Abstract
Background
Non-tuberculous mycobacteria recovered from respiratory tract specimens are emerging confounder organisms for the laboratory diagnosis of tuberculosis worldwide. There is an urgent need for new techniques to rapidly identify mycobacteria isolated in clinical practice. Matrix-assisted laser desorption time-of-flight mass spectrometry (MALDI-TOF MS) has previously been proven to effectively identify mycobacteria grown in high-concentration inocula from collections. However, a thorough evaluation of its use in routine laboratory practice has not been performed.
Methodology
We set up an original protocol for the MALDI-TOF MS identification of heat-inactivated mycobacteria after dissociation in Tween-20, mechanical breaking of the cell wall and protein extraction with formic acid and acetonitrile. By applying this protocol to as few as 105 colony-forming units of reference isolates of Mycobacterium tuberculosis, Mycobacterium avium, and 20 other Mycobacterium species, we obtained species-specific mass spectra for the creation of a local database. Using this database, our protocol enabled the identification by MALDI-TOF MS of 87 M. tuberculosis, 25 M. avium and 12 non-tuberculosis clinical isolates with identification scores ≥2 within 2.5 hours.
Conclusions
Our data indicate that MALDI-TOF MS can be used as a first-line method for the routine identification of heat-inactivated mycobacteria. MALDI-TOF MS is an attractive method for implementation in clinical microbiology laboratories in both developed and developing countries.
Introduction
Pulmonary tuberculosis is a deadly, contagious infection caused by mycobacteria belonging to the Mycobacterium tuberculosis complex (MTC) [1]. Tuberculosis remains in the top 10 health priorities in many developing countries, and with more than 1.3 million new cases yearly [2] and the emergence and rapid spread of multidrug-resistant and extensively drug-resistant M. tuberculosis strains in such countries, the rapid laboratory diagnosis of tuberculosis is essential. However, this task has been complicated by the emergence of non-tuberculous mycobacteria present in respiratory tract specimens from patients suspected of pulmonary tuberculosis, even in countries with a high incidence and burden of pulmonary tuberculosis [3]. For example, a recent study showed that 52.5% of non-tuberculous isolates in Taiwan were from respiratory tract specimens [4]. Indeed, the actinobacterial genus Mycobacterium comprises more than 120 species including both obligate human pathogens, such as the leprosy agent Mycobacterium leprae [5], and the MTC members and opportunistic pathogens, such as the Mycobacterium avium complex and the Mycobacterium abscessus group mycobacteria and harmless environmental organisms [6].
The identification of mycobacteria has long been based on conventional phenotypic methods requiring extensive incubation times and delaying the final diagnosis far beyond medical recommendations [3]. In some developing countries, these methods have been successfully replaced by the direct microscopic detection of cords specific for M. tuberculosis after a 5–10-day incubation of the specimen in either agar or miniaturized liquid medium [7], [8]. However, these techniques still require some degree of expertise and rely on microscopic examination, an operator-dependent method that is prone to errors. While some other techniques for the phenotypic analysis of mycobacteria, such as the high-pressure liquid chromatography of cell wall mycolic acids, proved efficient at identification, they have not been implemented as a routine technique in clinical microbiology laboratories [9], [10]. Molecular methods including PCR-based hybridization and sequencing methods [11], [12] are now routinely used for the identification of mycobacteria in laboratories in developed countries. Recently, confined real-time PCR has been shown to be effective for the rapid detection of tuberculosis, including rifampin-resistant tuberculosis, in respiratory tract specimens [13], [14]. These molecular techniques still require specific technical expertise and technically demanding, expensive equipment [15]–[17].
Several studies have provided the proof-of-concept that matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) could also identify mycobacteria [18]–[21]. Indeed, this technique has emerged over the last few years as a revolutionary technique for the routine identification of bacterial isolates [22]–[32]. MALDI-TOF MS has been shown to yield accurate results within hours at a lower price than any other method routinely used in clinical microbiology laboratories [32]. However, the studies supporting this method analyzed high-concentration inocula [18]–[21]. In a few studies, mycobacteria were analyzed without previous inactivation [21], [33], which does not comply with current standards for the manipulation of harmful organisms.
We therefore aimed to further explore various inactivation protocols and solvents that one might consider for mycobacteria with the perspective to decrease the inoculum required for the accurate MALDI-TOF MS identification of mycobacteria, thus speeding the diagnosis of tuberculosis and other non-tuberculosis, mycobacterial infections.
