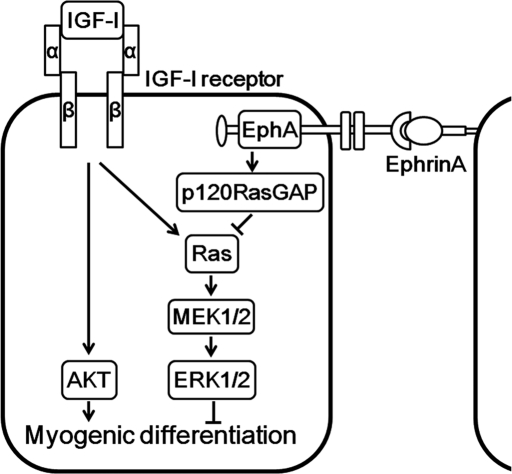
Insulin-like growth factor-I (IGF-I) activates not only the AKT pathway responsible for skeletal myogenesis but also the extracellular signal–regulated kinase (ERK) 1/2 cascade that inhibits myogenesis. The ephrinA/EphA signal facilitates IGF-I–induced myogenesis by inhibiting the Ras-ERK1/2 pathway via p120 Ras GTPase-activating protein.
Abstract
Insulin-like growth factor-I (IGF-I) activates not only the phosphatidylinositol 3-kinase (PI3K)–AKT cascade that is essential for myogenic differentiation but also the extracellular signal–regulated kinase (ERK) 1/2 cascade that inhibits myogenesis. We hypothesized that there must be a signal that inhibits ERK1/2 upon cell–cell contact required for skeletal myogenesis. Cell–cell contact–induced engagement of ephrin ligands and Eph receptors leads to downregulation of the Ras-ERK1/2 pathway through p120 Ras GTPase-activating protein (p120RasGAP). We therefore investigated the significance of the ephrin/Eph signal in IGF-I–induced myogenesis. EphrinA1-Fc suppressed IGF-I–induced activation of Ras and ERK1/2, but not that of AKT, in C2C12 myoblasts, whereas ephrinB1-Fc affected neither ERK1/2 nor AKT activated by IGF-I. IGF-I–dependent myogenic differentiation of C2C12 myoblasts was potentiated by ephrinA1-Fc. In p120RasGAP-depleted cells, ephrinA1-Fc failed to suppress the Ras-ERK1/2 cascade by IGF-I and to promote IGF-I–mediated myogenesis. EphrinA1-Fc did not promote IGF-I–dependent myogenesis when the ERK1/2 was constitutively activated. Furthermore, a dominant-negative EphA receptor blunted IGF-I–induced myogenesis in C2C12 and L6 myoblasts. However, the inhibition of IGF-I–mediated myogenesis by down-regulation of ephrinA/EphA signal was canceled by inactivation of the ERK1/2 pathway. Collectively, these findings demonstrate that the ephrinA/EphA signal facilitates IGF-I–induced myogenesis by suppressing the Ras-ERK1/2 cascade through p120RasGAP in myoblast cell lines.
INTRODUCTION
Skeletal myogenesis is a complex process that begins with the commitment of multipotent mesodermal precursor cells to the muscle fate (Andres and Walsh, 1996; Taylor, 2002). These committed cells—the myoblasts—subsequently withdraw from the cell cycle, differentiate, and fuse into multinucleated myotubes. In culture, most skeletal muscle cell lines proliferate under high serum conditions, whereas the cells placed under low serum conditions spontaneously undergo differentiation into myotubes (Florini et al., 1991; Ewton et al., 1994; Bach et al., 1995).
Differentiation of skeletal muscle cells is positively and negatively regulated by two major intracellular signaling pathways, namely the phosphatidylinositol 3-kinase (PI3K)–AKT cascade and the extracellular signal–regulated kinase (ERK) 1/2 cascade (Kaliman et al., 1996, 1998; Bennett and Tonks, 1997; Coolican et al., 1997; Tortorella et al., 2001; de Alvaro et al., 2005; Koyama et al., 2008). The latter involves Ras, Raf, mitogen-activated protein kinase (MAPK)/ERK kinase1/2 (MEK1/2), and ERK1/2. Activation of ERK1/2 cascade evoked by mitogens induces proliferation of cultured skeletal muscle cells but prevents their differentiation (Bennett and Tonks, 1997; Coolican et al., 1997; Tortorella et al., 2001; de Alvaro et al., 2005; Koyama et al., 2008). Among various growth factors, the insulin-like growth factors (IGFs), including IGF-I and IGF-II, have been reported to be quite singular, in that they stimulate both proliferation and differentiation of muscle cells in culture (Florini et al., 1991, 1996; Coolican et al., 1997; Kaliman et al., 1998; Koyama et al., 2008; Clemmons, 2009).
IGF-I receptor, which belongs to receptor tyrosine kinase (RTK) family, activates the PI3K–AKT signaling cascade in response to IGFs, thereby promoting differentiation of skeletal muscle cells (Florini et al., 1996; Coolican et al., 1997; Kaliman et al., 1998; White, 2003). The importance of the IGF/IGF-I receptor signal in muscle development is also illustrated by the poor muscle development and dystrophic phenotype of IGF-I receptor–deficient mice (Liu et al., 1993). However, the IGF/IGF-I receptor signal also induces activation of ERK1/2 through Grb2-associated binder 1–SHP2 signaling pathways in C2C12 cells, which counteracts PI3K/AKT signal-mediated myogenic differentiation (Coolican et al., 1997; Koyama et al., 2008). Thus the differentiation of myoblasts into myotubes is determined by the balance between the positive and negative signals mediated through PI3K–AKT and ERK1/2 cascades, respectively.
Myoblast differentiation is regulated not only by the signaling pathways induced by soluble myogenic growth factors but also by those initiated by cell–cell contacts (Krauss, 2010; Pavlath, 2010). Cell–cell adhesions are constituted by cadherin and other junctional molecules. Among them, N-cadherin (also known as cadherin-2) is expressed throughout skeletal myogenesis and was shown to be involved in myogenic differentiation in myoblast cell lines (Charrasse et al., 2002; Gavard et al., 2004; Lovett et al., 2006; Krauss, 2010). N-cadherin associates in cis with Cdo, a cell surface receptor of the immunoglobulin (Ig) superfamily in C2C12 myoblasts (Lu and Krauss, 2010). On N-cadherin ligation, the Cdo intracellular region binds to Bnip-2, a scaffold protein for Cdc42 small GTPase, and to JLP, a scaffold protein for the p38α/β MAPK, which results in Cdc42-dependent activation of p38α/β (Takaesu et al., 2006; Kang et al., 2008). In contrast to ERK1/2 MAPK, the p38α/β pathway promotes skeletal myogenesis by inducing the expression of muscle-specific genes in myoblast cell lines and primary myoblasts (Guasconi and Puri, 2009). Thus, upon cell–cell contact formation, the N-cadherin/Cdo complex promotes myogenic differentiation through activation of the p38α/β pathway. N-cadherin engagement also induces activation of RhoA, leading to serum response factor–dependent expression of muscle-specific genes in skeletal muscle cell lines (Carnac et al., 1998). It has also been suggested that M-cadherin, another member of the classic cadherin family, regulates myoblast fusion through Trio guanine nucleotide exchange factor–dependent activation of Rac1 in C2C12 myoblasts (Charrasse et al., 2007). In addition to cadherins, Ig superfamily members such as neogenin and neural cell adhesion molecule also regulate myogenic differentiation of cultured myoblasts in a cell–cell contact–dependent manner (Kang et al., 2004; Krauss, 2010). These results reveal the importance of the signaling pathways mediated by cell–cell contacts for myoblast differentiation.
Eph receptors and their ligands—ephrins—constitute the largest subfamily of RTKs, and have been implicated in diverse physiological and pathophysiological functions, such as neural development, angiogenesis, and tumorigenesis (Pasquale, 2005, 2008). The Eph receptors are divided into two classes, EphA and EphB, based on their ability to bind the ligands ephrinA and ephrinB, respectively. Unlike other RTK ligands, both ephrinA and ephrinB are membrane-bound proteins (Pasquale, 2005, 2008). EphrinAs anchor to the plasma membrane via a glycosylphosphatidylinositol moiety, whereas ephrinBs contain a transmembrane domain. Thus ephrin/Eph signaling is initiated by the formation of cell–cell contacts (Pasquale, 2010). On ephrin binding, Eph receptors undergo autophosphorylation at tyrosine residues in the cytoplasmic domain, which then triggers downstream signaling cascades through interaction with several signaling molecules, including p120 Ras GTPase-activating protein (p120RasGAP) (Pasquale, 2010). It has been shown that the ephrin/Eph signal negatively regulates the Ras-ERK1/2 cascade through inhibition of Ras by p120RasGAP in various types of cell lines (Elowe et al., 2001; Miao et al., 2001; Tong et al., 2003; Parri et al., 2005; Pasquale, 2010). The evidence that the Eph/ephrin signal depends on cell–cell contacts and its ability to down-regulate the Ras-ERK1/2 cascade prompted us to test our hypothesis that the ephrin/Eph signal promotes myogenic differentiation by reducing the myogenic inhibitory signal mediated by the Ras-ERK1/2 cascade. In this study, we found that the cell–cell contact–dependent ephrinA/EphA signal down-regulates the IGF-I–induced ERK1/2 pathway through p120RasGAP in myoblast cell lines, thereby accelerating myogenic differentiation.
