Abstract
The adaptor protein SH2B1β participates in regulation of the actin cytoskeleton during processes such as cell migration and differentiation. Here, we identify SH2B1β as a new focal adhesion protein. We provide evidence that SH2B1β is phosphorylated in response to phorbol 12-myristate 13-acetate (PMA)-induced protein kinase C (PKC) activation and show that PMA induces a rapid redistribution of SH2B1β out of focal adhesions. We also show that growth hormone (GH) increases cycling of SH2B1β into and out of focal adhesions. Ser161 and Ser165 in SH2B1β fall within consensus PKC substrate motifs. Mutating these two serine residues into alanine residues abrogates PMA-induced redistribution of SH2B1β out of focal adhesions, decreases SH2B1β cycling into and out of focal adhesions in control and GH-stimulated cells, and increases the size of focal adhesions. By contrast, mutating Ser165 into a glutamate residue decreases the amount of SH2B1β in focal adhesions and increases the number of focal adhesions per cell. These results suggest that activation of PKC regulates SH2B1β focal adhesion localization through phosphorylation of Ser161 and/or Ser165. The finding that phosphorylation of SH2B1β increases the number of focal adhesions suggests a mechanism for the stimulatory effect on cell motility of SH2B1β.
Key words: Focal adhesion, Phosphorylation, PKC, SH2 domain, SH2B1β
Introduction
SH2B1 is a member of the SH2B family of adaptor proteins that includes SH2B1 (also known as SH2-B and PSM), SH2B2 (also known as APS) and SH2B3 (also known as Lnk). Alternative splicing gives rise to α, β, γ and δ isoforms of SH2B1. The isoforms share a dimerization domain (DD), nuclear localization signal (NLS), nuclear export signal (NES), pleckstrin homology (PH) domain and Src homology 2 (SH2) domain, but differ at the extreme C-terminus (Nelms et al., 1999; Yousaf et al., 2001). SH2B1 isoforms are recruited, through their SH2 domain, to the activated form of cytokine receptor-associated JAK tyrosine kinases and multiple receptor tyrosine kinases. This enables them to serve as an adaptor and/or scaffolding protein for multiple hormones and growth factors, including growth hormone (GH), insulin, leptin, prolactin, fibroblast growth factor (FGF), insulin-like growth factor-1 (IGF1), platelet-derived growth factor (PDGF), nerve growth factor and glial-derived growth factor (Kong et al., 2002; Nelms et al., 1999; Qian et al., 1998; Ren et al., 2005; Ren et al., 2007; Rider et al., 2009; Riedel et al., 1997; Riedel et al., 2000; Rui and Carter-Su, 1998; Rui et al., 1997; Wang and Riedel, 1998; Zhang et al., 2006). Within the context of these signaling systems, SH2B1 enhances kinase activity, regulates gene transcription and/or modulates cytoskeletal dynamics (reviewed by Maures et al., 2007). These cellular effects contribute to the ability of SH2B1 to promote and maintain neuronal differentiation (Qian et al., 1998; Rui et al., 1999a), regulate energy and glucose homeostasis (Ren et al., 2007), and promote cell proliferation (Riedel et al., 2000) and motility (Diakonova et al., 2002; Herrington et al., 2000; Rider et al., 2009).
The processes of neuronal differentiation and cell motility both rely, in part, on regulation of focal adhesion dynamics. Focal adhesions are large integrin-based macromolecular complexes that mediate cell–extracellular-matrix (ECM) attachment, facilitate direct signaling between the ECM and the cell and facilitate cell anchorage and motility (reviewed by Geiger et al., 2009). The number of proteins known to localize to focal adhesions is large, and the number and degree of regulation of interactions between these focal adhesion proteins make these structures among the most complex and dynamic structures within a cell. Many focal adhesion proteins have been shown to continually cycle into and out of focal adhesions. Modulation of the cycling of focal adhesion proteins can directly affect the strength of focal adhesions (von Wichert et al., 2003), leading to altered rates of focal adhesion assembly and disassembly (termed focal adhesion ‘turnover’) and thus cell motility. Phosphorylation of focal adhesion proteins is a major mechanism by which focal adhesion assembly and disassembly are regulated. Numerous kinases are present in focal adhesions, including focal adhesion kinase (FAK), Src family kinases, extracellular regulated kinases (ERKs) and protein kinase C (PKC). When activated, these kinases phosphorylate nearby focal adhesion proteins, thereby regulating focal adhesion stability, turnover and cell motility (Besson et al., 2001; Webb et al., 2004). Multiple PKC isoforms (α, δ and ε) have been identified in focal adhesions (Barry and Critchley, 1994; Haller et al., 1998; Jaken et al., 1989). Active PKC isoforms phosphorylate focal adhesion proteins (Meenakshi et al., 1993; Tigges et al., 2003; Werth et al., 1983), regulate focal adhesion formation (Woods and Couchman, 1992), which leads to an increase in the overall number of focal adhesions per cell (Besson et al., 2001), and promote focal adhesion-dependent processes, such as cell adhesion, spreading and migration (Disatnik and Rando, 1999; Larsson, 2006; Vuori and Ruoslahti, 1993).
In this study, we identify SH2B1β as a new focal adhesion protein. We show that the SH2 domain of SH2B1β is necessary for both localization to focal adhesions and association with the focal adhesion protein talin. We provide evidence that PKC regulates the localization of SH2B1β to focal adhesions, through phosphorylation of Ser161 and/or Ser165 in SH2B1β, and that both GH and serum increase mobility of SH2B1β into and out of focal adhesions. Finally, we present evidence that phosphorylation of Ser161 and/or Ser165 increases overall focal adhesion number and decreases focal adhesion size, changes that are likely to contribute to SH2B1β-mediated regulation of cell motility.
Results
SH2B1β is a new focal adhesion protein
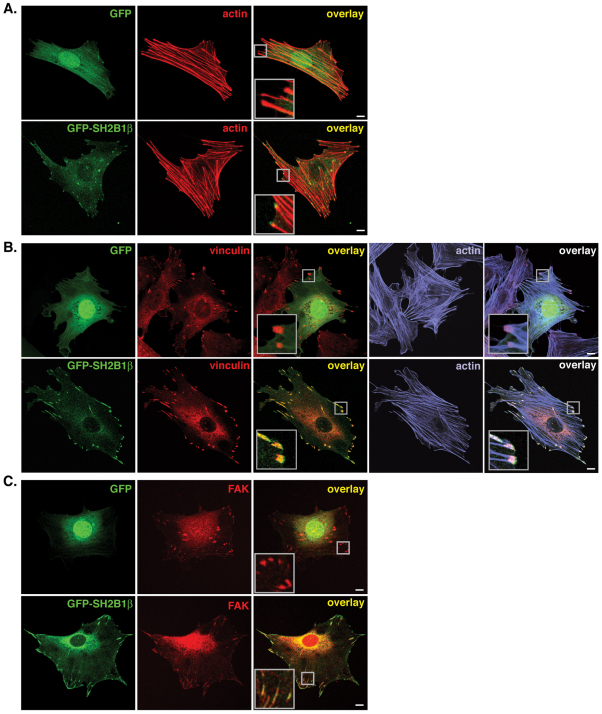
Our previous studies implicated SH2B1β in the control of cytoskeletal dynamics, showing that it enhances GH- and PDGF-dependent cell ruffling and lamellipodia formation and GH-induced pinocytosis and cell motility (Diakonova et al., 2002; Herrington et al., 2000). To gain further insight into the role of SH2B1β in cytoskeletal dynamics and cell motility, we visualized GFP–SH2B1β in 3T3-F442A fibroblasts, a highly GH-responsive cell line used previously to demonstrate a crucial role for SH2B1 in GH-induced membrane ruffling (Diakonova et al., 2002; Herrington et al., 2000; Rider et al., 2009). Images from near the bottom of the cell revealed that GFP–SH2B1β, but not GFP, colocalized with the termini of endogenous actin filaments (F-actin, identified by phalloidin staining) in a pattern characteristic of focal adhesion proteins (Fig. 1A). To confirm that SH2B1β is localized to focal adhesions, GFP- or GFP–SH2B1β-expressing 3T3-F442A fibroblasts were stained for the focal adhesion markers vinculin (Fig. 1B) and FAK (Fig. 1C). GFP–SH2B1β, but not GFP, colocalized with endogenous vinculin in focal adhesions at the termini of endogenous F-actin (Fig. 1B) as evidenced by the intense yellow color in structures resembling focal adhesions (caused by superimposition of the green GFP–SH2B1β signal with the red vinculin signal) (Fig. 1B, lower middle panel) and the white color revealing superimposition of the green GFP–SH2B1β signal with the red vinculin signal at the ends of the blue-stained F-actin (phalloidin stain) (Fig. 1B, lower right-hand panel). Similarly, GFP–SH2B1β, but not GFP, colocalized with endogenous FAK (Fig. 1C, lower right-hand panel). Line-scans across focal adhesions similarly showed superimposition of signals for GFP–SH2B1β and stained endogenous vinculin, confirming localization of GFP–SH2B1β at vinculin-positive focal adhesions (supplementary material Fig. S1A). Live-cell imaging revealed that GFP–SH2B1β also colocalized with small regions of intense mCherry–vinculin when both proteins were expressed in HeLa cells (supplementary material Fig. S1B), indicating that SH2B1β localizes to focal adhesions in multiple cell types.
