Abstract
Background
Morphine has been commonly used for postoperative pain control. We measured plasma concentrations of morphine and compared the efficacy and safety of continuous epidural analgesia (CEA) using morphine-bupivacaine with intravenous patient controlled analgesia (IV-PCA) with morphine for 48 hrs after the end of the operation.
Methods
Nineteen patients undergoing Mile's operation were assigned to receive a morphine loading dose of 5 mg followed by IV-PCA with 0.1% morphine (IV-PCA group, n = 9) or a morphine loading dose of 2 mg and 0.125% bupivacaine 10 ml, followed by CEA with 0.004% morphine and 0.075% bupivacaine at a rate of 5 ml/hr (CEA group, n = 10). The plasma concentrations of morphine were measured and visual analog scales (VAS) for pain were recorded at 1, 6, 12, 24, and 48 hr postoperatively and the effects on respiration and any other side effects were noted.
Results
The mean maximal and minimal levels of plasma morphine were 40.2 ± 21.2 ng/ml and 23.4 ± 9.7 ng/ml for the IV-PCA group and 11.8 ± 3.5 ng/ml and 8.2 ± 1.9 ng/ml for the CEA group, respectively. Resting and dynamic pain scores were significantly lower in the CEA group than in the IV-PCA group. There were no significant differences for the effects on respiration and for any side effects between the two groups.
Conclusions
We evaluated plasma concentrations of morphine with CEA using morphine-bupivacaine and IV-PCA using morphine for the postoperative pain control. The CEA group had better postoperative analgesia than that of the IV-PCA group and the incidence of side effects were not significantly different between the two groups.
Keywords: morphine, postoperative pain
INTRODUCTION
Until recently, it was a common practice for physician to administer opioids intermittently to patients with intramuscular (IM) injections for postoperative pain control. IM injections, however, often led to insufficient analgesia, and hence, other approaches have been used recently to provide better postoperative pain relief. Now some of the most commonly used methods are (a) continuous epidural analgesia (CEA) in which a catheter is placed into the epidural space, constantly administering opioids combined with local anesthetics; and (b) intravenous patient-controlled analgesia (IV-PCA), which uses a PCA pump that delivers opioids or nonsteroidal anti-inflammatory drugs (NSAIDs) intravenously.
Morphine is a commonly used postoperative pain control drug. Injecting it into the epidural space or vein through a PCA device is effective for postoperative pain relief. Nonetheless, there have been reports about higher postoperative pain control effects rendered by epidural morphine injections compared with IM injections or IV-PCA [1]. Several studies have reported attempting postoperative pain control through epidural injection of morphine. The positive effects included pain control efficacy such as a broader range of duration of action [2-6]. IV-PCA has been associated with fewer side effects and hence, greater safety and has a simple procedure, all of which contributes to its popularity and wide use [7,8].
Absorption of opioids and their analgesic effects can vary considerably from patient to patient. Since there are concerns over opioid-related adverse events, physicians and patients tend to favor smaller doses when administering opioids. Graves et al. [9] used IV morphine injections for postoperative pain control. They measured the plasma concentrations of morphine and observed its analgesic effects. They found that the plasma morphine concentration with minimal analgesia was 20-40 ng/ml, and that satisfactory pain control was achieved at a concentration level of 40 ng/ml or higher. Rawal et al. [10] injected 2 mg of morphine epidurally and observed its effects, which included adequate analgesial (despite the drug's low level of plasma concentrations) and an extended period of action, i.e., 10 hours or longer. Regarding epidural administration of morphine, some studies have found little co-relation between blood morphine concentration and the analgesic efficacy of morphine since they based their findings on the comparative data of the highest blood morphine concentration achieved under either epidural administration or IV administration [11]. In this study, we enrolled patients who had been diagnosed with rectal cancer and were scheduled to undergo Miles' operation at this hospital. We assigned them to either the IV-PCA group, receiving a morphine-based IV-PCA treatment, or to the CEA group, receiving a mixture of morphine and bupivacaine delivered continuously through epidural catheter. In each group, we measured plasma concentrations of morphine and observed the effects of each method on the patients' respiration, analgesic control efficacy, and association with adverse events.