Methods
Mycobacterium strains
As a first step, we used 11 strains of mycobacteria representative of 7 M. tuberculosis complex species, 12 strains of mycobacteria representative of 11 M. avium complex species and 20 additional mycobacteria strains representative of 16 additional non-tuberculous species grown from our collection to set up a reference database (Table 1). In a second step, all the mycobacteria isolated from February 2010 to May 2011 from patients in the Mycobacteria Reference Laboratory, Institut Hospitalier Universitaire POLMIT, Marseilles, France were prospectively analyzed by MALDI-TOF MS. The isolates were cultured in MGIT tubes (Becton Dickinson, Pont-De-Claix, France) or on a home-made 5% sheep blood-agar (BioMérieux, La Balme-les-Grottes, France) for 2–45 days at 32°C or 37°C as previously described [34], [35]. Gram staining was used to ensure the absence of any contaminant organisms in the culture, and standard identification of mycobacteria was performed by observing acid-fast organisms after Ziehl-Neelsen staining, rpoB sequencing for non-tuberculosis mycobacteria [36] and exact tandem repeat D sequencing (ETR-D) for MTC [12]. We tested two protocols for the inactivation of mycobacteria before the MALDI-TOF MS by incubating 1 mL of the mycobacterial suspension obtained by suspending colonies from solid medium or 3 mL of mycobacteria suspension of liquid medium either in a water bath at 95°C for one hour (Table 2, protocol A) [37] or in 70% ethanol for 10 minutes (Table 2, protocol B) [19]. The effectiveness of each inactivation protocol was tested in triplicate by inoculating 50 µl inactivated M. tuberculosis H37Rv, M. bovis BCG and M. fortuitum suspensions in MGIT tubes incubated in parallel at 37°C. The absence of any visible growth after incubation for 45 days was taken as an evidence for the effective inactivation of the mycobacteria.
Table 1. Reference mycobacteria strains used to create a MALDI-TOF MS database for the identification of mycobacteria.
| Mycobacterium tuberculosis complex | |
| M. tuberculosis | CIP 103471; H37Rv; CH6431 |
| M. africanum | CIP 105147 |
| M. bovis | CIP 671203; CIP 108541T; CIP105050 |
| M. bovis BCG Tokyo | ATCC 35737 |
| M. caprae | CIP105776 |
| M. microti | CRBIP7.40 |
| M. canettii | CIP 140010059T |
| M. pinnipedii | ATCC-BAA-688 |
*M. indicus pranii is not a validated species.
Table 2. Inactivation protocols and solvents used to treat mycobacteria for identification by MALDI-TOF MS.
| Protocol | Inactivation protocol | |
| A | 95°C/60 minutes | |
| B | 70% ethanol/10 minutes |
MALDI-TOF MS protocol for mycobacteria
Several protocols were tested before finalizing the protocol for the preparation of inactivated mycobacteria. Because some mycobacteria, including the MTC mycobacteria, are known to form cords which may hamper the MALDI-TOF MS analysis, we tested several compounds for their ability to solubilize such aggregates. We tested the incubation of mycobacteria in a detergent solution of 0.5% Tween-20 (Table 2, protocol 1) or β-mercaptoethanol, a chemical known to reduce disulfide bonds in proteins [38] (Table 2, protocol 2). We also tested the effects of using acetone (Table 2, protocol 3) or distilled water as a washing solution; absolute ethanol for protein precipitation (Table 2, protocol 4); and 0.1 M NaOH (Table 2, protocol 5), 10% v/v trifluoroacetic acid (TFA) (Table 2, protocol 6), 0.1 M HCl (Table 2, protocol 7) or 70% formic acid for protein elution (Table 2, protocol 8). Based on the results of these preliminary tests, protocol 8 was used for the final experiments. For each parameter, we compared the results obtained with M. tuberculosis H37Rv, M. bovis BCG and Mycobacterium fortuitum with the definitive reference profile, in terms of identification score (from 0 to 3), number of peaks in the 5,000–20,000 m/z range, and the intensity of the peaks, as determined by the Flex Analysis 3.0 software (Bruker Daltonics). Each test was done ten times.
Sensitivity of MALDI-TOF MS detection
In order to determine the sensitivity of our final protocol, serial 10-fold dilutions from 108 to 103 mycobacteria/mL were prepared for M. tuberculosis H37Rv, M. bovis BCG and Mycobacterium fortuitum. For each suspension, the inoculum was calibrated using two methods. First, by counting the mean number of mycobacteria observed in five areas on three slides of Ziehl-Neelsen stained mycobacteria observed at a 1,000× magnification. Second, by culturing the dilutions on blood agar plates for 5 weeks and counting the number of colonies. In order to facilitate this quantification, ImageJ colony-counting software (http://rsb.info.nih.gov/ij/) was used. The sensitivity was then calculated by reporting the colony number with the fold dilutions.
