Abstract
Background
Haiti is experiencing a cholera epidemic. Official epidemic projections, to date, have failed to incorporate existing disease trends or patterns of transmission, while proposed interventions have been debated without comparative estimates of their impact.
Methods
We designed mathematical models of cholera transmission and fit them to Haiti’s provincial incidence data. We then simulated future epidemic trajectories to estimate the impact of clean water, vaccination and enhanced antibiotic distribution programs.
Findings
The natural dynamics of cholera are expected to produce a prevalence decline by mid-January 2011. Between March and December 2011, we project 779,000 (95% CI: 599,000–914,000) cases and 11,100 (95% CI: 7,300–17,400) deaths from cholera in Haiti, over half of which would be expected to occur in the Artibonite and Oueste provinces. If contaminated water consumption were reduced by 1% per week, as per current efforts, we expect 105,000 cases (95% CI: 88,000–116,000) and 1,500 (95% CI: 1,100–2,300) deaths to be averted. A plan to vaccinate 10% of the population beginning on March 1 would be predicted to avert 63,000 (95% CI: 48,000–78,000) cases and 900 (95% CI: 600–1,500) deaths over the same period. By contrast, the proposal to extend antibiotic use to all patients with severe dehydration and half of patients with moderate dehydration would be expected to avert 9,000 (95% CI: 8,000–10,000) cases and 1,300 (95% CI: 900–2,000) deaths.
Interpretation
A decline in cholera prevalence in early 2011 is part of the natural history of the epidemic, and should not be interpreted as reflective of the success of human interventions. Vibrio cholerae in Haiti is expected to produce at least 750,000 cholera cases by November 2011, substantially higher than official estimates currently used for resource allocation. In addition to clean water provision and vaccination, expanded access to antibiotics may avert thousands of deaths.
Keywords: cholera, Haiti, mathematical models, vaccines, antibiotics, water
BACKGROUND
In October 2010, cholera was reported in Haiti for the first time in over 100 years. Within weeks, the disease had been identified in every Department (province), and by the end of the year, more than 150,000 cases and 3,500 deaths had been reported.1 While the country already faced poor water and sewage infrastructure,2,3 Haiti’s sanitation services had been further crippled by the devastating earthquake that had struck nine months prior, permitting rapid epidemic spread of Vibrio cholerae bacteria.4
The United Nations announced epidemic projections within a month of the first reported cases, arguing that 200,000 thousand cases were likely to occur within one year.5 While this estimate is being used for budgeting and response mobilization for the epidemic, the method of projection was crude at best: “Assuming all of the population (estimated at about 10 million for the purpose of this plan) is at risk of contracting cholera, and estimating a cholera attack rate of 2% (not a conservative estimate, given the prevalence of risk factors for cholera transmission including lack of safe water supply, poor sanitation conditions and the rainy season), the estimated number of cases would be 200,000 (10,000,000 pop × 2%)”. This estimate ignores the transmission dynamics and pathogenesis of cholera, such as where the bacteria is most likely to be transmitted, and how people can be asymptomatic carriers, acquire immunity, or receive vaccination and treatment. There is also no empiric basis for the estimated 2% attack rate. Two weeks later, the projection was doubled without explanation. This figure—400,000 cases—has been widely cited by the press and aid agencies.
As with the estimated burden of the epidemic, the projected impact of available interventions remains subject to considerable debate. Many regional and international bodies have focused on clean water provision for the population, mobilizing thousands of gallons of water. Other groups have argued for vaccination, but some experts have argued that vaccination will make little impact.6 In March, the World Health Organization (WHO) changed its position on the use of cholera vaccines,7 which held that vaccination campaigns would not be recommended after an outbreak had already begun, to instead allow for consideration of pre-emptive vaccination to halt the spread of outbreaks to new areas. While many groups now argue for vaccines to be deployed to Haiti, the paucity of vaccine supplies has prevented significant deployment to the country. Several experts have also recommended antibiotic use in moderate cases (5–10% bodyweight loss) to reduce morbidity, duration and cost of illness, and bacterial shedding.8,9 This recommendation, however, contradicts international treatment guidelines,10 which recommend antibiotics only for severe cases (>10% bodyweight loss), based on the premise that rehydration, not antibiotics, saves lives. With the recent decline in cases, some groups have also hypothesized that clean water provision efforts alone may already been addressing the epidemic sufficiently, such that vaccination and antibiotics may not make much additional impact.11
Early in such epidemics, mathematical models have been used to gain a sense of the potential size and duration of an epidemic, and to gain insights into the potential impact of alternative control strategies. Here, we apply a mathematical model of cholera to the epidemic in Haiti to provide projections of future morbidity and mortality, and to produce comparative impact estimates of proposed interventions.