RESULTS
EphrinA/EphA signal represses IGF-I–induced activation of ERK1/2, but not AKT, through p120RasGAP in mouse C2C12 and rat L6 myoblasts
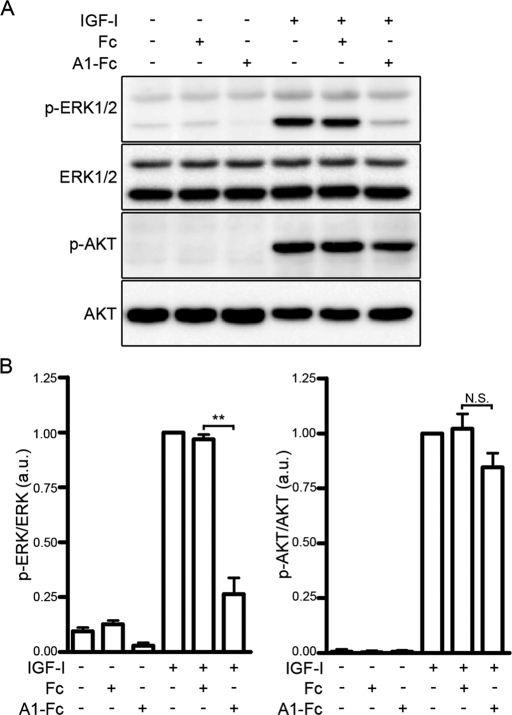
To investigate whether the ephrin/Eph signal downregulates the Ras-ERK1/2 cascade in myoblasts, we examined the effect of ephrinA1-Fc or ephrinB1-Fc on IGF-I–induced activation of ERK1/2 and AKT in myoblast cell lines. IGF-I induced activation of both ERK1/2 and AKT in sparse cultures of mouse C2C12 and rat L6 cells (Figure 1 and Supplemental Figure S1, A and B). EphrinA1-Fc, but not Fc, dramatically suppressed IGF-I–induced activation of ERK1/2 (Figure 1 and Supplemental Figure S1, A and B). In clear contrast, IGF-I–induced activation of AKT was unaffected by ephrinA1-Fc (Figure 1 and Supplemental Figure S1, A and B). EphrinB1-Fc did not affect IGF-I–induced activation of ERK1/2 and AKT in C2C12 cells (Supplemental Figure S1C). To confirm that ephrinB1-Fc used in this experiment is functional, we also examined the effect of ephrinB1-Fc on activation of ERK1/2 by cartilage oligomeric matrix protein (COMP)–angiopoietin-1 (Ang1), a potent Ang1 variant, in human umbilical vein endothelial cells, since it was reported that the ephrinB/EphB signal suppresses Ang1-induced activation of ERK1/2 in endothelial cells (Kim et al., 2002). COMP-Ang1–induced activation of ERK1/2 was clearly attenuated by the treatment with ephrinB1-Fc (Supplemental Figure S1D). These results indicate that the ephrinA/EphA signal, but not the ephrinB/EphB signal, downregulates the IGF-I–induced ERK1/2 cascade without affecting the AKT activation by IGF-I in C2C12 and L6 myoblasts.
FIGURE 1:
The ephrinA/EphA signal suppresses IGF-I–induced activation of ERK1/2 in C2C12 myoblasts. (A) Serum-starved C2C12 myoblasts were stimulated for 10 min with or without 10 nM IGF-I in the presence or absence of 1.7 nM Fc (Fc) or ephrinA1-Fc (A1-Fc) as indicated at the top. Cell lysates were subjected to Western blot analysis with anti–phospho-ERK1/2 (p-ERK1/2), anti-ERK1/2 (ERK1/2), anti–phospho-AKT (p-AKT), and anti-AKT (AKT) antibodies as indicated at the left. (B) Phosphorylation levels of ERK1/2 (left) and AKT (right) observed in A were quantified and represented by the ratio of phospho-ERK1/2 or phospho-AKT to total ERK1/2 or total AKT, respectively. The value for each group is expressed relative to the ratio observed in the cells stimulated with IGF-I in the absence of Fc and ephrinA1-Fc and shown as means ± SD of three independent experiments. **p < 0.01, significant difference between two groups. N.S., no significance between two groups. a.u., arbitrary unit(s).
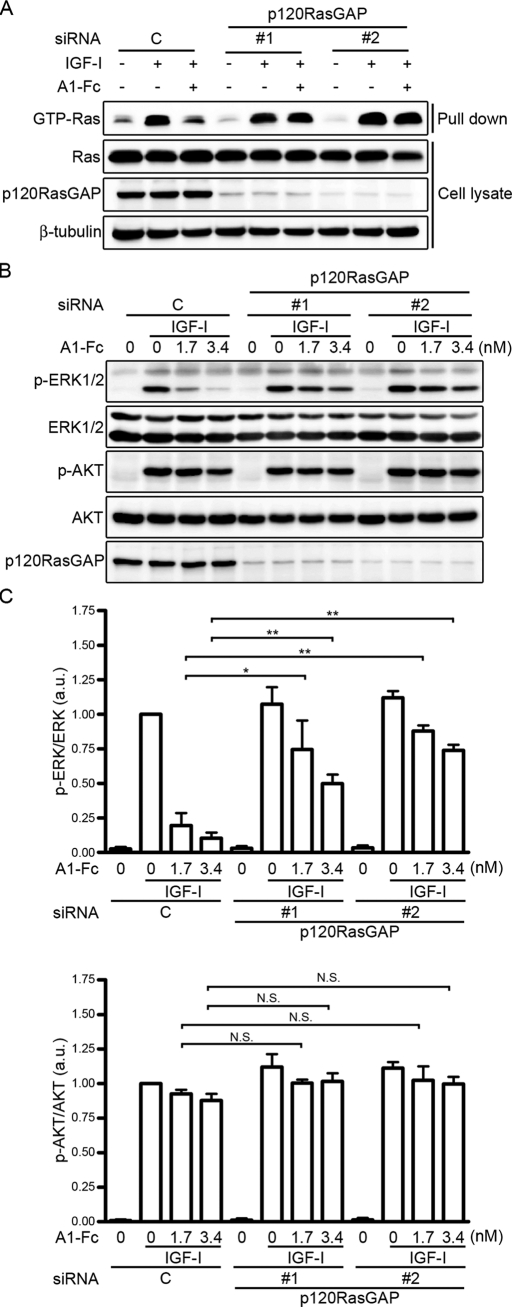
It is known that p120RasGAP is recruited to the ephrinA-stimulated EphA receptors, leading to down-regulation of the Ras–ERK1/2 pathway (Pasquale, 2010). Thus we next investigated whether the ephrinA/EphA signal down-regulates the IGF-I–induced ERK1/2 cascade by decreasing Ras activity through p120RasGAP. IGF-I induced activation of Ras in C2C12 myoblasts (Figure 2A). IGF-1–induced Ras activation was suppressed by ephrinA1-Fc (Figure 2A). However, ephrinA1-Fc failed to suppress the IGF-I–induced Ras activation when p120RasGAP was depleted by small interfering RNAs (siRNAs; Figure 2A). Consistently, depletion of p120RasGAP by siRNA blunted the inhibitory effect of ephrinA1-Fc on IGF-I–induced activation of ERK1/2, although it had no effect on IGF-I–induced activation of ERK1/2 and AKT (Figure 2, B and C). Collectively, these findings indicate that the ephrinA/EphA signal downregulates the IGF-I–evoked Ras–ERK1/2 cascade through p120RasGAP in C2C12 myoblasts.
FIGURE 2:
The ephrinA/EphA signal suppresses the IGF-I–induced Ras–ERK1/2 pathway through p120RasGAP. (A) Serum-starved C2C12 myoblasts transfected with either control siRNA (C) or two independent siRNAs targeting p120RasGAP (1 and 2) were stimulated for 5 min with or without 10 nM IGF-1 in the absence (–) or presence (+) of 1.7 nM ephrinA1-Fc (A1-Fc) as indicated at the top. GTP-bound Ras was collected as described in Materials and Methods and subjected to Western blot analysis with anti-Ras antibody (GTP-Ras). Aliquots of cell lysates were also subjected to Western blot analysis with anti-Ras (Ras), anti-p120RasGAP (p120RasGAP), and anti–β-tubulin (β-tubulin) antibodies as indicated at the left. (B) Serum-starved C2C12 myoblasts transfected with siRNA as described in A were stimulated for 10 min with or without 10 nM IGF-I in the absence or presence of 1.7 or 3.4 nM ephrinA1-Fc (A1-Fc) as indicated at the top. Cell lysates were subjected to Western blot analysis with anti–phospho-ERK1/2 (p-ERK1/2), anti-ERK1/2 (ERK1/2), anti–phospho-AKT (p-AKT), anti-AKT (AKT), and anti-p120RasGAP (p120RasGAP) antibodies as indicated at the left. (C) Phosphorylation levels of ERK1/2 (top) and AKT (bottom) observed in B were quantified and represented by the ratio of phospho-ERK1/2 or phospho-AKT to total ERK1/2 or total AKT, respectively. Values are expressed as explained in the legend of Figure 1B. Values are expressed relative to the ratio obtained from the IGF-I–treated cells transfected with control siRNA. *p < 0.05, **p < 0.01, significant differences between two groups. N.S., no significance between two groups. a.u., arbitrary unit(s).