MATERIALS AND METHODS
The participating patients were scheduled to receive Miles' operation at this hospital for rectal cancer; they expressed their interest in receiving postoperative pain relief. All patients were qualified for ASA physical status Class I or II. Exclusion criteria were those who were at advanced ages (i.e., 70 years or older), who had a psychiatric disorder, or who had shown dependency on or extra sensitivity to the drugs used in the study. Approval was obtained from the institutional review board (IRB) at this hospital. On the day before the surgery, we visited with the patients and explained to them how the two postoperative pain control methods (i.e., CEA and IV-PCA) are implemented. We informed them about the effects as well as possible adverse events of each method. We then let them choose a method and obtained their signed consent.
For pretreatment, all patients were given atropine sulfate (0.5 mg) in IM injection 1 hour prior to their surgery. With the CEA group, in the operating room before the surgery began, we used the loss of resistance technique to identify the epidural space while inserting the needle in the intervertebral space between L2 and L3. After identifying the epidural space, we inserted an epidural catheter into the epidural space, making sure the catheter did not come in contact with blood or cerebrospinal fluid (CSF). For testing, 0.375% bupivacaine (3 ml) combined with 0.0005% epinephrine was given through the epidural catheter. This test confirmed the positioning of the catheter inside the epidural space. For anesthesia, we injected the patients intravenously with thiopental sodium (5 mg/kg) and succinylcholine chloride (1.5 mg/kg) before conducting endobronchial intubation. The intubation was followed by typical general anesthesia using midazolam (5 mg), fentanyl (200 µg), N2O, and isoflurane. In addition, vecuronium was administered for continued muscle relaxation.
When the surgery was concluded, extubation took place. Patients were transferred to their recovery room. Upon their arrival in the recovery room, IV-PCA group received morphine (5 mg) intravenously; their IV line was hooked up to the PCA device (Walkmed, Medex, USA) which delivered a mixture of 0.1% morphine (100 ml) and ondansetron (4 mg). The bolus dose was 1 ml; the lockout time was 8 minutes. During the first hour into the morphine administration, patients were allowed to receive medication whenever they felt pain but only for a maximum of 5 times by pressing the button; from the second hour on, the number was lowered to 4 times per hour. In the meantime, patients in the CEA group received 0.125% bupivacaine (10 ml) combined with morphine (a 2 mg loading dose) immediately after they arrived in their recovery room. They then constantly received 0.075% bupivacaine solution containing 0.004% morphine through the PCA device at a rate of 5 ml/hr.
At 1, 6, 12, 24, and 48 hours after the surgery, a pain physician visited with the patients and collected blood samples for plasma morphine concentration measurements and did an arterial blood gas analysis. The physician monitored the patients' respiratory rate and checked the severity of pain. For pain monitoring, the visual analogue scale (VAS) was used while specifying the pain scores as either resting pain scores (i.e., lying down) or dynamic pain scores (i.e., coughing forcefully). In addition, inquiries were made on the incidence of adverse events such as nausea, vomiting, pruritis, sedation, hypotension, and respiratory depression (i.e., per-minute breathing rate of 8 or less), as well as on morphine consumption in the IV-PCA group. In case of nausea and vomiting, 10 mg of metoclopramide was given if requested by the patient. Blood samples collected at each of the above-mentioned postoperative hours for the analysis of the plasma morphine concentrations were deposited inside blood sample vials, free of anticoagulants. Within 30 minutes after the blood was collected, the samples were subjected to centrifugation at 4℃ to separate the serum. Immediately after this, the serum-free plasma was frozen at -70℃ and kept in cryopreservation until the plasma morphine concentrations were measured by means of radioimmunoassay.
