Abstract
The loss of sister chromatid cohesion triggers anaphase spindle movement. The budding yeast Mcd1/Scc1 protein, called cohesin, is required for associating chromatids, and proteins homologous to it exist in a variety of eukaryotes. Mcd1/Scc1 is removed from chromosomes in anaphase and degrades in G1. We show that the fission yeast protein, Mis4, which is required for equal sister chromatid separation in anaphase is a different chromatid cohesion molecule that behaves independent of cohesin and is conserved from yeast to human. Its inactivation in G1 results in cell lethality in S phase and subsequent premature sister chromatid separation. Inactivation in G2 leads to cell death in subsequent metaphase–anaphase progression but missegregation occurs only in the next round of mitosis. Mis4 is not essential for condensation, nor does it degrade in G1. Rather, it associates with chromosomes in a punctate fashion throughout the cell cycle. mis4 mutants are hypersensitive to hydroxyurea (HU) and UV irradiation but retain the ability to restrain cell cycle progression when damaged or sustaining a block to replication. The mis4 mutation results in synthetic lethality with a DNA ligase mutant. Mis4 may form a stable link between chromatids in S phase that is split rather than removed in anaphase.
Keywords: Cell cycle, cohesin, fission yeast, mitosis, UV irradiation, hydroxyurea
In eukaryotic cells, replication of chromosomal DNA occurs once during S phase followed by separation of duplicated DNAs into two daughter nuclei during M-phase. DNA replication is initiated at the onset of S phase in particular chromosomal regions, the origins, which are unwound and stabilized by single-strand-binding protein. The replication machinery is large and complex, containing a number of enzyme subunits including DNA polymerases and helicase. However, other factors are necessary for initiating replication such as the origin recognition complex, which is bound to origin sequences throughout the cell cycle. Cdc6 and the MCM complex are also needed and are loaded onto the origins to form the prereplicative complex (e.g., see Rowles and Blow 1997). Moreover, an essential protein kinase Cdc7/Dbf4 must act prior to replication, so that the replication machinery can interact with the prereplicative complex and thereby ensure continuing replication. The proteins described above are still part of the gene products required for the quality control of DNA replication. When chromosomal DNAs are damaged or replication is blocked, entry into mitosis is restrained until the damage is recognized and repaired (e.g., see Kitazono and Matsumoto 1998). A block of mitotic entry upon damage or inhibition of replication can be accomplished by inactivation of cyclin-dependent protein kinases (CDKs). This checkpoint system is important for maintaining the genome with high fidelity, otherwise damaged chromosomal information will be transmitted to the nuclei of progeny cells.
To maintain the genome at high fidelity, there is an enigmatic aspect in chromosomal structure, that is, the mechinism by which sister chromatids are linked after duplication of chromosomal DNA. This sister chromatid link, alternatively called sister chromatid cohesion, must be dissociated before chromosomes are segregated into daughter cells (Holloway et al. 1993). Sister cohesion is thought to oppose the splitting forces exerted by kinetochore microtubules. If such linkage is absent, precocious sister chromatid separation will take place. The loss of sister cohesion in normal anaphase may thus trigger spindle movement. Until quite recently, very little or nothing has been known about the proteins required for sister chromatid cohesion. The first such protein to be identified was the Drosophila MEI-S332 protein required for the maintenance of sister chromatid cohesion in male and female meiosis (Kerrebrock et al. 1995). This protein localizes to meiotic and mitotic centromeres (although it is not essential for mitosis) until sister chromatids separate and are dissociated from chromosomes at anaphase (Moore et al. 1998), suggesting that the destruction or the release of this protein might be required for sister chromatid separation. In fission yeast, meiotic sister chromatid cohesion is deficient in the recombination-defective rec8-110 mutant (Molnar et al. 1995). Aberrant segregation is caused by precocious segregation of sister chromatids at anaphase I. In the same organism, mitotic centromeres are separated in metaphase-arrested mis6 mutants defective in equal segregation of sister chromatids in mitosis (Saitoh et al. 1997). Mis6 is essential for the formation of a centromere-specific chromatin in the G1/S phase and maintains the connection of sister centromeres until anaphase.
In budding yeast, Mcd1p/Scc1p, a sister-chromatid cohesion protein called cohesin, was identified by genetic screening and found to form the complex with Smc1p, an SMC protein, known to be involved in the structural maintenance of chromosomes (Guacci et al. 1997; Michaelis et al. 1997). Mcd1p/Scc1p is similar to fission yeast Rad21 (Birkenbihl and Subramani 1992) and Rec8, and proteins homologous to Mcd1p/Scc1p are present in higher eukaryotes. The mcd1 mutant is defective in sister chromatid cohesion and chromosome condensation. Scc1p associates with chromosomes in an Smc1p-dependent manner, dissociates from chromosomes at the metaphase-to-anaphase transition, and is degraded in G1 by the anaphase promoting complex (APC)/cyclosome. Thus Rad21/Mcd1p/Scc1p-like proteins may form a common cohesion apparatus that forms a complex with two SMC proteins and is regulated by association with and removal from chromosomes in the cell cycle. The other protein, Scc2p, was discovered to be required for sister chromatid cohesion in budding yeast (Michaelis et al. 1997). Its behavior in the cell cycle and relationship to the cohesin complex has not been reported.