Mycobacterial MALDI-TOF database
To complement the Bruker Daltonics database, we analyzed a collection of reference strains for mycobacteria using protocol 8, as presented below. For each reference mycobacterial strain, one colony was selected from the agar plate for the solid medium, and approximately 3 ml of mycobacterial suspension was extracted for the liquid medium and was manipulated as described above. Each reference strain was analyzed four times using the same protocol. For each sample, 1.5 µL was deposited on a polished steel MSP 96 target plate (Bruker Daltonics, Bremen, Germany) and 1.5 µL of matrix solution (saturated α-cyano-4-hydroxycinnamic acid, 50% acetonitrile, 2.5% trifluoroacetic acid) was then added. The samples were air-dried for 5 minutes before being processed in the mass spectrometer. To validate the analysis of a whole MSP 96 target, Bacterial Test Standard (Escherichia coli DH5alpha protein extract; Bruker Daltonics ref 255343) was used as a positive control and non-inoculated matrix solution (alpha-cyano-4-Hydroxycinnamic acid) was used as a negative control on each plate. The analyses were performed using the Microflex MALDI-TOF MS spectrometer (Bruker Daltonics) at 337 nm with the Flex control software (Bruker Daltonics). Positive ions were extracted with an accelerating voltage of 20 KV in linear mode. The spectra were analyzed within an m/z range of 2,000 to 20,000. Four raw spectra were automatically acquired using the Flexcontrol 3.0 software and then compared with the Bruker Daltonics database using the MALDI Biotyper 2.0 Bruker Daltonics software. To validate the analysis using the MALDI Biotyper software, the identification of the positive control was required to be E. coli with an identification score ≥2, and the negative control had to yield a non-identifying score ≤1.7.
The profiles of the 11 reference strains for the M. tuberculosis complex, 12 reference strains for the M. avium complex and 20 reference strains for the other non-tuberculous mycobacteria were compared and analyzed using the Biotyper 2.0 software, and the MALDI-TOF MS spectra were entered into a local Mycobacterium database that was further merged with the 3,438-entry Bruker Daltonics database (June 2010).
MALDI-TOF MS identification of mycobacteria
All the clinical Mycobacterium isolates prospectively obtained from the Mycobacteria Reference Laboratory, Institut Hospitalier Universitaire POLMIT, Marseilles, France from February 2010 to May 2011 were prospectively analyzed using protocol 8, as presented below. To identify each clinical isolate, the corresponding spectra were then compared with those in the local Mycobacterium database merged with the Bruker Daltonics database using the Biotyper software 2.0.
Results
MALDI-TOF MS protocol for mycobacteria
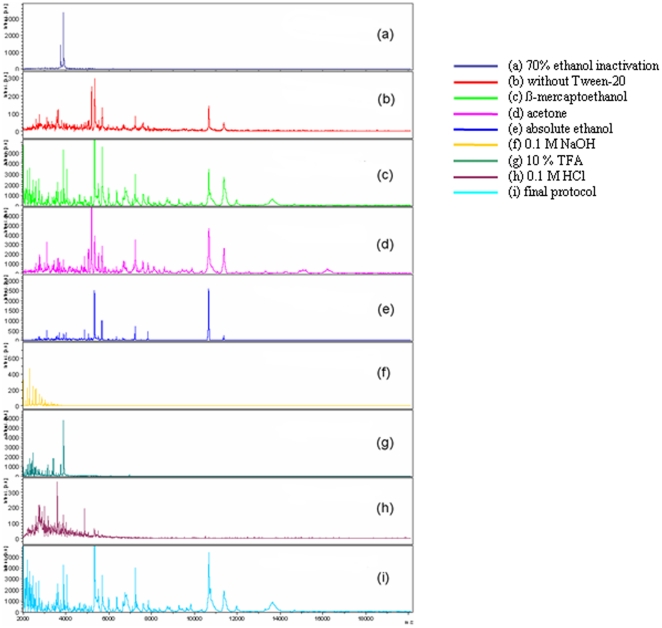
We observed no growth of M. tuberculosis, M. bovis BCG and M. fortuitum mycobacteria over a 45-day incubation after either heat inactivation or a 10-minute incubation with 70% ethanol. However, the incubation of Middlebrook 7H9-grown mycobacteria in ethanol yielded protein profiles of lower quality (Figure 1 part a) than those obtained after heat-inactivation. Therefore, the 1-hour incubation at 95°C was incorporated into our final protocol. Likewise, because incubating mycobacteria in 0.5% Tween-20 yielded spectra of better quality than mycobacteria run in parallel without Tween-20 treatment (Table 3.1; Figure 1 part b), 0.5% Tween-20 treatment was incorporated into our final protocol. We found that the suspension of mycobacteria in β-mercaptoethanol did not improve the quality of spectra (Table 3.2; Figure 1 part c), so β-mercaptoethanol was not used in our final protocol. We then tested the use of acetone and distilled water as washing solutions. No difference was observed between the spectra; both resulted in adequate identification with almost the same intensity and score (Table 3.3; Figure 1 part d). Acetone took longer to manipulate and to dry than distilled water, which was therefore retained in the final protocol. We observed that protein precipitation using absolute ethanol (Table 3.4; Figure 1 part e) did not improve the quality of the spectra when compared with distilled water alone. We also tested several protein extraction and elution solvents. No peak was observed in the 5,000–15,000 Kda range with 0.1 M NaOH (Table 3.5; Figure 1 part f) or 0.1 M HCl (Table 3.6; Figure 1 part.f), only one peak was observed with 10% TFA (Table 3.7; Figure 1 part g) and 11 peaks were obtained using 70% HCOOH (Table 3.8; Figure 1 part i). We therefore incorporated 70% HCOOH combined with 100% acetonitrile into our final protocol.
