Abstract
Adolescent rats show immaturities in executive function and are less able than adult rats to learn reinforcement reversals and shift attentional set. These two forms of executive function rely on the functional integrity of the orbitofrontal and prelimbic cortices respectively. Drugs used to treat attention deficit disorder, such as atomoxetine, that increase cortical catecholamine levels improve executive functions in humans, non-human primates and adult rats with prefrontal lesions. Cortical noradrenergic systems are some of the last to mature in primates and rats. Moreover, norepinephrine transporters (NET) are higher in juvenile rats than adults. The underdeveloped cortical noradrenergic system and higher number of NET are hypothesized to underlie the immaturities in executive function found in adolescents. We assessed executive function in male Long-Evans rats using an intra-dimensional/extra-dimensional set shifting task. We administered the NET blocker, atomoxetine (0.0, 0.1, 0.9 mg/kg/ml; i.p.), prior to the test of attentional set shift and a reinforcement reversal. The lowest dose of drug facilitated attentional set shifting but had no effect on reversal learning. These data demonstrate that NET blockade allows adolescent rats to more easily perform attentional set shifting.
Keywords: Executive function, Prefrontal cortex, Norepinephrine, Reversals, Set-shifting
Though the neurochemical and neuroanatomical basis of attentional function in adults has been elucidated by studies in humans (Chamberlain et al., 2007, Spencer et al., 1998) and through translational research using animals (Arnsten, 1998, Aston-Jones et al., 2000, Baxter et al., 1995, Birrell and Brown, 2000, Dalley et al., 2004, McGaughy et al., 1996, Sarter et al., 2005), the ontogeny of attention remains poorly understood. Much of the research on executive functions and adolescence has focused on changes in the dopaminergic systems (Black et al., 2006, Dow-Edwards et al., 2008, Greydanus et al., 2007, Kuczenski and Segal, 2002, Laviola et al., 1999, Loveland et al., 2008, Parker et al., 2007, Schulz et al., 2005). These systems have been implicated in response inhibition (Barkley, 1997, Dalley et al., 2004, Robbins, 2000) reward processing (Adriani et al., 2006, Brenhouse and Andersen, 2008, Brenhouse et al., 2008, Laboni et al., 1995) and may be dysfunctional in adolescents with attention deficit hyperactivity disorder (ADHD) (Castellanos and Tannock, 2002, Greydanus et al., 2007, Kieling et al., 2008, Sagvolden and Sergeant, 1998, Shafritz et al., 2004, Solanto, 1984, Sonuga-Barke et al., 2008, Viggiano et al., 2004) Tourette's syndrome (Andersen, 2008) and those individuals susceptible to substance abuse disorders (Berke and Hyman, 2000, Brenhouse et al., 2008, Burns et al., 1994, Robbins and Everitt, 1999). These data have provided important insights into one aspect of executive function that may develop during adolescence However, other aspects of executive function such as the ability to rapidly and appropriately re-direct attention has been shown to depend not on prefrontal dopamine, but on the functional integrity of prefrontal norepinephrine (NE: Lapiz and Morilak, 2006, McGaughy et al., 2008, Milstein et al., 2007, Newman et al., 2008, Tait et al., 2007).
Noradrenergic projections to frontal cortex have been hypothesized to be mature by post-natal day (PND) 25(Coyle and Axelrod, 1972, Harden et al., 1977, Levitt and Moore, 1979, Morrison et al., 1979, Wendlandt et al., 1977). Unlike fiber density which is mature by PND 25, norepinephrine transporter (NET) density dramatically decreases between PND 25 and PND 90 (Moll et al., 2000, Sanders et al., 2005). Recent data suggests that a polymorphism in the NET gene is associated with a higher incidence of attention deficit disorder in both Korean and U.S. populations (Joung et al., 2009, Xu et al., 2008). Drugs such as methylphenidate and atomoxetine that block NET, increase levels of prefrontal norepinephrine, (Bymaster et al., 2002, Christman et al., 2004, Tzavara et al., 2007) are used to treat ADHD (Biederman et al., 2002, Chamberlain et al., 2007, Christman et al., 2004, Kratochvil et al., 2003, Kratochvil et al., 2006, Newcorn et al., 2008, Newman et al., 2008) or improve cognition in normal subjects (Berridge and Waterhouse, 2003, Chamberlain et al., 2008, Mehta et al., 2000).
We hypothesize that the density of NET in prefrontal cortex is critical to executive function. Specifically, the higher density of NET in young rats is (Moll et al., 2000) hypothesized to decrease the amount of time NE is available in the synaptic cleft preventing shifts of attention (Aston-Jones et al., 2000, Aston-Jones et al., 2001, Aston-Jones and Cohen, 2005, Bouret and Sara, 2004, Bouret and Sara, 2005). Recent data from our lab have shown that adolescents tested at PND 40 and 43 were more cognitively rigid than adults (Newman and McGaughy, 2011). The behavior exhibited by normal adolescents is similar to that of adult rats following a loss of noradrenergic afferents to the prelimbic cortex (Newman et al., 2008, Tait et al., 2007). Previous work has shown that the administration of atomoxetine restores normal attentional set shifting abilities in rats after noradrenergic deafferentation (Newman et al., 2008), improves behavioral flexibility in rats and monkeys (Seu et al., 2008), and response inhibition in patients with ADHD (Chamberlain et al., 2007, Chamberlain et al., 2008, Christman et al., 2004, Kratochvil et al., 2003). In the present study, we test the hypothesis that the higher density of NET in the prefrontal cortex of adolescent rats produces attentional rigidity that may remediated by the systemic administration of the selective NET blocker atomoxetine. Executive abilities were assessed in a test of cognitive flexibility that allowed the assessment of complex, conditional discriminations, reversal learning, as well as the formation and shifting of an attentional set (Birrell and Brown, 2000, Lapiz-Bluhm et al., 2009, Lapiz et al., 2007, McAlonan and Brown, 2003, McGaughy et al., 2008). We hypothesized that the administration of a low dose of atomoxetine that increases prefrontal NE selectively would be more effective at producing flexible cognitive performance than a higher dose of the drug that may increase levels of norepinephrine beyond the range optimal for behavioral flexibility (Aston-Jones and Cohen, 2005, Arnsten, 2006a, Arnsten, 2006b, Bouret and Sara, 2005).
1. Methods
1.1. Subjects
Twelve male, Long-Evans adolescent rats (Charles River, Wilmington, MA) were used in this study. Rats were PND 39 and had achieved pre-putial separation prior to the onset of behavioral training. Each rat was housed individually and maintained on a 12 h light/dark cycle with lights on at 7:00am. Testing occurred between 9am and 1pm daily. Rats were moderately food restricted (12–15 g/day) and given water ad libitum. All procedures described were approved by the University of New Hampshire Institutional Animal Care and Use Committee in accordance the Guide for the Care and Use of Laboratory Animal.