METHODS
Model Structure
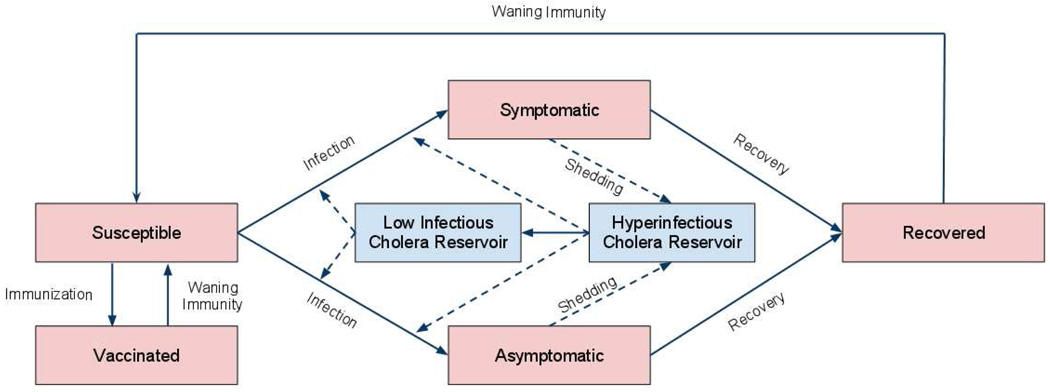
We constructed a mathematical model of cholera transmission based on prior models by Codeço, King, Hartley, and Miller Neilan.12–15 The model is compartmental, describing how individuals can transition between different states of susceptibility or infection with cholera. In the model (Fig. 1), individuals begin as persons susceptible to infection, and are at risk for consuming water contaminated with Vibrio cholerae. When an individual consumes an infectious dose of Vibrio, they experience either symptomatic or asymptomatic infection. Symptomatic individuals have a higher risk of mortality until they recover from infection; during this period, they excrete Vibrio into the water reservoirs around them, posing infection risks to susceptible individuals. Asymptomatic individuals have no increased mortality risk, and they excrete cholera at a much lower rate than symptomatic individuals. Recovered individuals are immune to re-infection, but immunity wanes over time, returning individuals to the susceptible compartment where they can experience re-infection.
Figure 1.
Model of cholera transmission.
Consistent with recent discoveries about cholera pathogenesis, we incorporate how V. cholera has a brief period of hyper-infectiousness upon excretion into the environment.16,17 After this hyper-infectious period, the bacteria return to a “low infectious” state, whereby a larger dose is required to cause infection. Vibrio also have a particular death rate within the environment that is incorporated into the model.
The model is described by a series of seven differential equations (see Table S1 in Appendix for detailed equations). Parameters describing the pathogenesis of cholera were defined by literature estimates used in previously published models (see Table S2 in Appendix).
Model Calibration
We calibrated the model to data describing cholera-related hospitalizations, hospital deaths and community deaths reported by the Haitian Ministry of Health in each Department from October 31, 2010 through January 24, 2011.1 Some Departments only reported hospitalized cases and did not report community cases. To estimate total cases in these provinces, we multiplied their reported number of hospitalized cases by the highest ratio of community to hospitalized cases among Departments that did report community cases (a ratio of 0.38).
Models were fit to the daily reported hospitalized cases and total deaths from cholera in each department. Port-au-Prince was modeled separately from Oueste Department, in which it lies, because data for the capital city have been reported separately.
The key parameters of uncertainty that were fit to the data were the rate of contaminated water consumption, the mortality rate from symptomatic cholera, and the concentration of cholera in the environmental reservoir at model initiation. We let these parameters’ values be sampled from broad prior distributions and used 100,000 iterations of Markov Chain Monte Carlo sampling using an Adaptive Metropolis algorithm to estimate their posterior distributions by fitting the model to the data in each province.
The model was programmed in Python, and PyMC was used for MCMC parameter estimation (version 2.0, MIT Open Source Initiative, Cambridge, MA).
Epidemic Projections
We projected the number of cases occurring over the nine-month period from March 1 through November 30, 2011. For uncertainty analysis, we used Latin Hypercube Sampling to draw 1000 times from the distributions of parameter values to generate 95% credible intervals around our estimates of future projected cases and deaths. We also performed one-way sensitivity analysis by varying each parameter over its range of possible values to determine which parameters had the greatest influence on model projections. In particular, we varied the proportion of hospitalized to total cases across a wide range based on evidence from prior studies.
Simulated Interventions
We simulated the impact of clean water projects, vaccination and expanded antibiotic use on the projected numbers of future cholera cases and deaths. All interventions were simulated as being started on March 1, 2011. Given current estimates of clean water provision,18,19 we simulated the impact of 1% per week reduction in the proportion of the population consuming contaminated water.
For the vaccination simulation, we estimated that 67% of vaccine recipients would be fully protected from the disease, having a mean duration of immunity of 2 years, based on data from prospective clinical trials.20,21 We simulated the impact of 10% vaccination (approximately 2 million doses, or 1 million recipients, total), being carried out over the course of one month after March 1, based on current estimates of vaccine availability and production capability.6,22 In sensitivity analysis, we varied the availability of vaccines from 1 million to 5 million doses.