Figure 1. MALDI-TOF MS spectra obtained from the M. tuberculosis H37Rv strain using the protocols tested herein.
Protocol A was used throughout; protocol B was used where mentioned (Line 2).
Table 3. Spectra obtained from Mycobacterium tuberculosis H37Rv using the tested protocols.
| Protocol | Number of peaks | Mean intensity (+/− standard deviation) of the most prevalent peaks | Identification score average | |||||
| 5340 m/z | 5500 m/z | 5690 m/z | 10670 m/z | 10700 m/z | 11380 m/z | |||
| (1) | 8 | 270(+/−78) | 171(+/−57) | 139(+/−45) | 118(+/−37) | 61(+/−14) | 44(+/−12) | 1.45 |
| (2) | 8 | 5846(+/−2797) | 1804(+/−1007) | 2544(+/−1248) | 1968(+/−960) | 1400(+/−594) | 1518(+/−213) | 1.87 |
| (3) | 11 | 5349(+/−4850) | 4037(+/−3835) | 4314(+/−2912) | 2847(+/−2851) | 2077(+/−2115) | 2557(+/−2229) | 2.17 |
| (4) | 11 | 4798(+/−5400) | 2531(+/−2324) | 1950(+/−2252) | 1681(+/−1709) | 949(+/−1258) | 675(+/−1380) | 2.43 |
| (5) | 0 | − | − | |||||
| (6) | 0 | − | − | |||||
| (7) | 1 | 1007 (+/− 260) | − | |||||
| (8) | 11 | 6166(+/−2477) | 2910(+/−2503) | 3122(+/−2212) | 2276(+/−1580) | 2435(+/−1539) | 2148(+/−1708) | 2.51 |
Tests were done 10 times by using inactivation protocol A.
Protocol 8 (Table 3.9; Figure 1 part i) was used as the final protocol. Colonies from each isolate were collected in a screw-cap Eppendorf tube containing 500 µl of HPLC-grade water and 0.5% Tween-20 and inactivated by heating at 95°C for one hour [37]. The mycobacterial suspensions were then washed twice with 500 µL of HPLC-grade water and centrifuged at 13,000×g for 10 min. The pellet was vortexed along with 500 µL of HPLC-grade water and 0.3 g acid-washed glass beads (diameter ≤106 µm, Sigma) in a BIO 101 FastPrep instrument (Qbiogene, Strasbourg, France) at level 6.5 (full speed) for 3 min. The suspension was centrifuged at 13,000×g for 10 min. The pellet was then resuspended in 5 to 50 µl 70% HCOOH and 5 to 50 µl 100% acetonitrile, depending on the volume of the pellet. The suspension was centrifuged at 11,000×g for 1 min and 1.5 µl of the supernatant was deposited on a target plate (Bruker Daltonics, Bremen, Germany) in four replicates. Finally, 1.5 µl matrix solution (saturated α cyano-4-hydroxycinnamic acid, 50% acetonitrile, 2.5% TFA) was added and allowed to co-crystallize with the sample pellet at room temperature.
Mycobacterium MALDI-TOF database
Using our optimized protocol, the lower limit of detection of MALDI-TOF MS was about 105 CFU/mL, which corresponds to 103 mycobacteria deposited on the MALDI-TOF MS plate. All of the mycobacteria reference strains tested by mass spectrometry had a species-specific spectrum profile. Spectrum profiles were deposited in a spectrum database that is an internal MALDI-TOF database of La Timone hospital Which combined with the Bruker database yielded a total of 141 reference spectra comprising of 62 Mycobacterium species (Table S1).
MALDI-TOF identification of mycobacteria
In all cases, the negative control wells yielded either no visible peaks or faint profiles that were not interpreted by the system, and the positive controls yielded the expected protein profile. A total of 124 clinical isolates were prospectively analyzed within a 16-month period by MALDI-TOF MS and all tested mycobacterial isolates yielded a visible protein profile with an identification score of 2 to 2.7. Of the clinical isolates, 87 were identified as M. tuberculosis, 17 as M. avium subsp. hominisuis, 7 as M. intracellulare, one as M. marsiliense, 7 M. chelonae, 2 as M. abscessus, 2 as M. kansasii and one as M. fortuitum. In all cases, the MALDI-TOF MS identification agreed with the molecular identification routinely performed in our laboratory using either ETR-D sequencing for the identification of MTC species [12] or partial rpoB sequencing for the other mycobacteria [36].