Fig. 1.
SH2B1β localizes to focal adhesions. (A) 3T3-F442A cells expressing GFP (top panels) or GFP–SH2B1β (bottom panels), were fixed and stained with phalloidin to visualize F-actin. Overlay of the GFP (green) and phalloidin (red) signals is shown in the right-hand panel. (B) 3T3-F442A cells expressing GFP (top panels) or GFP–SH2B1β (bottom panels) were fixed and stained for endogenous vinculin (to visualize focal adhesions) and F-actin (phalloidin). Overlays of the GFP (green) and vinculin (red) signals (middle panels) or GFP, vinculin, and phalloidin (blue) signal (right-hand panels) are shown. (C) 3T3-F442A cells expressing GFP (top panels) or GFP–SH2B1β (bottom panels) were fixed and stained for endogenous FAK (to visualize focal adhesions). Overlay of the GFP (green) and FAK (red) signals is shown in the right-hand panel. All images were obtained by confocal microscopy. Insets in the overlay images are magnifications of the boxed areas. Images are representative of images obtained from two to six replicates of each experiment. Scale bars: 10 μm.
The SH2 domain of SH2B1β is necessary and sufficient for focal adhesion localization
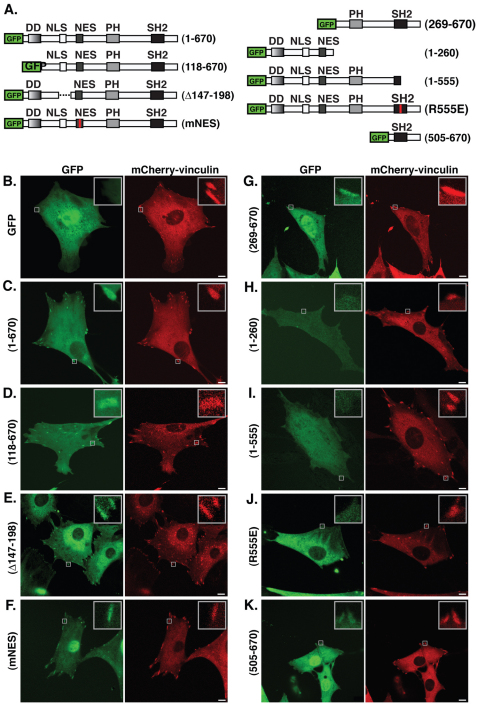
To determine the region of SH2B1β responsible for focal adhesion localization, a series of GFP-tagged SH2B1β truncation, deletion and point mutants, which delete or disrupt SH2B1β signaling domains (Fig. 2A), were coexpressed with mCherry–vinculin in 3T3-F442A fibroblasts and visualized in live cells using confocal microscopy (Fig. 2B–K). Because the dimerization domain and the polybasic NLS have been implicated in localization of SH2B1β to the plasma membrane (Maures et al., 2011), we first examined the effect of deleting these domains on the subcellular localization of SH2B1β. Deletion of the dimerization domain [SH2B1β (118–670), Fig. 2D] or the NLS (SH2B1β Δ148–198, Fig. 2E) did not prevent GFP–SH2B1β from localizing to focal adhesions. Similarly, mutation of the NES (SH2B1β mNES, Fig. 2F) did not prevent GFP–SH2B1β from localizing to focal adhesions. GFP–SH2B1β (269–670), which lacks all three of these domains but contains the SH2 and PH domains, also retained its ability to localize to focal adhesions (Fig. 2G). By contrast, GFP–SH2B1β (1–260), which lacks both the PH and SH2 domains, failed to localize to focal adhesions (Fig. 2H). Both GFP–SH2B1β (1–555), which contains the PH domain but lacks an intact SH2 domain (Fig. 2I) and GFP–SH2B1β (R555E), which lacks a functional SH2 domain, owing to a single point mutation (Rui and Carter-Su, 1998) (Fig. 2J), failed to localize to focal adhesions. However, GFP–SH2B1β (505–670), which consists primarily of the SH2 domain, localized to focal adhesions (Fig. 2K). Taken together, these data indicate that the SH2 domain of SH2B1β is both necessary and sufficient for localization of SH2B1β to focal adhesions.
Fig. 2.
The SH2 domain of SH2B1β is necessary and sufficient for focal adhesion localization. (A) Schematic representations of SH2B1β truncation, deletion and point mutations. Red lines indicate point mutations that render the respective domains nonfunctional (Chen and Carter-Su, 2004; Rui and Carter-Su, 1998). The broken line indicates a deleted region. (B–K) Live 3T3-F442A cells coexpressing mCherry–vinculin with GFP, GFP–SH2B1β or the indicated mutant GFP–SH2B1β were imaged by confocal microscopy. Insets in the images are magnifications of the boxed areas. Images are representative of images obtained in two to three replicates of each experiment. Scale bars: 10 μm.
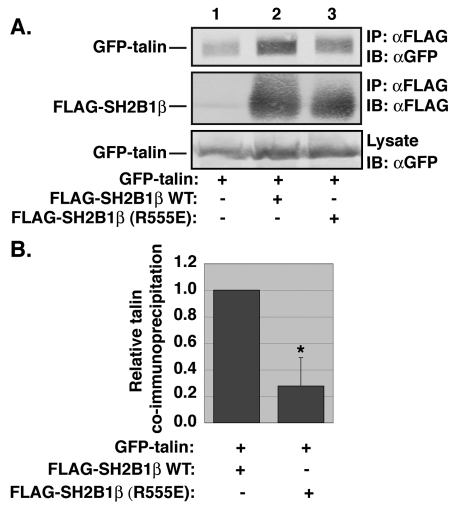
SH2 domains bind preferentially to phosphorylated tyrosine residues. We reasoned that focal adhesion-localized SH2B1β would bind to one or more focal adhesion proteins that were phosphorylated on tyrosine residues. We therefore tested whether SH2B1β would co-immunoprecipitate with several well-characterized focal adhesion proteins known to be phosphorylated on tyrosine residues [e.g. FAK (Panetti, 2002), vinculin (Subauste et al., 2004; Zhang et al., 2004), paxillin (Panetti, 2002) and talin (Pasquale et al., 1986)]. SH2B1β failed to co-immunoprecipitate with FAK, vinculin or paxillin (data not shown). However, GFP-tagged talin co-immunoprecipitated with wild-type (WT) SH2B1β. Furthermore, co-immunoprecipitation was significantly (P<0.05) decreased when the SH2 domain was mutated (Fig. 3). This finding provides further evidence that SH2B1β localizes to focal adhesions and that the SH2 domain of SH2B1β is important for that localization.
Fig. 3.
SH2B1β co-immunoprecipitates with talin. (A) HEK-293T cells expressing GFP–talin and FLAG, FLAG–SH2B1β or FLAG–SH2B1β (R555E), as indicated, were immunoprecipitated (IP) with anti-FLAG-agarose (αFLAG) and immunoblotted (IB) with anti-FLAG or anti-GFP antibody (αGFP). Cell lysates were blotted with anti-GFP antibody to show relative levels of GFP–talin expression. (B) The GFP–talin and FLAG–SH2B1β (top two panels of A) were quantified and the non-specific binding to the anti-FLAG-agarose of GFP–talin (lane 1, top panel of A) and FLAG–SH2B1β (lane 1, middle panel of A) were subtracted from the GFP–talin and FLAG–SH2B1β signals in lanes 2 and 3, respectively. The ratio of GFP–talin to FLAG–SH2B1β was then determined and normalized to the ratio calculated for FLAG–SH2B1β WT. Error bars indicate s.e.m. (n=3). *P<0.05 by two-tailed Student's t-test compared with SH2B1β WT.