To simulate the impact of antibiotics, we assumed that individuals received antibiotics on the day of symptom onset. In accordance with cohort studies of antibiotic use among cholera patients, we simulated antibiotics as shortening the duration of disease and the duration of bacterial shedding, as well as reducing the quantity of cholera shed per day.23 Based on prior clinical studies of V. cholera O1 infection,24 we estimated that 8% of patients had severe diarrhea and were receiving antibiotics and that an additional 20% of patients had moderate diarrhea and were not receiving antibiotics. We simulated the use of early antibiotics in all severe and half of moderate diarrhea cases achieved over the course of 30 days from March 1, 2011 onwards based on current descriptions of the proportion of moderately-ill patients who would present to health facilities and antibiotic availability there.18 In sensitivity analysis, we projected the impact of providing antibiotics in 100% of moderate cases. Additionally, we simulated interventions in conjunction by running the model with multiple simulated interventions acting at the same time.
Funding: JRA is supported by the National Institutes of Health (NIGMS Grant No. U54 GM088558) and had responsibility for the decision to submit the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, which played no role in the study design, methods, interpretation of results, the content of this manuscript, or the decision to submit it for publication.
RESULTS
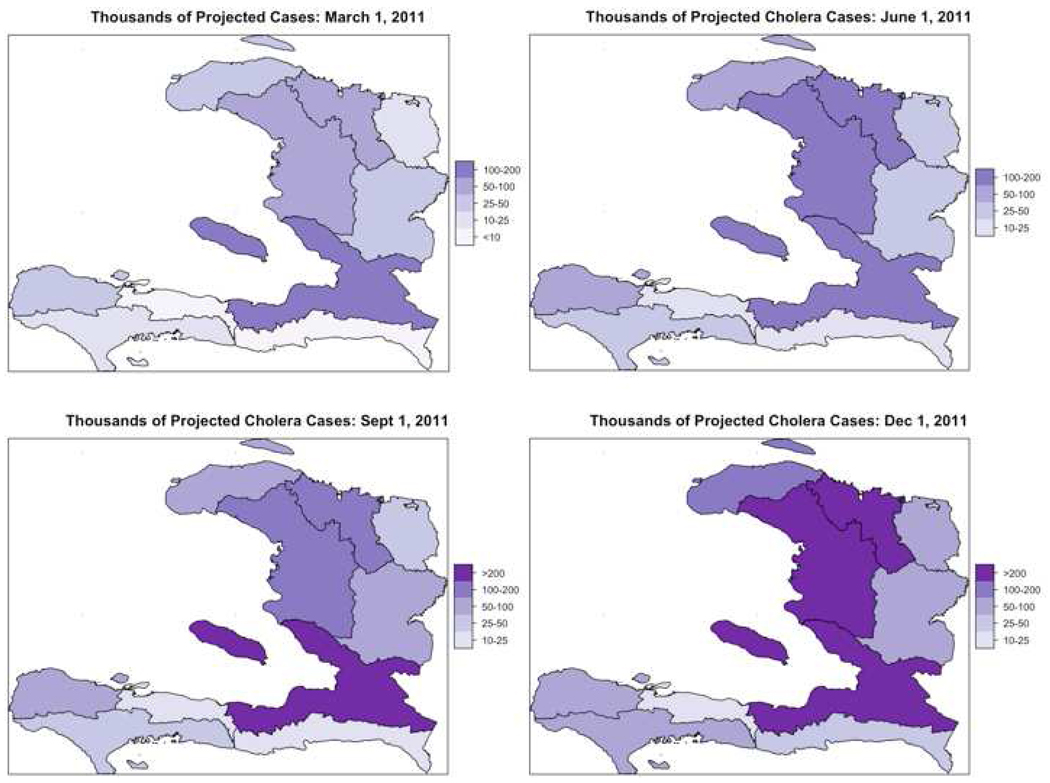
Our model projected 779,000 cases (95% CI: 599,000–914,000) of cholera to occur in Haiti in the nine months following March 1, 2011, in the absence of new interventions (Table 1). More than half of cases occurred in two Departments, Oueste and Artibonite, which are the hotspots for transmission (Fig. 2). The prevalence of active cases naturally fell in most Departments by mid-January because of the gradual accumulation of immunity and loss of susceptible persons. The model projected 11,100 deaths (95% CI: 7,300–17,400) due to cholera during the period from March through November 2011.
Table 1.
Thousands of cases (1a) and hundreds of deaths (1b) from cholera projected between March 1 and November 30, 2011 by Department under base case and under each intervention scenario.