EphrinA/EphA signal enhances IGF-I–induced myogenic differentiation
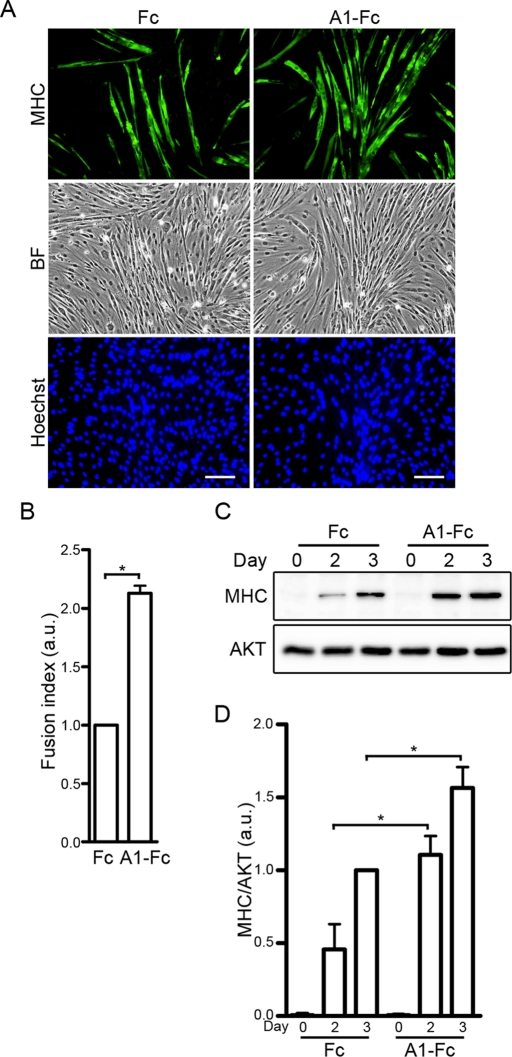
Myogenic differentiation is negatively and positively regulated by the ERK1/2 and AKT cascades, respectively (Kaliman et al., 1996, 1998; Bennett and Tonks, 1997; Coolican et al., 1997; Tortorella et al., 2001; de Alvaro et al., 2005; Koyama et al., 2008). Because the ephrinA/EphA signal downregulates the ERK1/2 cascade without affecting the AKT pathway in myoblast cell lines, we hypothesized that myogenic differentiation might be enhanced by the ephrinA/EphA signal. To address this possibility, confluent C2C12 myoblasts were differentiated into myotubes by being incubated in DMEM containing 1% fetal bovine serum (FBS) and 10 nM IGF-I in the presence of either Fc or ephrinA1-Fc. To evaluate myogenic differentiation, the expression of muscle-specific myosin heavy chain (MHC) protein was examined by immunocytochemical and Western blot analyses. On the third day after induction of myogenic differentiation, formation of MHC-positive myotubes was greater in the cells differentiated in the presence of ephrinA1-Fc than in those in the presence of Fc (control; Figure 3, A and B). Consistently, expression of MHC protein was 2.4 and 1.6 times higher in the ephrinA1-Fc–treated cells than in those treated with Fc on the second and third days after induction of differentiation, respectively (Figure 3, C and D). Similarly, myogenic differentiation of C2C12 cells induced by low concentrations of IGF-I was also potentiated by the treatment with ephrinA1-Fc (Supplemental Figure S2). These results indicate that activation of the ephrinA/EphA signal results in enhancement of IGF-I–dependent myogenic differentiation in C2C12 cells.
FIGURE 3:
The ephrinA/EphA signal promotes IGF-I–induced myogenic differentiation. (A) Immunocytochemical analysis of C2C12 myoblasts using anti-MHC antibody. Confluent cells were differentiated into myotubes in DMEM containing 1% FBS and 10 nM IGF-I in the presence of either 6.4 nM Fc (Fc) or ephrinA1-Fc (A1-Fc) for 3 d. The cells were then fixed, immunostained with anti-MHC antibody, and visualized with Alexa Fluor 488–conjugated secondary antibody. The cells were also poststained with Hoechst 33342 to visualize the nuclei. Alexa 488 (MHC), bright-field (BF), and Hoechst 33342 (Hoechst) images are shown as indicated at the left. Experiments were repeated three times with similar results. Scale bars, 100 μm. (B) The fusion index observed in A was determined by dividing the number of nuclei within multinucleated myotubes by the total number of nuclei analyzed. Values are expressed relative to that observed in the cells differentiated in the presence of Fc and shown as means ± SD of three independent experiments. (C) Confluent C2C12 myoblasts were differentiated into myotubes in DMEM containing 1% FBS and 10 nM IGF-I in the presence of either 6.4 nM Fc (Fc) or ephrinA1-Fc (A1-Fc) for time periods (days) indicated at the top. Cell lysates were subjected to Western blot analysis using anti-MHC (MHC) and anti-AKT (AKT) antibodies as indicated at the left. (D) The expression level of MHC observed in C was quantified by normalizing the expression of MHC by that of AKT. Values are expressed relative to that observed in the cells differentiated in the presence of Fc for 3 d and shown as means ± SD of three independent experiments. *p < 0.05, significant differences between two groups. a.u., arbitrary unit(s).
EphrinA/EphA signal promotes IGF-I–induced myogenic differentiation by suppressing the Ras–ERK1/2 cascade through p120RasGAP
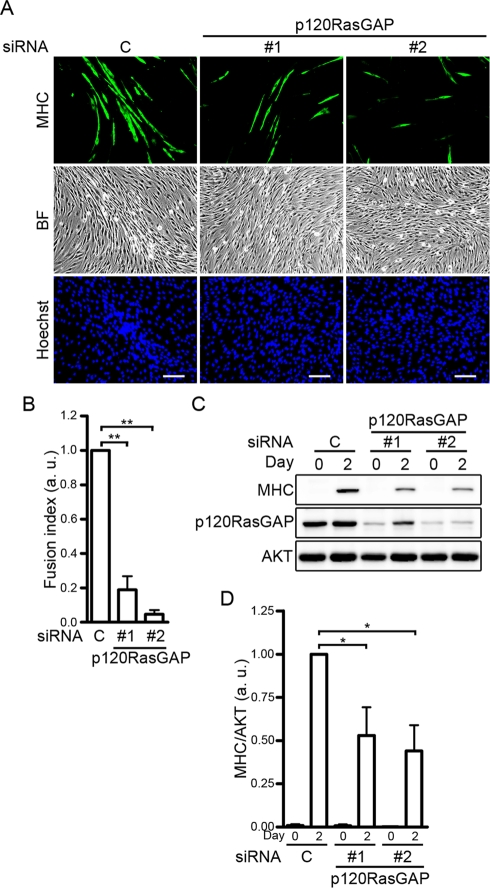
To investigate whether the ephrinA/EphA signal promotes IGF-I–dependent myogenic differentiation by suppressing the Ras–ERK1/2 pathway through p120RasGAP, we examined the effect of p120RasGAP depletion on the enhancement of IGF-I–induced myogenic differentiation by the ephrinA/EphA signal. C2C12 myoblasts transfected with either control siRNA or two independent siRNAs targeting p120RasGAP were differentiated in the media containing both IGF-I and ephrinA1-Fc for 2 d. Myogenic differentiation of C2C12 cells was significantly suppressed in the p120RasGAP-depleted cells compared to the cells transfected with control siRNA, as assessed by immunocytochemical analysis with anti-MHC antibody (Figure 4, A and B). Consistently, the expression of MHC was decreased by the depletion of p120RasGAP (Figure 4, C and D). These findings show that the ephrinA/EphA signal implicates p120RasGAP in the enhancement of the IGF-I–induced myogenic differentiation of C2C12 cells.
FIGURE 4:
The ephrinA/EphA signal promotes IGF-I–dependent myogenic differentiation through p120RasGAP. (A) C2C12 myoblasts transfected with either control siRNA (C) or two independent siRNAs targeting p120RasGAP (1 and 2) were differentiated into myotubes in DMEM containing 1% FBS and 10 nM IGF-I in the presence of 6.4 nM ephrinA1-Fc for 2 d. The cells were immunostained with anti-MHC antibody and visualized with Alexa Fluor 488–conjugated secondary antibody. The cells were also poststained with Hoechst 33342 to visualize the nuclei. Alexa 488 (MHC), bright-field (BF), and Hoechst 33342 (Hoechst) images are shown as indicated at the left. Experiments were repeated three times with similar results. Scale bars, 100 μm. (B) The fusion index observed in A was determined as described in the legend of Figure 3B. Values are expressed relative to that observed in the cells transfected with control siRNA and shown as means ± SD of three independent experiments. (C) siRNA-transfected C2C12 myoblasts were differentiated into myotubes as described in A for time periods (days) indicated at the top. Cell lysates were subjected to Western blot analysis using anti-MHC (MHC), anti-p120RasGAP (p120RasGAP), and anti-AKT (AKT) antibodies as indicated at the left. (D) Expression level of MHC observed in C was quantified by normalizing the expression of MHC by that of AKT. Values are expressed relative to that observed in the control siRNA-transfected cells differentiated for 2 d and shown as means ± SD of three independent experiments. *p < 0.05, **p < 0.01, significant differences between two groups. a.u., arbitrary unit(s).