PKC activation by PMA induces SH2B1β phosphorylation
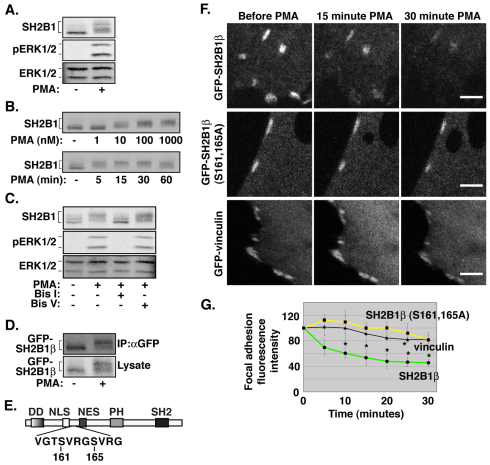
Phosphorylation has been shown to regulate the localization of proteins to focal adhesions (Vadlamudi et al., 1999; Webb et al., 2004). Several PKC isoforms have been shown to localize to focal adhesions (Barry and Critchley, 1994; Haller et al., 1998; Jaken et al., 1989) and we have previously provided substantial evidence that SH2B1 is phosphorylated on serine and threonine residues in response to the PKC agonist, phorbol 12-myristate 13-acetate (PMA), in the PC12 neuronal cell line (Rui et al., 1999a). We therefore examined whether phosphorylation of SH2B1β by PKC might regulate the localization of SH2B1β at focal adhesions. We first confirmed that PMA was active in 3T3-F442A cells by showing that PMA activated ERKs 1 and 2 (Fig. 4A, middle panel). PKC is known to be a potent activator of the Raf-MEK-ERK pathway (Hoshino et al., 1998; Marais et al., 1998; Schonwasser et al., 1998). PMA stimulation also caused a substantial upward shift in the mobility of endogenous SH2B1 in western blots (Fig. 4A, top panel). We have previously shown that this upward shift in mobility is indicative of increased serine and/or threonine residue phosphorylation of SH2B1 (Rui et al., 1999b; Rui et al., 1997). Dose–response experiments revealed that PMA stimulation for 15 minutes resulted in substantial phosphorylation of SH2B1 (assessed by the degree of decreased mobility) with 10 nM PMA with maximal stimulation detected with 1 μM PMA. At a fixed dose of 100 nM PMA, maximal stimulation occurred after 15–30 minutes (Fig. 4B). Pre-treating cells before PMA stimulation with the PKC inhibitor, bisindolylmaleimide I (bis I), but not its inactive analogue, bisindolylmaleimide V (bis V), inhibited the upward mobility shift of SH2B1 (Fig. 4C), consistent with PKC or a kinase downstream of PKC mediating the PMA-induced SH2B1 phosphorylation. Treatment of 3T3-F442A fibroblasts expressing GFP-tagged SH2B1β with PMA also resulted in an upward shift in migration of GFP–SH2B1β (Fig. 4D), consistent with ectopically expressed GFP–SH2B1β being phosphorylated in response to PKC activation.
Fig. 4.
PMA stimulation induces a loss of GFP–SH2B1β, but not GFP–SH2B1β (S161A, S165A), from focal adhesions. (A) 3T3-F442A cells were treated with vehicle or 100 nM PMA for 15 minutes. Lysates were blotted for total SH2B1, phosphorylated ERK1/2 (pERK1/2) or total ERK1/2 as indicated (n=4). (B) 3T3-F442A cells were treated with increasing concentrations of PMA for 15 minutes (top panel) or with 100 nM PMA for increasing time periods (bottom panel). Lysates were blotted with anti-SH2B1 antibody (n=3). (C) 3T3-F442A cells were pretreated with DMSO, bis I or bis V for 1 hour, then treated with vehicle or 100 nM PMA for 15 minutes. Lysates were blotted for total SH2B1, phosphorylated ERK1/2 (pERK1/2) or total ERK1/2, as indicated (n=4). (D) 3T3-F442A cells expressing GFP–SH2B1β were treated with vehicle or 100 nM PMA for 15 minutes. Proteins from lysates were immunoprecipitated (IP) with anti-GFP antibody (αGFP). Both immunoprecipitated proteins and cell lysates were blotted for GFP (n=4). (E) Schematic representation of Ser161 and Ser165 in SH2B1β. (F) Live 3T3-F442A cells expressing GFP-tagged SH2B1β (top panels), SH2B1β (S161A, S165A) (middle panels) or vinculin (bottom panels) were imaged by confocal microscopy before and after 100 nM PMA stimulation for up to 30 minutes. (G) Metamorph (Sunnyvale, CA) imaging software was used to quantify the fluorescence intensity of individual focal adhesions treated as in F. Data were normalized to the t=0 time point. Four independent experiments assessing focal adhesions from 2–4 cells per experiment were performed for GFP-tagged WT and mutant SH2B1β and three independent experiments assessing focal adhesions from 2–4 cells per experiment were performed for GFP–vinculin. Error bars indicate s.e.m. *P<0.05 by two-tailed Student's t-test compared with t=0. Images were taken at the same level on the z-axis and at the same intensity. Scale bars: 10 μm.
Phosphorylation of Ser161 and Ser165 decreases the localization of SH2B1β to focal adhesions
To determine whether phosphorylation of SH2B1β in response to PMA influences SH2B1β localization at focal adhesions, GFP–SH2B1β was expressed in 3T3-F442A fibroblasts and live cells were imaged by confocal microscopy before and after PMA treatment. Images were taken every 5 minutes for 30 minutes (Fig. 4F) and the GFP fluorescence intensity of individual focal adhesions was quantified at each time-point (Fig. 4G). PMA treatment resulted in a significant reduction (>50%; P<0.05) in the amount of GFP–SH2B1β at focal adhesions over 30 minutes (Fig. 4F, top panel; Fig. 4G). PMA treatment reduced GFP–vinculin localization at focal adhesions to a statistically significant extent at only the 25-minute time-point (Fig. 4F, bottom panel; Fig. 4G), indicating that PMA does not induce general focal adhesion disassembly in this context.
We have shown previously by mass spectrometry that Ser161 in SH2B1β is phosphorylated in PMA-treated HEK-293T cells and have substantial indirect evidence that Ser165 is also phosphorylated (Maures et al., 2011). Both serine residues lie within the consensus PKC-substrate motif x(S/T)x(R/K) (Center for biological sequence analysis, http://www.cbs.dtu.dk/index.shtml) Fig. 4E). When Ser161 and Ser165 were mutated into alanine residues, GFP–SH2B1β did not exhibit a statistically significant PMA-induced depletion from focal adhesions (Fig. 4F, middle panel; Fig. 4G). Taken together, the data in Fig. 4 suggest that PMA-dependent phosphorylation of SH2B1β at Ser161 and/or Ser165 leads to SH2B1β dissociation from focal adhesions.
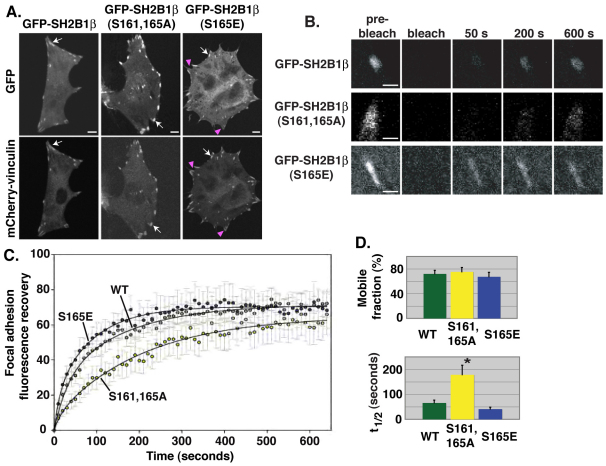
Ser161 and/or Ser165 regulates the focal adhesion dynamics of SH2B1β
The PMA-induced decrease in focal adhesion localization of GFP–SH2B1β WT but not of GFP–SH2B1β (S161A, S165A) suggests that phosphorylation of Ser161 and/or Ser165 modulates the dynamic cycling of SH2B1β into and out of focal adhesions. To investigate this further, we first assessed the localization to focal adhesions of GFP-tagged SH2B1β WT, SH2B1β (S161A, S165A) and the phosphomimetic SH2B1β (S165E) in which Ser165 is mutated into a glutamate residue to mimic phosphorylation. Expression levels of GFP–SH2B1β WT, GFP–SH2B1β (S161A, S165A) and GFP–SH2B1β (S165E) were similar throughout all experiments (supplementary material Fig. S2A). GFP-tagged SH2B1β WT, SH2B1β (S161A, S165A) and SH2B1β (S165E) all localized to focal adhesions, but to various extents (Fig. 5A). Compared with GFP–SH2B1β WT, which localizes to focal adhesions and the cytoplasm, GFP–SH2B1β (S161A, S165A) appeared to localize more exclusively to focal adhesions, whereas the phosphomimetic GFP–SH2B1β (S165E) localized to focal adhesions to a lesser extent and more to the cytoplasm. Whereas all vinculin-positive focal adhesions in cells expressing GFP–SH2B1β WT or GFP–SH2B1β (S161A, S165A) were also positive for SH2B1β (Fig. 5A, arrows), in GFP–SH2B1β (S165E) cells, some vinculin-positive focal adhesions did not contain detectable levels of SH2B1β (S165E) (Fig. 5A, magenta arrowheads). Quantification of GFP fluorescence intensity revealed a decrease in the amount of GFP–SH2B1β (S165E) in focal adhesions compared with that of GFP–SH2B1β WT and GFP–SH2B1β (S161A, S165A) (supplementary material Fig. S2B). These results with PMA and the Ser161 and/or Ser165 mutants are consistent with phosphorylation of Ser161 and/or Ser165 (e.g. in response to PMA) decreasing SH2B1β localization at focal adhesions.