| 1a. | Thousands of Cases | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Artibonite | Centre | Grande Anse | Nippes | Nord | Nord Oueste | Nord Est | Ouest | Port au Prince | Sud | Sud Est | Total | ||
| Base Case | Mean 95% CI | 137 (105–161) | 49 (36–59) | 55 (47–59) | 17 (12–21) | 121 (105–128) | 65 (51–74) | 38 (30–43) | 77 (56–94) | 154 (110–191) | 43 (31–53) | 23 (16–30) | 779 (599–914) |
| Expanded Antibiotic Use (50% moderate cases) | Mean 95% CI | 136 (104–160) | 48 (35–59) | 54 (46–58) | 16 (11–21) | 120 (104–127) | 65 (51–74) | 38 (30–43) | 76 (54–93) | 152 (108–189) | 42 (30–53) | 23 (15–30) | 770 (589–905) |
| Vaccination (10% of population) | Mean 95% CI | 126 (97–148) | 45 (33–55) | 50 (43–53) | 15 (11–19) | 109 (96–114) | 60 (47–68) | 35 (28–39) | 71 (51–86) | 142 (102–176) | 40 (28–49) | 22 (14–28) | 716 (551–836) |
| Expanded Antibiotics and Vaccination | Mean 95% CI | 125 (96–146) | 44 (33–54) | 50 (43–53) | 15 (10–19) | 109 (95–114) | 59 (47–67) | 35 (28–39) | 70 (50–85) | 140 (99–174) | 39 (28–49) | 21 (14–27) | 707 (542–827) |
| Improvement in water (1% per week) | Mean 95% CI | 119 (90–140) | 42 (30–51) | 49 (41–53) | 14 (10–18) | 108 (92–114) | 57 (44–65) | 33 (26–38) | 65 (47–81) | 131 (92–164) | 37 (26–46) | 20 (13–26) | 674 (511–797) |
| All three interventions | Mean 95% CI | 108 (81–127) | 38 (27–47) | 44 (37–48) | 13 (9–16) | 97 (83–102) | 52 (40–59) | 30 (24–35) | 59 (42–73) | 118 (83–148) | 33 (23–42) | 17 (11–23) | 609 (461–720) |
| 1b. | Thousands of Cases | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Artibonite | Centre | Grande Anse | Nippes | Nord | Nord Oueste | Nord Est | Ouest | Port au Prince | Sud | Sud Est | Total | ||
| Base Case | Mean 95% CI | 14 (9–21) | 9 (6–15) | 17 (12–24) | 7 (4–11) | 10 (8–16) | 6 (4–10) | 7 (4–11) | 8 (5–12) | 13 (8–23) | 7 (5–12) | 13 (7–20) | 111 (73–174) |
| Expanded Antibiotic Use (50% moderate cases) | Mean 95% CI | 13 (8–18) | 8 (5–13) | 15 (10–21) | 6 (4–10) | 9 (7–15) | 5 (4–8) | 6 (4–9) | 7 (4–11) | 12 (7–21) | 6 (4–10) | 11 (6–18) | 98 (64–154) |
| Vaccination (10% of population) | Mean 95% CI | 13 (9–19) | 9 (5–14) | 15 (11–22) | 6 (4–10) | 9 (7–15) | 6 (4–9) | 6 (4–10) | 7 (5–11) | 12 (8–21) | 7 (4–11) | 12 (7–18) | 102 (67–159) |
| Expanded Antibiotics and Vaccination | Mean 95% CI | 12 (8–17) | 8 (5–12) | 14 (9–19) | 5 (3–9) | 8 (6–13) | 5 (4–8) | 6 (4–9) | 6 (4–10) | 11 (7–19) | 6 (4–9) | 10 (6–16) | 90 (59–141) |
| Improvement in water (1% per week) | Mean 95% CI | 12 (8–18) | 8 (5–13) | 15 (10–21) | 6 (3–9) | 9 (7–15) | 5 (4–8) | 6 (4–9) | 7 (4–10) | 11 (7–20) | 6 (4–10) | 10 (6–17) | 96 (62–151) |
| All three interventions | Mean 95% CI | 10 (7–15) | 7 (4–10) | 12 (8–17) | 4 (3–7) | 7 (6–12) | 4 (3–7) | 5 (3–8) | 5 (3–8) | 9 (6–16) | 5 (3–8) | 8 (5–14) | 77 (50–122) |
Figure 2.
Model projections of cumulative cases of cholera from the start of the epidemic by Department (scale: thousands of cholera cases). Cases from Port-au-Prince were combined with those from the remainder of Oueste Department here.