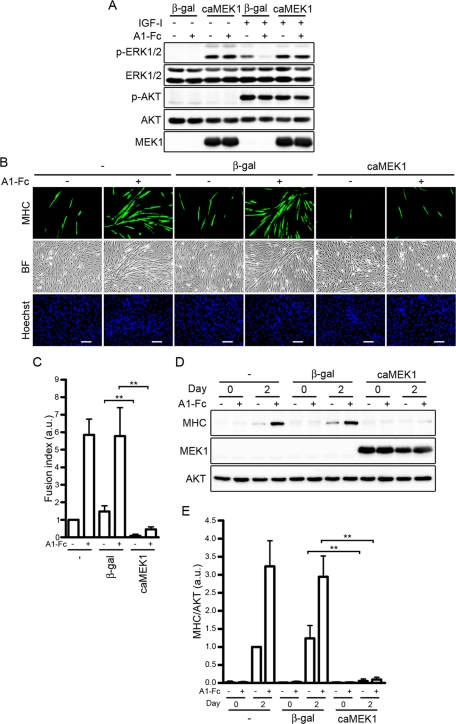
To further clarify whether the ephrinA/EphA signal promotes IGF-I–dependent myogenic differentiation through inactivation of ERK1/2 pathway, we examined the effect of adenovirus-mediated overexpression of a constitutively active mutant of MEK1 (caMEK1). As expected, overexpression of caMEK1 in C2C12 cells resulted in constitutive activation of ERK1/2 (Figure 5A). Inactivation of ERK1/2 in response to IGF-I by ephrinA1-Fc was canceled in the cells expressing caMEK1, although overexpression of caMEK1 did not affect IGF-I–induced AKT activation in the presence or absence of ephrinA1-Fc (Figure 5A). In the C2C12 myoblasts expressing caMEK1, IGF-I failed to induce formation of MHC-positive myotubes even in the presence of ephrinA1-Fc (Figure 5, B and C). Consistently, IGF-I–induced expression of MHC was prevented by overexpression of caMEK1 irrespective of the presence or absence of ephrinA1-Fc (Figure 5, D and E). Collectively, these findings indicate that the ephrinA/EphA signal potentiates IGF-I–induced myogenic differentiation of C2C12 cells through p120RasGAP-mediated down-regulation of the Ras–ERK1/2 cascade.
FIGURE 5:
Constitutive activation of the ERK1/2 pathway inhibits the promotion of IGF-I–mediated myogenic differentiation by the ephrinA/EphA signal. (A) Serum-starved C2C12 myoblasts infected with adenoviruses encoding either β-gal (β-gal) or constitutive active mutant of MEK1 (caMEK1) were stimulated with or without 10 nM IGF-I for 10 min in the presence of 1.7 nM Fc (−) or ephrinA1-Fc (A1-Fc) as indicated at the top. Cell lysates were subjected to Western blot analysis with anti–phospho-ERK1/2 (p-ERK1/2), anti-ERK1/2 (ERK1/2), anti–phospho-AKT (p-AKT), anti-AKT (AKT), and MEK1 (MEK1) antibodies as indicated at the left. (B) C2C12 myoblasts infected without (–) or with adenoviruses encoding either β-gal (β-gal) or constitutive active mutant of MEK1 (caMEK1) were differentiated into myotubes in DMEM containing 1% FBS and 10 nM IGF-I in the presence of 6.4 nM Fc (–) or ephrinA1-Fc (A1-Fc) for 2 d. The cells were immunostained with anti-MHC antibody, and visualized with Alexa Fluor 488–conjugated secondary antibody. The cells were also poststained with Hoechst 33342 to visualize the nuclei. Alexa 488 (MHC), bright-field (BF), and Hoechst 33342 (Hoechst) images are shown as indicated at the left. Experiments were repeated three times with similar results. Scale bars, 100 μm. (C) The fusion index observed in B was determined as described in the legend of Figure 3B. Values are expressed relative to that observed in the uninfected cells differentiated in the presence of Fc and shown as means ± SD of three independent experiments. (D) Adenovirus-infected C2C12 myoblasts were differentiated into myotubes as described in B for time periods (days) indicated at the top. Cell lysates were subjected to Western blot analysis using anti-MHC (MHC), anti-MEK1 (MEK1), and anti-AKT (AKT) antibodies as indicated at the left. (E) The expression level of MHC observed in D was quantified by normalizing the expression of MHC by that of AKT. Values are expressed relative to that observed in the uninfected cells differentiated in the presence of Fc for 2 d and shown as means ± SD of three independent experiments. **p < 0.01, significant differences between two groups. a.u., arbitrary unit(s).
EphA2 receptor that lacks the cytoplasmic domain acts as a dominant-negative mutant for EphA family members
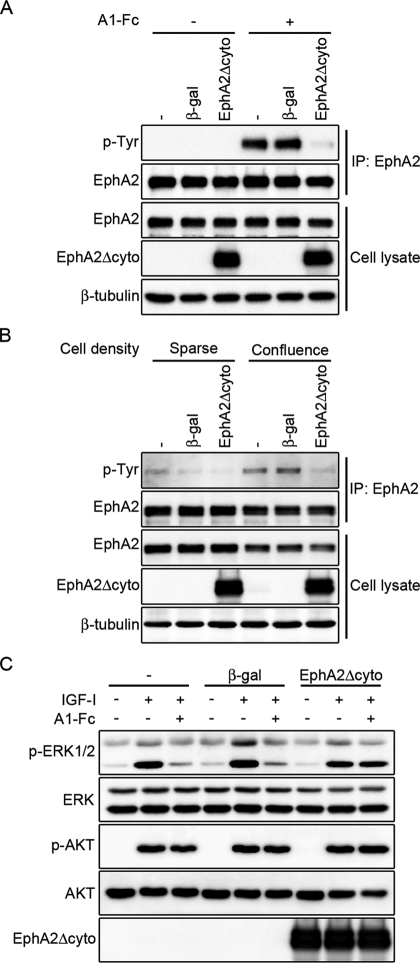
We further investigated whether the ephrinA/EphA signal is required for IGF-I–dependent myogenic differentiation. Because reverse transcription (RT)–PCR analysis revealed that C2C12 myoblasts express multiple members of ephrinA ligands such as ephrinA1, ephrinA3, ephrinA4, and ephrinA5 and those of EphA receptors, which include EphA1, EphA2, EphA3, and EphA4 (Supplemental Figure S3), we decided to use a dominant-negative approach instead of performing siRNA-mediated silencing of ephrinA ligands and EphA receptors. We prepared the adenovirus encoding the EphA2 receptor mutant lacking the cytoplasmic region (EphA2Δcyto), which was previously reported to exhibit dominant-negative activity (Cheng et al., 2003; Taddei et al., 2009). Overexpression of EphA2Δcyto blunted the ephrinA1-Fc–mediated phosphorylation of EphA2 in C2C12 cells (Figure 6A). It is significant that phosphorylation of EphA2 was greater in the confluent C2C12 cells than in the sparse cells (Figure 6B). Phosphorylation of EphA2 was likely to be induced by the binding of EphA2 to endogenous ephrinA upon cell–cell contact. EphA2 phosphorylation observed in the confluent cells was decreased by overexpression of EphA2Δcyto (Figure 6B), indicating that the EphA2 signal functions under confluent culture conditions and can be blocked by overexpression of EphA2Δcyto. Because C2C12 cells express several members of the EphA receptor family, as indicated by RT-PCR analysis, it can be assumed that ephrinA1-Fc suppresses IGF-I–induced activation of ERK1/2 through multiple members of EphA receptors. In C2C12 and L6 myoblasts, the inhibitory effect of ephrinA1-Fc on ERK1/2 activation in response to IGF-I was canceled by overexpression of EphA2Δcyto (Figure 6C and Supplemental Figure S4), implying that EphA2Δcyto acts as a dominant-negative mutant not only for EphA2, but also for other members of the EphA receptor family.
FIGURE 6:
EphA2 mutant lacking the cytoplasmic region acts as a dominant-negative EphA receptor. (A) C2C12 myoblasts infected without (–) or with adenoviruses encoding either β-gal (β-gal) or C-terminal HA-tagged EphA2 mutant that lacks the cytoplasmic region (EphA2Δcyto) were serum starved and stimulated for 10 min in the presence of 2.2 nM Fc (–) or ephrinA1-Fc (A1-Fc) as indicated at the top. EphA2 was immunoprecipitated with anti-EphA2 antibody and subjected to Western blot analysis with anti-phosphotyrosine (p-Tyr) and anti-EphA2 (EphA2) antibodies. Aliquots of total-cell lysate were also subjected to Western blot analysis with anti-EphA2 (EphA2), anti-HA (EphA2Δcyto), and anti–β-tubulin (β-tubulin) antibodies as indicated at the left. (B) C2C12 myoblasts infected with adenoviruses as described in A were placed on the culture dishes for 4 h under either sparse or confluent culture condition as indicated at the top. Immunoprecipitation of EphA2 and Western blot analysis were performed as described in A. (C) C2C12 myoblasts infected with adenoviruses as described in A were serum starved and stimulated for 10 min with or without 10 nM IGF-I in the presence of 1.7 nM Fc (–) or ephrinA1-Fc (A1-Fc) as indicated at the top. Cell lysates were subjected to Western blot analysis with anti–phospho-ERK1/2 (p-ERK1/2), anti-ERK1/2 (ERK1/2), anti–phospho-AKT (p-AKT), anti-AKT (AKT), and anti-HA (EphA2Δcyto) antibodies as indicated at the left.