Fig. 5.
Ser161 and/or Ser165 regulate the focal adhesion dynamics of SH2B1β. (A) Live 3T3-F442A cells expressing mCherry–vinculin and GFP-tagged SH2B1β, SH2B1β (S161A, S165A) or SH2B1β (S165E) were imaged by confocal microscopy. Arrows indicate focal adhesions that are positive for both GFP–SH2B1β and mCherry–vinculin. Magenta arrowheads indicate focal adhesions that are positive only for mCherry–vinculin. Images were taken at the same level on the z-axis and at the same intensity. (B) Confocal microscopy images of GFP-tagged SH2B1β WT, SH2B1β (S161A, S165A) or SH2B1β (S165E) in focal adhesions of live 3T3-F442A cells before and after photobleaching. (C) FRAP values were obtained using Olympus Fluoview software. Data were normalized and best-fit curves were determined as described in the Materials and Methods. (D) The mobile fraction (left-hand panel) and t1/2 (right-hand panel) values were calculated as described in the Materials and Methods. n=9 [for SH2B1β WT and SH2B1β (S161A, S165A)] or 10 [SH2B1β (S165E)]. Error bars indicate s.e.m. *P<0.05 by two-tailed Student's t-test compared with SH2B1β WT. Scale bars: 10 μm (A); 2 μm (B).
To test whether phosphorylation of S161A, S165A increases the dynamic cycling of SH2B1β into as well as out of focal adhesions, we expressed GFP-tagged SH2B1β WT, SH2B1β (S161A, S165A) and SH2B1β (S165E) in 3T3-F442A cells and performed fluorescence recovery after photobleaching (FRAP) experiments on individual focal adhesions. Compared with recovery of GFP–SH2B1β WT, recovery of GFP–SH2B1β (S161A, S165A) was substantially delayed, whereas recovery of GFP–SH2B1β (S165E) appeared slightly accelerated (Fig. 5B,C). The fraction of focal adhesion-localized GFP–SH2B1β that was mobile (maximal fluorescent intensity recovered in the bleached focal adhesion as a fraction of initial intensity, designated the ‘mobile fraction’, Fig. 5D, left-hand panel) and the time needed for GFP–SH2B1β to recover 50% of maximal recovered fluorescence intensity in the bleached focal adhesions (t1/2, Fig. 5D, right-hand panel) were calculated for multiple focal adhesions for all three forms of GFP–SH2B1β. Although the mobile fraction of both mutants was similar to that of SH2B1β WT, the t1/2 of GFP–SH2B1β (S161A, S165A) was significantly increased compared with GFP–SH2B1β WT, whereas the t1/2 of GFP–SH2B1β (S165E) was lower (although statistical significance was not achieved, P=0.09) (Fig. 5D). These data indicate that although the total fraction of focal adhesion-localized SH2B1β able to dynamically cycle in and out of focal adhesions is not affected by mutating Ser161 and/or Ser165, the rate of cycling both into and out of focal adhesions was significantly decreased when Ser161 and Ser165 could not be phosphorylated.
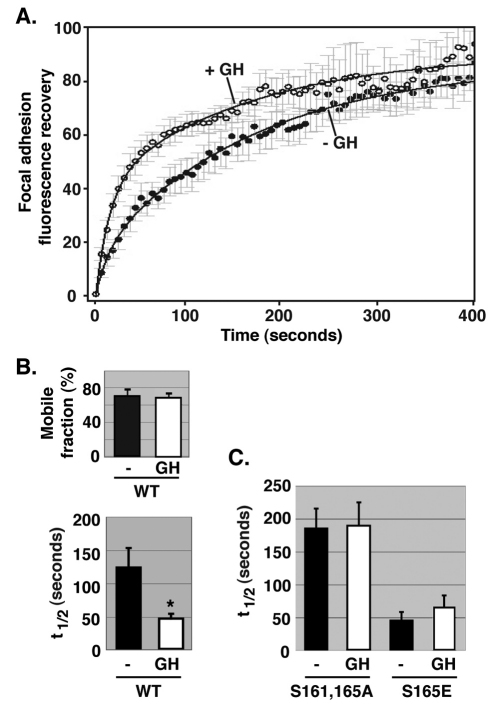
GH regulates the focal adhesion dynamics of SH2B1β
Multiple cytokines and growth factors utilize SH2B1 for signaling (reviewed by Maures et al., 2007), raising the possibility that cytokines and growth factors regulate the focal adhesion dynamics of SH2B1β. To determine whether factors present in serum influence the focal adhesion dynamics of SH2B1β, FRAP analysis was performed for GFP-tagged SH2B1β WT and SH2B1β (S161A, S165A) in cells deprived of serum overnight and in cells grown in serum. Supplementary material Fig. S3 demonstrates that serum deprivation reduced the rate of recovery of GFP–SH2B1β WT to that of GFP–SH2B1β (S161A, S165A) in the presence of serum. Serum-deprivation did not alter recovery of GFP–SH2B1β (S161A, S165A). These data indicate that serum factors regulate the dynamic cycling of SH2B1β into and out of focal adhesions, and suggest that the primary mechanism of regulation is through phosphorylation of Ser161 and/or Ser165.
GH is one factor present in serum that can activate PKC (reviewed by Argetsinger and Carter-Su, 1996; Tripathi and Sodhi, 2009) and that utilizes SH2B1β for signaling (Maures et al., 2007). GH stimulation significantly increased the rate of recovery of GFP–SH2B1β (decreased the t1/2) at focal adhesions (Fig. 6), thus identifying at least one specific factor (i.e. GH) that increases the dynamic cycling of SH2B1β into and out of focal adhesions. No GH-dependent change in the mobile fraction was detected. Consistent with our results suggesting that phosphorylation of Ser161 and/or Ser165 increases the dynamic cycling of SH2B1β at focal adhesions, GH lost its ability to decrease the t1/2 of SH2B1 fluorescence recovery at focal adhesions when Ser161 and Ser165 were mutated into alanine residues, whereas there was no further decrease in the t1/2 when Ser165 was mutated into a glutamate residue (Fig. 6C) raising the possibility that phosphorylation of Ser161 and/or Ser165 mediates the stimulatory effect of GH on SH2B1β cycling into and out of focal adhesions. Like the mobile fraction of GFP–SH2B1β WT (Fig. 6B), the mobile fraction of GFP–SH2B1β (S161A, S165A) or GFP–SH2B1β (S165E) was not influenced by GH stimulation (data not shown).
Fig. 6.
Growth hormone regulates the focal adhesion dynamics of SH2B1β. (A) 3T3-F442A cells expressing GFP–SH2B1β were incubated in serum-free medium overnight and then individual focal adhesions were photobleached. During the photobleaching scans, cells were treated with (n=5) or without (n=8) 500 ng/ml GH. FRAP analysis was carried out for 400 seconds. FRAP values were obtained using Olympus Fluoview software. Data were normalized and best-fit curves were determined as described in the Materials and Methods. (B) Mobile fraction and t1/2 values were calculated as described in the Materials and Methods. Error bars indicate s.e.m. *P<0.05 by two-tailed Student's t-test compared with the data from cells treated without GH. (C) 3T3-F442A cells expressing GFP–SH2B1β (S161A, S165A) or GFP–SH2B1β (S165E) were treated and the results were analyzed as in A. t1/2 values were calculated as in B. Error bars indicate s.e.m. (n=3). The t1/2 for GFP–SH2B1β (S165E) (+ GH or − GH) is less than that of GFP–SH2B1β (S161A, S165A) (+ GH or − GH) (P<0.05 by two-tailed Student's t-test).