1.2. Materials
Animals were tested in a plastic testing box (91.44 cm × 45.72 cm × 25.40 cm, L × W × H; Sterilite, Townsend, MA) and trained to dig in terra cotta pots with a height of 6.1 cm and diameter of 6.1 cm that varied in three dimensions: texture, digging media, and odor. A removable divider was placed in the plastic testing box to control access to stimuli.
1.3. Training to dig
Each rat was trained to dig for a food reward (Research Diets, New Brunswick, NJ; 45 mg pellet) before starting testing. An unscented terra cotta pot was filled with pine chip bedding and placed in one end of the testing box and separated from the rat by a divider. A reinforcer was placed on top of the bedding and the rat was given a 90 s limited hold. A stopwatch was started when the divider was removed. The response latency was recorded for each trial and defined as the length of time between the removal of the divider and displacement of the digging media by the rat using either its forepaw or nose. Subjects were required to retrieve 5 reinforcers from atop the digging media, then shaped to retrieve fully buried reinforcers until they successfully retrieved 10 fully buried reinforcers.
1.4. Exemplar training
The methods described in the present study are similar to those previously used (Birrell and Brown, 2000, Newman et al., 2008, Newman and McGaughy, 2011). Rats were trained to perform three separate simple, conditional discriminations using sets of exemplar pots, i.e. one set in each of the dimensions the rat would be required to attend to during the subsequent attentional set shifting task (ASST). The pots were wrapped in fabric to give different textures (fake fur vs. reverse of fake fur), filled with different digging media (white vs. green shredded paper), or scented with different odors (cherry vs. pine). A pair of pots was placed in the box behind a divider to prevent the rat's access to testing stimuli prior to the onset of a trial. The experimenter removed the divider and started the timer. A response was defined as displacement of digging media by either the forepaw or nose of a rat. After a response was made, the timer was stopped then the response latency and accuracy were recorded. The first four trials of every discrimination were designated discovery trials with a limited hold of 90 s. If they responded incorrectly the latency was recorded, the trial was scored as a miss, but they were allowed to explore the correct pot to retrieve the reinforcement. On trials subsequent to the discovery trials, the limited hold was reduced to 60 s and the animal was no longer allowed to explore the correct pot if an incorrect choice was made. Because a criterion of six correct consecutive trials could be achieved by chance alone in 64 trials and pilot studies showed that adolescent rats required more than 64 trials to master a particular subtest, a more stringent criterion of seven consecutive correct trials was applied to all stages of shaping and testing. Previous work has shown that this more stringent criterion when applied to training adult rats does not alter task acquisition (Newman and McGaughy, 2011). These exemplars were not used again.
1.5. Attentional set shifting task
1.5.1. Simple discrimination
The first discrimination in the attentional set shifting task was a simple discrimination (SD). As in the exemplar testing, the pots differed on one dimension (texture, digging medium, or odor). The animal was again given four discovery trials in which they were permitted to dig in both pots, and again only one pot was baited (e.g. cinnamon scented pot). Once a rat reached criterion performance, it began training in the compound discrimination.
1.5.2. Compound discrimination (CD1)
At this stage, two additional dimensions were introduced, e.g. texture and digging media, but the rat was still rewarded for attending to the previously reinforced dimension (e.g. cinnamon). One of these two irrelevant dimensions varies across a stimulus pairwhile the other is held constant. This additional attribute allowed a test of learned irrelevance after completion of the first ASST. The reinforced, non-reinforced and invariant dimensions were counterbalanced across subjects with one example of a testing schedule provided in Table 1.
Table 1.
An example of a testing sequence is given below with reinforced stimuli shown in bold. In this example, the subject was first reinforced for attending to an odor stimulus in the SD and maintaining attention to this same exemplar when distracting attributes were introduced in CD1. During reversal trials, the modality reinforced was the same as before but the alternate exemplar in a stimulus pair predicted reinforcement, e.g. patchouli. The formation of attentional set was assessed during ID1 where subjects were presented with a novel set of stimuli and reinforced for maintaining attentional focus to the dimension that previously predicting reinforcement, e.g. odor. Another reinforcement reversal followed this test and preceded the test of attentional set shifting (ED1). A novel set of stimuli were introduced and subjects had to learn that a different stimulus dimension predicted reinforcement. At the same time, subjects had to inhibit responding to the stimulus dimension that had been paired with reinforcement in the tests of SD-IDR1.
| Task | Testing pair 1 | Testing pair 2 | Not relevant attribute |
|---|---|---|---|
| SD | Cinnamon vs. patchouli | Cinnamon vs. patchouli | |
| CD1 | Cinnamon/light shapes vs. patchouli/dark shapes | Cinnamon/dark shapes vs. patchouli/light shapes | Fake fur |
| CDR1 | Patchouli/light shapes vs. cinnamon/dark shapes | Patchouli/dark shapes vs. cinnamon/light shapes | Fake fur |
| ID1 | Lilac/gold buttons vs. rose/black buttons | Lilac/black buttons vs. rose/gold buttons | Ribbed side corduroy |
| IDR1 | Rose/gold buttons vs. lilac/black buttons | Rose/black buttons vs. lilac/gold buttons | Ribbed side corduroy |
| ED1 | Metal beads/gardenia vs. plastic beads/jasmine | Metal beads/jasmine vs. plastic beads/gardenia | Terrycloth |
| EDR1 | Plastic beads/gardenia vs. metal beads/jasmine | Plastic beads/jasmine vs. metal beads/gardenia | Terrycloth |
| LI 1 | Plastic beads/terrycloth vs. metal beads/reverse terrycloth | Plastic beads/reverse terrycloth vs. metal beads/terrycloth | Gardenia |
1.5.3. CDR1
After completion of CD1, the reinforcement contingencies within a modality were reversed (e.g. patchouli, not cinnamon, scented pot was rewarded, CDR1).
1.5.4. Intra-dimensional shift (ID1)/IDR1
Upon successful completion of the CDR 1, a new set of stimuli (new odors, digging media, textures) was introduced. As before, the animal was reinforced for attending to the same dimension, e.g. odor. This discrimination was known as the intra-dimensional shift (ID1). Learning at this stage should have been facilitated by the animal maintaining attentional focus to the previously reinforced stimulus dimension so that fewer trials should be required to reach criterion performance on ID1 than CD1. After the successful completion of the ID1, another reinforcement reversal was performed (IDR1; e.g. a rat was reinforced for digging in rose scented pot)
1.5.5. Extra-dimensional shift/EDR
The next discrimination was the extra-dimensional shift (ED1) which assessed the ability to shift attentional set which began with the introduction of a third set of novel stimuli. The rat was reinforced for attending to a previously irrelevant, variable dimension, e.g. digging medium. If the animal had formed an attentional set, then they should require significantly more trials to achieve criterion performance on ED1 than on ID1. After successful completion of the ED1, the final reversal of this session was given (EDR1; plastic beads)
1.5.6. Next day, learned irrelevance
After completing the EDR, the next day's testing began with an assessment of learned irrelevance that measured the rat's ability to ignore the dimension never paired with reinforcement. This design allowed the experimenter to maintain continuity in the complexity of the stimuli rather than returning to stimuli that differed on only one dimension, i.e. a SD. For this example, all pots were texturized but the texture did not vary among stimulus pots. In the test of learned irrelevance, changes are introduced in this dimension but all other aspects of the testing stimuli are the same as the last reversal of the previous testing day.