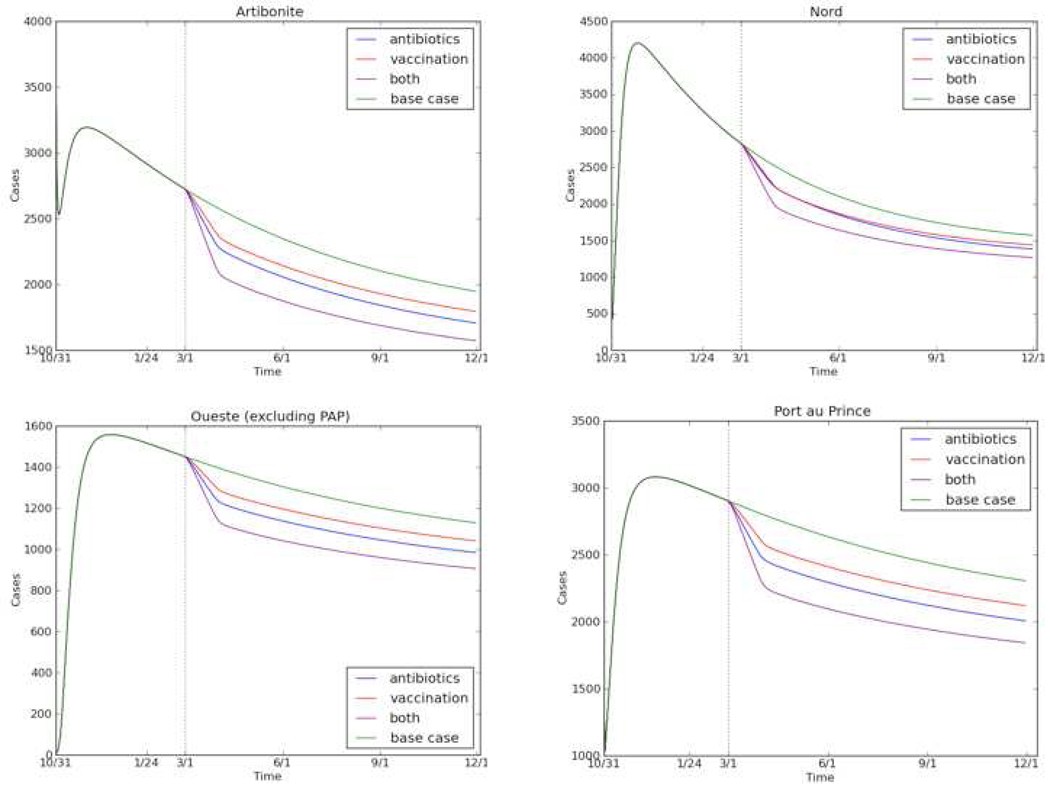
Improvements in access to clean water, modeled as a 1% per week reduction in the proportion of the population consuming contaminated water, were projected to avert 105,000 cases (95% CI: 88,000–116,000) and 1,500 deaths (95% CI: 1,100–2,300) from March through November 2011. Vaccinating 10% of the population was projected to avert 63,000 cases (95% CI: 48,000–78,000) and 900 deaths (95% CI: 600–1,500). Varying the vaccinated proportion from 5% to 25% averted 32,000 cases (95% CI: 24,000–35,000) to 159,000 cases (95% CI: 118,000–193,000), respectively. Expanding early antibiotic use to all severe and half of moderate cases was projected to avert 9,000 cases (95% CI: 8,000–10,000) and 1,300 (95% CI: 900–2,000) deaths. Implementing both vaccination of 10% of the population and antibiotic expansion would be expected to avert 73,000 cases (95% CI: 57,000–86,000) and 2,200 deaths (95% CI: 1,400–3,300). Vaccination averted more new cholera cases, while antibiotics had a greater impact on the prevalence of cases by reducing the duration of illness (Fig 3.). All three interventions were estimated to avert 170,000 cases (95% CI: 138,000–193,000) and 3,400 deaths (95% CI: 2,300–5,200).
Figure 3.
Impact of interventions on cholera prevalence in the four most heavily-burdened Departments in Haiti. Interventions begin March 1, 2011 (dashed vertical line). Note the different scale on the vertical axis for each Department.
If hospitalized cases represented 50% of cases, the total number of projected cholera cases from March through November would reduce to 664,000 (in the base case, hospitalized cases were estimated to represent 38% of all cases); if hospitalized cases represented only 10% of total cases, the projected total case load would increase to 1,276,000 (see Appendix, Figure A2). Across the ranges of parameters assessed, the projections were most sensitive to the proportion of symptomatic patients hospitalized, proportion of cases that are asymptomatic (case projections varied from 32% lower to 22% higher than the base case). They were modestly sensitive to immunity duration (11% lower to 16% higher) and fairly robust to the dose of cholera needed to cause infection (6% lower to 21% higher) and the water reservoir size (6% lower to 13% higher).
Discussion
In contrast to the current UN projection of 400,000 cases from December 2010 to December 2011, a dynamic model of cholera incorporating key features of disease transmission and pathogenesis projected over 750,000 to occur in just the 9-month period from March to December 2011. While the prevalence of cholera is declining in Haiti, model projections also reveal that this is an expected natural course of the epidemic, and does not necessarily reflect the impact of human interventions.
The model demonstrates that the cholera epidemic in Haiti can be critically affected by water distribution, vaccination and antibiotic expansion. Water has been the current focus of most efforts, and consistent with the presumed impact of clean water provision, our model found that efforts to distribute clean water may avert 105,000 cases and 1,600 deaths, more than the individual impacts of antibiotics or vaccines. However, antibiotics and vaccines had considerable additional impact beyond the impact of water alone; all three interventions were projected to avert 170,000 cases and 3,400 deaths between March and December 2011.
The use of vaccines after the start of cholera epidemics has historically been controversial. Until recently, the WHO had not recommended their use under such circumstances. WHO’s recently revised guidelines now state that “reactive vaccination could be considered… as an additional control measure” after thorough review of the epidemiology and infrastructure of the setting.7 Given that cholera has spread throughout Haiti, we could not project the impact of “pre-emptive” vaccination to avert spread to new areas. However, recent studies have supported the use of “reactive” cholera vaccination after the onset of an outbreak.25,26 We projected the random use of reactive cholera vaccination rather than the targeting of high-risk communities, as data about the size and comparative hazard ratio for high-risk populations in Haiti is not available. Hence, our model could under-estimate the impact of vaccination if high-risk targeting is more potent than general distribution vaccination, but this a limitation inherent in the type of model employed in this study. Alternatively, the model may overestimate the effect of vaccination if a large proportion of the population has had asymptomatic infection, as vaccination programs cannot distinguish susceptible individuals from those with natural immunity through asymptomatic infection; however, the same is true in field studies of vaccines from which vaccine parameters were taken. Nevertheless vaccinating 10% of the population in this model was projected to avert 63,000 cases and 900 deaths.