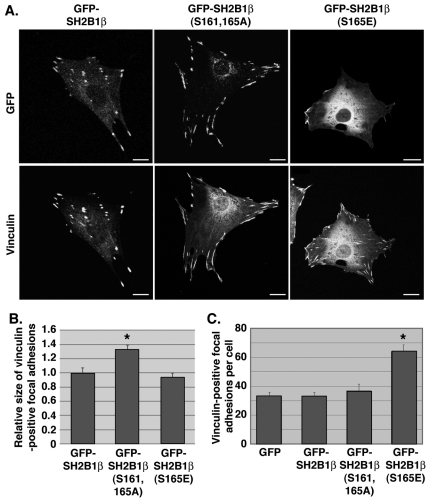
Ser161 and/or Ser165 in SH2B1β regulate focal adhesion size and number
Focal adhesions in cells expressing GFP–SH2B1β (S161A, S165A) appeared larger than in cells expressing GFP–SH2B1β WT or GFP–SH2B1β (S165E) (Fig. 5A,B; Fig. 7A). Quantification of the size of focal adhesions from multiple cells revealed a >30% increase in focal adhesion size in cells expressing GFP–SH2B1β (S161A, S165A) (Fig. 7B). This raises the possibility that the presence and/or dynamics of SH2B1β within focal adhesions modulates focal adhesion size, as has been shown for some other focal adhesion proteins (Ziegler et al., 2006). PKC activation is associated with increased focal adhesion formation (Besson et al., 2001). Intriguingly, expression of GFP–SH2B1β (S165E) almost doubled the number of focal adhesions per cell (assessed by staining with antibody to vinculin) compared with cells expressing GFP, GFP–SH2B1β WT or GFP–SH2B1β (S161A, S165A) (Fig. 7A,C). These results raise the exciting possibility that in addition to regulating SH2B1β cycling into and out of focal adhesions, phosphorylation of SH2B1β at Ser165 is a key event in PKC-mediated regulation of focal adhesion formation.
Fig. 7.
Ser161 and Ser165 in SH2B1β regulate focal adhesion size and number. (A) 3T3-F442A cells expressing GFP-tagged WT or mutant SH2B1β were fixed and stained for endogenous vinculin. GFP and vinculin were imaged by confocal microscopy. Scale bars: 10 μm. (B) The size of vinculin-positive focal adhesions in live 3T3-F442A cells expressing mCherry–vinculin and GFP-tagged WT or mutant SH2B1β was quantified using Nikon NIS-Elements Advanced Research imaging software. Three independent experiments assessing at least 130 vinculin-positive focal adhesions were performed for each condition. Error bars indicate s.e.m. *P<0.05 by two-tailed Student's t-test compared with GFP–SH2B1β WT. (C) Quantification of number of focal adhesions per cell. Three independent experiments assessing vinculin-positive focal adhesions from at least ten cells were performed for each condition. Error bars indicate s.e.m. *P<0.05 by two-tailed Student's t-test compared with GFP–SH2B1β.
Discussion
Here, we have identified SH2B1β as a new focal adhesion protein that is targeted to focal adhesions through its SH2 domain. Our finding that its SH2 domain is necessary and sufficient for SH2B1β localization at focal adhesions, and that it is also necessary for co-immunoprecipitation with the focal adhesion protein talin, provides strong evidence that SH2B1β binds a protein within the focal adhesion complex that is phosphorylated on tyrosine residues. In addition to talin (Pasquale et al., 1986), focal adhesions contain numerous tyrosine-phosphorylated proteins, including paxillin (Panetti, 2002), vinculin (Subauste et al., 2004; Zhang et al., 2004), p130Cas (also known as BCAR1) (Vuori and Ruoslahti, 1995), tensin (Bockholt and Burridge, 1993) and FAK (Panetti, 2002). SH2B1β did not co-immunoprecipitate with FAK, vinculin or paxillin (data not shown), raising the possibility that the interaction between SH2B1β and talin is direct. Consistent with a direct interaction, talin contains two phosphorylated tyrosine residues (Tyr70-P, Tyr2530-P) that lie within an YxxL motif (PhosphoSitePlus, http://www.phosphosite.org), a motif that SH2B1β is known to bind (Kurzer et al., 2004). The interaction of talin with the cytoplasmic tails of integrin increases integrin ligand affinity, thereby strengthening integrin-based cell–ECM interactions (reviewed by Moser et al., 2009). Thus, it will be interesting to see whether future studies reveal that SH2B1β modulates talin–integrin interactions or integrin-based cell–ECM adherence.
Our finding that SH2B1β localizes to focal adhesions through its SH2 domain is consistent with previous work showing that the SH2 domains of some other focal adhesion proteins are important for localization to focal adhesions (Machida et al., 2007; Salgia et al., 1995). It suggests that mechanisms regulating phosphorylation at tyrosine residues of the specific proteins in focal adhesions that bind SH2B1β will be found to affect the recruitment of SH2B1β to focal adhesions.
Intriguingly, our studies provide substantial evidence that SH2B1β localization at focal adhesions is also regulated by phosphorylation of Ser161 and/or Ser165. Ser161 and Ser165 fall within consensus PKC substrate sequences and are phosphorylated in PMA-treated cells (Maures et al., 2011). We show that PMA promotes dissociation of SH2B1β from focal adhesions, a dissociation that is prevented by mutating Ser161 and Ser165 into alanine residues. This same mutation decreases the rate at which GFP–SH2B1β is able to enter focal adhesions in FRAP experiments. Precedence exists for phosphorylation of serine residues regulating the localization of focal adhesion proteins; phosphorylation of serine residues in paxillin has been shown to cause dissociation of paxillin from focal adhesions (Vadlamudi et al., 1999) and to increase dynamic turnover of paxillin at focal adhesions (Webb et al., 2004). PKC is an important factor in integrin-mediated signaling (reviewed in Larsson, 2006) and has been shown to directly phosphorylate focal adhesion proteins [e.g. vinculin (Werth et al., 1983), talin (Meenakshi et al., 1993), filamin (Tigges et al., 2003) and integrins (Rabinovitz et al., 2004)]. PKC isoforms have also been shown to induce activation of focal adhesion-localized ERK1/2 (Besson et al., 2001; Howe and Juliano, 1998; Rigot et al., 1998), RhoA (Dovas et al., 2006) and FAK (Mogi et al., 1995), events that lead to an increase in focal adhesion number, cell adhesion and/or cell migration. Similar to what we report here for PKC-mediated SH2B1β release from focal adhesions, our recent work suggests that activation of PKC with subsequent phosphorylation of SH2B1β at Ser161 and Ser165 also releases SH2B1β from the plasma membrane (Maures et al., 2011). However, the basis for the release of SH2B1β appears to differ between plasma membranes and focal adhesions. Positively charged amino acids within the NLS of SH2B1β are thought to recruit SH2B1β to the negatively charged plasma membrane. Phosphorylation of Ser161 and/or Ser165, which are proximate to the positively charged NLS (residues 146-152), appears to neutralize the ionic interaction between the positively charged NLS and the negatively charged plasma membrane, leading to dissociation of SH2B1β from the plasma membrane (Maures et al., 2011). By contrast, we show in the current study that the polybasic NLS is dispensable for association of SH2B1β with focal adhesions. Thus, phosphorylation of Ser161 and/or Ser165 causes SH2B1β to dissociate from focal adhesions by some other mechanism than a neutralization of an electrostatic interaction involving the NLS of SH2B1β (e.g. a phosphorylation-induced conformational change leading to decreased affinity of SH2B1β for a binding partner).
Our findings that focal adhesion size is increased by expressing SH2B1β in which Ser161 and Ser165 are mutated into alanine residues and that focal adhesion number is increased by expressing SH2B1β in which Ser165 is mutated into a phosphomimetic glutamate residue suggest that phosphorylation of SH2B1β at Ser161 and/or Ser165, and/or the resulting increased mobility of SH2B1β, is a key step in the turnover and formation of new focal adhesions. A link between SH2B1β mobility and focal adhesion number was also observed when cells were deprived of serum. Serum-deprivation inhibited the mobility of SH2B1β WT (supplementary material Fig. S3) and decreased the number of vinculin-positive focal adhesions (data not shown) in cells expressing SH2B1β WT, but it did not alter the mobility of SH2B1β (S161A, S165A) or the number of vinculin-positive focal adhesions in cells expressing SH2B1β (S161A, S165A). Because SH2B1β is thought to function as an adapter protein, one mechanism by which SH2B1β might regulate focal adhesion number is by modulating the dynamic turnover of other focal adhesion proteins. There is evidence indicating that turnover of individual focal adhesion proteins might be directly related to the turnover of focal adhesions themselves (von Wichert et al., 2003; Webb et al., 2004), a process that is linked to the formation of new focal adhesions. After localizing to focal adhesions through its SH2 domain, SH2B1β might bind and stabilize complexes of other focal adhesion proteins, thus providing additional integrity to the focal adhesion complex. Taken together, these observations suggest that PKC-mediated phosphorylation of SH2B1β and/or subsequent redistribution of SH2B1β (accompanied by some or all of its binding partners) out of focal adhesions could initiate partial destabilization of focal adhesions, leading to smaller, and an increased number of, focal adhesions. This, in turn, would then result in the increased cell motility seen with either activation of PKC (Besson, et al., 2001; Besson et al., 2002) or overexpression of SH2B1β (Diakonova et al., 2002; Herrington et al., 2000; Rider et al., 2009).
