Abstract
The commonly used triethylsilyl per-fluoro-tetraphenylborate salt, [Et3Si+][F20-BPh4−], has been misidentified. As prepared, the cation is a hydride-bridged silane adduct [R3Si-H-SiR3+]. Under favorable circumstances it can be an effective source of the triethylsilylium ion Et3Si+ but in the absence of a stabilizing base the potent electrophilicity of Et3Si+ decomposes the “inert” F20-BPh4− counterion.
Over the past decade, R3Si+ silylium ion chemistry has progressed from controversy over their existence1–6 to an exploitation of their potent electrophilicity in stoichiometric reactions7 and, most impressively, in catalytic chemistry.8 These advances owe much to the availability of very weakly coordinating anions,9,10 particularly carborane anions7 and the perfluorinated tetraphenylborate anion11 (abbrev. F20-BPh4− or PFTPB−).
A frequently used reagent in this silylation chemistry is the so-called triethylsilyl perfluorotetraphenylborate salt [Et3Si+][F20-BPh4−], first reported by Lambert.12,13 It is a versatile reagent for the abstraction of halide ions from transition metal complexes and from main group element compounds to give reactive, coordinatively unsaturated cations with F20-BPh4− counterions. As liquid clathrates or “swirls”, such salts have high effective solubilities in low dielectric solvents making them very suitable for cationic catalysis. The reagent is readily prepared by reaction of the trityl salt of the F20-BPh4− anion with triethylsilane, both starting materials being commercially available (Eq. 1).
| (1) |
Given that all solvents, even those as weakly coordinating as benzene,13 dichlorobenzene and liquid SO214 coordinate to the Et3Si+ ion forming [Et3Si(solvent)]+ cations, [Et3Si+][F20-BPh4−] was prepared in the “absence” of solvent, or more correctly, using excess triethylsilane reagent as solvent.13 Evaporation of the excess silane gave a white powder which was characterized by CPMAS NMR as [Et3Si+][F20-BPh4−]. The lack of any detectable difference in the 19F NMR of the anion between the presumed [Et3Si+][F20-BPh4−] and the corresponding trityl ion salt of F20-BPh4− led to the conclusion that the F20-BPh4− anion was not coordinated to silicon.13 Lending some support to this ionic formulation is the more recent report of the X-ray crystal structure of the Me3Si+ moiety partnered with the most weakly coordinating carborane anion, the undeca-fluorinated RCB11F11− ion.15 The sum of the three C-Si-C angles in the Me3Si moiety in Me3Si(RCB11F11) is 354.4°, only ca. 6° short of the planarity expected for a Me3Si+ ion. The Si atom is 0.25 Å out of the plane of the three C atoms towards the carborane anion. While perhaps not strictly ionic, this compound is certainly ion-like.
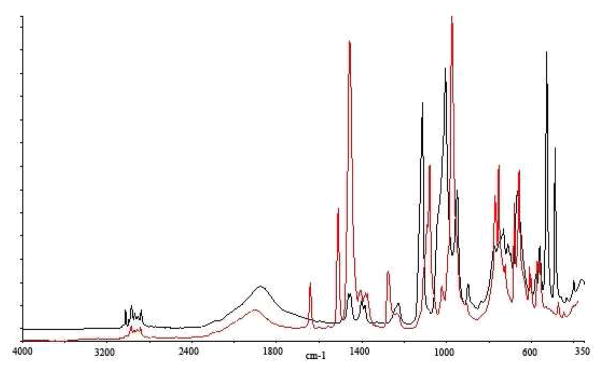
However, during routine characterization of the presumed [Et3Si+][F20-BPh4−] salt we noticed that its ATR-IR spectrum contained an unanticipated strong absorption at ca. 1900 cm−1. We had noticed this band earlier while characterizing the hydride-bridged disilyl cation [Et3Si-H-SiEt3]+ as a CHB11Cl11− carborane salt and assigned it to νasSiHSi.14 Even the distinctive shape of the band was reproduced (see Fig. 1). It is immediately evident that the so-called silylium ion salt [Et3Si+][F20-BPh4−] salt is, in fact, isolated as a silane adduct thereof: [Et3Si-H-SiEt3][F20-BPh4]. Once again, the solvent is coordinated to the silylium ion (Eq. 2).
Fig. 1.
ATR IR spectra of [Et3Si-H-SiEt3]+Y− for Y = F20-BPh4− (red) and CHB11Cl11− (black) showing the distinctive νasSiHSi band near 1900 cm−1.
| (2) |
The same result is obtained whether pure silane or a silane/hexane mixture is used as solvent, as long as at least 2 equiv. of silane are present. When the formation reaction is carried out with only one equivalent of Et3SiH, 1H NMR indicates that only half of the trityl ion is consumed. The reaction appears to be general. When trimethyl- and tri-i-propylsilane are substituted for triethylsilane, the products have the same characteristic IR band of a [R3Si-H-SiR3]+ cation (see Supp. Info.). The products have the correct elemental analyses for [R3Si-H-SiR3][F20-BPh4] salts. In addition, the presence of a displaceable equivalent of volatile silane was established by 1H NMR for the isolated triethylsilane product by dissolving it in benzene and integrating [Et3Si(benzene)]+ against a hexamethylbenzene internal standard.