All results were expressed as mean ± standard deviation. Statistical analyses were done with SPSS ver. 12.0 software (SPSS Inc., USA). For inter-group comparison, the non-parametric Mann-Whitney U test was used. Incidences of adverse events were analyzed with Fisher's exact test; the results were considered statistically significant if the P value was less than 0.05.
RESULTS
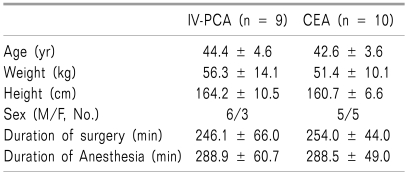
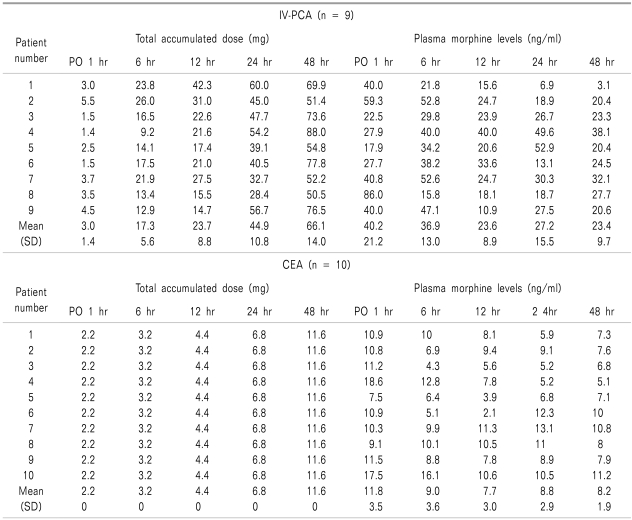
No significant differences were found between the IV-PCA and CEA groups in terms of age, weight, duration of surgery, and duration of anesthesia (Table 1). The mean total morphine consumption during the 48-hour postoperative period was 66.1 ± 14.0 mg and 11.6 mg in the IV-PCA and CEA groups, respectively. In the IV-PCA group, the mean maximal and minimal values for the plasma morphine concentration were 40.2 ± 21.2 ng/ml and 23.4 ± 9.7 ng/ml, respectively. The corresponding values in the CEA group were 11.8 ± 3.5 ng/ml and 8.2 ± 1.9 ng/ml, respectively (Table 2).
Table 1.
Demographic Data
Values are mean ± SD. There were no significant differences between the teo groups.
Table 2.
Total Accumulated Dose and Plasma Morphine Levels
PO: postoperative.
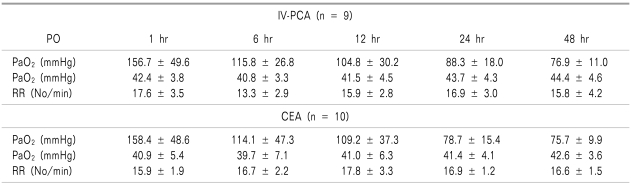
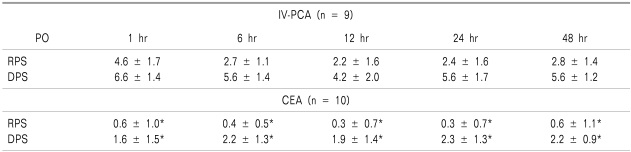
There were no significant differences in the data upon inter-group comparison for the respiratory rate and arterial blood gas analysis, which were collected to examine the effects of the drugs used in this study on respiration (Table 3). In terms of the VAS pain scores, the CEA group scored significantly lower than that of the IV-PCA group, for both resting pain scores and dynamic pain scores measured at 1, 6, 12, 24, and 48 hours after the surgery (P < 0.05) (Table 4).
Table 3.
Arterial Blood Gases and Respiratory Rates (RR)
Values are mean ± SD. PO: postoperative. There were no significant differences between the two groups.
Table 4.
The Pain Scores (VAS)
Values are mean ± SD. PO: postoperative, RPS: rest pain score, DPS: dynamic pain score. *P < 0.05 compared with Group IV-PCA.