Discussion
The protein spectra obtained from the reference and clinical mycobacterial strains in this study were interpreted as accurate and specific. In each case, the positive controls yielded the expected and identifying profiles, whereas the negative controls yielded either no detectable peaks or faint profiles that were not identified by the system. Moreover, the identity of both the reference strains and the clinical isolates were validated by partial rpoB sequencing in parallel with the MALDI-TOF MS identification [36].
In this study, MALDI-TOF MS spectra were obtained after the inactivation of the isolates. M. tuberculosis complex mycobacteria are harmful organisms that have previously been found to be responsible for cases of laboratory-acquired tuberculosis in laboratory staff [39]. M. tuberculosis species are Biosafety Level 3 organisms that must be inactivated prior to manipulation outside a biological safety cabinet to avoid potentially exposing the laboratory staff to contamination [39], [40]. Because the tuberculous nature of AFB organisms cannot be determined by microscopic observation, we suggest that systematic inactivation of AFB organisms is warranted prior to their identification by MALDI-TOF MS. Although some previous studies did not mention how the mycobacteria were inactivated prior to MALDI-TOF MS analysis [21], [33], ethanol has been used for this purpose [19], [41]. We noticed that the latter protocol relied on the centrifugation of mycobacteria prior to ethanol inactivation, which carries the risk of generating MTC mycobacteria aerosols that are potentially harmful for the laboratory staff. We aimed to avoid any centrifugation of non-inactivated mycobacteria and therefore chose to inactivate the mycobacteria by heating, a procedure previously reported to effectively inactivate mycobacteria, including MTC mycobacteria, after 30 minutes at 95°C [18], [37], [42], [43]. We were also concerned that the use of ethanol could interfere with the quality of the spectra because ethanol has a protein precipitating action. Indeed, when we used ethanol to kill mycobacteria in liquid medium, it precipitated all the proteins in the medium, including those of the mycobacteria. As previously reported [21], the composition of the medium (either Middlebrook 7H9 liquid medium or solid blood-agar medium) did not interfere with identification by mass spectrometry, especially after the incorporation of washing steps.
In this study, the addition of Tween-20 during the inactivation phase increased the quality of the MALDI-TOF MS spectra of mycobacteria. Tween-20 is a detergent that disrupts the cords formed by mycobacteria. Bruker and the authors of a later work [18] used a micropestle for this step. However, we chose to use Tween-20, which is easier to manipulate and more cost-effective. Breaking the cell wall was another crucial step that facilitated the extraction of mycobacterial proteins. Indeed, we previously observed that disrupting the strong cell wall of mycobacteria was necessary for their proper molecular detection [44]. This was also the case for the molecular detection of methanogenic Archaea, another group of prokaryotes with a strong cell wall, using either a molecular approach [45] or MALDI-TOF MS (Dridi and Drancourt, unpublished data). Using the protocol described here allowed us to decrease the required inoculum of mycobacteria down to 105 CFU/mL, which is the lowest inoculum reported for the efficient identification of mycobacteria by MALDI-TOF MS. Although the initial studies and some recent reports used a non-calibrated inoculum [18], [19], [21], more recent studies incorporated a high-concentration (108–1010 CFU/mL) inoculum [33]. Obtaining such a high-concentration inoculum requires extensive, time-consuming culture and exposes laboratory personnel to potentially harmful pathogens for extended periods of time.
Over the past five years, our Mycobacteriology Reference Laboratory, serving a cosmopolitan area with 1–2 million inhabitants identified 354 new cases of Mycobacterium infection, including 295 new cases of M. tuberculosis infection and 33 new cases of MAC infections (2005 to 2009). Other non-tuberculous mycobacteria isolated in our laboratory during this period were M. chelonae (7 cases), M. lentiflavum (5 cases), M. fortuitum (3 cases), M. xenopi (3 cases), M. gordonae (3 cases), M. kansasii (2 cases), M. marinum (2 cases) and M. phocaicum (1 case). We set up a MALDI-TOF MS database that included the Mycobacterium organisms most frequently identified over the last several years. This local database perfectly completed the original Bruker Daltonics database, which contained approximately 80 Mycobacterium spectra representative of 40 Mycobacterium species (June 2010 version) (Table S1). Although this database is enriched in Mycobacterium species most frequently encountered in our geographical region, nevertheless it is the largest Mycobacterium MALDI-TOF database ever published, comprising of 62 species and 177 references to be compared with previous reports comprising of 4–53 species and 5–53 reference spectra [18]–[21]. Therefore, present database could likely serve as an efficient tool for the identification of mycobacteria in other geographical regions. Although the M. tuberculosis complex and M. avium complex organisms together represented more than 90% of Mycobacterium isolates in our laboratory, the Bruker Daltonics database included only eight M. tuberculosis spectra and six M. avium spectra out of a total of 40 Mycobacterium spectra. In contrast, we previously noted that a library of ≥10 reference spectra was necessary for the accurate MALDI-TOF MS identification of cultured bacteria [25]. In this work, we therefore expanded the Bruker Daltonics database by 37% by adding a total of 43 reference MALDI-TOF MS spectra, and we doubled the number of MTC references and tripled those of MAC.