The use of antibiotics in moderate cholera has likewise been the subject of controversy. WHO guidelines recommend reserving antibiotics for severe cases, while many centers and cholera experts recommend their use in moderate cases to reduce mortality, repeat admissions, and shedding at home. Our findings support the latter position, suggesting that expanding access to antibiotics to half of moderate-severity patients could avert 9,000 cases and 1,300 deaths. We assume that individuals receiving antibiotics began them on the day of symptom onset; we believe that this assumption is reasonable in light of prior clinical studies reporting that patients typically present within the first day of symptoms, but acknowledge that there may be variability across communities. A longer duration between symptom onset and antibiotic initiation would diminish the modeled benefit of antibiotics. We did not account for the possibility of increased resistance of circulating strains caused by the antibiotic strategy.
As with all mathematical models, the results of this model are based upon assumptions inherent to the modeling exercise. The scale up of interventions simulated, while plausible, may be overly ambitious given the current healthcare infrastructure. Access to clean water has undoubtedly been changing and will continue to do so over the course of the next year in Haiti. Our estimates are projections based on fitting data to the current epidemiologic scenario of sanitation and water provision, which hopefully—though not certainly—will provide the worst-case simulation. Whether the clean water intervention simulated is plausible is uncertain; prior studies have shown that even simple and efficacious clean water interventions may not be maintained.27 Even if achievable in some locations, such as Port-au-Prince, it may not be plausible in more remote parts of the country. Furthermore, we did not estimate the impact of decontaminating water reservoirs, as this would be difficult to reflect in the type of model currently used to simulate cholera transmission. Recent evidence has suggested that newer strains of cholera may cause more severe disease.28 We utilized prior data for V. cholera O1 and examined the impact of more virulent strains in sensitivity analysis.
Many other factors not incorporated in this analysis, such as supply chain, nutrition, hygiene practices, education, active case finding and overall socioeconomic development, undoubtedly will impact the burden of cholera in both the short and long-term.29 Additionally, it has been observed in many other settings that cholera prevalence increases during rainy seasons, but in the absence of historical data on the interaction between the rainy season and cholera in Haiti, our model could estimate the precise impact of these factors. Should the rainy season worsen water contamination, our projections may be taken as conservative, lower-bound estimates of future cholera burden.
Finally, since we use reported incidence to fit our models, under-reporting of cases could alter model projections. Our model adjusted for the fact that a significant proportion of symptomatic patients may not be hospitalized; the proportion estimated here (38%) through analysis of Ministry of Health data is consistent with estimates from prior studies.30,31 However, differences in hospitalization rates and reporting practices between Departments represent important parameters for future analysis as data becomes available, though it is unlikely for such data to be available before the end of the epidemic, when such information will be least useful for resource allocation or averting future deaths.
It is thought that the size of a water reservoir may be an important component of cholera transmission models, in which the reproductive number (number of secondary cases produced by each primary case) can be inversely proportional to the size of the water reservoir (a dilution effect).32 In sensitivity analysis, we found that the water reservoir size parameter in our model did not significantly impact our case projections, because our model adjusted the water consumption rate to fit available data when the reservoir size was changed, producing similar projections over a range of plausible water reservoir sizes. This indicates that our results are robust to a wide variety of potential water-use settings in Haiti, from smaller towns to larger cities.
The modeling of cholera transmission remains challenging and relatively primitive compared to the modeling of many other infectious diseases. Human-environment-pathogen interactions are not well described, particularly for the processes of contamination, water consumption, and Vibrio cholerae behavior within the environment. Further studies are needed to improve the parameterization of mathematical models of cholera. Nevertheless, model-based analyses have yielded important insights into cholera epidemiology, particularly early in epidemics when we can project the natural history of an outbreak and provide estimates of how much impact hotly-debated interventions or policy changes may produce. The alternative currently available for Haiti is a “best guess” that ignores current disease rates or cholera pathogenesis and transmission, and may underestimate the resources needed to avert future cases and deaths.