This finding offers a note of caution to those using so-called “[Et3Si][F20-BPh4]” in synthesis.16–33 When prepared using excess silane as the sole solvent (or when using excess silane mixed with a non-coordinating solvent for the Et3Si+ ion, e.g. an alkane) the isolated product will be [Et3Si-H-SiEt3][F20-BPh4] not [Et3Si][F20-BPh4]. Because of its higher molecular weight, stoichiometries in subsequent metathesis reactions may therefore be miscalculated. Fortunately, the “extra” equivalent of silane is usually displaced during a silylation reaction and escapes the reaction as an innocent volatile byproduct. Indeed, those22–33 who pre-dissolve [Et3Si-H-SiEt3][F20-BPh4] in a solvent that is coordinating to the Et3Si+ ion, e.g. arenes, ethers etc., will displace the silane forming R3Si(solvent)+ cations as the active silylating agents. Silane may be seen bubbling off.
These observations beg the question “Does [Et3Si][F20-BPh4] really exist?” The answer appears to be no. When a film of [Et3Si-H-SiEt3][F20-BPh4] is deposited on the windows of an evacuable IR cell and pumped at 10−6 torr for 6 hours, no loss of intensity of the νasSiHSi band is observed, i.e., silane is not removed from the [Et3Si-H-SiEt3]+ cation under high vacuum at room temperature. When heated under vacuum to 65 °C the colorless product turns black and tris(pentafluorophenyl)boron34 is an identifiable sublimate. The cleavage of a pentafluorophenyl group from the F20-BPh4− anion is consistent with a growing number of reports that this anion is unstable towards strong electrophiles including H+,35 “naked” Ag+,36 and R3Si+.17 Nevertheless, there is one report of [Me3Si][F20-BPh4] as a colorless solid with Mp. 137 °C, no IR band in the region expected for νasSiHSi, and an acceptable C,H elemental analysis.37 When we repeated the described synthetic procedure we observed only the formation of [Me3Si-H-SiMe3][F20 -BPh4].38
In summary, there is no evidence for the existence of [Et3Si][F20-BPh4]. The “as prepared” material is the silane adduct [Et3Si-H-SiEt3][F20-BPh4] and our attempts to remove the silane under vacuum lead to decomposition of the F20-BPh4− anion. Only halogenated carborane anions are stable to the fierce electrophilicity of the Et3Si+ silylium ion.7,15,39 A truly free trialkylsilylium ion exists only in the gas phase.
Supplementary Material
Acknowledgments
This work was supported by the National Science Foundation (CHE 0841428) and the National Institutes of Health (ARRA 3RO1GM023851-30S1).
ABBREVIATIONS
- F20-BPh4−
perfluorinated tetraphenylborate anion
Footnotes
Supporting Information. Synthetic details and spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Reed CA. Acc Chem Res. 1998;31:325–332. [Google Scholar]
- 2.Lambert JB, Zhao Y, Zhang SM. J Phys Org Chem. 2001;14:370–379. [Google Scholar]
- 3.Kim KC, Reed CA, Elliott DW, Mueller LJ, Tham F, Lin L, Lambert JB. Science. 2002;297:825–827. doi: 10.1126/science.1073540. [DOI] [PubMed] [Google Scholar]
- 4.Maerker C, Schleyer PvR. In: The Chemistry of Organic Silicon Compounds. Rappaport Z, Apeilog Y, editors. Vol. 2. Wiley; Chichester: 1998. pp. 513–359. [Google Scholar]
- 5.Mueller T. Adv Organomet Chem. 2005;53:155–215. [Google Scholar]
- 6.Lee VYa, Sekiguchi A. In: Reviews of Reactive Intermediate Chemistry. Platz MS, Moss RA, Jones M Jr, editors. Wiley; New York: 2007. pp. 47–120. [Google Scholar]
- 7.Reed CA. Acc Chem Res. 2010;43:121–128. doi: 10.1021/ar900159e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Review: Klare HFT, Oestreich M. Dalton Trans. 2010;39:9176–9184. doi: 10.1039/c003097j.