In this study, we evaluated the prospective use of MALDI-TOF MS for the routine identification of mycobacteria isolates from specimens in patients suspected of pulmonary tuberculosis. While previous studies provided the proof-of-concept that MALDI-TOF MS could be used for the first-line identification of mycobacteria [18], [19], [21], these studies were based on the retrospective analysis of mycobacteria retrieved from collections. The few studies incorporating both M. tuberculosis complex isolates and non-tuberculous mycobacteria suffered from limitations that would prevent their application to routine laboratory use.
In conclusion, MALDI-TOF MS has been successfully adapted for the routine identification of mycobacteria. This revolutionary technique allows for the easier and faster diagnosis of mycobacterial pathogens compared with conventional phenotypic identification methods. MALDI-TOF MS therefore appears to be an alternative first-line approach to the routine identification of the vast majority of bacteria commonly cultured in the clinical microbiology laboratory [25], [32].
Supporting Information
List of 62 Mycobacterium species included in the MALDI-TOF database. This database combined Bruker and home-made databases. For each species/sub-species, the number of reference spectra is indicated, for a total of 177 references.
(DOC)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors have no support or funding to report.
References
- 1.Djelaoudji Z, Raoult D, Drancourt M. Palaeogenomics of Mycobacterium tuberculosis: epidemic bursts with a degrading genome. Lancet Infect Dis. 2011 doi: 10.1016/S1473-3099(11)70093-7. in press. [DOI] [PubMed] [Google Scholar]
- 2.Guy ES, Mallampalli A. Managing tuberculosis in the 21st century: existing and novel drug therapies. Ther Adv Respir Dis. 2008;2:401–408. doi: 10.1177/1753465808099522. [DOI] [PubMed] [Google Scholar]
- 3.McGrath EE, McCabe J, Anderson PB. Guidelines on the diagnosis and treatment of pulmonary non-tuberculous mycobacteria infection. Int J Clin Pract. 2008;62:1947–1955. doi: 10.1111/j.1742-1241.2008.01891.x. [DOI] [PubMed] [Google Scholar]
- 4.Chou CH, Chen HY, Chen CY, Huang CT, Lai CC, et al. Clinical features and outcomes of disseminated infections caused by non-tuberculous mycobacteria in a university hospital in Taiwan, 2004–2008. Scand J Infect Dis. 2011;43:8–14. doi: 10.3109/00365548.2010.519345. [DOI] [PubMed] [Google Scholar]
- 5.Truman RW, Singh P, Sharma R, Busso P, Rougemont J, et al. Probable zoonotic leprosy in the southern United States. N Engl J Med. 2011;28:1626–1633. doi: 10.1056/NEJMoa1010536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfyffer G. Murray PR, Barron EJ, Jorgensen JH, Landry ML, Pfaller MA, editors. Mycobacterium: general characteristics, laboratory detection and staining procedures. Manual of clinical Microbiology. 2007. pp. 543–572. American Society for Microbiology, Washington DC.