Panel
Research in Context
Systematic Review
The field of cholera modeling has been advancing substantially over the past decade. In 2001, Codeço extended a 1979 model by Capasso to qualitatively, describing how endemic and epidemic cholera could be simulated through a series of differential equations describing the interaction between concentrations of cholera bacteria in water reservoirs and human hosts who consumed contaminated water.12 This model has been extended by King to assess the impact of “inapparent infections” in Bengal; King’s model found inapparent infections to be more common than previously thought and concluded that these undiagnosed cholera cases could amplify the transmission and mortality caused by cholera epidemics.14 Hartley adapted the Codeço model to also explore the importance of a hyperinfectious state of cholera transmission, finding that a model incorporating the highly-infectious state of cholera bacteria soon after shedding allowed for better predictions of epidemics in Bangladesh.13 Applying these insights into epidemics in Calcutta, Miller Neilan simulated alternative interventions during a cholera epidemic, arguing that simultaneously enacting multiple interventions, such as vaccination, sanitation and rehydration, was more effective that pooling all resources into one intervention such as vaccination.15
More recently, cholera models have been used to anticipate epidemics and design future prevention strategies. Pascual and colleagues have published a series of papers assessing the role of El Niño weather effects on cholera outbreaks.33 Longini et al. has contributed longitudinal analyses of cholera data from Bangladesh to assess different types of immunity, which may inform cholera vaccine design.34,35 A recent model by Reyburn et al. took such information and showed that reactive vaccination during epidemics could have averted many cases in Zimbabwe, India and Tanzania.26
There have been no epidemiologic models or analyses of cholera published to date that assess the cholera epidemic in Haiti.
Interpretation
This analysis adapts prior models of cholera transmission to the epidemic in Haiti. While current global estimates of the epidemic are based on the assumption that the epidemic will attack 4% of the population, this assumption is essentially a guess—based on no data, and ignoring the dynamics of cholera epidemics such as where people acquire the infection, how they gain immunity, and what role human interventions such as water allocation or vaccination may play. Our model-based analysis provides a framework for making more reasonable predictions of epidemic size by fitting the model to Haiti’s data, and provides comparative estimates of the potential impact of vaccination, antibiotics, and clean water provision.
Supplementary Material
Acknowledgements
We thank Marc Lipsitch for helpful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
We declare that we have no conflict of interest.
Author Contributions: JA and SB developed the models and conceptual framework and analyzed the results. JA wrote the first draft of the manuscript. Both authors were involved in drafting the final version of the manuscript.
References
- 1.Ministere de la Sante Publique et de la Population. [Accessed January 15, 2011];Rapport de cas journalier et cumulatif. 2011 Available at: www.mspp.gouv.ht/site/index.php.
- 2.WHO/UNICEF Joint Monitoring Programme (JMP) for Water Supply and Sanitation. Progress on sanitation and drinking water: 2010 update. 2010 Available at: http://whqlibdoc.who.int/publications/2010/9789241563956_eng_full_text.pdf.
- 3.Sullivan C, Meigh J, Giacomello A. The Water Poverty Index: Development and application at the community scale. Natural Resources Forum. 2003;27(3):189–199. [Google Scholar]
- 4.Walton DA, Ivers LC. Responding to cholera in post-earthquake Haiti. N. Engl. J. Med. 2011;364(1):3–5. doi: 10.1056/NEJMp1012997. [DOI] [PubMed] [Google Scholar]
- 5.United Nations Office for the Coordination of Humanitarian Affairs. Cholera Inter-Sector Response Strategy for Haiti. Nov 2010 – Dec 2010. 2010 [Google Scholar]
- 6.Cyranowski D. Cholera vaccine plan splits experts. Nature. 2011;469:273–274. doi: 10.1038/469273a. [DOI] [PubMed] [Google Scholar]
- 7.Cholera vaccines: WHO position paper. Wkly. Epidemiol. Rec. 2010;85(13):117–128. [PubMed]
- 8.Nelson EJ, Nelson DS, Salam MA, Sack DA. Antibiotics for both moderate and severe cholera. N. Engl. J. Med. 2011;364(1):5–7. doi: 10.1056/NEJMp1013771. [DOI] [PubMed] [Google Scholar]
- 9.Ivers LC, Farmer P, Almazor CP, Léandre F. Five complementary interventions to slow cholera: Haiti. Lancet. 2010;376(9758):2048–2051. doi: 10.1016/S0140-6736(10)62243-X. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Cholera. 2010 Available at: http://www.who.int/mediacentre/factsheets/fs107/en/index.html.