In this report we show that the Mis4 protein, initially identified by its requirement for stable maintenance of minichromosomes in fission yeast (Takahashi et al. 1994), is required for sister chromatid cohesion, and its function is quite distinct from cohesin. Fission yeast mis mutants are temperature-sensitive lethal at the restrictive temperature (36°C) and can grow at the permissive temperature (26°C), but show high loss rates of minichromosomes at 26°C or semi-permissive temperatures. Twelve mis genes have been identified. The mis5+ gene encodes one of the MCM subunits essential for replication (Takahashi et al. 1994; Adachi et al. 1997) whereas mis6+ codes for a centromere protein (Saitoh et al. 1997). The mis1+ and mis10+ genes encode the subunits of DNA polymerase δ (H. Tatebe, H. Kondoh, M. Yanagida, unpubl.). The phenotype of mis4 is similar to that of mis6, as not only minichromosomes but also regular chromosomes were missegregated at 36°C (Takahashi et al. 1994). In addition to the mis phenotype, the mis4 mutant displays a low frequency of the cut phenotype (the occurrence of septation or cytokinesis in the absence of normal nuclear division; Hirano et al. 1986), and hypersensitivity to hydroxyurea (HU) and UV irradiation at permissive and semi-permissive temperatures (Takahashi et al. 1994). Mis4 thus appears to play an important role in maintaining the quality of the genome against cell cycle aberration, damage, and replication inhibition.
Results
Missegregation in mis4-242 is preceded by a prior lethal event
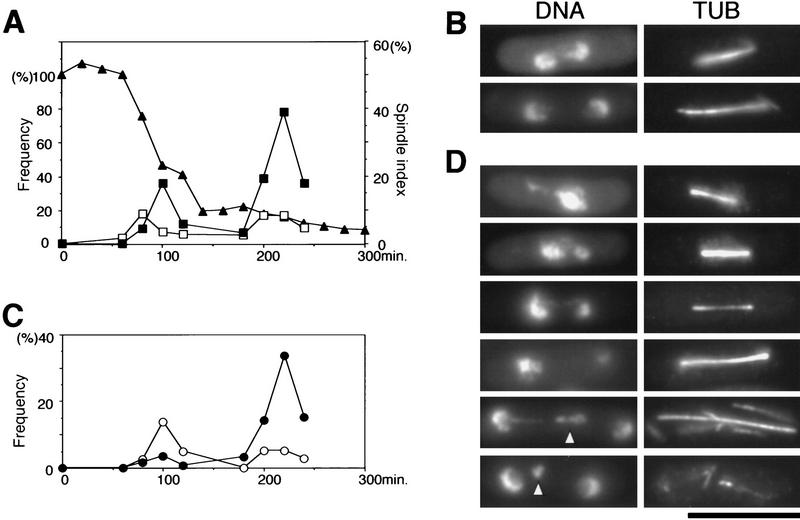
To determine which stage is defective during the cell cycle, cultures of mis4-242 were synchronized. Small, early G2 cells were collected by use of an elutriator rotor from an exponentially grown culture at 26°C, the permissive temperature. Then the culture was transferred to 36°C, the restrictive temperature (0 min, Fig. 1A). Viability of the mutant cells remained high until 60 min but then decreased and reached 50% at about 100 min while the cells proceeded across the first mitosis at around 80–100 min. To distinguish the timing of metaphase and anaphase, the frequencies of cells showing short or elongated spindles were measured; cells containing short spindles (<2.5 μm in length), which corresponded to prophase through metaphase, reached a maximum at 80 min, whereas those displaying elongated anaphase spindles (>2.5 μm and up to 14 μm) peaked at 100 min. Thus mis4-242 cells synchronized culture in early G2 at 36°C appeared to lose viability as they progress from metaphase through anaphase. Further decrease of viability occurred in the succeeding S phase and cell separation (around 120–140 min). The G1 phase is very short in fission yeast.
Figure 1.
A lethal event in the first cell cycle precedes the abnormal second mitosis in a G2-synchronized culture of mis4-242 cells. Mutant cells were grown at 26°C, and small early G2 cells were collected by an elutriator rotor and then cultured at 36°C for 5 hr in synthetic EMM2 medium. (A) Cell viability (%; ▴) was measured by plating of cells at 26°C. Spindle indexes (%) were obtained by estimation of the frequencies of cells that contained the spindle stained by anti-tubulin antibody. The length of the spindle was classified into short (<2.5 μm; □) or long (>2.5 μm; █). Short spindles peaked at 80 and 200 min, whereas long spindles peaked at 100 and 220 min. The decrease of cell viability occurred when the two spindle indexes were high in the first mitosis. Abnormal mitosis occurred only in the second mitosis. (B) Mitotic cells in the first mitosis at 36°C showing normal anaphase. Micro graphs taken at 100 min. (Left) Chromatin DNA staining by DAPI; (right) spindle staining by anti-tubulin antibody. (C) Frequencies of cells showing equal sister chromatid separation in normal anaphase (○; examples shown in B) and unequal abnormal anaphase (•; examples in D). (D) Mitotic cells in the second mitosis at 36°C showing unequal sister chromatid separation. Micrographs were taken at 220 min. (Arrowheads) Chromatids remaining along the spindle. Bar, 10 μm.
In sharp contrast to the above observations, chromosome segregation and spindle movements were apparently normal during the first mitosis (micrographs in Fig. 1B taken at 100 min: left, DNA stain; right, anti-tubulin antibody stain). Missegregation defects were quite frequently produced only later during the second round of mitosis at 200–220 min (Fig. 1C). Different types of missegregation are shown in Figure 1D (taken at 220 min). Unequal segregation of sister chromatids was most common. Chromatids remaining along the anaphase spindle (indicated by arrowheads) were also seen. The kinetochore microtubules might fail to pull the chromatids toward the poles.
mis4 mutants lose viability during S phase
We examined whether the missegregation defect could be produced in the first mitosis, if mutant cells previously arrested in G1 by nutrient starvation were transferred to the growing medium at 36°C. Viability of the mutant cells sharply decreased between 3 and 4 hr (Fig. 2A), while the cells traversed S phase (Fig. 2C, right panel). Abnormal missegregation phenotypes were frequently produced during the following first mitosis (7 hr, Fig. 2B,D). These results showed that mis4-242 cells cultured from G1 at 36°C became inviable during the S phase, and chromosome missegregation occurred in the succeeding mitosis.