Endogenous ephrinA/EphA signal is required for efficient myogenic differentiation induced by IGF-I
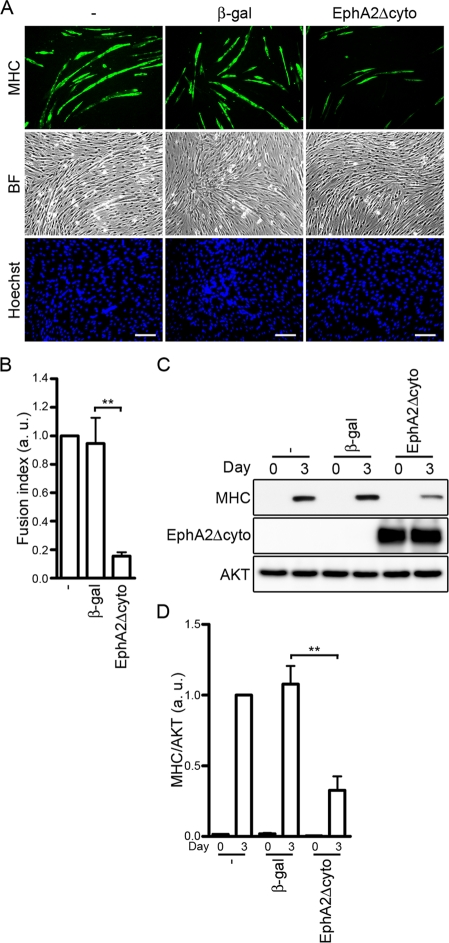
To clarify the role of the ephrinA/EphA signal in IGF-I–induced myogenic differentiation, the EphA signal was abrogated by EphA2Δcyto overexpression during IGF-I–induced myogenic differentiation. C2C12 and L6 myoblasts infected with adenoviruses encoding EphA2Δcyto exhibited decreased formation of MHC-positive myotubes compared to the uninfected cells or the cells infected with β-galactosidase (β-gal)–encoding adenoviruses (Figure 7, A and B, and Supplemental Figure S5A). Consistently, IGF-I–mediated expression of MHC protein was significantly reduced by EphA2Δcyto overexpression (Figure 7, C and D, and Supplemental Figure S5B). These findings indicate that the ephrinA/EphA signal is required for efficient myogenic differentiation induced by IGF-I in myoblast cell lines.
FIGURE 7:
IGF-I–induced myogenic differentiation is blunted by blocking the ephrinA/EphA signal. (A) C2C12 myoblasts infected without (−) or with adenoviruses encoding either β-gal or EphA2Δcyto were differentiated into myotubes in DMEM containing 1% FBS and 10 nM IGF-I for 3 d. The cells were immunostained with anti-MHC antibody and visualized with Alexa Fluor 488–conjugated secondary antibody. The cells were also poststained with Hoechst 33342 to visualize the nuclei. Alexa 488 (MHC), bright-field (BF), and Hoechst 33342 (Hoechst) images are shown as indicated at the left. Experiments were repeated three times with similar results. Scale bars, 100 μm. (B) The fusion index observed in A was determined as described in the legend of Figure 3B. Values are expressed relative to that observed in the uninfected cells and shown as means ± SD of three independent experiments. (C) Adenovirus-infected C2C12 myoblasts were differentiated into myotubes as described in A for time periods (days) indicated at the top. Cell lysates were subjected to Western blot analysis using anti-MHC (MHC), anti-HA (EphA2Δcyto), and anti-AKT (AKT) antibodies as indicated at the left. (D) The expression level of MHC observed in C was quantified by normalizing the expression of MHC by that of AKT. Values are expressed relative to that observed in the uninfected cells differentiated for 3 d and shown as means ± SD of three independent experiments. **p < 0.01, significant differences between two groups. a.u., arbitrary unit(s).
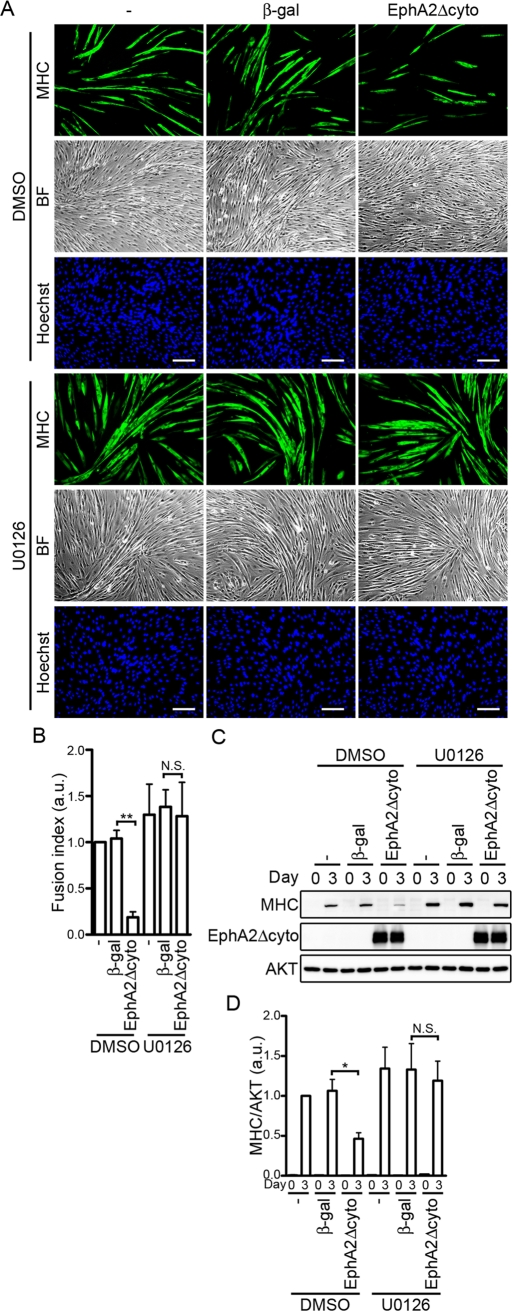
To investigate whether myogenic differentiation induced by IGF-I requires the suppression of ERK1/2 activity by the ephrinA/EphA signal, we first examined the effect of EphA2Δcyto overexpression on ERK1/2 activity by inducing differentiation with IGF-I. The ERK1/2 activity of the C2C12 cells expressing EphA2Δcyto varied at the several time points during differentiation and was not always significantly increased compared with either the parental cells or those expressing β-gal. Therefore, to further assess the effect of EphA2Δcyto on the ERK1/2 activity during the differentiation, we examined it using the cells in the presence of ephrinA1-Fc in addition to IGF-1. C2C12 cells infected with adenoviruses expressing EphA2Δcyto exhibited a high level of ERK1/2 activity compared to those infected without or with β-gal–expressing adenoviruses during myogenic differentiation (Supplemental Figure S6). Furthermore, we investigated the effect of a MEK1/2 inhibitor, U0126, on the IGF-I–induced myogenic differentiation. U0126 slightly enhanced formation of MHC-positive myotubes and increased the expression of MHC in C2C12 and L6 myoblasts infected with or without β-gal–encoding adenoviruses (Figure 8 and Supplemental Figure S5). As observed in Figure 7, overexpression of EphA2Δcyto partially inhibited myotube formation and decreased the expression of MHC (Figure 8 and Supplemental Figure S5). However, the inhibitory effect of EphA2Δcyto on IGF-I-mediated myogenic differentiation was completely suppressed by the treatment with U0126 (Figure 8 and Supplemental Figure S5). Collectively, these findings suggest that IGF-I–induced myogenic differentiation is facilitated by the ephrinA/EphA signal, leading to down-regulation of the ERK1/2 cascade in myoblast cell lines.
FIGURE 8:
Inhibition of IGF-I–induced myogenic differentiation by a dominant-negative EphA receptor mutant is canceled by inhibiting the ERK1/2 pathway. (A) C2C12 myoblasts infected without (–) or with adenoviruses encoding either β-gal or EphA2Δcyto were differentiated into myotubes in DMEM containing 1% FBS and 10 nM IGF-I in the presence of vehicle (dimethyl sulfoxide [DMSO]) or 3 μM U0126 for 3 d. The cells were immunostained with anti-MHC antibody and visualized with Alexa Fluor 488–conjugated secondary antibody. The cells were also poststained with Hoechst 33342 to visualize the nuclei. Alexa 488 (MHC), bright-field (BF), and Hoechst 33342 (Hoechst) images are shown as indicated at the left. Experiments were repeated three times with similar results. Scale bars, 100 μm. (B) The fusion index observed in A was determined as described in the legend of Figure 3B. Values are expressed relative to that observed in the uninfected cells differentiated in the presence of DMSO and shown as means ± SD of three independent experiments. (C) Adenovirus-infected C2C12 myoblasts were differentiated into myotubes as described in A for time periods (days) indicated at the top. Cell lysates were subjected to Western blot analysis using anti-MHC (MHC), anti-HA (EphA2Δcyto), and anti-AKT (AKT) antibodies as indicated at the left. (D) The expression level of MHC observed in C was quantified by normalizing the expression of MHC by that of AKT. Values are expressed relative to that observed in the uninfected cells differentiated in the presence of DMSO for 3 d and shown as means ± SD of three independent experiments. *p < 0.05, **p < 0.01, significant differences between two groups. N.S., no significance between two groups. a.u., arbitrary unit(s).
DISCUSSION
In the present study, we investigated the role of ephrin/Eph signaling in skeletal muscle differentiation and found that the ephrinA/EphA signal facilitates IGF-I–induced myogenic differentiation by repressing the Ras–ERK1/2 signaling cascade through p120RasGAP in myoblast cell lines (Figure 9). To our knowledge, this study reveals for the first time the potential role of the ephrinA/EphA signal in skeletal myogenesis.
FIGURE 9:
Schematic representation of a proposed model for how the ephrinA/EphA signal potentiates IGF-I–induced myogenic differentiation. IGF-I not only induces myogenic differentiation through the PI3K/AKT pathway but also stimulates the myogenic inhibitory signal mediated by the Ras–ERK1/2 cascade. The cell–cell contact–dependent ephrinA/EphA signal suppresses the RasxERK1/2 cascade by IGF-I through p120RasGAP, thereby facilitating IGF-I–mediated myogenic differentiation.