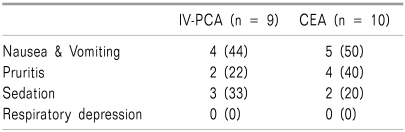
Side effects experienced by the patients included nausea, vomiting, pruritis, and sedation. No severe adverse events were reported. In each of the above mentioned side effects, no significant inter-group difference was found (Table 5).
Table 5.
The Incidence of Side Effects
Values represent number of patients. Values in parentheses are percentages. There were no significant differences between the two groups.
DISCUSSION
Recently, both epidural and IV administrations of opioids have been widely used in postoperative pain relief. An example of epidural administration is CEA, and opioids reduce the occurrence of dose-related adverse events when used in combination with local anesthetics and produce synergistic effects for pain control. In the study by de Leon-Casasola et al. [12], 4,227 cancer patients received 0.05-0.1% bupivacaine and 0.01% morphine with epidural injections at a rate of 5-10 ml/hr postoperatively. The results demonstrated the pain control efficacy of the bupivacaine-morphine regimen. Other researchers have reported on epidural administration of morphine (2 mg) in patients with pain. The maximal pain-relieving effect was observed at 10-15 minutes after the administration; the action of morphine lasted 6-24 hours [13]. In this study, where a mixture of 0.004% morphine and 0.075% bupivacaine was delivered into the epidural space, we, too, observed positive effects from the infusion throughout the study period since the patients invariably rated their pain as '3' or lower on the VAS. Notwithstanding the reported frequency and popularity of epidural administration of morphine as an excellent pain control method, the possibilities of adverse events, such as late respiratory depression, still exist due to the poor lipid solubility of morphine [14]. The side effects of epidural opioid administration include nausea, vomiting, pruritis, urinary retention, sedation, and respiratory depression. These effects are attributable to opioids which, once injected into the epidural space, enter into the CSF, travel in a cephalad direction, and become active within the brain [11]. The aforementioned respiratory depression is seen, though rarely, in elderly or high-risk patients, as well as with varying doses of opioids. Thus, physicians must take into consideration the patient's age and overall physical condition, and the type of surgery when deciding upon an optimal dose of morphine.
IV-PCA is an effective way to control pain because it is easy to use and is associated with fewer side effects and higher patient satisfaction. When compared with IM injection for opioid administration, IV-PCA has greater analgesia, helps reduce the consumption of opioids, leads to less sedation and better nighttime sleep, facilitates early ambulation, results in fewer pulmonary complications, and ensures higher patient satisfaction [15]. In terms of the analgesia of IV-PCA alone, a previous study has reported satisfactory pain control results, indicated by VAS scores of '4' or lower [14]. In that regard, dynamic pain scores in this study, which were '4' or higher on the VAS, indicate that pain relief of the IV-PCA alone was not too effective. Similar to what we found, some studies have reported a relative weakness in analgesia for IV-PCA compared to epidural administration of opioids [14,16].
Depending on personal preference, patients may or may not feel comfortable about undergoing CEA, and may therefore choose IV-PCA as an alternative for themselves. A previous study showed high levels of patient satisfaction with both CEA and IV-PCA using morphine [16]. The determining factors of the patients response included analgesia, incidence of adverse events, less invasive care, awareness of pain management importance, and treatment efficacy of the physician. The reported strengths of CEA were the ability for outstanding pain control and lucidity in patients. CEA was also favored by older patients, who generally prefer their medication administered by their physician rather than by themselves and whose probability of developing sedation is higher. The strength of IV-PCA, on the other hand, was that it could be operated easily. Postoperative pain is reportedly most intense during the first 24 hours into recovery. It lessens gradually over the next 24-hour period, and is nearly non-existent in 3 to 4 days [17]. In this study, we continued to monitor for pain until 48 hours (or for full 2 days) following the surgery.