- 9.Strauss SH. Chem Rev. 1993;93:927–942. [Google Scholar]
- 10.Krossing I, Raabe I. Angew Chem Int Ed. 2004;43:2066–2090. doi: 10.1002/anie.200300620. [DOI] [PubMed] [Google Scholar]
- 11.Massey AG, Park AJ. J Organometallic Chem. 1964;2:245–250. [Google Scholar]
- 12.Lambert JB, Zhang S. J Chem Soc Chem Commun. 1993:383–384. [Google Scholar]
- 13.Lambert JB, Zhang S, Ciro SM. Organometallics. 1994;13:2430–2443. [Google Scholar]
- 14.Hoffmann SP, Kato T, Tham FS, Reed CA. Chem Commun. 2006:767–769. doi: 10.1039/b511344j. [DOI] [PubMed] [Google Scholar]
- 15.Kuppers T, Bernhardt E, Eujen R, Willner H, Lehmann CW. Angew Chem Int Ed. 2007;46:6346–6349. doi: 10.1002/anie.200701136. [DOI] [PubMed] [Google Scholar]
- 16.Cypryk M, Kurjata J, Chojnowski J. J Organometal Chem. 2003;686:373–378. [Google Scholar]
- 17.Scott VJ, Celenligil R, Ozerov OV. J Am Chem Soc. 2005;127:2852–2853. doi: 10.1021/ja0426138. [DOI] [PubMed] [Google Scholar]
- 18.Egbert JD, Bullock MR, Heinekey M. Organometallics. 2007;26:2291–2295. [Google Scholar]
- 19.Bonnier C, Piers WE, Parvez M, Sorensen TS. Chem Commun. 2008:4593–4595. doi: 10.1039/b808739c. [DOI] [PubMed] [Google Scholar]
- 20.Shultz A, Thomas J, Villinger A. Chem Commun. 2010:3696–3698. doi: 10.1039/c0cc00013b. [DOI] [PubMed] [Google Scholar]
- 21.Adams JJ, Lau A, Arulsamy N, Roddick DM. Organometallics. 2011;30:689–696. [Google Scholar]
- 22.Reed CA, Fackler NLP, Kim KC, Stasko D, Evans DR. J Am Chem Soc. 1999;121:6314–6315. [Google Scholar]
- 23.Driess M, Barmeyer R, Monse C, Merz K. Angew Chem Int Ed. 2001;40:2308–2310. [PubMed] [Google Scholar]
- 24.Lambert JB, Liu C, Kouliev T. J Phys Org Chem. 2002;15:667–671. [Google Scholar]
- 25.Zhang Y, Sita LRJ. Am Chem Soc. 2004;126:7776–7777. doi: 10.1021/ja0492889. [DOI] [PubMed] [Google Scholar]
- 26.Hara K, Akiyama R, Sawamura M. Org Lett. 2005;25:5621–5623. doi: 10.1021/ol052206g. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka S, Takashina M, Tokimoto H, Fujimoto Y, Tanaka K, Fukase Synlett. 2005:2325–2328. [Google Scholar]
- 28.Lavallo V, Frey GD, Kousar S, Donnadieu B, Bertand G. PNAS. 2007;104:13569–13573. doi: 10.1073/pnas.0705809104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Del Grosso A, Pritchard RG, Muryn CA, Ingleson M. J Organometallics. 2009;29:241–249. [Google Scholar]
- 30.Rivers JH, Jones RA. Chem Commun. 2010:4300–4302. doi: 10.1039/c0cc00892c. [DOI] [PubMed] [Google Scholar]
- 31.Matthews SL, Heinekey DM. Inorg Chem. 2010;49:9746–9748. doi: 10.1021/ic1017328. [DOI] [PubMed] [Google Scholar]
- 32.Schulz A, Villinger A. Chem Europ J. 2010;16:7276–7281. doi: 10.1002/chem.201000289. [DOI] [PubMed] [Google Scholar]
- 33.Basuli F, Wicker B, Huffman JC, Mindiola DJ. J Organometal Chem. 2011;696:235–243. [Google Scholar]
- 34.Massey AG, Park AJ. J Organometal Chem. 1966;5:218–225. [Google Scholar]
- 35.Reed CA, KimK-CStoyanov ES, Stasko D, Tham FS, Mueller LJ, Boyd PDW. J Am Chem Soc. 2002;125:1796–1804. doi: 10.1021/ja027336o. [DOI] [PubMed] [Google Scholar]
- 36.Kuprat M, Lehmann M, Schulz A, Villinger A. Organometallics. 2010;29:1421–1427. [Google Scholar]
- 37.Lehmann M, Schulz A, Villinger A. Angew Chem Int Ed. 2009;48:7444–7447. doi: 10.1002/anie.200902992. [DOI] [PubMed] [Google Scholar]
- 38.While the present paper was in review, Prof. Schulz confirmed in private communication that [Me3Si][F20-BPh4] reported by his group in ref 37 must indeed be [Me3Si-H-SiMe3][F20-BPh4].
- 39.Douvris C, Ozerov OV. Science. 2008;321:1188–1190. doi: 10.1126/science.1159979. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.