Our findings that GH stimulation significantly increases the cycling of SH2B1β into and out of focal adhesions and that increased focal adhesion cycling of SH2B1β is associated with decreased focal adhesion size and increased focal adhesion number suggests a mechanism by which SH2B1β could enhance GH-induced cell motility. In support of phosphorylation of SH2B1β playing a role in the GH-induced increased motility of SH2B1β, GH responsiveness was lost when SH2B1β mobility was inhibited by mutating Ser161 and Ser165 into alanine residues. Additionally, no further GH-induced increase in mobility was detected when Ser161 and/or Ser165 were mutated into a phosphomimetic glutamate residue. It will therefore be of interest to determine whether GH enhances the phosphorylation of Ser161 and/or Ser165 in SH2B1β. Because SH2B1 is also recruited to a variety of other receptors whose ligands stimulate cell motility (e.g. PDGF, FGF and IGF1), it seems probable that cycling of SH2B1 into and out of focal adhesions would also contribute to their ability to regulate cell motility. Thus, it will be of great interest in the future to determine whether mutations to SH2B1β that suppress focal adhesion turnover dynamics also suppress cell motility in response to GH and other growth factors.
The regulation of cell signaling, cytoskeletal dynamics and cell motility through focal adhesions is crucial for numerous physiological and pathophysiological processes. A growing number of studies implicate SH2B family members in an array of physiological processes that are dependent on cytoskeletal dynamics and/or cell motility. Our finding of SH2B1β as a new focal adhesion protein whose phosphorylation dynamically regulates focal adhesion number provides the grounds for further study into the precise function and regulation of SH2B1β in focal adhesions.
Materials and Methods
Antibodies
Anti-vinculin mouse monoclonal antibody was from Sigma-Aldrich (cat. no. V9131) and anti-FAK mouse monoclonal antibody was from Transduction Laboratories (cat. no. F15020). Both were used at a dilution of 1:100 for immunofluorescence. Polyclonal antibody to rat SH2B1 [a gift from Liangyou Rui (University of Michigan, Ann Arbor, MI)] was raised against an SH2B1β–GST fusion protein and used at a dilution of 1:1000 (Duan et al., 2004). Anti-phosphorylated-MAPK 44/42 antibody that recognizes both ERK1 and ERK2 that are doubly phosphorylated on Thr202 and Tyr204 and anti-total ERK were from Cell Signaling Technology and were used at a dilution of 1:1000. IRDye-800- and IRDye-700-conjugated affinity-purified anti-mouse-IgG and anti-rabbit-IgG (1:20,000 dilution) and anti-GFP IRDye-800-congujated goat polyclonal antibody (1:5000 dilution) were from Rockland Immunochemicals. Anti-GFP mouse monoclonal antibody for immunoprecipitation (1:100 dilution) was from Clontech. Alexa-Fluor-568-conjugated phalloidin (dilution 1:100) and anti-mouse-IgG Alexa-Fluor-405 and -594-conjugated secondary antibodies (dilution 1:1000) for confocal immunofluorescence were from Invitrogen.
Reagents
Recombinant 22 kDa human GH was a gift from Eli Lilly & Co. PMA (Sigma) was diluted in DMSO. Dulbecco's modified Eagle's medium (DMEM) was from Cambrex. Fetal bovine serum (FBS) was from Hyclone. Calf serum was from Atlanta Biologicals. The antibiotic–antimycotic solution, trypsin-EDTA and Magic Mark XP western standards were from Invitrogen. Aprotinin, leupeptin and Triton X-100 were from Roche. Recombinant protein A–agarose was from Repligen. Hybond-C Extra nitrocellulose was from Amersham Biosciences. Bisindolylmaleimide I and bisindolylmaleimide V were from Calbiochem. Human fibronectin was from BD Biosciences. Paraformaldehyde was from Electron Microscopy Sciences.
Cell culture, transfection and stimulation
The stock of murine 3T3-F442A fibroblasts was provided by Howard Green (Harvard University, Cambridge, MA). 3T3-F442A cells were grown in DMEM supplemented with 1 mM L-glutamine, 100 units of penicillin per ml, 100 μg of streptomycin per ml, 0.25 μg of amphotericin per ml and 8% FBS and transiently transfected using Amaxa nucleofector technology from Lonza (Cologne, Germany) using solution V and setting U24. HeLa cells, from ATCC, were grown in DMEM supplemented with 100 units of penicillin per ml, 100 μg of streptomycin per ml, 0.25 μg of amphotericin per ml and 10% FBS. They were transiently transfected using FuGene HD from Roche. All cells were incubated overnight in serum-free medium containing 1% BSA before treatment with PMA or GH.
Plasmids, cloning and mutagenesis
All cDNAs encoding GFP-tagged SH2B1β and SH2B1β point, deletion and truncation mutants were as described previously (Chen and Carter-Su, 2004; Diakonova et al., 2002; Herrington et al., 2000; Maures et al., 2009; Maures et al., 2011; Rui et al., 1999a). GFP–vinculin was a kind gift from Kenneth Yamada (National Institutes of Health, Bethesda, MD). mCherry–vinculin was constructed by excising mCherry from pmCherry-C1 (Clontech) and GFP from GFP–vinculin using the AgeI and BspEI restriction sites for both restriction digestions. mCherry was then ligated in front of vinculin. GFP–talin (Franco et al., 2004) was a kind gift from Anna Huttenlocher (University of Wisconsin-Madison, Madison, WI).
Immunofluorescence
For fixed-cell imaging, cells were fixed in 4% paraformaldehyde in PBS, gently washed 3× in PBS, permeabilized in 0.1% Triton X-100 in PBS and blocked for 30 minutes in PBS containing 5% normal serum from the species used for secondary antibody production. Cells were then incubated for 1 hour with primary antibody diluted 1:100 in blocking solution. Cells were gently washed 3× in PBS, and then incubated for 1 hour with secondary antibody diluted 1:1000 in PBS or Alexa-Fluor-conjugated phalloidin diluted 1:100 in PBS. Cells were gently washed 3× in double-distilled water, and then mounted on Fisherfinest Premium Microscope Slides using Prolong Gold Antifade mounting reagent (Invitrogen). For live-cell imaging, cells were grown on glass-bottomed dishes (MatTek) and imaged in Ringer's buffer (155 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2.6H2O, 2 mM NaH2PO4.H2O, 10 mM HEPES pH 7.2 and 10 mM glucose). All images were obtained with an Olympus FluoView 500 laser-scanning confocal microscope. For focal adhesion counting, cells were fixed and stained for vinculin using anti-vinculin antibody; 15–20 random images were obtained in each of three independent experiments for each condition, and vinculin-positive focal adhesions in each cell were counted.
Fluorescence recovery after photobleaching
GFP–SH2B1β, GFP–SH2B1β (S161A, S165A) or GFP–SH2B1β (S165E) were transiently expressed in 3T3-F442A fibroblasts. The GFP signal in individual focal adhesions was bleached by three iterations of 100% laser power. Fluorescent intensity measurements were taken every 6 or 10 seconds from 30 individual focal adhesions in ten individual cells for each condition. Data were normalized to unbleached sections of cytosol after background subtraction. SigmaPlot 11.0 was used to fit curves to FRAP data by applying the nonlinear regression of exponential rise to maximum and the double fours best fit equation, y=a(1 − e−bx)+c(1 − e−dx). The fraction of mobile SH2B1β (determined as the percentage of fluorescence recovery) and t1/2 (time needed to recover to 50% of the mobile fraction) were calculated from the equations.