Figure 2.
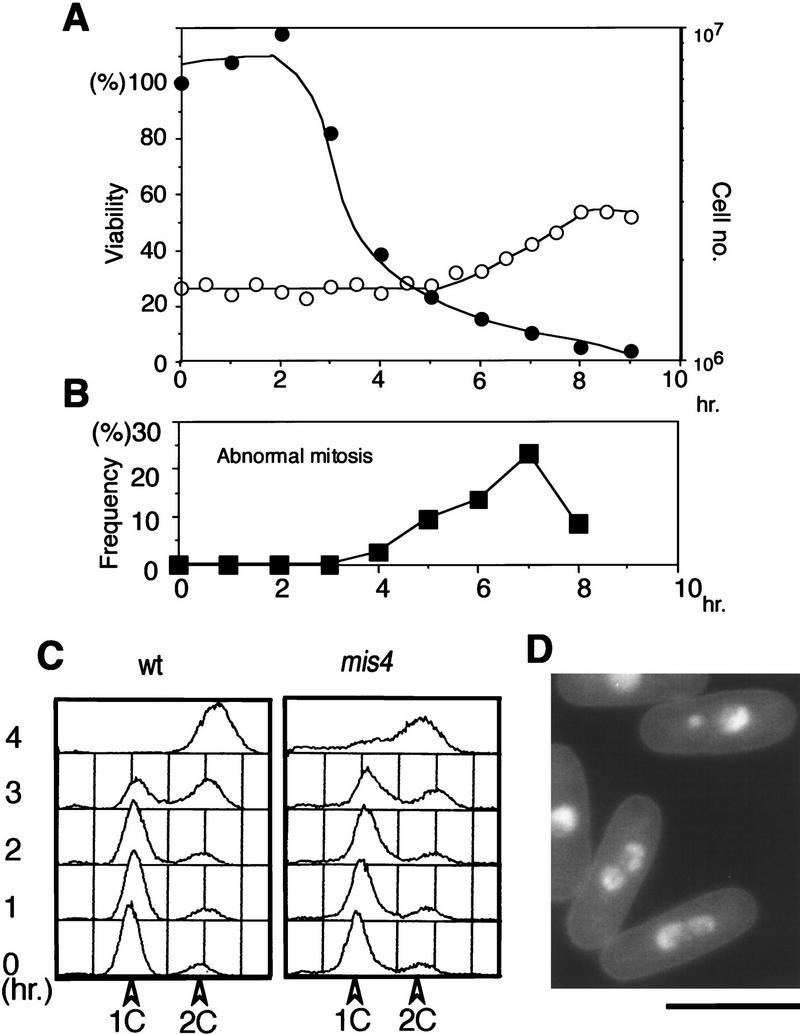
A lethal event occurs in S phase when mis4-242 mutant cells are released from G1 arrest. Mutant cells were nitrogen source-starved at 26°C to arrest then in G1. Cells were then released in complete medium at 36°C. (A) Cell viability (•; decreasing from 3 to 4 hr after the shift) and cell number (○; increasing around 8–9 hr). (B) Frequency of cells showing unequal chromosome segregation (peaking at 7 hr). (C) DNA content measured by FACScan analysis showed that DNA replication in mis4-242 cells took place around 3–4 hr, coinciding with the occurrence of cell inviability. (wt) Wild-type control. (D) Examples of mutant cells showing abnormal anaphase. Cells stained by DAPI. Bar, 10 μm.
The 160-kD product of mis4+is an evolutionarily conserved protein
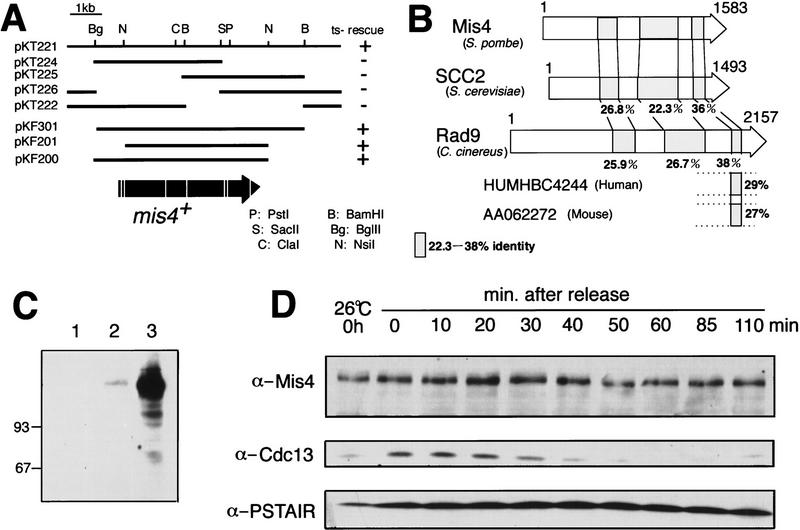
The mis4+ gene was isolated by a search of plasmids that fully suppressed the temperature-sensitive phenotype of mis4-242 by use of a genomic library of Schizosaccharomyces pombe. Thirty-nine independent clones were obtained, and they were all found to overlap, indicating that they were derived from a single genetic locus. The cloned DNA was integrated into the chromosome by homologous recombination with the ura4+ gene marker (see Materials and Methods), followed by tetrad analysis with the mis4-242 mutant, and verified to be derived from the mis4+ locus. The plasmid pKT221 was further subcloned, and the resulting pKF201 contained a minimal functional sequence (5.1 kb). The nucleotide sequence was determined, and one coding region representing the mis4+ gene was present. The predicted polypeptide contained 1583 amino acids interrupted with seven presumed introns (Fig. 3A). This coding region was also present in the S. pombe genome database.