After completion of this stage, testing was the same as before beginning with a repetition of the previous order of testing from the CD-EDR using novel stimuli. The third day's test began with a second test of learned irrelevance as shown in Table 2. Each rat received every dose of atomoxetine (0.0; 0.1 and 0.9 mg/kg/ml) with the order of doses counterbalanced across subjects so that neither age nor order of testing confounded the effects of dose. Similarly, the sequences of testing were counterbalanced across all subjects so that 1/3 of the subjects were first reinforced for attending to odor, 1/3 for attending to texture and 1/3 for attending to digging media. ED2 always required rats to shift attention to the previously irrelevant, constant dimension in the tests of CD1-EDR1. ED3 required rats to shift attention to the dimension reinforced in the original SD.
Table 2.
An overview of testing is provided and shows the age at which each series of test was conducted. Drug administration began 30 min prior to the ED on days 3, 4 and 5. The order of drug doses was counterbalanced across subjects so that the order of drug administration was not confounded by age. Additionally testing sequences were counterbalanced so that the modality that was being reinforced on a given day was not the same for all rats or for all doses of drug.
| Day | Testing sequence | Age |
|---|---|---|
| 1 | Training to dig | PND 39 |
| 2 | Exemplar 1; exemplar 2; exemplar 3; SD | PND 40 |
| 3 | CD1; CDR; ID1; IDR1; ED1; EDR1 | PND 41 |
| 4 | LI1; CD2; CDR2; ID2; IDR2; ED2; EDR2 | PND 42 |
| 5 | LI2; CD3; CDR3; ID3; IDR3; ED3; EDR3 | PND 43 |
1.6. Injection procedures
In order to acclimate rats to injection procedures, each rat was given a saline injection thirty minutes prior to the onset of training to dig and the exemplar testing. On subsequent days, rats were given intra-peritoneal (i.p.) injections of atomoxetine hydrochloride (Tocris Cookson, Ellisville, MO) thirty minutes prior to the onset of the ED in order to test the hypothesis that it would improve the ability of adolescent rats to shift attentional set. The order of doses of atomoxetine, 0.0, 0.1, and 0.9 mg/kg/ml was counterbalanced across subjects.
1.7. Statistical analysis
All statistical analyses were performed with SPSS v. 17.0 (SPSS, Chicago, IL, USA). The number of trials required to reach criterion performance and correct response latencies were analyzed in separate ANOVA's. Data from one animal were excluded because he omitted responding after all injections including placebo. The first analysis assessed the effects of atomoxetine on how well adolescent rats formed and shifted attentional set using a repeated measures ANOVA of dose (3) × test (2). A series of planned comparisons determined if there was a cost to shift attentional set by comparing the number of trials to reach criterion performance on the ED after vehicle injection to those required to reach criterion on the ID. Additionally, the effectiveness of atomoxetine in improving the performance of adolescent rats on the EDs was assessed by a series of planned comparisons. The effects of atomoxetine on reversals occurring after the EDs’ were analyzed using a repeated measure ANOVA of dose (3). Additionally to confirm that the formation of an attentional set facilitated performance the number of trials needed to reach criterion performance on the IDs was compared to that needed on the CDs in repeated measures ANOVA of test (2) × repetition (3). Performance on reversal trials prior to the administration of drug was compared using a repeated measures ANOVA with the factors test (2) × day (3). Performance on LI1 and LI2 was compared using a paired t-test.
2. Behavioral results
There was no difference in performance of discrimination based on the modality as the number of trials to reach criterion was similar across all three tests performed during exemplar training (F(2.20) = 2.75; p = 0.11). As a result, this factor was not included in subsequent analyses.
2.1. Effects of atomoxetine
2.1.1. The formation and shifting of attentional set
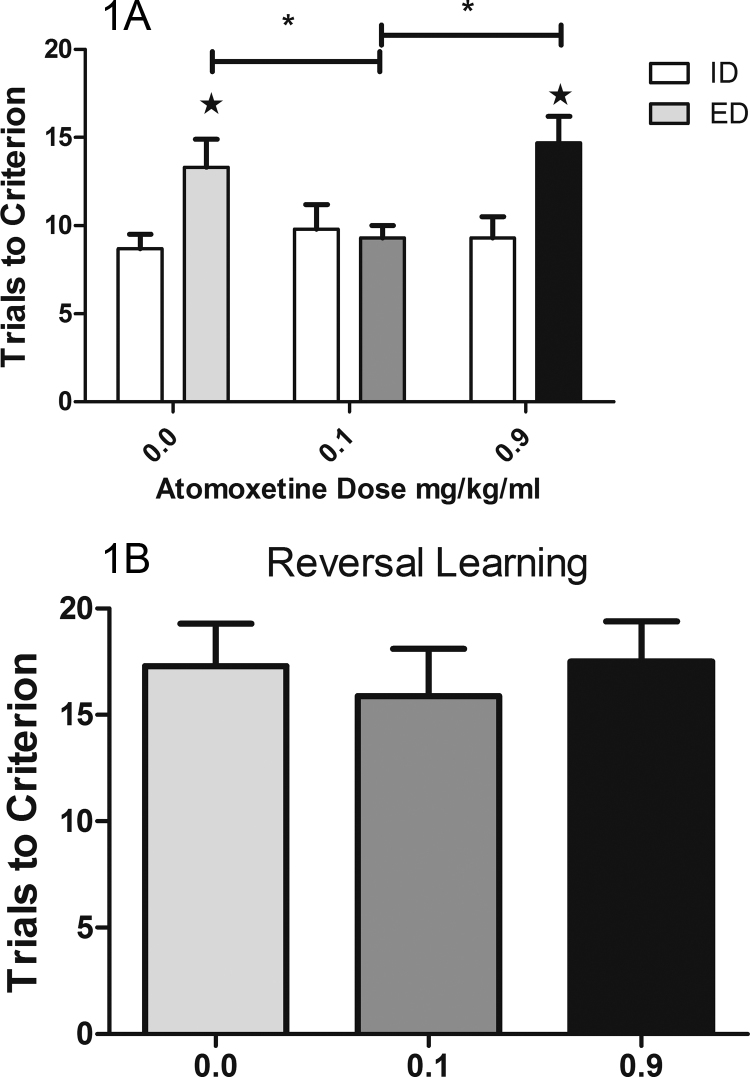
All rats required more trials to reach criterion performance on the EDs than the IDs supporting the hypothesis that there was a cognitive cost to shifting attentional set (Test: F(1,10) = 6.37; p = 0.03; vehicle ID vs. ED: t(10) = 2.74; p = 0.02). As shown in Fig. 1A, the lower dose, but not the higher dose, of atomoxetine was effective in reducing the number of trials needed to reach criterion performance during the ED (0.0 vs. 0.1 mg/kg/ml: t(10) = 2.44; p = 0.03; 0.0 vs. 0.9 mg/kg/ml; t(10) = 0.76; p = 0.47; test × dose: F(2,20) = 3.87; p = 0.04; ɛ = 0.94). There was no main effect of dose (F(2,20) = 1.97; p = 0.17). All subjects took longer to make a correct response on the EDs than the IDs (F(1,10) = 5.94; p = 0.04; Mean ± SEM; IDs: 3.69 ± 0.34 s; EDs: 5.94 ± 0.83 s). There were no effects of atomoxetine on correct response latencies (F(2,20) = 0.55; p = 0.55).