Supplementary Material
Acknowledgments
We thank Kenneth Yamada for vinculin cDNA, Anna Huttenlocher for talin cDNA and Liangyou Rui for the anti-SH2B1 antibody; James Herrington for the initial observation of SH2B1β in focal adhesions; Travis Maures and Joel Cline for help with constructs; Stephen Lentz for assistance with confocal microscopy; Barbara Hawkins for help with the manuscript; and Jessica Schwartz, Ram Menon, Jun-Lin Guan, Edward Stuenkel and Arthur Alberts for helpful discussions. Confocal microscopy was performed in the Morphology and Imaging Core of the Michigan Diabetes Research and Training Center (P60-DK20572). cDNAs were sequenced by the University of Michigan DNA Sequencing Core with partial support from the University of Michigan Multipurpose Arthritis Center (P60-AR20557) and the University of Michigan Comprehensive Cancer Center (NIH P30 CA46592). This work was supported by NIH grant RO1-DK54222 (C.C.S.), a University of Michigan Rackham Regents Fellowship (N.J.L.) and a predoctoral fellowship from the NIH-funded Organogenesis Training Grant (T32-HD007505) (N.J.L.). Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.081547/-/DC1
References
- Argetsinger L. S., Carter-Su C. 1996. Mechanism of signaling by growth hormone receptor. Physiol. Rev. 76, 1089-1107 [DOI] [PubMed] [Google Scholar]
- Barry S. T., Critchley D. R. 1994. The RhoA-dependent assembly of focal adhesions in Swiss 3T3 cells is associated with increased tyrosine phosphorylation and the recruitment of both pp125FAK and protein kinase C-delta to focal adhesions. J. Cell Sci. 107, 2033-2045 [DOI] [PubMed] [Google Scholar]
- Bersenev A., Wu C., Balcerek J., Tong W. 2008. Lnk controls mouse hematopoietic stem cell self-renewal and quiescence through direct interactions with JAK2. J. Clin. Invest. 118, 2832-2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson A., Davy A., Robbins S. M., Yong V. W. 2001. Differential activation of ERKs to focal adhesions by PKC ε is required for PMA-induced adhesion and migration of human glioma cells. Oncogene 20, 7398-7407 [DOI] [PubMed] [Google Scholar]
- Besson A., Wilson T. L., Yong V. W. 2002. The anchoring protein RACK1 links protein kinase Cepsilon to integrin beta chains. Requirements for adhesion and motility. J. Biol. Chem. 227, 22073-22084 [DOI] [PubMed] [Google Scholar]
- Bockholt S. M., Burridge K. 1993. Cell spreading on extracellular matrix proteins induces tyrosine phosphorylation of tensin. J. Biol. Chem. 268, 14565-14567 [PubMed] [Google Scholar]
- Chen L., Carter-Su C. 2004. Adapter protein SH2-Bβ undergoes nucleocyto-plasmic shuttling: implications for nerve growth factor induction of neuronal differentiation. Mol. Cell. Biol. 24, 3633-3647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diakonova M., Gunter D. R., Herrington J., Carter-Su C. 2002. SH2-Bβ is a Rac-binding protein that regulates cell motility. J. Biol. Chem. 277, 10669-10677 [DOI] [PubMed] [Google Scholar]
- Disatnik M. H., Rando T. A. 1999. Integrin-mediated muscle cell spreading. The role of protein kinase c in outside-in and inside-out signaling and evidence of integrin cross-talk. J. Biol. Chem. 274, 32486-32492 [DOI] [PubMed] [Google Scholar]
- Dovas A., Yoneda A., Couchman J. R. 2006. PKCbeta-dependent activation of RhoA by syndecan-4 during focal adhesion formation. J. Cell Sci. 119, 2837-2846 [DOI] [PubMed] [Google Scholar]
- Duan C., Li M., Rui L. 2004. SH2-B promotes insulin receptor substrate 1 (IRS1)- and IRS2-mediated activation of the phosphatidylinositol 3-kinase pathway in response to leptin. J. Biol. Chem. 279, 43684-43691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco S. J., Rodgers M. A., Perrin B. J., Han J., Bennin D. A., Critchley D. R., Huttenlocher A. 2004. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat. Cell Biol. 6, 977-983 [DOI] [PubMed] [Google Scholar]
- Geiger B., Spatz J. P., Bershadsky A. D. 2009. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 10, 21-33 [DOI] [PubMed] [Google Scholar]
- Haller H., Lindschau C., Maasch C., Olthoff H., Kurscheid D., Luft F. C. 1998. Integrin-induced protein kinase Cα and Cε translocation to focal adhesions mediates vascular smooth muscle cell spreading. Circ. Res. 82, 157-165 [DOI] [PubMed] [Google Scholar]
- e X., Li Y., Schembri-King J., Jakes S., Hayashi J. 2000. Identification of actin binding protein, ABP-280, as a binding partner of human Lnk adaptor protein. Mol. Immunol. 37, 603-612 [DOI] [PubMed] [Google Scholar]
- Herrington J., Diakonova M., Rui L., Gunter D. R., Carter-Su C. 2000. SH2-B is required for growth hormone-induced actin reorganization. J. Biol. Chem. 275, 13126-13133 [DOI] [PubMed] [Google Scholar]
- Hoshino M., Izumi T., Shimizu T. 1998. Leukotriene D4 activates mitogen-activated protein kinase through a protein kinase Cα-Raf-1-dependent pathway in human monocytic leukemia THP-1 cells. J. Biol. Chem. 273, 4878-4882 [DOI] [PubMed] [Google Scholar]
- Howe A. K., Juliano R. L. 1998. Distinct mechanisms mediate the initial and sustained phases of integrin-mediated activation of the Raf/MEK/mitogen-activated protein kinase cascade. J. Biol. Chem. 273, 27268-27274 [DOI] [PubMed] [Google Scholar]
- Jaken S., Leach K., Klauck T. 1989. Association of type 3 protein kinase C with focal contacts in rat embryo fibroblasts. J. Cell Biol. 109, 697-704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong M., Wang C. S., Donoghue D. J. 2002. Interaction of fibroblast growth factor receptor 3 and the adapter protein SH2-B. J. Biol. Chem. 277, 15962-15970 [DOI] [PubMed] [Google Scholar]
- Kurzer J. H., Argetsinger L. S., Zhou Y.-J., Kouadio J.-L., O'Shea J. J., Carter-Su C. 2004. Tyrosine 813 is a site of JAK2 autophosphorylation critical for activation of JAK2 by SH2-Bβ. Mol. Cell. Biol. 24, 4557-4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzer J. H., Saharinen P., Silvennoinen O., Carter-Su C. 2006. Binding of SH2-B family members within a potential negative regulatory region maintains JAK2 in an active state. Mol. Cell. Biol. 26, 6381-6394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson C. 2006. Protein kinase C and the regulation of the actin cytoskeleton. Cell. Signal. 18, 276-284 [DOI] [PubMed] [Google Scholar]
- Machida K., Thompson C. M., Dierck K., Jablonowski K., Karkkainen S., Liu B., Zhang H., Nash P. D., Newman D. K., Nollau P., et al. 2007. High-throughput phosphotyrosine profiling using SH2 domains. Mol. Cell 26, 899-915 [DOI] [PubMed] [Google Scholar]
- Marais R., Light Y., Mason C., Paterson H., Olson M. F., Marshall C. J. 1998. Requirement of Ras-GTP-Raf complexes for activation of Raf-1 by protein kinase C. Science 280, 109-112 [DOI] [PubMed] [Google Scholar]
- Maures T. J., Kurzer J. H., Carter-Su C. 2007. SH2B1 (SH2-B) and JAK2: a multifunctional adaptor protein and kinase made for each other. Trends Endocrinol. Metab. 18, 38-45 [DOI] [PubMed] [Google Scholar]
- Maures T. J., Chen L., Carter-Su C. 2009. Nucleocytoplasmic shuttling of the adapter protein SH2B1β (SH2-Bβ) is required for nerve growth factor (NGF)-dependent neurite outgrowth and enhancement of expression of a subset of NGF-responsive genes Mol. Endocrinol. 23, 1077-1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maures T. J., Su H.-W., Argetsinger L. A., Grinstein S., Carter-Su C. 2011. Phosphorylation controls a dual-function polybasic nuclear localization sequence in the adapter protein SH2B1β to regulate its cellular function and distribution. J. Cell Sci. 124, 1542-1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meenakshi T., Ross F. P., Martin J., Teitelbaum S. L. 1993. 1,25-Dihydroxyvitamin D3 and macrophage colony-stimulating factor-1 synergistically phosphorylate talin. J. Cell. Biochem. 53, 145-155 [DOI] [PubMed] [Google Scholar]