Figure 3.
The gene product of mis4+ is similar to that of C. cinereus Rad9 and proteins present in yeast and mammals. (A) Isolation of plasmid carrying the mis4+ gene. The temperature-sensitive phenotype of mis4-242 was rescued (indicated by +) by pKT221 and some of its subclones. (Arrow) Coding region; (vertical white lines) introns. pKF201 is the minimal functional clone that contains the whole coding region. (B) Schematic representation of S. pombe Mis4, S. cerevisiae Scc2, and C. cinereus Rad9. Partial sequences derived from human and mouse cDNAs are also shown. The conserved regions (22% to 38% identity) are hatched. Mouse AA062272 and human HMHBC4244 sequences are similar to the carboxyl termini of Mis4/Scc2/Rad9. The DDBJ accession number for the nucleotide sequences of Mis4 is AB016866. (C) Immunoblot of S. pombe extracts containing Mis4–HA integrated into the chromosome (lane 2) or a plasmid containing Mis4–HA (lane 3). (lane 1) Control extracts of cells without integration and carrying the vector. (D) The immunoblot patterns of cdc25 mutant block and release experiments, showing the levels of Mis4, Cdc2 (PSTAIR), and mitotic cyclin (Cdc13). Cyclin destruction occurred 30 min after the shift to the permissive temperature (26°C). The Mis4 protein band slightly increased its mobility after 50 min (when Cdc13 levels dropped), but this was not reproducible.
A database search revealed that the fungus Coprinus cinereus Rad9 (2157 amino acids; Seitz et al. 1996) and the budding yeast Scc2 (1493 amino acids; Michaelis et al. 1997) showed a significant similarity from the central to the carboxy-terminal region of the polypeptides as illustrated in Figure 3B. Their overall identity was not high, but three regions showed significant sequence identity (22%–38%). It was also noted that mouse and human cDNA sequences (HUMHBC4244 and AA062272) similar to the carboxy-terminal regions of these proteins exist in the databases, indicating that Mis4-like proteins are present in mammalian organisms.
The hemagglutinin antigen (HA) sequence was fused to the carboxyl terminus of Mis4. The resulting fusion protein was expressed in S. pombe cells either after integration into the chromosome or introduced as a multicopy plasmid. As shown in Figure 3C, HA-tagged Mis4 was detected at the expected molecular mass of 160 kD (lane 2, integrated single copy; lane 3, overproducing plasmid). Nonintegrated cells carrying the vector plasmid did not show the band (lane 1). Comparison of the immunoblotting intensity of Cut2 also tagged with the same HA construct indicated that the amount of Mis4 in cells was approximately four-fold higher than Cut2, that is, ∼30,000 molecules per cell.
In block and release experiments carried out with a cdc25 mutant, cells were arrested in late G2 and then released to the permissive temperature. The amount of Mis4 protein did not greatly fluctuate during the cell cycle; it showed an increase (1.5-fold) in late mitosis, but otherwise the level remained roughly constant. The control mitotic cyclin (Cdc13) decreased in exiting of mitosis (Fig. 3D). Mis4 did not degrade in the G1-arrested cdc10 mutant cells, and the level did not change in the cyclosome subunit mutants (data not shown). Mis4 is thus present throughout the cell cycle, whereas budding yeast Mcd1p/Scc1p is destroyed in G1.
The mis4+ gene is essential for viability
Gene disruption of mis4+ was achieved by a single-step replacement (see Materials and Methods). The coding region was replaced with the S. pombe ura4+ gene. The presence of the disrupted gene in the genomes of heterozygous diploids was verified by genomic Southern hybridization (data not shown). Tetrad analysis of the sporulated diploids indicated that only two spores were viable and that all viable spores were nondisrupted Ura−. The mis4+ gene was therefore absolutely required for viability. The mis4-disrupted cells were observed after DAPI staining, chromosome condensation was observed in mitosis, and missegregation phenotypes resembling mis4-242 were seen (data not shown).
HU-sensitive mis4-242 maintains replication checkpoint control
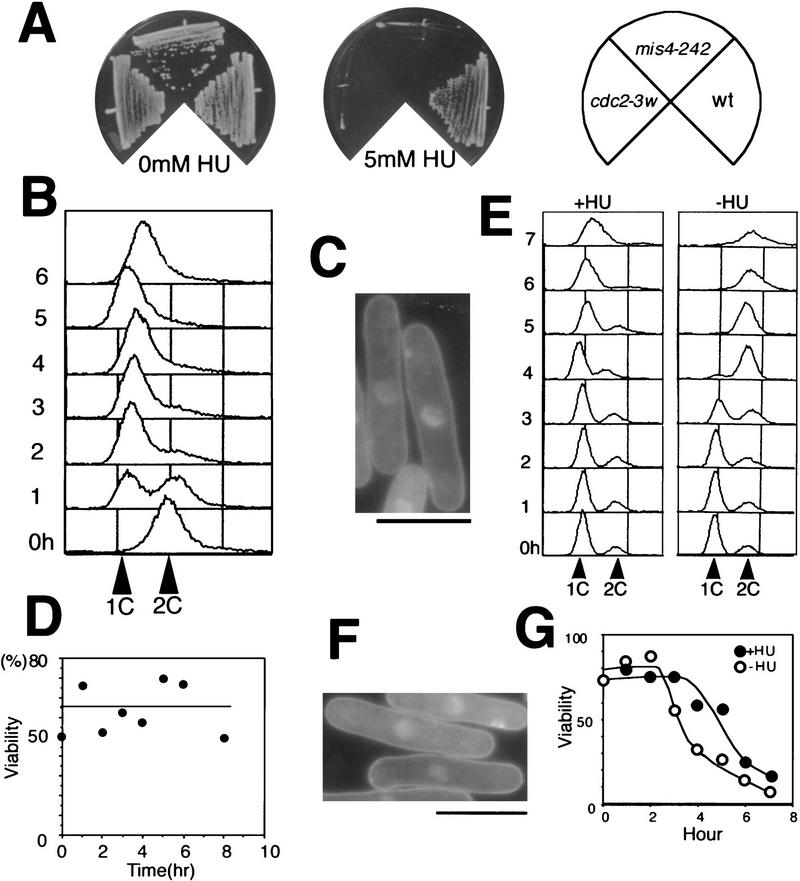
mis4-242 was found to be hypersensitive to HU; like cdc2-3w it did not form colonies in 5 mm HU at 26°C (Fig. 4A). Mutant cells cultured at 26°C in liquid medium containing 15 mm HU were greatly elongated (Fig. 4C), although they contained a 1C DNA content determined by FACS analysis (Fig. 4B), showing that HU-induced replication checkpoint control, which restrained entry into mitosis, was maintained in the mutant. Note that cell viability did not decrease under these conditions for up to 8 hr (Fig. 4D).