Fig. 1.
(A) Subjects require fewer trials to reach criterion performance on IDs’ (white bars) than EDs’ after the 0.0 mg/kg/ml dose (light gray bar) and the 0.9 mg/kg/ml dose (black bar). In contrast, performance on the EDs after the 0.1 mg/kg/ml dose (dark gray bars) shows no cost of attentional set shifting. Performance on the EDs after the 0.1 mg/kg/ml dose is significantly better than after either of the other doses. ★ Indicates that ED performance is significantly worse that ID at the same dose of drug. (B) Previous work from our laboratory has shown that adolescents are less able to perform reinforcement reversals than adults but neither dose of atomoxetine facilitated this type of learning. Performance on this stage of testing depends upon the functional integrity of the orbitofrontal cortex and may be more reliant on serotonergic than noradrenergic function.
2.1.2. Reversal learning
There were no effects of atomoxetine on tests of reversal learning in the analyses of trials needed to reach criterion (Fig. 1B; F(2,20) = 0.42; p = 0.66) or correct response latencies (F(2, 20) = 0.49; p = 0.62).
2.2. Pre-injection performance
2.2.1. Reversal learning
All rats required fewer trials to reach criterion performance on the second reversal of the day when it was compared to the first reversal of the testing session (F(1,10) = 13.49; p = 0.004; Mean ± SEM; REV 1: 25.3 ± 1.9; REV 2: 15.3 ± 1.6) and took longer to emit correct responses on the second reversal of the day when compared to the first (F(1,10) = 12.85; p = 0.005;Mean ± SEM; REV 1: 2.4 ± 0.2; REV 2: 4.1 ± 0.5 s). When the number of trials needed to reach criterion on the first reversal of the day was compared to the second on subsequent testing days, the effect was the same (all p > 0.66).
2.2.2. Performance on compound discriminations
There was no difference in the number of trials to reach criterion performance when CD1, CD2 and CD3 were compared (F(2,20) = 0.26; p = 0.75; ɛ = 0.89). Moreover, rats required fewer trials to reach criterion performance on IDs than CDs supporting the hypothesis that rats used an attentional set to determine which stimulus attribute predicted reinforcement (F(2,20) = 21.76; p = 0.001). There were no differences in response latencies between the ID and CD nor any differences in response latencies among CD1, CD2 and CD3 (all p > 0.64). There was a trend for improved performance on tests of learned irrelevance with repeated testing (t(10) = 2.09; p > 0.06).
3. Discussion
Adolescent rats’ ability to perform an attentional set shift is facilitated by the administration of a low dose of atomoxetine. The ability to perform shifts of attentional set in adult rats has been shown to depend on optimal levels of norepinephrine in the prelimbic cortex (Danet et al., 2010, Lapiz et al., 2007, Lapiz and Morilak, 2006, Milstein et al., 2007, Newman et al., 2008, Tait et al., 2007). The lowest dose used in the present study was selected because it has been shown to increase levels of prefrontal NE without concurrent increases in other neuromodulators (Bymaster et al., 2002, Tzavara et al., 2007, Wong et al., 1982). The higher dose used in the present study was not effective in facilitating attentional set shifting or reversal learning. This supports the hypothesis that increased levels of NE produced by the lower dose of atomoxetine are sufficient to improve executive function as previously demonstrated (Newman et al., 2008). Though higher levels of norepinephrine have been shown impair cognition (Bouret and Sara, 2005, Arnsten, 2006a, Arnsten, 2006b), the higher dose used here did not impair cognition rather it failed to improve it. Higher doses of atomoxetine, e.g. 0.9 mg/kg/ml produce less selective effects on NET and have been shown to increase cortical NE, dopamine and acetylcholine (Bymaster et al., 2002, Tzavara et al., 2007). It is unclear whether the lack of effect after the higher dose results from concomitant release of other neuromodulators counteracting the beneficial increase in NE or is a result of supraoptimal levels of this neuromodulator. Improvements in cognitive function produced by atomoxetine are hypothesized to depend upon binding at post-synaptic α2 receptors (Gamo et al., 2010). The present data and previous work (Murrin et al., 2007) support the hypothesis these receptors exist in the adolescent brain in sufficient numbers to produce beneficial effects.
Recent, unpublished data from our lab shows that rats have a higher density of NET and lower density of DBH at PND 40 than PND 60. These age-related differences have been found in prelimbic, infralimbic and orbitofrontal cortices. We hypothesize that this high density of NET in the prefrontal cortices of adolescents decreases the amount of NE available in adolescents to produce immaturities in executive function. Increased cognitive flexibility results from higher levels of prefrontal NE (Aston-Jones and Cohen, 2005, Berridge and Waterhouse, 2003) as produced by changes in the firing of the locus coeruleus (Bouret and Sara, 2005, Usher et al., 1999) or through the administration of NE enhancing drugs (Arnsten, 2006a, Arnsten, 2006b, Berridge et al., 2006, Devilbiss and Berridge, 2008). High density of NET in adolescent prefrontal cortex is hypothesized to prevent increases in prefrontal NE and limit cognitive flexibility. NET blockade by atomoxetine prolongs the action of NE in the synapse and is hypothesized to increase NE in the prefrontal cortex of adolescent rats to allow attentional set shifts.