We delineated the molecular mechanism by which an endogenous ephrinA/EphA signal is involved in the inactivation of ERK1/2 that is preferable for myogenic differentiation induced by IGF-I. Although it is well known that IGFs promote myogenic differentiation through the PI3K/AKT pathway, IGFs also induces activation of myogenic inhibitory signal mediated by the ERK1/2. Activation of the PI3K/AKT pathway is responsible for myogenic differentiation, whereas the ERK1/2 pathway counteracts the PI3K/AKT pathway–dependent myogenic differentiation (Kaliman et al., 1996, 1998; Bennett and Tonks, 1997; Coolican et al., 1997; Tortorella et al., 2001; de Alvaro et al., 2005; Koyama et al., 2008). Thus it is reasonable that the mechanisms that suppress the ERK1/2 pathway might exist to facilitate myogenic differentiation during skeletal myogenesis.
The EphrinA/EphA signal in C2C12 cells suppresses ERK1/2 during myogenesis. EphA and EphB receptors inhibit the Ras–ERK1/2 pathway by decreasing the Ras activity through p120RasGAP in various types of cells (Elowe et al., 2001; Miao et al., 2001; Tong et al., 2003; Parri et al., 2005; Pasquale, 2010). Thus, we assumed that the ephrin/Eph signal is favorable for skeletal myogenesis and found that the ephrinA/EphA signal down-regulates the Ras–ERK1/2 pathway induced by IGF-I through p120RasGAP in myoblast cell lines, thereby facilitating myogenic differentiation. In addition, it was previously reported that DA-Raf1, a splicing isoform of A-Raf, is expressed during skeletal myogenesis and enhances myogenic differentiation of C2C12 cells by acting as a dominant-negative antagonist of the Ras–ERK1/2 pathway (Yokoyama et al., 2007). Recently glypican, a heparin sulfate proteoglycan expressed in myoblasts, was reported to sequester basic fibroblast growth factor (bFGF) in lipid rafts away from its receptors (Gutierrez and Brandan, 2010). Because bFGF represses myogenic differentiation through the activation of the ERK1/2 pathway (Tortorella et al., 2001), sequestration of bFGF by glypican promotes myogenic differentiation. Therefore the ERK1/2 activity in myoblasts is suppressed by various mechanisms to facilitate myogenic differentiation during skeletal myogenesis.
Temporal ERK1/2 inactivation and activation might be important for myogenesis. The ERK1/2 cascade is essential for proliferation of myoblasts but negatively regulates myogenic differentiation by suppressing the expression of muscle-specific genes. Of interest, it was also shown that the late stage of skeletal muscle differentiation requires the ERK1/2 activity (Bennett and Tonks, 1997). Expression of MAPK phosphatase-1, which inhibits ERK1/2 activity, is down-regulated during the late stage of myogenesis, and its overexpression prevents myotube formation without affecting the expression of muscle-specific genes. Thus the ERK1/2 activity is temporarily controlled during myogenic differentiation. On induction of myogenic differentiation, the cell–cell contact–dependent ephrinA/EphA signal suppresses ERK1/2 activity, as we demonstrated in this study. However, the ephrinA/EphA signal may be down-regulated to reactivate the ERK1/2 cascade at the late stage of skeletal muscle differentiation. Because EphA2 expression is induced by activation of the Ras–ERK1/2 pathway (Macrae et al., 2005), EphA-signal–dependent inhibition of the Ras–ERK1/2 pathway may lead to down-regulation of EphA2 expression. Therefore the ERK1/2 activity may be temporarily controlled by an EphA2–Ras–ERK1/2 negative feedback loop during skeletal myogenesis.
There is only limited information on the role of the ephrin/Eph signal in muscle development. It was shown that the ephrinA5/EphA4 signal is involved in migration of muscle precursor cells (Swartz et al., 2001). EphrinA5 expressed in mesoderm tissue prevents EphA4-positive muscle precursor cells from migrating into abnormal embryonic regions. Furthermore, a role of EphA4 signal in maintenance of neuromuscular junctions was suggested (Lai et al., 2004). EphA4 localized at the neuromuscular junctions of adult muscle induces expression of acetylcholinesterase, an enzyme that degrades the neurotransmitter acetylcholine, through Janus kinase/signal transducers and activators of transcription pathway. However, the role of ephrin/Eph signal in differentiation of myoblasts into myocytes has never been reported. Therefore the present study is the first to unravel the potential role of the ephrinA/EphA signal in myogenic differentiation of myoblast cell lines. However, further study is required to clarify whether the ephrinA/EphA signal is implicated in differentiation of primary myoblasts or in myogenic differentiation in vivo.
The present study apparently indicates that p120RasGAP-mediated inhibition of the Ras–ERK1/2 pathway is responsible for the promotion of myogenic differentiation by the ephrinA/EphA signal in myoblast cell lines. However, since the ephrinA/EphA signal not only activates p120RasGAP but also stimulates various intracellular signaling molecules (Pasquale, 2010), myogenic differentiation might also be regulated by other signaling pathways. EphA2 signaling is known to promote the intercellular junctions mediated by cadherins such as E-cadherin and N-cadherin (Miao et al., 2003; Cooper et al., 2008; Jun et al., 2009; Miura et al., 2009). Because engagement of N-cadherin and M-cadherin leads to the activation of promyogenic signaling pathways in myoblasts (Krauss, 2010), the ephrinA/EphA signal may promote skeletal myogenesis by enhancing cadherin-based cell–cell junctions. RhoA is activated by EphA receptors through ephexin (Shamah et al., 2001) and induces expression of muscle-specific genes through serum response factor (Carnac et al., 1998). Thus the ephrinA/EphA signal may also regulate myogenesis through RhoA-dependent expression of muscle-specific genes. In addition, it has been shown that ephrinA-mediated reverse signaling also regulates various biological functions, such as insulin secretion from β cells, neurogenesis, tumor suppression, and tumor progression (Pasquale, 2010). Thus it would be interesting to examine the involvement of ephrinA reverse signaling in myogenic differentiation.
In conclusion, we have demonstrated that the cell–cell contact–dependent ephrinA/EphA signal suppresses IGF-I–induced activation of the Ras–ERK1/2 cascade by decreasing the Ras activity through p120RasGAP in myoblast cell lines. This molecular mechanism accounts for IGF-I–induced myogenic differentiation facilitated by ephrinA/EphA.
MATERIALS AND METHODS
Reagents and antibodies
COMP-Ang1 was kindly provided by G. Y. Koh (Korea Advanced Institute of Science and Technology, Daejeon, South Korea). Human recombinant IGF-I was kindly provided by Astellas Pharma (Tokyo, Japan). Other reagents were purchased as follows: Hoechst 33342 nuclear dye from Sigma-Aldrich (St. Louis, MO); human Fc, mouse ephrinA1-Fc, and mouse ephrinB1-Fc from R&D Systems (Minneapolis, MN); and U0126 from Cell Signaling Technology (Beverly, MA). Antibodies were purchased as follows: anti–phospho-p44/42 ERK1/2 (Thr-202/Tyr-204), anti–phospho-AKT (Thr-308), and anti-AKT from Cell Signaling Technology; anti-MEK1, anti-ERK1/2, anti-EphA2, and anti-phosphotyrosine (PY99) from Santa Cruz Biotechnology (Santa Cruz, CA); anti-HA tag from Roche Diagnostics (Indianapolis, IN); anti–pan Ras from Calbiochem (La Jolla, CA); anti-MHC (MF20) from the Developmental Studies Hybridoma Bank (Iowa City, IA); anti–β-tubulin from Sigma-Aldrich; horseradish peroxidase–conjugated anti-mouse, anti-rabbit, and anti-rat from GE Healthcare Life Sciences (Piscataway, NJ); and Alexa Fluor 488–labeled goat anti–mouse IgG from Molecular Probes (Eugene, OR).
Cell culture, stimulation, and myogenic differentiation
Mouse C2C12 and rat L6 myoblasts were maintained as subconfluent monolayers in DMEM (Nissui, Tokyo, Japan) supplemented with FBS (20% for C2C12 cells, 10% for L6 cells), 4.5 g/l glucose, 0.58 g/l l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. To examine the IGF-I–induced activation of ERK1/2, AKT, and Ras, the cells were serum starved overnight and stimulated with IGF-I in the presence or absence of nonclustered Fc, ephrinA1-Fc, or ephrinB1-Fc as indicated in the figure legends. For the induction of myogenic differentiation, cultured medium was replaced with DMEM containing 1% FBS and IGF-I at the concentrations described in the figure legends, when cell density reached confluency. The differentiation medium was exchanged every day. Human umbilical vein endothelial cells were purchased from Kurabo (Osaka, Japan) and maintained as described previously (Fukuhara et al., 2005).
siRNA-mediated protein knockdown
Stealth siRNAs targeting mouse p120RasGAP (no. 1, 5′-UGUCCAACACCUAACAACCAGUUUA-3′; no. 2, 5′-CACUACUGGCCAGCAUCCUACUAAA-3′) and siRNA duplexes with irrelevant sequences (Stealth RNAi negative control) as a control were purchased from Invitrogen (Carlsbad, CA). For siRNA-mediated gene silencing, 10 nM siRNA duplexes were introduced into C2C12 myoblasts by reverse transfection using Lipofectamine RNAiMAX reagent (Invitrogen) according to the manufacturer's instruction. The cells were replated 24–30 h after the transfection and subjected to the experiments.