Figure 4.
Mis4 is sensitive to HU and becomes lethal in the S-phase block induced by HU. (A) like Cdc2-3w cells mis4-242 cells failed to form colonies in the presence of 5 mm HU at 26°C. (B) FACS analysis indicating that replication was blocked (DNA content is 1C) for 6 hr in mis4-242 in the presence of 15 mm HU at 26°C. (C) Mutant cells elongated with the interphase single nucleus. Bar, 10 μm. (D) Viability of mutant cells blocked by 15 mm HU remained high for 6 hr at 26°C. (E–G) Mutant cells were nitrogen starved to cause arrest in G1 at 26°C and then released in complete medium at 36°C in the presence or the absence of 15 mm HU. (E) The DNA content of mutant cells remained 1C for 7 hr in the presence of HU but increased to 2C after 4 hr in the absence of HU. (F) Mutant cells were elongated at 36°C in the presence of HU. (G) Viability of mutant cells decreased to 50% and 20% after 3 and 5 hr, respectively, in the absence of HU (○), whereas viability decreased to 50% and 20%, respectively, after 5 and 7 hr in the presence of HU (•). Mutant cells became inviable while the DNA content was still 1C.
The HU-sensitive phenotype of the mutant was further examined at 36°C. Mutant cells initially arrested in G1 at 26°C were released in rich medium at 36°C either in the presence or the absence of HU. These cells contained 1C DNA even after 7 hr in the presence of HU whereas cells without HU completed S-phase at 4 hr (Fig. 4E). Cells were highly elongated in the presence of HU for 7 hr at 36°C (Fig. 4F), indicating that replication checkpoint control remained in the mutant even at that temperature. Cell viability in the presence of HU remained high until 4 hr but then decreased and reached 10% after 6 hr (Fig. 4G). This timing of inviability was 1–2 hr slower than that of mutant cells that lost viability during the progression through S phase in the absence of HU. Notably, however, most mutant cell nuclei after 6 hr in the presence of HU contained only a 1C DNA content. The prolonged S-phase block by HU in mis4-242 thus caused severe cell lethality.
mis4-242 cells are sensitive to UV irradiation
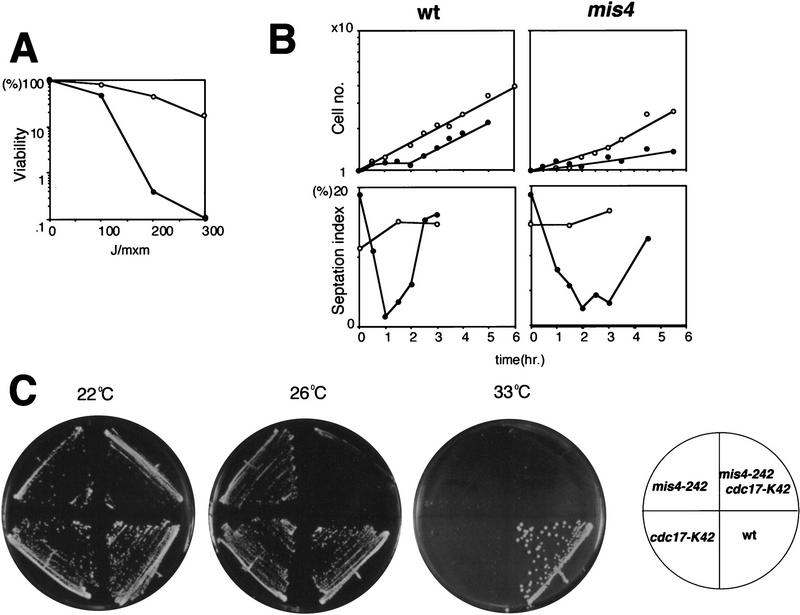
In addition to HU-sensitivity, mis4-242 cells were UV-irradiation sensitive at 26°C (Fig. 5A). To determine whether the DNA damage checkpoint control was retained after irradiation, the frequency of septum formation after damage was measured. Wild-type cells treated with UV irradiation showed a rapid decrease in the septum index as a result of the great delay for mitotic entry (Fig. 5B, left). Cells were elongated with a single nucleus (data not shown). The septation index was also reduced in mis4-242 mutants after irradiation (right panel). The recovery of this index, however, was delayed (the mutant took 2–3 hr, rather than 1.5 hr as shown by the wild type). These results show that mis4-242 mutants retain damage-sensing checkpoint control, but appear to be impaired in the process of repair or recovery.
Figure 5.