Recent work by Finlay and colleagues has shown that prefrontal NE efflux is similar in adolescents and adults (Boyce and Finlay, 2009). This may seem in disagreement with the present theoretical framework. One possible explanation for this discrepancy may lie in the fact that these assessments were not made while rats were performing a cognitively demanding task and this type of cognitive demand may be necessary to reveal lower levels of NE in adolescent rats. In other words, though adolescent rats may show similar levels of NE during conditions of low cognitive demand, they may be unable to sustain release in situations that require high levels of cognitive flexibility. Neuromodulator levels in prefrontal cortex substantially increase during conditions of high cognitive demand (Dalley et al., 2001, Himmelheber et al., 1997, Himmelheber et al., 2000, Himmelheber et al., 2001, McGaughy et al., 2002). Rats with pre-existing damage to neuromodulatory systems may show normal efflux when cognitive demand is low but are unable to maintain neuromodulatory levels when assessments are made during conditions of high demand (McGaughy et al., 2002, Parikh et al., 2007). We hypothesize that adolescent rats would show lower levels of cortical NE during attentional testing than adults, but additional studies are required to address this question.
Systemic administration of atomoxetine did not improve reversal learning in adolescent rats. This seems unlikely to be due to a ceiling effect as adolescent rats have previously been shown to be less proficient at reversal learning than adults (Newman and McGaughy, 2011). In humans, adolescents with behavioral disorders (Ernst et al., 2003, Romer et al., 2009), mood disorders (Dickstein et al., 2009) and psychopathic traits (Finger et al., 2008) show an inability to modify responding based on changes in reinforcement contingencies so understanding this form of executive function in adolescents is likely to have wide-ranging implications. One explanation for the lack of effect of atomoxetine on reversal learning is that it is more dependent upon serotonin (Clarke et al., 2004, Clarke et al., 2005, Lapiz-Bluhm et al., 2009, Murphy et al., 2002) and dopamine (Cools et al., 2001a, Cools et al., 2001b, Cools et al., 2004, Dalley et al., 2004, Takahashi et al., 2009) than norepinephrine. Maturation of serotonergic systems has been shown to precede that of noradrenergic systems in rats (Murrin et al., 2007), humans (Verney, 1999) and non-human primates (Lambe et al., 2000). Based on these findings, adolescents should be expected to be proficient at reversal learning sooner than attentional set shifting. However, recent data do not support this hypothesis. Adolescent rats could successfully perform attentional set shifts at a younger age than reversals (Newman and McGaughy, 2011). One theoretical framework suggests that NE is critical to learning in situations of unexpected, but not expected, uncertainty (Yu and Dayan, 2005). This framework suggests that the cognitive demands of the first reversal encountered maydepend upon NE, but subsequent reversal learning may be more dependent upon other neuromodulators. Unpublished data from our laboratory suggests that noradrenergic deafferentation of the orbitofrontal cortex in adult rats produces deficits in the first test of reversal learning (CDR), but not subsequent tests of reversals. In the present study the effects of atomoxetine were tested not on the first reversal but the third which may be less dependent upon NE. It is unclear whether the administration of atomoxetine would improve performance on the test of the first reversal but future studies should explore this possibility.
Behavioral studies have shown that the attentional performance of adolescent rats is qualitatively similar to that of adult rats after noradrenergic lesions (McGaughy et al., 2008, Newman et al., 2008, Tait et al., 2007). The fact that performance in both groups is improved by doses of a selective norepinephrine reuptake blocker suggest that adolescents have functional deficits in prefrontal noradrenergic systems similar to that produced by selective deafferentation. Studies have shown that juvenile rats have higher densities of NET in the frontal cortex than adults (Moll et al., 2000, Sanders et al., 2005). These studies assessed frontal cortex NET density as a whole without regard for functionally distinct subregions and did not assess NET density in adolescents. We have recently found that NET density is higher at PND 40 than PND 60 in prelimbic and orbitofrontal cortices (Agster et al., unpublished data). We have also found that dopamine beta hydroxylase levels in the same prefrontal subregions assessed at the same ages are lower. Together both the lower levels of synthetic enzyme and higher NET are hypothesized to produce a functional NE deficit in the prefrontal cortices of adolescents. At present, it is unclear whether strategies aimed at increasing this synthetic precursor would also produce more flexible attentional performance. Finally, it is important to remember that while the present study supports the hypothesis that adolescent attention differs from adult because of higher densities of prefrontal NET more studies are required to understand how chronic NET blockade may impact the development of executive function and its biological substrates.
Acknowledgement
This work is supported by NIMH, MH087921 and MH074811.
References
- Adriani W., Leo D., Greco D., Rea M., di Porzio U., Laviola G. Methylphenidate administration to adolescent rats determines plastic changes on reward-related behavior and striatal gene expression. Neuropsychopharmacology. 2006;31:1946–1956. doi: 10.1038/sj.npp.1300962. [DOI] [PubMed] [Google Scholar]
- Andersen S.L. Trajectories of brain developnment: point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev. 2008;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Arnsten A.F.T. Catecholamine modulation of prefrontal cortical cognitive function. Trends Cogn. Neurosci. 1998;2:436–447. doi: 10.1016/s1364-6613(98)01240-6. [DOI] [PubMed] [Google Scholar]
- Arnsten A.F.T. Fundamentals of attention-deficit/hyperactivity disorder: circuits and pathways. J. Clin. Psychiatry. 2006;67:4–9. [PubMed] [Google Scholar]
- Arnsten A.F.T. Stimulants: therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31:2376–2383. doi: 10.1038/sj.npp.1301164. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G., Chen S., Zhu Y., Oshinsky M.L. A neural circuit for circadian regulation of arousal. Nat. Neurosci. 2001;4:732–738. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G., Cohen J.D. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G., Rajkowski J., Cohen J. Locus coeruleus and regulation of behavioral flexibility and attention. Prog. Brain Res. 2000;126:165–182. doi: 10.1016/S0079-6123(00)26013-5. [DOI] [PubMed] [Google Scholar]