- Mogi A., Hatai M., Soga H., Takenoshita S., Nagamachi Y., Fujimoto J., Yamamoto T., Yokota J., Yaoi Y. 1995. Possible role of protein kinase C in the regulation of intracellular stability of focal adhesion kinase in mouse 3T3 cells. FEBS Lett. 373, 135-140 [DOI] [PubMed] [Google Scholar]
- Moser M., Legate K. R., Zent R., Fassler R. 2009. The tail of integrins, talin, and kindlins. Science 324, 895-899 [DOI] [PubMed] [Google Scholar]
- Nelms K., O'Neill T. J., Li S., Hubbard S. R., Gustafson T. A., Paul W. E. 1999. Alternative splicing, gene localization, and binding of SH2-B to the insulin receptor kinase domain. Mamm. Genome 10, 1160-1167 [DOI] [PubMed] [Google Scholar]
- Nishi M., Werner E. D., Oh B. C., Frantz J. D., Dhe-Paganon S., Hansen L., Lee J., Shoelson S. E. 2005. Kinase activation through dimerization by human SH2-B. Mol. Cell. Biol. 25, 2607-2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panetti T. S. 2002. Tyrosine phosphorylation of paxillin, FAK, and p130CAS: effects on cell spreading and migration. Front. Biosci. 7, d143-d150 [DOI] [PubMed] [Google Scholar]
- Pasquale E. B., Maher P. A., Singer S. J. 1986. Talin is phosphorylated on tyrosine in chicken embryo fibroblasts transformed by Rous sarcoma virus. Proc. Natl. Acad. Sci. USA 83, 5507-5511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X., Riccio A., Zhang Y., Ginty D. D. 1998. Identification and characterization of novel substrates of Trk receptors in developing neurons. Neuron 21, 1017-1029 [DOI] [PubMed] [Google Scholar]
- Rabinovitz I., Tsomo L., Mercurio A. M. 2004. Protein kinase C-α phosphorylation of specific serines in the connecting segment of the beta 4 integrin regulates the dynamics of type II hemidesmosomes. Mol. Cell. Biol. 24, 4351-4360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D., Li M., Duan C., Rui L. 2005. Identification of SH2-B as a key regulator of leptin sensitivity, energy balance and body weight in mice. Cell Metab. 2, 95-104 [DOI] [PubMed] [Google Scholar]
- Ren D., Zhou Y., Morris D., Li M., Li Z., Rui L. 2007. Neuronal SH2B1 is essential for controlling energy and glucose homeostasis. J. Clin. Invest. 117, 397-406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider L., Tao J., Snyder S., Brinley B., Lu J., Diakonova M. 2009. Adapter protein SH2B1β cross-links actin filaments and regulates actin cytoskeleton. Mol. Endocrinol. 23, 1065-1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel H., Wang J., Hansen H., Yousaf N. 1997. PSM, an insulin-dependent, pro-rich, PH, SH2 domain containing partner of the insulin receptor. J. Biochem. 122, 1105-1113 [DOI] [PubMed] [Google Scholar]
- Riedel H., Yousaf N., Zhao Y., Dai H., Deng Y., Wang J. 2000. PSM, a mediator of PDGF-BB-, IGF-I-, and insulin-stimulated mitogenesis. Oncogene 19, 39-50 [DOI] [PubMed] [Google Scholar]
- Rigot V., Lehmann M., Andre F., Daemi N., Marvaldi J., Luis J. 1998. Integrin ligation and PKC activation are required for migration of colon carcinoma cells. J. Cell Sci. 111, 3119-3127 [DOI] [PubMed] [Google Scholar]
- Rui L., Carter-Su C. 1998. Platelet-derived growth factor (PDGF) stimulates the association of SH2-Bβ with PDGF receptor and phosphorylation of SH2-Bβ. J. Biol. Chem. 273, 21239-21245 [DOI] [PubMed] [Google Scholar]
- Rui L., Mathews L. S., Hotta K., Gustafson T. A., Carter-Su C. 1997. Identification of SH2-Bβ as a substrate of the tyrosine kinase JAK2 involved in growth hormone signaling. Mol. Cell. Biol. 17, 6633-6644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui L., Herrington J., Carter-Su C. (1999a). SH2-B is required for nerve growth factor-induced neuronal differentiation. J. Biol. Chem. 274, 10590-10594 [DOI] [PubMed] [Google Scholar]
- Rui L., Herrington J., Carter-Su C. (1999b). SH2-B, a membrane-associated adapter, is phosphorylated on multiple serines/threonines in response to nerve growth factor by kinases within the MEK/ERK cascade. J. Biol. Chem. 274, 26485-26492 [DOI] [PubMed] [Google Scholar]
- Salgia R., Uemura N., Okuda K., Li J. L., Pisick E., Sattler M., de Jong R., Druker B., Heisterkamp N., Chen L. B. 1995. CRKL links p210BCR/ABL with paxillin in chronic myelogenous leukemia cells. J. Biol. Chem. 270, 29145-29150 [DOI] [PubMed] [Google Scholar]
- Schonwasser D. C., Marais R. M., Marshall C. J., Parker P. J. 1998. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol. Cell. Biol. 18, 790-798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subauste M. C., Pertz O., Adamson E. D., Turner C. E., Junger S., Hahn K. M. 2004. Vinculin modulation of paxillin-FAK interactions regulates ERK to control survival and motility. J. Cell Biol. 165, 371-381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigges U., Koch B., Wissing J., Jockusch B. M., Ziegler W. H. 2003. The F-actin cross-linking and focal adhesion protein filamin A is a ligand and in vivo substrate for protein kinase Cα. J. Biol. Chem. 278, 23561-23569 [DOI] [PubMed] [Google Scholar]
- Tripathi A., Sodhi A. 2009. Growth hormone-induced production of cytokines in murine peritoneal macrophages in vitro: role of JAK/STAT, PI3K, PKC and MAP kinases. Immunobiology 214, 430-440 [DOI] [PubMed] [Google Scholar]
- Vadlamudi R., Adam L., Talukder A., Mendelsohn J., Kumar R. 1999. Serine phosphorylation of paxillin by heregulin-β1: role of p38 mitogen activated protein kinase. Oncogene 18, 7253-7264 [DOI] [PubMed] [Google Scholar]
- von Wichert G., Haimovich B., Feng G. S., Sheetz M. P. 2003. Force-dependent integrin-cytoskeleton linkage formation requires downregulation of focal complex dynamics by Shp2. EMBO J. 22, 5023-5035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuori K., Ruoslahti E. 1993. Activation of protein kinase C precedes α5β1 integrin-mediated cell spreading on fibronectin. J. Biol. Chem. 268, 21459-21462 [PubMed] [Google Scholar]
- Vuori K., Ruoslahti E. 1995. Tyrosine phosphorylation of p130Cas and cortactin accompanies integrin-mediated cell adhesion to extracellular matrix. J. Biol. Chem. 270, 22259-22262 [DOI] [PubMed] [Google Scholar]
- Wang J., Riedel H. 1998. Insulin-like growth factor-I receptor and insulin receptor association with a Src homology-2 domain-containing putative adapter. J. Biol. Chem. 273, 3136-3139 [DOI] [PubMed] [Google Scholar]
- Webb D. J., Donais K., Whitmore L. A., Thomas S. M., Turner C. E., Parsons J. T., Horwitz A. F. 2004. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 6, 154-161 [DOI] [PubMed] [Google Scholar]
- Werth D. K., Niedel J. E., Pastan I. 1983. Vinculin, a cytoskeletal substrate of protein kinase C. J. Biol. Chem. 258, 11423-11426 [PubMed] [Google Scholar]
- Woods A., Couchman J. R. 1992. Protein kinase C involvement in focal adhesion formation. J. Cell Sci. 101, 277-290 [DOI] [PubMed] [Google Scholar]
- Yousaf N., Deng Y., Kang Y., Riedel H. 2001. Four PSM/SH2-B alternative splice variants and their differential roles in mitogenesis. J. Biol. Chem. 276, 40940-40948 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhu W., Wang Y. G., Liu X. J., Jiao L., Liu X., Zhang Z. H., Lu C. L., He C. 2006. Interaction of SH2-Bβ with RET is involved in signaling of GDNF-induced neurite outgrowth. J. Cell. Sci. 119, 1666-1676 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Izaguirre G., Lin S. Y., Lee H. Y., Schaefer E., Haimovich B. 2004. The phosphorylation of vinculin on tyrosine residues 100 and 1065, mediated by SRC kinases, affects cell spreading. Mol. Biol. Cell 15, 4234-4247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler W. H., Liddington R. C., Critchley D. R. 2006. The structure and regulation of vinculin. Trends Cell Biol. 16, 453-460 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.