5 UV sensitivity and instability of chromosomes in mis4-242. (A) mis4-242 cells are (•) sensitive to UV irradiation at 26°C. (○) Wild-type control. (B) The septation indices of wild-type and mis4-242 cells were equally reduced by 100 joules of UV irradiation at 26°C (•), but the recovery of the septation index to the level of normal growing cells was significantly delayed in mutant cells (○) 0 joules of UV irradiation. (C) The double mutant mis4-242–cdc17-K42 did not form colonies even at 26°C.
mis4 is synthetic lethal with a DNA ligase mutation at 26°C
We looked for mutations that interacted with mis4-242. For this purpose, a number of genetic crossings were done between mis4-242 and various cut or cdc mutations, and the phenotypes were tested. A strong interaction with the cdc17 mutation, which is defective in DNA ligase, was found. The mis4–cdc17 double mutant did not form colonies at 26°C, but formed small colonies at 22°C (Fig. 5C). Weak interactions were found for other double mutants with mis5, cdc20, or cdc22, all defective in replication; the double mutants failed to form colonies below the restrictive temperature of single mutants.
Hypercondensation of chromosome occurs, but whole sister chromatids are separated in metaphase-arrested mis4-242 cells
Incorrect chromosome segregation in mis4 mutants strongly suggested that Mis4 may act to hold sister chromatids together until anaphase. Therefore, we examined the phenotypes of mitotically arrested mis4 mutants by introducing a second mutation that blocked cells in metaphase. Mis4 sister chromatids were condensed and held together in metaphase-arrested cut9 mutant cells; Cut9 is an essential component of the 20S APC/cyclosome complex required for anaphase-promoting proteolysis (Yamada et al. 1997). The double mutant cut9–mis4 was constructed and examined. Single cut9 and double cut9–mis4 mutants were arrested in G1 by nitrogen starvation and then released in rich medium at 36°C. In both mutant strains, Cdc2 protein kinase activity was measured and found to be high for 5–8 hr (Fig. 6A), and neither Cut2 nor Cdc13 was degraded (Fig. 6B). In single cut9 mutants at 36°C, chromosomes were highly condensed (Samejima and Yanagida 1994) whereas in the double cut9–mis4 mutants, they were also highly condensed but scattered (Fig. 6C). mis4-242 thus differs from budding yeast mcd1-1, which was shown to be defective in chromosome condensation (Guacci et al. 1997).
Figure 6.

Sister chromatid separation occurs in metaphase-arrested mis4-242 cells. (A) Single cut9 and double cut9–mis4 mutant cells initially arrested in G1 by nitrogen starvation and then released in complete medium and cultured at 36°C for 8 hr. The H1 kinase activity was high in both single cut9 and double cut9–mis4 mutant cells. (B) Cut2 and Cdc13 proteolysis was absent. (C) DAPI-stained single cut9 and double cut9–mis4 mutant cells. Both mutant strains were arrested at metaphase with condensed chromosomes. (D–F) FISH analysis applied to arrested single cut9 and double cut9–mis4 cells with probes from rDNA (D) the unique centromere region cenII (E), and the unique telomere region telII (F). Counter staining by anti-SPB antibody or DAPI is also shown with the merged image. Bar, 10 μm. (G) Localization of Mis4–GFP in the nucleus. The mis4+ gene was tagged with GFP and integrated into the chromosome as a single-copy gene with the native promoter. In living cells, the GFP signal was seen in nuclear chromatin. Signals were seen in the nucleus throughout the cell cycle. Punctate nuclear signals were also seen, which appeared to localize to rDNA and telomeres. (H) Mis4–GFP signals were seen on condensed chromosomes produced in the mitotically arrested β-tubulin mutant nda3-311 at 20°C.
To show that sister chromatids were separated in double mutant cells, FISH analysis (Uzawa and Yanagida 1992; Funabiki et al. 1993) was performed with three kinds of probes, namely, sequences of rDNA located on chromosome III, unique sequences derived from the centromere II (cen II), and the telomere of the short arm of chromosome II (tel II). As seen in a typical example of cells in Figure 6D–F, the regions of rDNA, centromeres, and telomeres were all separated in double mutant cells, but never in single cut9 mutants. These results definitively demonstrate that Mis4 is needed to associate all the regions of sister chromatids in metaphase-arrested cells.
Localization of Mis4 to the nuclear chromatin region
The results described above suggest that Mis4 is a nuclear chromosomal protein. Mis4 is indeed localized to the nucleus. The single copy gene of mis4+ was tagged with jellyfish green fluorescent protein (GFP) and integrated into the chromosome (see Materials and Methods). The carboxyl terminus of Mis4 was tagged with GFP and the promoter was kept native. This construct was functional, as cells substituting the temperature-sensitive mutant gene with the GFP construct could grow normally. As shown in Figure 6G, the nuclear chromatin in cells expressing GFP–Mis4 was fluorescent with intense nuclear punctate signals. Note that fluorescence signals are enriched in rDNA (Uzawa and Yanagida 1992) as well as other restricted chromatin regions. No decrease of the signals was detected during mitosis. Punctate GFP signal was also observed in anaphase nuclear chromatin. In nda3 mutant cells expressing Mis4–GFP, the signals were localized to condensed chromosomes (Fig. 6H). Some of the chromosomal punctate signals were also seen. Mis4, at least its fraction, was associated with chromosomes throughout the cell cycle.