- Barkley R.A. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol. Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Baxter M., Bucci D., Gorman L., Wiley R., Gallagher M. Selective immunotoxic lesions of basal forebrain cholinergic cells: effects on learning and memory in rats. Behav. Neurosci. 1995;109:714–722. doi: 10.1037//0735-7044.109.4.714. [DOI] [PubMed] [Google Scholar]
- Berke J., Hyman S.E. Addiction, dopamine and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Berridge C., Waterhouse B. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res. Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Berridge C.W., Devilbiss D.M., Andrzejewski M.E., Arnsten A.F., Kelley A.E., Schmeichel B. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol. Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Biederman J., Heiligenstein J.H., Faries D.E., Galil N., Dittman R., Emslie G.J. Efficacy of atomoxetine versus placebo in school-age girls with attention-deficit/hyperactivity disorder. Pediatrics. 2002;110:e75. doi: 10.1542/peds.110.6.e75. [DOI] [PubMed] [Google Scholar]
- Birrell J., Brown V. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J. Neurosci. 2000;20(11):4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black Y.D., Maclaren F.R., Naydenov A.V., Carlezon W.A.J., Baxter M., Konradi C. Altered attention and prefrontal cortex gene expression in rats after binge-like exposure to cocaine during adolescence. J. Neurosci. 2006;26:9656–9665. doi: 10.1523/JNEUROSCI.2391-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret S., Sara S.J. Reward expectation, orientation of attention and locus coeruleus-medial frontal cortex interplay during learning. Eur. J. Neurosci. 2004;20:791–802. doi: 10.1111/j.1460-9568.2004.03526.x. [DOI] [PubMed] [Google Scholar]
- Bouret S., Sara S.J. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 2005;28:574–582. doi: 10.1016/j.tins.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Boyce P.J., Finlay J.M. Extracellular dopamine and norepinephrine in the developing rat prefrontal cortex: transient effects of early partial loss of dopamine. Brain Res Bull. 2009;79(2):104–110. doi: 10.1016/j.brainresbull.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse H.C., Andersen S.L. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behav. Neurosci. 2008;122:460–465. doi: 10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse H.C., Sonntag K.C., Andersen S.L. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J. Neurosci. 2008;28(10):2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns L., Everitt B.J., Kelley A.E., Robbins T.W. Glutamate-dopamine interactions in the ventral striatum: role in locomotor activity and responding with conditioned reinforcement. Psychopharmacology (Berl) 1994;115:516–528. doi: 10.1007/BF02245576. [DOI] [PubMed] [Google Scholar]
- Bymaster F.P., Katner J.S., Nelson D.L., Hemrick-Luecke S.K., Threlkeld P.G., Heiligenstein J.H. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Castellanos F.X., Tannock R. Neuroscience of attention deficit/hyperactivity disorder: the search for endophenotypes. Neuroscience. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Chamberlain S., Hampshire A., Müller U., Rubia K., Campo N.D., Craig K. Atomoxetine modulates right inferior frontal activation during inhibitory control: a pharmacological functional magnetic resonance imaging study. Biol. Psychiatry. 2008;2009(65):550–555. doi: 10.1016/j.biopsych.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Chamberlain S.R., del Campo N., Dowson J., Muller U., Clark L., Robbins T.W. Atomoxetine improved response inhibition in adults with attention deficit/hyperactivity disorder. Biol. Psychiatry. 2007;62:977–984. doi: 10.1016/j.biopsych.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Christman A.K., Fermo J.D., Markowitz J.S. Atomoxetine, a novel treatment for attention-deficit-hyperactivity disorder. Pharmacotherapy. 2004;24:1020–1036. doi: 10.1592/phco.24.11.1020.36146. [DOI] [PubMed] [Google Scholar]
- Clarke H.F., Dalley J.W., Crofts H.S., Robbins T.W., Roberts A.C. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;204:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Clarke H.F., Walker S.C., Crofts H.S., Dalley J.W., Robbins T.W., Roberts A.C. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. J. Neurosci. 2005;25:532–538. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R., Barker R.A., Sahakian B.J., Robbins T.W. Enhanced or impaired cognitive function in Parkinson's disease as a function of dopaminergic medication and task demands. Cereb. Cortex. 2001;11:1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- Cools R., Barker R.A., Sahakian B.J., Robbins T.W. Mechanisms of cognitive set flexibility in Parkinson's disease. Brain. 2001;124:2503–2512. doi: 10.1093/brain/124.12.2503. [DOI] [PubMed] [Google Scholar]
- Cools R., Clark L., Robbins T.W. Differential responses in human striatum and prefrontal cortex to changes in object and rule relevance. J. Neurosci. 2004;24:1129–1135. doi: 10.1523/JNEUROSCI.4312-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle J.T., Axelrod J. Dopamine-β-hydroxylase in the rat brain: developmental characteristics. J. Neurochem. 1972;19:449–459. doi: 10.1111/j.1471-4159.1972.tb01354.x. [DOI] [PubMed] [Google Scholar]
- Dalley J.W., Cardinal R.N., Robbins T.W. Prefrontal executive functions in rodents: neural and neurochemical substrates. Neurosci. Biobehav. Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Dalley J.W., McGaughy J., O’Connell M.T., Cardinal R.N., Levita L., Robbins T.W. Distinct changes in cortical acetylcholine and noradrenaline efflux during contingent and non-contingent performance of a visual attentional task. J. Neurosci. 2001;21:4908–4914. doi: 10.1523/JNEUROSCI.21-13-04908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danet M., Lapiz-Bluhm S., Morilak D.A. A cognitive deficit induced in rats by chronic intermittent cold stress is reversed by chronic antidepressant treatment. Int. J. Psychopharmacol. 2010;11:1–13. doi: 10.1017/S1461145710000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilbiss D.M., Berridge C.W. Cognition-enhancing doses of methylphenidate preferentially increase prefrontal cortex neuronal responsiveness. Biol. Psychiatry. 2008;64:626–635. doi: 10.1016/j.biopsych.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein D.P., Finger E.C., Brotman M.A., Rich B.A., Pine D.S., Blair J.R. Impaired probabilistic reversal learning in youths with mood and anxiety disorders. Psychol. Med. 2009;40:1089–1100. doi: 10.1017/S0033291709991462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow-Edwards D.L., Weedon J.C., Hellman E. Methylphenidate improves performance on the radial arm in periadolescent rats. Neurotoxicol. Teratol. 2008;30:419–427. doi: 10.1016/j.ntt.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Grant S.J., London E.D., Contoreggi C.S., Kimes A.S., Spurgeon L. Decision making in adolescents with behavior disorders and adults with substance abuse. Am. J. Psychiatry. 2003;160:33–40. doi: 10.1176/appi.ajp.160.1.33. [DOI] [PubMed] [Google Scholar]