Using morphine for pain control in various clinical situations requires knowledge about the metabolization of the drug, a thorough understanding of its pharmacological mechanisms, and the ability to predict possible adverse events. We reviewed several studies that measured plasma concentrations of morphine. Berkowitz et al. [18] reported that the mean half-life of morphine, injected either IV or IM, was 2 hours. They reported that sufficient analgesia involved fast absorption of the opioid into the systemic circulation (even through IM injection), achieving maximal blood morphine levels within 10 to 20 minutes, and plasma morphine concentrations of 50 ng/ml or higher. In another study [9], the plasma morphine concentration with minimal efficacy was observed in postoperative patients who received morphine through IV-PCA. The concentration in question was found to be 20-40 ng/ml; satisfactory pain control occurred at 40 ng/ml or higher; the respiratory rate, severity of pain, and sedation were all found to be related to the morphine plasma levels. In this study, we found that the mean maximal and minimal values of the morphine plasma level in the IV-PCA group were 40.2 ± 21.2 ng/ml and 23.4 ± 9.7 ng/ml, respectively. These numbers are consistent with previously reported morphine plasma levels with minimal efficacy, i.e., 20-40 ng/ml. Based on these data, we concluded that the patients' resting pain scores were satisfactory while their dynamic pain scores (invariably '4' or higher) were not so satisfactory.
Murakawa et al. [19] in their study on CEA evaluated morphine plasma levels for postoperative pain relief. They injected 3 mg of morphine into the epidural space immediately before concluding the surgery, and then continued to give morphine through CEA after the surgery. The postoperative plasma concentrations of morphine were found to be 4.6 ng/ml. Its analgesic effects were at an appropriate level and remained as such throughout the study period without resulting in any severe complications, such as respiratory depression. This CEA morphine plasma level was lower than the morphine plasma level with minimal analgesia (i.e., 10-40 ng/ml) [18]. In this study, we found that the mean value of the CEA group's plasma morphine concentrations ranged between 11.8 ± 3.5 ng/ml (maximal value) and 8.2 ± 1.9 ng/ml (minimal value). The CEA group invariably rated their pain as '3' or lower on the VAS throughout the study period, possibly indicating pain control was adequate.
When injected into the epidural space, morphine produces its analgesic effect, as one study [13] suggests, by entering into the subarachnoid space and acting directly on specific opioid receptors in the substantia gelatinosa of the posterior horn cells of the spinal cord. Several other studies, too, have reported on opioids' direct action on the spinal cord, and on their analgesic efficacy when injected into the epidural or subarachnoid space. Morphine, when administered epidurally, enters into the systemic circulation and travels rapidly. Some previous studies reported that epidural injection of morphine resulted in a similar level of plasma morphine concentration when compared to IM injection of morphine [20,21]. In a study by Weddel and Ritter on morphine (5 mg/70 kg) that was injected epidurally [22], the mean time to onset of significant effects was 15 minutes; an adequate level of analgesia was found to last 37.9 hours. Based on what they found about the analgesic efficacy of morphine and its plasma concentration levels, researchers have supported the concept of the selective action of morphine on the spinal cord. Rawal et al. [10] reported that morphine, in most patients, achieved its maximal plasma concentration (2.6-8.2 ng/ml) within 10 minutes. The results, they argued, indicate morphine's fast absorption into the blood stream. In addition, based on its extended period of action, epidurally injected morphine might act on specific sites in the spinal cord. Kalman et al. [23] went further by measuring the level of morphine in the CSF. They found that the concentration of morphine (4 mg), injected epidurally, had increased rapidly in the CSF, with the mean maximal concentration achieved after 10 to 30 minutes. However, morphine concentration levels varied considerably in the patients, and particularly so, in the CSF rather than in the plasma. They also found that CSF morphine concentrations at 30 minutes were not significantly related to VAS pain scores at 10 hours to 24 hours.