Premature sister chromatid separation occurs in mis4-242 cells during the post-replicative interphase
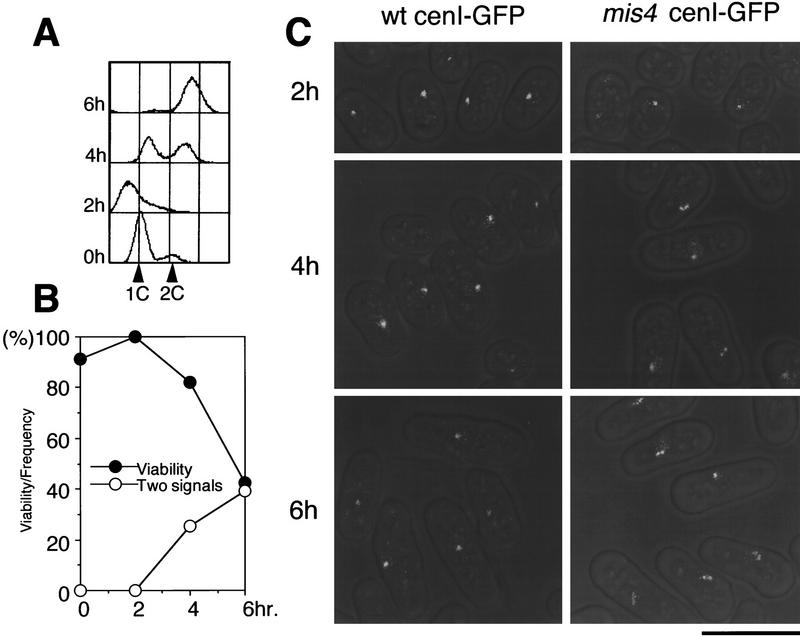
A question was raised whether sister chromatids were prematurely separated in mis4-242 interphase cells after replication. The mutant cells were initially arrested in G1 by nitrogen starvation and then released in complete medium at 36°C. DNA replication occurred about 4 hr after the shift (Fig. 7A). Mitosis took place around 7 hr (see Fig. 2). Cells were collected at 2, 4, and 6 hr, and to visualize behavior of the centromere DNA of chromosome I (cen1) in living cells (see Materials and Methods; Straight et al. 1996; Nabeshima et al. 1998) the cen1–GFP technique was applied to score the frequencies of cells revealing the two separated signals of the unique cen1 DNA. A large fraction of interphase cells showed the two split cen1 signals after 4–6 hr (∼30% and 40% after 4 and 6 hr, respectively). Examples of cells showing the split cen1–GFP dots in interphase are shown in Figure 7C. Hence, we concluded that the sister chromatids were prematurely separated after the S phase and continued to be split until the following mitosis in mis4 mutant cells. Note that these interphase cells revealing the two split signals did not contain the spindle at all, so that separation was not attributable to the spindle force. The decrease of viability coincided with the increase of split signals, suggesting that the cause for the loss of viability was the failure to form the sister chromatid cohesion.
Figure 7.
Sister centromeres are prematurely separated in the interphase state of mis4-242 after replication. The cen1 DNA was visualized by use of the LacI system; (Straight et al. 1996). Lac repressor was tagged with GFP and an NLS and expressed in a S. pombe strain containing LacO repeats integrated near cen1 (Nabeshima et al. 1998) mis4-242 cells (and a wild-type control) expressing the LacI–NLS–GFP and integrated with the LacO repeat were arrested in G1 by nitrogen starvation at 26°C, shifted to complete medium, and cultured at 36°C for 8 hr. (A) FACS analysis indicating that replication occurred in mis4-242 cells after 4 hr and was completed before 6 hr as in the wild type (not shown). (B) cen1-GFP signals were visualized, and the frequencies of cells revealing two signals (○) graphed with the frequencies of viable cells (•). Because mitotic cells started to increase after 7 hr, data for the two split signals were not taken after that time. (C) Example of mis4-242 cells showing the two split cen1 signals. The wild-type control is also shown. Bar, 10 μm.
Viability of metaphase-arrested mis4 mutant cells is quickly lost
As S phase in growing fission yeast cells was initiated immediately after mitotic anaphase, we wanted to assure that mis4 cells truely lost viability during the first mitosis when cells were shifted from early G2 (see Fig. 1). For this purpose, viability of the double mutants cut9–mis4 and cut7–mis4 was measured and compared with that of single mutants (cut7 mutant was arrested at prophase; Hagan and Yanagida 1990). Viability of the double mutants, which were cultured from G2 and arrested at metaphase at 36°C, decreased more rapidly (reduced to 10%–15% after 2 hr) than single cut9 and cut7 mutants (60%–70%), suggesting that Mis4 was needed to maintain viability in mitotically arrested cells. The viability of double mutant cdc25–mis4 cells (similarly cultured as described above but arrested in late G2), on the other hand, decreased much more slowly (60% after 2 hr) than single mis4 mutants (30%), indicating that viability is lost in mis4 mutant much faster in metaphase- rather than in G2-arrested cells.
Discussion
This study describes results demonstrating that fission yeast Mis4 is required for sister chromatid cohesion, its loss leading to premature sister chromatid separation. Properties of Mis4 differ from those of the previously identified Rad21/Mcd1p/Scc1p cohesin. In Xenopus, XRAD21 forms a complex with the two SMC subunits (XSMC1 and XSMC3) and two other unknown subunits, p155 and p95 (Losada et al. 1998). This five-member Xenopus cohesin complex is required for sister chromatid cohesion: Immunodepletion of the complex causes defects in sister chromatid cohesion in subsequent mitosis. In S. pombe, besides Rad21 (Birkenbihl and Subramani 1992), proteins highly similar to Smc1 and Smc3 are present, and their complex products were obtained (T. Sutani, J. Morishita, T. Tomonaga, M. Yanagida, unpubl.). Mis4 does not seem to be one of the unknown subunits in the cohesin complex. Immunoprecipitation with antibodies against Smc3 or Rad21 did not coprecipitate Mis4 (K. Furuya, T. Sutani and M. Yanagida, unpubl.). In other experiments with anti-Cut3 antibodies, Mis4 was not coprecipitated with Cut3, indicating that Mis4 is the subunit of neither cohesin nor condensin. Mis4 may exist in an oligomeric complex, as it sediments at ∼10S in sucrose gradient centrifugation (K. Furuya, K. Takahashi, and M. Yanagida, unpubl.), distinct from the 14S peak of the five-member cohesin.
