treated Mtb 20S oriented to view H bonds stabilizing new position of S4-H1 loop in the Mtb β chain after oxathiazol-2-one treatment. S4-H1 loop as upper surface of the constricted substrate-binding pocket is stabilized by an H-bond between Glu54 and Trp129 of the neighboring βsubunit and by 3 pairs of water-mediated H-bonds with the neighboring β-subunit and with Ser20 at the lower substrate-binding surface of the same subunit. Panel at right compares Mtb’s H-bonding residues with the human counterparts. The 2Fo-Fc electron density map contoured at 1.2 σ is superimposed at the oxazolidin-2-one ring site in (c) and (d). Thr1 modification and β-subunit conformational changes upon exposure to GL1 or HT1171 in the wild type and the open gate Mtb 20S are virtually the same.