Morphine, when delivered by lumbar epidural injections, travels in the cephalad direction and arrives, mixed with the CSF, at the brain stem and fourth ventricle within 6 hours. Its blood concentration has not been found to be related to incidences of adverse events [20]. The CSF-morphine mixture slowly travels cephalad, once it reaches the fourth ventricle, it achieves equilibrium fast within 15 to 30 minutes [11]. When injected in a single, large-quantity dose, morphine reaches a high level of concentration inside the CSF; its concentration increases rapidly as it travels to the brain and reaches the fourth ventricle. In contrast, when injected in a small quantity or in a certain quantity over a period of time, morphine will show little change in concentration level in the fourth ventricle. In one study, morphine was administered at a consistent rate and produced more positive effects, including the absence of respiratory depression [24]. In this study, we did not measure CSF concentrations of morphine. Pharmacodynamic results that are observed in the CSF will play, we suspect, a more direct role in explaining the long duration of action for epidurally injected morphine and its association with adverse events.
In summary, we compared the efficacy of two morphine-based postoperative pain control methods in rectal cancer patients who underwent Miles' operation. We measured plasma morphine concentrations in IV-PCA (receiving morphine only) and CEA (receiving a morphine-bupivacaine mixture) groups over 48 hours into the recovery. In addition, we observed the effects of the pain relief regimens on the patients' respiration, their pain control efficacy and their association with adverse events. Regarding respiration and adverse events, we found no significant differences between the groups. As for pain control efficacy measured by means of VAS pain scores, the pain scores for the CEA group were lower indicating a more effective pain relief. We recommend that further research be done on the concentrations of morphine used in postoperative pain control, and on its action mechanisms and effects.
References
- 1.Eisenach JC, Grice SC, Dewan DM. Patient-controlled analgesia following cesarean section: a comparison with epidural and intramuscular narcotics. Anesthesiology. 1988;68:444–448. doi: 10.1097/00000542-198803000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Blumenthal S, Min K, Nadig M, Borgeat A. Double epidural catheter with ropivacaine versus intravenous morphine: a comparison for postoperative analgesia after scoliosis correction surgery. Anesthesiology. 2005;102:175–180. doi: 10.1097/00000542-200501000-00026. [DOI] [PubMed] [Google Scholar]
- 3.Gustafsson LL, Friberg-Nielsen S, Garle M, Mohall A, Rane A, Schildt B, et al. Extradural and parenteral morphine: kinetics and effects in postoperative pain. A controlled clinical study. Br J Anaesth. 1982;54:1167–1174. doi: 10.1093/bja/54.11.1167. [DOI] [PubMed] [Google Scholar]
- 4.Block BM, Liu SS, Rowlingson AJ, Cowan AR, Cowan JA, Jr, Wu CL. Efficacy of postoperative epidural analgesia: a meta-analysis. JAMA. 2003;290:2455–2463. doi: 10.1001/jama.290.18.2455. [DOI] [PubMed] [Google Scholar]
- 5.Taenzer AH, Clark C. Efficacy of postoperative epidural analgesia in adolescent scoliosis surgery: a meta-analysis. Paediatr Anaesth. 2010;20:135–143. doi: 10.1111/j.1460-9592.2009.03226.x. [DOI] [PubMed] [Google Scholar]
- 6.Ha HS, Park YC, Kim HK, Baik SW, Chung KS. The effect of low dose bupivacaine on epidural morphine analgesia for postoperative pain. J Korean Pain Soc. 1994;7:188–192. [Google Scholar]
- 7.Cohen SE, Subak LL, Brose WG, Halpern J. Analgesia after cesarean delivery: patient evaluations and costs of five opioid techniques. Reg Anesth. 1991;16:141–149. [PubMed] [Google Scholar]