- Finger E.C., Marsh A.A., Mitchell D.G., Reid M.E., Sims C., Budhani S. Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Arch. Gen. Psychiatry. 2008;65(5):586–594. doi: 10.1001/archpsyc.65.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamo N.J., Wang M., Arnsten A.F.T. Methylphenidate and atomoxetine enhance prefrontal function through α2-adrenergic and dopamine D1 receptors. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49:1011–1029. doi: 10.1016/j.jaac.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greydanus D.E., Pratt H.D., Patel D.R. Attention deficit hyperactivity disorder across the lifespan: the child, adolescent, and adult. Dis. Mon. 2007;53:70–131. doi: 10.1016/j.disamonth.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Harden T.K., Wolfe B.B., Sporn J.R., Perkins J.P., Molinoff P.B. Ontogeny of B -adrenergic receptors in rat cerebral cortex. Brain Res. 1977;125:99–108. doi: 10.1016/0006-8993(77)90362-6. [DOI] [PubMed] [Google Scholar]
- Himmelheber A.M., Sarter M., Bruno J.P. Operant performance and cortical acetylcholine release: role of response rate, reward density, and non-contingent stimuli. Cogn. Brain Res. 1997;6:23–36. doi: 10.1016/s0926-6410(97)00014-1. [DOI] [PubMed] [Google Scholar]
- Himmelheber A.M., Sarter M., Bruno J.P. Increases in cortical acetylcholine release during sustained attention performance in rats. Cogn. Brain Res. 2000;9:313–325. doi: 10.1016/s0926-6410(00)00012-4. [DOI] [PubMed] [Google Scholar]
- Himmelheber A.M., Sarter M., Bruno J.P. The effects of manipulations of attentional demand on cortical acetylcholine release. Cogn. Brain Res. 2001;12:353–370. doi: 10.1016/s0926-6410(01)00064-7. [DOI] [PubMed] [Google Scholar]
- Joung Y., Kim C.-H., Moon J., Jang W.-S., Yang J., Shin D. Association studies of −3081(A/T) polymorphism of norepinephrine transporter gene with attention deficit/hyperactivity disorder in Korean population. Am. J. Med. Genet. B Neuropsychitr. Genet. 2009;153B:691–694. doi: 10.1002/ajmg.b.31012. [DOI] [PubMed] [Google Scholar]
- Kieling C., Goncalves R.R.F., Tannock R., Castellanos F.X. Neurobiology of attention deficit hyperactivity disorder. Child Adolesc. Psychiatr. Clin. N. Am. 2008;17:285–307. doi: 10.1016/j.chc.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Kratochvil C.J., Vaughn B.S., Harrington M.J., Burke W.J. Atomoxetine: a selective noradrenergic reuptake inhibitor for the treatment of attention-deficit/hyperactivity disorder. Exp. Opin. Pharmacother. 2003;4:1165–1174. doi: 10.1517/14656566.4.7.1165. [DOI] [PubMed] [Google Scholar]
- Kratochvil C.J., Wilens T.E., Greenhill L.L., Gao H., Baker K.D., Feldman P.D. Effects of long-term atomoxetine treatment for young children with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2006;45:919–927. doi: 10.1097/01.chi.0000222788.34229.68. [DOI] [PubMed] [Google Scholar]
- Kuczenski R., Segal D.S. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J. Neurosci. 2002;22:7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laboni F., Douglas V.I., Baker A.G. Effects of reward and response costs on inhibition in ADHD children. J. Abnorm. Psychol. 1995;104:232–240. doi: 10.1037/0021-843X.104.1.232. [DOI] [PubMed] [Google Scholar]
- Lambe E.K., Krimer L.S., Goldman-Rakic P.S. Differential postnatal development of catecholamine and serotonin inputs to identified neurons in prefrontal cortex of rhesus monkey. J. Neurosci. 2000;20:8780–8787. doi: 10.1523/JNEUROSCI.20-23-08780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapiz-Bluhm M.D., Soto-Piña A.E., Hensler J.G., Morilak D.A. Chronic intermittent cold stress and serotonin depletion induce deficits of reversal learning in an attentional set-shifting test in rats. Psychopharmacology (Berl) 2009;202:329–341. doi: 10.1007/s00213-008-1224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapiz M.D.S., Bondi C.O., Morilak D. Chronic treatment with desipramine improves cognitive performance of rats in an attentional set-shifting test. Neuropsychopharmacology. 2007;32:1000–1010. doi: 10.1038/sj.npp.1301235. [DOI] [PubMed] [Google Scholar]
- Lapiz M.D.S., Morilak D. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience. 2006;137:1039–1049. doi: 10.1016/j.neuroscience.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Laviola G., Adriania W., Livia-Terranova M., Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci. Biobehav. Rev. 1999;23:993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- Levitt P., Moore R.Y. Development of the noradrenergic innervation of the neocortex. Brain Res. 1979;162:243–259. doi: 10.1016/0006-8993(79)90287-7. [DOI] [PubMed] [Google Scholar]
- Loveland K.A., Bachevalier J., Pearson D.A., Lane D.M. Fronto-limbic functioning in children and adolescents with and without autism. Neuropsychologia. 2008;46:49–62. doi: 10.1016/j.neuropsychologia.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K., Brown V.J. Orbital prefrontal cortex mediates reversal learning and not attentional set-shifting. Behav. Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- McGaughy J., Dalley J.W., Morrison C.H., Everitt B.J., Robbins T.W. Selective behavioral and neurochemical effects of cholinergic lesions produced by intrabasalis infusions of 192 IgG-saporin on attentional performance in a 5 choice serial reaction time task. J. Neurosci. 2002;22:1905–1913. doi: 10.1523/JNEUROSCI.22-05-01905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughy J., Kaiser T., Sarter M. Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behav. Neurosci. 1996;110:247–265. doi: 10.1037//0735-7044.110.2.247. [DOI] [PubMed] [Google Scholar]
- McGaughy J., Ross R.S., Eichenbaum H. Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set-shifting. Neuroscience. 2008;153:63–71. doi: 10.1016/j.neuroscience.2008.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta M.A., Owen A.M., Sahakian B.J., Mavaddat N., Pickard J.D., Robbins T.W. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J. Neurosci. 2000;20:RC65. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein J.A., Lehmann O., Theobald D.E.H., Dalley J.W., Robbins T.W. Selective depletion of cortical noradrenaline by anti-dopamine beta-hydroxylase impairs attentional function and enhances the effects of guanfacine in the rat. Psychopharmacology (Berl) 2007;190:51–63. doi: 10.1007/s00213-006-0594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll G.H., Mehnert C., Wicker M., Bock N., Rothenberger A., Rüther E. Age-associated changes in the densities of presynaptic monoamine transporters in different regions of the rat brain from early juvenile life to late adulthood. Brain Res. Dev. Brain Res. 2000;7:251–257. doi: 10.1016/s0165-3806(99)00182-0. [DOI] [PubMed] [Google Scholar]
- Morrison J.H., Molliver M.E., Grzanna R., Coyle J.T. Noradrenergic innervation patterns in three regions of medial cortex: an immunofluorescence characterization. Brain Res. Bull. 1979;4:849–857. doi: 10.1016/0361-9230(79)90022-4. [DOI] [PubMed] [Google Scholar]