- 11.Fox B. Cholera takes a breather, but could surge. Associated Press; 2011. Available at: http://www.google.com/hostednews/ap/article/ALeqM5hAU1yn4wO2WcE5B5Mg7i87js4hOg?docId=f98e143afc8243febc75d19846bf9b9e. [Google Scholar]
- 12.Codeço CT. Endemic and epidemic dynamics of cholera: the role of the aquatic reservoir. BMC Infect. Dis. 2001;1:1. doi: 10.1186/1471-2334-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartley DM, Morris JG, Smith DL. Hyperinfectivity: a critical element in the ability of V. cholerae to cause epidemics? PLoS Med. 2006;3(1):e7. doi: 10.1371/journal.pmed.0030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King AA, Ionides EL, Pascual M, Bouma MJ. Inapparent infections and cholera dynamics. Nature. 2008;454(7206):877–880. doi: 10.1038/nature07084. [DOI] [PubMed] [Google Scholar]
- 15.Miller Neilan RL, Schaefer E, Gaff H, Fister KR, Lenhart S. Modeling optimal intervention strategies for cholera. Bull. Math. Biol. 2010;72(8):2004–2018. doi: 10.1007/s11538-010-9521-8. [DOI] [PubMed] [Google Scholar]
- 16.Merrell DS, Butler SM, Qadri F, et al. Host-induced epidemic spread of the cholera bacterium. Nature. 2002;417(6889):642–645. doi: 10.1038/nature00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alam A, Larocque RC, Harris JB, et al. Hyperinfectivity of human-passaged Vibrio cholerae can be modeled by growth in the infant mouse. Infect. Immun. 2005;73(10):6674–6679. doi: 10.1128/IAI.73.10.6674-6679.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.International Federaion of Red Cross and Red Crescent Societies. Haiti and the Dominican Republic: Cholera outbreak - response and preparedness. 2011 Available at: http://reliefweb.int/rw/RWFiles2011.nsf/FilesByRWDocUnidFilename/MUMA- 8DK2KC-full_report.pdf/$File/full_report.pdf. [Google Scholar]
- 19.Oxfam. Haiti Progress Report 2010. Oxfam; 2011. Available at: http://www.oxfam.org.uk/resources/policy/conflict_disasters/downloads/haiti-progress-report-2010-en.pdf. [Google Scholar]
- 20.Sur D, Lopez AL, Kanungo S, et al. Efficacy and safety of a modified killed-whole-cell oral cholera vaccine in India: an interim analysis of a cluster-randomised, double-blind, placebo-controlled trial. Lancet. 2009;374(9702):1694–1702. doi: 10.1016/S0140-6736(09)61297-6. [DOI] [PubMed] [Google Scholar]
- 21.Thiem VD, Deen JL, von Seidlein L, et al. Long-term effectiveness against cholera of oral killed whole-cell vaccine produced in Vietnam. Vaccine. 2006;24(20):4297–4303. doi: 10.1016/j.vaccine.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Zarocostas J. Experts urge vaccination to try to control cholera outbreak in Haiti. BMJ. 2011;342:d23. doi: 10.1136/bmj.d23. [DOI] [PubMed] [Google Scholar]
- 23.Rahaman MM, Majid MA, Alam AKMJ, Islam MR. Effects of doxycycline in actively purging cholera patients: a double-blind clinical trial. Antimicrob. Agents Chemother. 1976;10(4):610–612. doi: 10.1128/aac.10.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaper JB, Morris JG, Jr, Levine MM. Cholera. Clinical Microbiology Reviews. 1995;8(1):48–86. doi: 10.1128/cmr.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anh DD, Lopez AL, Thiem VD, et al. Use of Oral Cholera Vaccines in an Outbreak in Vietnam: A Case Control Study. PLoS Negl Trop Dis. 2011;5(1):e1006. doi: 10.1371/journal.pntd.0001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reyburn R, Deen JL, Grais RF, et al. The Case for Reactive Mass Oral Cholera Vaccinations. PLoS Negl Trop Dis. 2011;5(1):e952. doi: 10.1371/journal.pntd.0000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luby SP, Mendoza C, Keswick BH, Chiller TM, Hoekstra RM. Difficulties in bringing point-of-use water treatment to scale in rural Guatemala. Am. J. Trop. Med. Hyg. 2008;78(3):382–387. [PubMed] [Google Scholar]
- 28.Siddique AK, Nair GB, Alam M, et al. El Tor cholera with severe disease: a new threat to Asia and beyond. Epidemiol. Infect. 2010;138(3):347–352. doi: 10.1017/S0950268809990550. [DOI] [PubMed] [Google Scholar]
- 29.Dowell SF, Tappero JW, Frieden TR. Public Health in Haiti — Challenges and Progress. N Engl J Med. 2011;364(4):300–301. doi: 10.1056/NEJMp1100118. [DOI] [PubMed] [Google Scholar]
- 30.Lawoyin TO, Ogunbodede NA, Olumide EA, Onadeko MO. Outbreak of cholera in Ibadan, Nigeria. Eur. J. Epidemiol. 1999;15(4):367–370. doi: 10.1023/a:1007547117763. [DOI] [PubMed] [Google Scholar]
- 31.Ryan ET, Dhar U, Khan WA, et al. Mortality, morbidity, and microbiology of endemic cholera among hospitalized patients in Dhaka, Bangladesh. Am. J. Trop. Med. Hyg. 2000;63(1–2):12–20. doi: 10.4269/ajtmh.2000.63.12. [DOI] [PubMed] [Google Scholar]
- 32.Pascual M, Bouma MJ, Dobson AP. Cholera and climate: revisiting the quantitative evidence. Microbes Infect. 2002;4(2):237–245. doi: 10.1016/s1286-4579(01)01533-7. [DOI] [PubMed] [Google Scholar]
- 33.Pascual M, Rodó X, Ellner SP, Colwell R, Bouma MJ. Cholera dynamics and El Niño-Southern Oscillation. Science. 2000;289(5485):1766–1769. doi: 10.1126/science.289.5485.1766. [DOI] [PubMed] [Google Scholar]
- 34.Longini IM, Nizam A, Ali M, et al. Controlling endemic cholera with oral vaccines. PLoS Med. 2007;4(11):e336. doi: 10.1371/journal.pmed.0040336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Longini IM, Yunus M, Zaman K, et al. Epidemic and endemic cholera trends over a 33-year period in Bangladesh. J. Infect. Dis. 2002;186(2):246–251. doi: 10.1086/341206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.