- 8.Harrison DM, Sinatra R, Morgese L, Chung JH. Epidural narcotic and patient-controlled analgesia for post-cesarean section pain relief. Anesthesiology. 1988;68:454–457. doi: 10.1097/00000542-198803000-00025. [DOI] [PubMed] [Google Scholar]
- 9.Graves DA, Arrigo JM, Foster TS, Baumann TJ, Batenhorst RL. Relationship between plasma morphine concentrations and pharmacologic effects in postoperative patients using patient-controlled analgesia. Clin Pharm. 1985;4:41–47. [PubMed] [Google Scholar]
- 10.Rawal N, Sjöstrand U, Dahlström B. Postoperative pain relief by epidural morphine. Anesth Analg. 1981;60:726–731. [PubMed] [Google Scholar]
- 11.Cousins MJ, Mather LE. Intrathecal and epidural administration of opioids. Anesthesiology. 1984;61:276–310. [PubMed] [Google Scholar]
- 12.de Leon-Casasola OA, Parker B, Lema MJ, Harrison P, Massey J. Postoperative epidural bupivacaine-morphine therapy. Experience with 4,227 surgical cancer patients. Anesthesiology. 1994;81:368–375. doi: 10.1097/00000542-199408000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Behar M, Magora F, Olshwang D, Davidson JT. Epidural morphine in treatment of pain. Lancet. 1979;1:527–529. doi: 10.1016/s0140-6736(79)90947-4. [DOI] [PubMed] [Google Scholar]
- 14.Eriksson-Mjöberg M, Svensson JO, Almkvist O, Olund A, Gustafsson LL. Extradural morphine gives better pain relief than patient-controlled i.v. morphine after hysterectomy. Br J Anaesth. 1997;78:10–16. doi: 10.1093/bja/78.1.10. [DOI] [PubMed] [Google Scholar]
- 15.Wasylak TJ, Abbott FV, English MJ, Jeans ME. Reduction of postoperative morbidity following patient-controlled morphine. Can J Anaesth. 1990;37:726–731. doi: 10.1007/BF03006529. [DOI] [PubMed] [Google Scholar]
- 16.Egan KJ, Ready LB. Patient satisfaction with intravenous PCA or epidural morphine. Can J Anaesth. 1994;41:6–11. doi: 10.1007/BF03009653. [DOI] [PubMed] [Google Scholar]
- 17.Wallace PG, Norris W. The management of postoperative pain. Br J Anaesth. 1975;47:113–120. doi: 10.1093/bja/47.2.113. [DOI] [PubMed] [Google Scholar]
- 18.Berkowitz BA, Ngai SH, Yang JC, Hempstead J, Spector S. The diposi tion of morphine in surgical patients. Clin Pharmacol Ther. 1975;17:629–635. doi: 10.1002/cpt1975176629. [DOI] [PubMed] [Google Scholar]
- 19.Murakawa T, Baba S, Isozaki KI, Kudo T, Kudo M, Matsuki A, et al. Plasma morphine levels during its continuous epidural infusion. Masui. 1989;38:1166–1170. [PubMed] [Google Scholar]
- 20.Bromage PR, Camporesi EM, Durant PA, Nielsen CH. Rostral spread of epidural morphine. Anesthesiology. 1982;56:431–436. doi: 10.1097/00000542-198206000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Chauvin M, Samii K, Schermann JM, Sandouk P, Bourdon R, Viars P. Plasma concentration of morphine after i.m., extradural and intrathecal administration. Br J Anaesth. 1981;53:911–913. doi: 10.1093/bja/53.9.911. [DOI] [PubMed] [Google Scholar]
- 22.Weddel SJ, Ritter RR. Serum levels following epidural administration of morphine and correlation with relief of postsurgical pain. Anesthesiology. 1981;54:210–214. doi: 10.1097/00000542-198103000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Kalman S, Metcalf K, Eintrei C. Morphine, morphine-6-glucuronide, and morphine-3-glucuronide in cerebrospinal fluid and plasma after epidural administration of morphine. Reg Anesth. 1997;22:131–136. doi: 10.1016/s1098-7339(06)80031-3. [DOI] [PubMed] [Google Scholar]
- 24.Rauck RL, Raj PP, Knarr DC, Denson DD, Speight KL. Comparison of the efficacy of epidural morphine given by intermittent injection or continuous infusion for the management of postoperative pain. Reg Anesth. 1994;19:316–324. [PubMed] [Google Scholar]