- Murphy F.C., Smith K.A., Cowen P.J., Robbins T.W., Sahakian B.J. The effects of tryptophan depletion on cognitive and affective processing in healthy volunteers. Psychopharmacology (Berl) 2002;163:42–53. doi: 10.1007/s00213-002-1128-9. [DOI] [PubMed] [Google Scholar]
- Murrin L.C., Sanders J.D., Bylund D.B. Comparison of the maturation of the adrenergic and serotonergic neurotransmitter systems in the brain: implications for differential drug effects on juveniles and adults. Biochem. Pharmacol. 2007;73:1225–1236. doi: 10.1016/j.bcp.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcorn J.H., Kratochvil C.J., Allen A.J., Casat C.D., Ruff D.D., Moore R.J. Atomoxetine and osmotically released methylphenidate for the treatment of attention deficit hyperactivity disorder: acute comparison and differential response. Am. J. Psychiatry. 2008;165:721–730. doi: 10.1176/appi.ajp.2007.05091676. [DOI] [PubMed] [Google Scholar]
- Newman L.A., Darling J., McGaughy J. Atomoxetine reverses attentional deficits produced by noradrenergic deafferentation of medial prefrontal cortex. Psychopharmacology (Berl) 2008;200:39–50. doi: 10.1007/s00213-008-1097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman L.A., McGaughy J. Adolescent rats show cognitive rigidity in a test of attentional set shifting. Dev. Psychobiol. 2011;53(4):391–401. doi: 10.1002/dev.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V., Kozak R., Martinez V., Sarter M. Prefrontal acetylcholine release controls cue detection on multiple time scales. Neuron. 2007;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker K.J., Rainwater K.L., Buckmaster C.L., Schatzberg A.F., Lindley S.E., Lyons D.M. Early life stress and novelty seeking behavior in adolescent monkeys. Psychoneuroendocrinology. 2007;32:785–792. doi: 10.1016/j.psyneuen.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins T.W. Chemical neuromodulation of frontal-executive functions in humans and other animals. Exp. Brain Res. 2000;133:130–138. doi: 10.1007/s002210000407. [DOI] [PubMed] [Google Scholar]
- Robbins T.W., Everitt B.J. Drug addiction: bad habits add up. Nature. 1999;398:567–570. doi: 10.1038/19208. [DOI] [PubMed] [Google Scholar]
- Romer D., Betancourt L., Giannetta J.M., Brodsky N.L., Farah M., Hurt H. Executive cognitive functions and impulsivity as correlates of risk taking and problem behavior in preadolescents. Neuropsychologia. 2009;47:2916–2926. doi: 10.1016/j.neuropsychologia.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagvolden T., Sergeant J. Attention deficit/hyperactivity disorder-from branin dysfuncitons to behaviour. Behav. Brain Res. 1998;94:1–10. [PubMed] [Google Scholar]
- Sanders J.D., Happer H.K., Bylund D.B., Murrin L.C. Development of the norepinephrine transporter in the rat CNS. Neuroscience. 2005;130:107–117. doi: 10.1016/j.neuroscience.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Sarter M., Hasselmo M.E., Bruno J.P., Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res. Brain Res. Rev. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Schulz K.P., Tang C.Y., Fan J., Marks D.J., Cheung A.M., Newcorn J.H. Differential prefrontal cortex activation during inhibitory control in adolescents with and without childhood attention-deficit/hyperactivity disorder. Neuropsychology. 2005;19:390–402. doi: 10.1037/0894-4105.19.3.390. [DOI] [PubMed] [Google Scholar]
- Seu E., Lang A., Rivera R.J., Jentsch J.D. Inhibition of the norepinephrine transporter improves behavioral flexibility in rats and monkeys. Psychopharmacology (Berl) 2008;202:505–519. doi: 10.1007/s00213-008-1250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafritz K.M., Marchione K.E., Gore J.C., Shaywitz S.E., Shaywitz B.A. The effects of methylphenidate on neural systems of attention in attention deficit hyperactivity disorder. Am. J. Psychiatry. 2004;161:1990–1997. doi: 10.1176/appi.ajp.161.11.1990. [DOI] [PubMed] [Google Scholar]
- Solanto M.V. Neuropharmacological basis of stimulant drug action in attention deficit disorder with hyperactivity: a review and synthesis. Psychol. Bull. 1984;95:387–409. [PubMed] [Google Scholar]
- Sonuga-Barke E.J.S., Sergeant J.A., Nigg J., Willcutt E. Executive dysfunction and delay aversion in attention deficit hyperactivity disorder: nosologic and diagnostic implications. Child Adolesc. Psychiatr. Clin. N. Am. 2008;17:367–384. doi: 10.1016/j.chc.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Spencer T., Biederman J., Wilens T.E., Prince J., Hatch M., Jones J. Effectiveness and tolerability of tomoxetine in adults with attention deficit hyperactivity disorder. Am. J. Psychiatry. 1998;155:93–95. doi: 10.1176/ajp.155.5.693. [DOI] [PubMed] [Google Scholar]
- Tait D.S., Brown V.J., Farovik A., Theobald D.E., Dalley J.W., Robbins T.W. Lesions of the dorsal noradrenergic bundle impair attentional set shifting in the rat. Eur. J. Neurosci. 2007;25:3719–3724. doi: 10.1111/j.1460-9568.2007.05612.x. [DOI] [PubMed] [Google Scholar]
- Takahashi Y.K., Roesch M.R., Stalnaker T.A., Haney R.Z., Calu D.J., Taylor A.R. The orbitofrontal cortex and ventral tegmental area are necessary for learning from unexpected outcomes. Neuron. 2009;62:269–280. doi: 10.1016/j.neuron.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzavara E.T., Bymaster F.P., Overshiner C.D., Davis R.J., Perry K.W., Wolff M. Procholinergic and memory enhancing properties of the selective norepinephrine uptake inhibitor atomoxetine. Mol. Psychiatry. 2007;11:187–195. doi: 10.1038/sj.mp.4001763. [DOI] [PubMed] [Google Scholar]
- Usher M., Cohen J.D., Servan-Schreiber D., Rajkowski J., Aston-Jones G. The role of locus coeruleus in the regulation of cognitive performance. Science. 1999;283:549–554. doi: 10.1126/science.283.5401.549. [DOI] [PubMed] [Google Scholar]
- Verney C. Distribution of the catecholaminergic neurons in the central nervous system of human embryos and fetuses. Microsc. Res. Tech. 1999;46:24–47. doi: 10.1002/(SICI)1097-0029(19990701)46:1<24::AID-JEMT3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Viggiano D., Vallone D., Sadile A.G. Dysfunction in dopamine systems and ADHD: evidence from animals and modeling. Neural Plast. 2004;11:97–114. doi: 10.1155/NP.2004.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendlandt S., Crow T.J., Stirling R.V. The involvement of the noradrenergic system arising from the locus coeruleus in the postnatal development of the cortex in rat brain. Brain Res. 1977;125:1–9. doi: 10.1016/0006-8993(77)90355-9. [DOI] [PubMed] [Google Scholar]
- Wong D.T., Threlkeld P.G., Best K.L., Bymaster F.P. A new inhibitor of norepinephrine uptake devoid of affinity for receptors in rat brain. J. Pharmacol. Exp. Ther. 1982;222:61–65. [PubMed] [Google Scholar]
- Xu X., Hawi Z., Brookes K.J., Anney R., Bellgrove M., Franke B. Replication of a rare protective allele in the noradrenaline transporter gene and ADHD. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008;147B:1564–1567. doi: 10.1002/ajmg.b.30872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A.J., Dayan P. Uncertainty neuromodulation and attention. Neuron. 2005;46:681–692. doi: 10.1016/j.neuron.2005.04.026. [DOI] [PubMed] [Google Scholar]