Adenovirus vector construction and infection
To generate adenovirus vector expressing the human EphA2 mutant that lacks the cytoplasmic region (EphA2Δcyto), a cDNA fragment encoding amino acids 1–574 of human EphA2 was amplified by PCR using an expression vector for human EphA2 (a gift from A. Sakakibara, Nagoya University, Nagoya, Japan) as a template and was subcloned into pCMV-HA vector (Fukuhara et al., 2008). Then, a cDNA fragment encoding C-terminal HA-tagged EphA2Δcyto was excised and inserted into the pShuttle vector (Clontech, Mountain View, CA). The adenovirus was produced by using the Adeno-X system according to the manufacturer's protocol (Clontech). Recombinant adenovirus vectors encoding β-gal and caMEK1 were kindly provided by M. Matsuda (Kyoto University, Kyoto, Japan) and S. Kawashima (Kobe University, Kobe, Japan), respectively. For adenoviral infection, subconfluent C2C12 cells were infected with adenoviruses at the multiplicity of infection of 30 for 24 h and replated for the experiments.
Immunoprecipitation and Western blot analysis
Cells were washed with ice-cold phosphate-buffered saline (PBS), lysed in lysis buffer containing 50 mM Tris, pH 7.5, 150 mM NaCl, 1 mM sodium orthovanadate, 20 mM sodium fluoride, 1% Nonidet P-40, 0.1% SDS, 0.5% sodium deoxycholate, and 1× protease inhibitor cocktail (Roche Diagnostics), and centrifuged at 15,000 × g for 20 min at 4°C. The supernatant was used as precleared total-cell lysate. For the immunoprecipitation of EphA2, the cells were lysed in lysis buffer containing 20 mM Tris, pH 7.5, 150 mM NaCl, 3 mM EDTA, 1% Nonidet P-40, and 1× protease inhibitor cocktail. EphA2 was immunoprecipitated from the cleared lysates by incubation with anti-EphA2 antibody for 2 h at 4°C. Immunocomplexes were recovered with the aid of protein A–Sepharose beads (GE Healthcare Life Sciences). Aliquots of cell lysate and the immunoprecipitates were subjected to SDS–PAGE and Western blot analysis with the antibodies as indicated in the figure legends.
Immunocytochemistry
Cells plated on 3.5-cm collagen type I–coated plastic dishes (Iwaki Asahi Glass, Tokyo, Japan) were fixed with 2% formaldehyde in PBS for 15 min, permeabilized with 0.1% Triton X-100 for 5 min, and blocked with PBS containing 4% bovine serum albumin for 1 h. The cells were then stained with anti-MHC antibody for 1 h at room temperature. Protein reacting with the antibody was visualized with Alexa Fluor 488–conjugated secondary antibody. The nucleus was also poststained with Hoechst 33342 nuclear dye. Fluorescent images of Alexa Fluor 488 and Hoechst 33342 and phase-contrast images were recorded with an Olympus IX-81 inverted fluorescence microscope (Olympus Corporation, Tokyo, Japan) as described previously (Noda et al., 2010).
Reverse transcription-PCR
Total RNA was prepared from C2C12 myoblasts using TRIzol reagent (Invitrogen), and reverse-transcribed by random hexamer primers using Superscript II (Invitrogen) according to the manufacturer's instruction. PCR was performed using the gene-specific primers listed in the Supplemental Table S1. Amplification of glyceraldehyde-3-phosphate dehydrogenase was also performed in parallel as a control.
Detection of GTP-bound form of Ras
Ras activation was assessed using a pull-down technique. Cells were lysed at 4°C in a pull-down lysis buffer containing 20 mM Tris, pH 7.5, 100 mM NaCl, 10 mM MgCl2, 1% Triton X-100, 1 mM ethylene glycol tetraacetic acid, 1 mM dithiothreitol, 1 mM sodium orthovanadate, and 1× protease inhibitor cocktail. GTP-bound Ras was collected on the glutathione transferase-tagged Ras binding domain of Raf precoupled to glutathione-Sepharose beads. GTP-bound Ras and aliquots of total cell lysate were subjected to Western blot analysis with the antibodies as indicated in the figure legends.
Statistical analysis
The signal intensity of the band of Western blot analysis was calculated using Scion Image software. All data are expressed as means ± SD. Differences among multiple groups were compared by one-way analysis of variance followed by a post hoc comparison tested with the Bonferroni method. Values of p < 0.05 were considered significant.
Supplementary Material
Acknowledgments
We are grateful to G. Y. Koh (Korea Advanced Institute of Science and Technology) for COMP-Ang1, to A. Sakakibara (Nagoya University) for an expression vector for human EphA2, and to M. Matsuda (Kyoto University) and S. Kawashima (Kobe University) for the adenovirus encoding β-gal and caMEK1, respectively. We also thank K. Nakaoka (Osaka University, Osaka, Japan) and K. Miura for helpful discussion and M. Sone, K. Hiratomi, H. Yonekawa, and Y. Matsuura for technical assistance. This work was supported in part by grants from the Ministry of Education, Science, Sports and Culture of Japan (to S.F. and N.M.), the Ministry of Health, Labor, and Welfare of Japan (to N.M.), the Program for the Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (to S.F. and N.M.), the Naito Foundation (to S.F.), the Takeda Science Foundation (to S.F. and N.M.), the Sagawa Foundation for Promotion of Cancer Research (to S.F.), the Mochida Memorial Foundation for Medical and Pharmaceutical Research (to S.F.), the Kowa Life Science Foundation (to S.F.), the Kanae Foundation for the Promotion of Medical Science (to S.F.), the Novartis Foundation (Japan) for the Promotion of Science (to S.F.), the Senri Life Science Foundation (to S.F.), the Mitsubishi Foundation (to N.M.), the Japan Cardiovascular Research Foundation (to S.F.), and an AstraZeneca Research Grant (to N.M.).
Abbreviations used:
- Ang1
angiopoietin-1
- β-gal
β-galactosidase
- bFGF
basic fibroblast growth factor
- caMEK1
constitutively active form of MEK1
- COMP
cartilage oligomeric matrix protein
- EphA2Δcyto
EphA2 mutant that lacks the cytoplasmic region
- ERK
extracellular signal–regulated kinase
- FBS
fetal bovine serum
- Ig
immunoglobulin
- IGF
insulin-like growth factor
- MAPK
mitogen-activated protein kinase
- MEK1/2
MAPK/ERK kinase1/2
- MHC
myosin heavy chain
- p120RasGAP
p120 Ras GTPase-activating protein
- PI3K
phosphatidylinositol 3-kinase
- RTK
receptor tyrosine kinase
- siRNA
small interfering RNA.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-03-0183) on July 27, 2011.
REFERENCES
- Andres V, Walsh K. Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J Cell Biol. 1996;132:657–666. doi: 10.1083/jcb.132.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach LA, Salemi R, Leeding KS. Roles of insulin-like growth factor (IGF) receptors and IGF-binding proteins in IGF-II-induced proliferation and differentiation of L6A1 rat myoblasts. Endocrinology. 1995;136:5061–5069. doi: 10.1210/endo.136.11.7588242. [DOI] [PubMed] [Google Scholar]
- Bennett AM, Tonks NK. Regulation of distinct stages of skeletal muscle differentiation by mitogen-activated protein kinases. Science. 1997;278:1288–1291. doi: 10.1126/science.278.5341.1288. [DOI] [PubMed] [Google Scholar]
- Carnac G, Primig M, Kitzmann M, Chafey P, Tuil D, Lamb N, Fernandez A. RhoA GTPase and serum response factor control selectively the expression of MyoD without affecting Myf5 in mouse myoblasts. Mol Biol Cell. 1998;9:1891–1902. doi: 10.1091/mbc.9.7.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrasse S, Comunale F, Fortier M, Portales-Casamar E, Debant A, Gauthier-Rouviere C. M-cadherin activates Rac1 GTPase through the Rho-GEF trio during myoblast fusion. Mol Biol Cell. 2007;18:1734–1743. doi: 10.1091/mbc.E06-08-0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrasse S, Meriane M, Comunale F, Blangy A, Gauthier-Rouviere C. N-cadherin-dependent cell-cell contact regulates Rho GTPases and beta-catenin localization in mouse C2C12 myoblasts. J Cell Biol. 2002;158:953–965. doi: 10.1083/jcb.200202034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N, Brantley D, Fang WB, Liu H, Fanslow W, Cerretti DP, Bussell KN, Reith A, Jackson D, Chen J. Inhibition of VEGF-dependent multistage carcinogenesis by soluble EphA receptors. Neoplasia. 2003;5:445–456. doi: 10.1016/s1476-5586(03)80047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmons DR. Role of IGF-I in skeletal muscle mass maintenance. Trends Endocrinol Metab. 2009;20:349–356. doi: 10.1016/j.tem.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Coolican SA, Samuel DS, Ewton DZ, McWade FJ, Florini JR. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J Biol Chem. 1997;272:6653–6662. doi: 10.1074/jbc.272.10.6653. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Son AI, Komlos D, Sun Y, Kleiman NJ, Zhou R. Loss of ephrin-A5 function disrupts lens fiber cell packing and leads to cataract. Proc Natl Acad Sci USA. 2008;105:16620–16625. doi: 10.1073/pnas.0808987105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Alvaro C, Martinez N, Rojas JM, Lorenzo M. Sprouty-2 overexpression in C2C12 cells confers myogenic differentiation properties in the presence of FGF2. Mol Biol Cell. 2005;16:4454–4461. doi: 10.1091/mbc.E05-05-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elowe S, Holland SJ, Kulkarni S, Pawson T. Downregulation of the Ras-mitogen-activated protein kinase pathway by the EphB2 receptor tyrosine kinase is required for ephrin-induced neurite retraction. Mol Cell Biol. 2001;21:7429–7441. doi: 10.1128/MCB.21.21.7429-7441.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewton DZ, Roof SL, Magri KA, McWade FJ, Florini JR. IGF-II is more active than IGF-I in stimulating L6A1 myogenesis: greater mitogenic actions of IGF-I delay differentiation. J Cell Physiol. 1994;161:277–284. doi: 10.1002/jcp.1041610212. [DOI] [PubMed] [Google Scholar]