- 7.Moore DA, Evans CA, Gilman RH, Caviedes L, Coronel J, et al. Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N Eng J Med. 2006;355:539–550. doi: 10.1056/NEJMoa055524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robledo JA, Mejía GI, Morcillo N, Chacón L, Camacho M, et al. Evaluation of a rapid culture method for tuberculosis diagnosis: a Latin American multi-center study. Int J Tuberc Lung Dis. 2006;10:613–619. [PubMed] [Google Scholar]
- 9.Butler WR, Jost KC, Jr, Kilburn JO. Identification of mycobacteria by high-performance liquid chromatography. J Clin Microbiol. 1991;29:2468–2472. doi: 10.1128/jcm.29.11.2468-2472.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lévy-Frébault VV, Portaels F. Proposed minimal standards for the genus Mycobacterium and for description of new slowly growing Mycobacterium species. Int J Syst Bacteriol. 1992;42:315–323. doi: 10.1099/00207713-42-2-315. [DOI] [PubMed] [Google Scholar]
- 11.Djelouadji Z, Raoult D, Daffé M, Drancourt M. A single-step sequencing method for the identification of Mycobacterium tuberculosis complex species. PLoS Negl Trop Dis. 2008;2:e253. doi: 10.1371/journal.pntd.0000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Djelouadji Z, Arnold C, Gharbia S, Raoult D, Drancourt M. Multispacer sequence typing for Mycobacterium tuberculosis genotyping. PLoS ONE. 2008;3:e2433. doi: 10.1371/journal.pone.0002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armand S, Vanhuls P, Delcroix G, Courcol R, Lemaître N. Comparison of the Xpert MTB/RIF Test with an IS6110-TaqMan Real-Time PCR Assay for Direct Detection of Mycobacterium tuberculosis in Respiratory and Nonrespiratory Specimens. J Clin Microbiol. 2011;49:1772–1776. doi: 10.1128/JCM.02157-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Eng J Med. 2010;9:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki K, Higuchi T. Improvement of routine works and quality control in mycobacterial laboratory. Kekkaku. 2007;82:217–227. [PubMed] [Google Scholar]
- 16.Martín A, Herranz M, Lirola MM, Fernández RF INDAL-TB group. Optimized molecular resolution of cross-contamination alerts in clinical mycobacteriology laboratories. BMC Microbiol. 2008;14:30. doi: 10.1186/1471-2180-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castro C, González L, Rozo JC, Puerto G, Ribón W. Biosafety evaluation of the DNA extraction protocol for Mycobacterium tuberculosis complex species, as implemented at the Instituto Nacional de Salud, Colombia. Biomedica. 2009;29:561–566. [PubMed] [Google Scholar]
- 18.Saleeb PG, Drake SK, Murray PR, Zelazny AM. Identification of mycobacteria in solid-culture media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2011;49:1790–1794. doi: 10.1128/JCM.02135-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lotz A, Ferroni A, Beretti JL, Dauphin B, Carbonnelle E, et al. Rapid identification of mycobacterial whole cells in solid and liquid culture media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2010;48:4481–4486. doi: 10.1128/JCM.01397-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hettick JM, Kashon ML, Slaven JE, Ma Y, Simpson JP, et al. Discrimination of intact mycobacteria at the strain level: a combined MALDI-TOF MS and biostatical analysis. Proteomics. 2006;6:6416–6425. doi: 10.1002/pmic.200600335. [DOI] [PubMed] [Google Scholar]
- 21.Pignone M, Greth KM, Cooper J, Emerson D, Tang J. Identification of mycobacteria by matrix-assisted laser desorption ionization-time-of-flight mass spectrometry. J Clin Microbiol. 2006;44:1963–1970. doi: 10.1128/JCM.01959-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anhalt J, Fenselau C. Identification of bacteria using mass spectrometry. Anal Chem. 1975;47:219–225. [Google Scholar]
- 23.Bittar F, Ouchenane Z, Smati F, Raoult D, Rolain JM. MALDI-TOF-MS for rapid detection of staphylococcal Panton-Valentine leukocidin. Int J Antimicrob Agents. 2009;34:467–470. doi: 10.1016/j.ijantimicag.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 24.Degand N, Carbonnelle E, Dauphin B, Beretti JL, Le Bourgeois M, et al. Matrix assisted laser desorption ionization time of flight mass spectrometry for identification of nonfermenting gram-negative bacilli isolated from cystic fibrosis patients. J Clin Microbiol. 2008;46:3361–3367. doi: 10.1128/JCM.00569-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seng P, Drancourt M, Gouriet F, La Scola B, Fournier PE, et al. Ongoing revolution in bacteriology: Routine identification of bacteria by matrix assisted laser desorption ionization time of flight mass spectrometry. Clin Infect Dis. 2009;49:543–551. doi: 10.1086/600885. [DOI] [PubMed] [Google Scholar]