- Florini JR, Ewton DZ, Coolican SA. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev. 1996;17:481–517. doi: 10.1210/edrv-17-5-481. [DOI] [PubMed] [Google Scholar]
- Florini JR, Magri KA, Ewton DZ, James PL, Grindstaff K, Rotwein PS. “Spontaneous” differentiation of skeletal myoblasts is dependent upon autocrine secretion of insulin-like growth factor-II. J Biol Chem. 1991;266:15917–15923. [PubMed] [Google Scholar]
- Fukuhara S, Sako K, Minami T, Noda K, Kim HZ, Kodama T, Shibuya M, Takakura N, Koh GY, Mochizuki N. Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat Cell Biol. 2008;10:513–526. doi: 10.1038/ncb1714. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol. 2005;25:136–146. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavard J, Marthiens V, Monnet C, Lambert M, Mege RM. N-cadherin activation substitutes for the cell contact control in cell cycle arrest and myogenic differentiation: involvement of p120 and beta-catenin. J Biol Chem. 2004;279:36795–36802. doi: 10.1074/jbc.M401705200. [DOI] [PubMed] [Google Scholar]
- Guasconi V, Puri PL. Chromatin: the interface between extrinsic cues and the epigenetic regulation of muscle regeneration. Trends Cell Biol. 2009;19:286–294. doi: 10.1016/j.tcb.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez J, Brandan E. A novel mechanism of sequestering fibroblast growth factor 2 by glypican in lipid rafts, allowing skeletal muscle differentiation. Mol Cell Biol. 2010;30:1634–1649. doi: 10.1128/MCB.01164-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun G, et al. EPHA2 is associated with age-related cortical cataract in mice and humans. PLoSGenet. 2009;5:e1000584. doi: 10.1371/journal.pgen.1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliman P, Canicio J, Shepherd PR, Beeton CA, Testar X, Palacin M, Zorzano A. Insulin-like growth factors require phosphatidylinositol 3-kinase to signal myogenesis: dominant negative p85 expression blocks differentiation of L6E9 muscle cells. Mol Endocrinol. 1998;12:66–77. doi: 10.1210/mend.12.1.0047. [DOI] [PubMed] [Google Scholar]
- Kaliman P, Vinals F, Testar X, Palacin M, Zorzano A. Phosphatidylinositol 3-kinase inhibitors block differentiation of skeletal muscle cells. J Biol Chem. 1996;271:19146–19151. doi: 10.1074/jbc.271.32.19146. [DOI] [PubMed] [Google Scholar]
- Kang JS, Bae GU, Yi MJ, Yang YJ, Oh JE, Takaesu G, Zhou YT, Low BC, Krauss RS. A Cdo-Bnip-2-Cdc42 signaling pathway regulates p38alpha/beta MAPK activity and myogenic differentiation. J Cell Biol. 2008;182:497–507. doi: 10.1083/jcb.200801119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JS, Yi MJ, Zhang W, Feinleib JL, Cole F, Krauss RS. Netrins and neogenin promote myotube formation. J Cell Biol. 2004;167:493–504. doi: 10.1083/jcb.200405039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Ryu YS, Kwak HJ, Ahn SY, Oh JL, Yancopoulos GD, Gale NW, Koh GY. EphB ligand, ephrinB2, suppresses the VEGF- and angiopoietin-1-induced Ras/mitogen-activated protein kinase in venous endothelial cells. FASEB J. 2002;16:1126–1128. doi: 10.1096/fj.01-0805fje. [DOI] [PubMed] [Google Scholar]
- Koyama T, et al. Interaction of scaffolding adaptor protein Gab1 with tyrosine phosphatase SHP2 negatively regulates IGF-I-dependent myogenic differentiation via the ERK1/2 signaling pathway. J Biol Chem. 2008;283:24234–24244. doi: 10.1074/jbc.M803907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss RS. Regulation of promyogenic signal transduction by cell-cell contact and adhesion. Exp Cell Res. 2010;316:3042–3049. doi: 10.1016/j.yexcr.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai KO, Chen Y, Po HM, Lok KC, Gong K, Ip NY. Identification of the Jak/Stat proteins as novel downstream targets of EphA4 signaling in muscle: implications in the regulation of acetylcholinesterase expression. J Biol Chem. 2004;279:13383–13392. doi: 10.1074/jbc.M313356200. [DOI] [PubMed] [Google Scholar]
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- Lovett FA, Gonzalez I, Salih DA, Cobb LJ, Tripathi G, Cosgrove RA, Murrell A, Kilshaw PJ, Pell JM. Convergence of Igf2 expression and adhesion signalling via RhoA and p38 MAPK enhances myogenic differentiation. J Cell Sci. 2006;119:4828–4840. doi: 10.1242/jcs.03278. [DOI] [PubMed] [Google Scholar]
- Lu M, Krauss RS. N-cadherin ligation, but not Sonic hedgehog binding, initiates Cdo-dependent p38alpha/beta MAPK signaling in skeletal myoblasts. Proc Natl Acad Sci USA. 2010;107:4212–4217. doi: 10.1073/pnas.0908883107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae M, Neve RM, Rodriguez-Viciana P, Haqq C, Yeh J, Chen C, Gray JW, McCormick F. A conditional feedback loop regulates Ras activity through EphA2. Cancer Cell. 2005;8:111–118. doi: 10.1016/j.ccr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Miao H, Nickel CH, Cantley LG, Bruggeman LA, Bennardo LN, Wang B. EphA kinase activation regulates HGF-induced epithelial branching morphogenesis. J Cell Biol. 2003;162:1281–1292. doi: 10.1083/jcb.200304018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H, Wei BR, Peehl DM, Li Q, Alexandrou T, Schelling JR, Rhim JS, Sedor JR, Burnett E, Wang B. Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK pathway. Nat Cell Biol. 2001;3:527–530. doi: 10.1038/35074604. [DOI] [PubMed] [Google Scholar]
- Miura K, Nam JM, Kojima C, Mochizuki N, Sabe H. EphA2 engages Git1 to suppress Arf6 activity modulating epithelial cell-cell contacts. Mol Biol Cell. 2009;20:1949–1959. doi: 10.1091/mbc.E08-06-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda K, Zhang J, Fukuhara S, Kunimoto S, Yoshimura M, Mochizuki N. Vascular endothelial-cadherin stabilizes at cell-cell junctions by anchoring to circumferential actin bundles through α- and β-catenins in cyclic AMP-Epac-Rap1 signal-activated endothelial cells. Mol Biol Cell. 2010;21:584–596. doi: 10.1091/mbc.E09-07-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parri M, Buricchi F, Taddei ML, Giannoni E, Raugei G, Ramponi G, Chiarugi P. EphrinA1 repulsive response is regulated by an EphA2 tyrosine phosphatase. J Biol Chem. 2005;280:34008–34018. doi: 10.1074/jbc.M502879200. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer. 2010;10:165–180. doi: 10.1038/nrc2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlath GK. Spatial and functional restriction of regulatory molecules during mammalian myoblast fusion. Exp Cell Res. 2010;316:3067–3072. doi: 10.1016/j.yexcr.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamah SM, et al. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell. 2001;105:233–244. doi: 10.1016/s0092-8674(01)00314-2. [DOI] [PubMed] [Google Scholar]
- Swartz ME, Eberhart J, Pasquale EB, Krull CE. EphA4/ephrin-A5 interactions in muscle precursor cell migration in the avian forelimb. Development. 2001;128:4669–4680. doi: 10.1242/dev.128.23.4669. [DOI] [PubMed] [Google Scholar]
- Taddei ML, et al. Kinase-dependent and -independent roles of EphA2 in the regulation of prostate cancer invasion and metastasis. Am J Pathol. 2009;174:1492–1503. doi: 10.2353/ajpath.2009.080473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaesu G, Kang JS, Bae GU, Yi MJ, Lee CM, Reddy EP, Krauss RS. Activation of p38alpha/beta MAPK in myogenesis via binding of the scaffold protein JLP to the cell surface protein Cdo. J Cell Biol. 2006;175:383–388. doi: 10.1083/jcb.200608031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MV. Muscle differentiation: how two cells become one. Curr Biol. 2002;12:R224–R228. doi: 10.1016/s0960-9822(02)00757-1. [DOI] [PubMed] [Google Scholar]
- Tong J, Elowe S, Nash P, Pawson T. Manipulation of EphB2 regulatory motifs and SH2 binding sites switches MAPK signaling and biological activity. J Biol Chem. 2003;278:6111–6119. doi: 10.1074/jbc.M208972200. [DOI] [PubMed] [Google Scholar]
- Tortorella LL, Milasincic DJ, Pilch PF. Critical proliferation-independent window for basic fibroblast growth factor repression of myogenesis via the p42/p44 MAPK signaling pathway. J Biol Chem. 2001;276:13709–13717. doi: 10.1074/jbc.M100091200. [DOI] [PubMed] [Google Scholar]
- White MF. Insulin signaling in health and disease. Science. 2003;302:1710–1711. doi: 10.1126/science.1092952. [DOI] [PubMed] [Google Scholar]
- Yokoyama T, Takano K, Yoshida A, Katada F, Sun P, Takenawa T, Andoh T, Endo T. DA-Raf1, a competent intrinsic dominant-negative antagonist of the Ras-ERK pathway, is required for myogenic differentiation. J Cell Biol. 2007;177:781–793. doi: 10.1083/jcb.200703195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.