- 26.La Scola B, Raoult D. Direct identification of bacteria in positive blood culture bottles by matrix assisted laser desorption ionisation time of flight mass spectrometry. Plos ONE. 2009;4:e8041. doi: 10.1371/journal.pone.0008041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevenson LG, Drake SK, Murray PR. Rapid identification of bacteria in positive blood culture broths by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2010;48:444–447. doi: 10.1128/JCM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferreira L, Sánchez-Juanes F, Muñoz-Bellido JL, González-Buitrago JM. Rapid method for direct identification of bacteria in urine and blood culture samples by matrix-assisted laser desorption ionization time-of-flight mass spectrometry: intact cell vs. extraction method. Clin Microbiol Infect. 2010;12 doi: 10.1111/j.1469-0691.2010.03339.x. doi: 10.1111/j.1469–0691.2010.03339.x. [DOI] [PubMed] [Google Scholar]
- 29.Prod'hom G, Bizzini A, Durussel C, Bille J, Greub G. Matrix-assisted laser desorption ionization-time of flight mass spectrometry for direct bacterial identification from positive blood culture pellets. J Clin Microbiol. 2010;48:1481–1483. doi: 10.1128/JCM.01780-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Veen SQ, Claas EC, Kuijper EJ. High-throughput identification of bacteria and yeast by matrix-assisted laser desorption ionization-time of flight mass spectrometry in conventional medical microbiology laboratories. J Clin Microbiol. 2010;48:900–907. doi: 10.1128/JCM.02071-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferroni A, Suarez S, Beretti JL, Dauphin B, Bille E, et al. Real-time identification of bacteria and Candida species in positive blood culture broths by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2010;48:1542–1548. doi: 10.1128/JCM.02485-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seng P, Rolain JM, Fournier PE, La Scola B, Drancourt M, Raoult D. MALDI-TOF-mass spectrometry applications in clinical microbiology. Future Microbiol. 2010;5:1733–1754. doi: 10.2217/fmb.10.127. [DOI] [PubMed] [Google Scholar]
- 33.Hettick JM, Kashon ML, Simpson JP, Siegel PD, Maruzek GH, et al. Proteomic profiling of intact mycobacteria by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal Chem. 2004;76:5769–5776. doi: 10.1021/ac049410m. [DOI] [PubMed] [Google Scholar]
- 34.Drancourt M, Raoult D. Cost-effectiveness of blood agar for isolation of mycobacteria. PLoS Negl Trop Dis. 2007;28:e83. doi: 10.1371/journal.pntd.0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drancourt M, Carrieri P, Gévaudan MJ, Raoult D. Blood agar and Mycobacterium tuberculosis: the end of a dogma. J Clin Microbiol. 2003;41:1710–1711. doi: 10.1128/JCM.41.4.1710-1711.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adékambi T, Berger P, Raoult D, Drancourt M. rpoB gene sequence-based characterization of emerging non-tuberculous mycobacteria with descriptions of Mycobacterium bolletii sp. nov., Mycobacterium phocaicum sp. nov. and Mycobacterium aubagnense sp. nov. Int J Syst Evol Microbiol. 2006;56:133–143. doi: 10.1099/ijs.0.63969-0. [DOI] [PubMed] [Google Scholar]
- 37.Djelouagji Z, Drancourt M. Inactivation of cultured Mycobacterium tuberculosis organisms prior to DNA extraction. J Clin Microbiol. 2006;44:1594–1595. doi: 10.1128/JCM.44.4.1594-1595.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McAlpin CR, Cox CR, Matyi SA, Voorhees KJ. Enhanced matrix-assisted laser desorption/ionization time-of-flight mass spectromic analysis of bacteriophage major capsid proteins with beta-mercaptoethanol pretreatment. Rapid Commun Mass Spectrom. 2010;24:11–14. doi: 10.1002/rcm.4349. [DOI] [PubMed] [Google Scholar]
- 39.Miller AK, Tepper A, Sieber K. Historical risks of tuberculin skin test conversion among non-physician staff at a large urban hospital. Am J Ind Med. 2002;42:228–235. doi: 10.1002/ajim.10108. [DOI] [PubMed] [Google Scholar]
- 40.Woith WM, Volchenkov G, Larson JL. Russian health care workers' knowledge of tuberculosis and infection control. Int J Tuberc Lung Dis. 2010;14:1489–1492. [PMC free article] [PubMed] [Google Scholar]
- 41.Elbir H, Abdel-Muhsin AM, Babiker A. A one-step DNA PCR-based method for the detection of Mycobacterium tuberculosis complex grown on Lowenstein-Jensen media. Am J Trop Med Hyg. 2008;78:316–317. [PubMed] [Google Scholar]
- 42.Bemer-Melchior P, Drugeon HB. Inactivation of Mycobacterium tuberculosis for DNA typing analysis. J Clin Microbiol. 1999;37:2350–2351. doi: 10.1128/jcm.37.7.2350-2351.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doig C, Seagar AL, Watt B, Forbes KJ. The efficacy of the heat killing of Mycobacterium tuberculosis. J Clin Pathol. 1992;55:778–779. doi: 10.1136/jcp.55.10.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El Khéchine A, Henry M, Raoult D, Drancourt M. Detection of Mycobacterium tuberculosis complex organisms in the stools of patients with pulmonary tuberculosis. Microbiology. 2009;155:2384–2389. doi: 10.1099/mic.0.026484-0. [DOI] [PubMed] [Google Scholar]
- 45.Dridi B, Henry M, El Khéchine A, Raoult D, Drancourt M. High prevalence of Methanobrevibacter smithii and Methanosphaera stadtmanae detected in the human gut using an improved DNA detection protocol. PLoS One. 2009;4:e7063. doi: 10.1371/journal.pone.0007063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of 62 Mycobacterium species included in the MALDI-TOF database. This database combined Bruker and home-made databases. For each species/sub-species, the number of reference spectra is indicated, for a total of 177 references.
(DOC)