Properties of Mis4 suggest that the manner in which Mis4 is involved in sister chromatid cohesion is distinct from Rad21/Scc1/Mcd1, the subunit of cohesin. First, Mis4 is not directly related to condensation whereas Mcd1 is shown to be required for chromosome condensation (Guacci et al. 1997). However, the requirement of cohesin (Mcd1p) for chromosome condensation has recently been challenged in a Xenopus system by Losada et al. (1998). Second, Mis4 is not the target of the APC/cyclosome whereas Mcd1/Scc1 requires the APC/cyclosome for its destruction (Michaelis et al. 1997). Mis4 is present in anaphase- and G1-arrested cells; Mis4–GFP signals were clearly visualized in anaphase chromosomes. Third, Mis4 is required to maintain viability during the S phase, though replication per se is not blocked in mis4 mutants. Synthetic lethality with a DNA ligase mutant suggests that gaps or nicks, causing chromosome instability or recombination, might be produced in mis4 mutant cells. UV and HU hypersensitivity also suggest the presence of chromosomal defects generated by mis4 mutation. During ongoing replication and G2, correction of damage on one sister is normally facilitated by the close proximity of the undamaged sister, but loss of cohesion interferes with such interactions. Recombinational repair might also be defective in mis4 mutant as the sisters are farther away. But these possibilities remain to be determined. Taken together, we conclude that Mis4 belongs to a distinct class of proteins required for sister chromatid cohesion. It functions differently from Rad21/Scc1/Mcd1, and is unrelated to the amino acid sequences of SMC proteins. Mis4, together with C. cinereus Rad9, budding yeast Scc2, mouse AA062272, and human HUMHBC4244 may constitute a novel family of sister chromatid adhesion molecules. We propose naming Rad9/Scc2/Mis4 adherin to distinguish it from the original SMC-interacting cohesin. We discuss possible roles of Mis4 for sister chromatid cohesion, ad propose a simple model.
Mis4 is an essential component of sister chromatid cohesion
Punctate nuclear signals of Mis4–GFP suggest that Mis4 may preferentially associate with distinct chromosomal sites. In mitotically arrested cells, the dotted images were also seen on hypercondensed chromosomes. The punctate signals might colocalize with repetitive regions such as rDNA. As the rDNA repeats are located at the ends of chromosome III, Mis4 may be enriched in other repetitive chromosomal ends. Mis4 did not appear to be enriched in the centromeric regions, however. Mis4 may recognize DNA sequence motifs or protein(s) enriched in such particular chromosomal regions. Further investigation is needed to precisely identify Mis4 localization, however. The conserved carboxy-terminal regions in Rad9/Scc2/Mis4 adherin molecules form no nucleotide binding sequence nor known DNA-binding motif, and their molecular function is unknown. Three Cdc2 kinase phosphorylation sites are present in the amino termini of Mis4 and Rad9, but not in Scc2. The amino-terminal region may have a regulatory function, as that of Mis4 also contains the nuclear localization signal (Y. Toyoda, K. Furuya, and M. Yanagida, unpubl.).
We show that whole chromatids are separated in metaphase-arrested mis4 mutant cells using three different hybridization probes for the FISH analysis. Premature sister chromatid separation in mis4 mutants is also demonstrated in interphase G2 cells by inactivation of mutant Mis4 protein in G1. Separation is not caused by the spindle force, as G2 cells in fission yeast are completely devoid of spindles. Great reduction in the level of Mis4 protein in mutant cells probably causes the loss of connection between sister chromatids and results in the premature separation phenotype. Mis4 may thus provide a direct link between chromatids or may alter chromatids so that they have a structure capable of interacting with cohesin. No genetic interaction, however, has been found between mis4 and rad21 mutations so far. It is of considerable interest to determine whether these two chromatid cohesion molecules are functionally interrelated.
Requirement for Mis4 in S phase and M phase
When mis4 mutants had been inactivated in G1 or G2, a cell cycle defect was produced in the subsequent S or M phase, respectively, suggesting that Mis4 was required for both these phases. Viability was kept to be relatively high in the gap stages between S phase and M phase. The failure to form sister chromatid cohesion is probably the cause of mutant cell death during S phase; this defect could not be restored when cells had passed this phase. The requirement for Mis4 in M phase is intriguing, as the progression of the first mitosis is an unrecoverable lethal event but cytologically indistinguishable from normal mitosis. One explanation of this phenotype is that mutant cells could not establish, during the first mitosis, a particular chromosome conformation that is specifically required for the reforming of sister chromatid cohesion in the subsequent S phase. The major chromosome reorganization reported to take place at the onset of mitosis in vertebrate cells (Losada et al. 1988) may also take place in S. pombe. The lack of Mis4 in the reorganization step may not affect sister chromatid separation immediately thereafter but may lead to the loss of the ability to reform cohesion in the subsequent S phase. If sister cohesion is not made in S phase, premature sister chromatid separation inevitably occurs in G2 and gives rise to nearly random chromosome segregation in the following mitosis. Bi-orientation of sister centromeres will not be achieved in metaphase if sister chromatid cohesion is entirely abolished. A similar loss of cohesion, in the restricted region, was reported for the mutant of Mis6, a centromere-specific connector protein essential for association of sister centromeres that acts during the G1/S phase (Saitoh et al. 1997).
Mis4 and the onset of anaphase
The removal of cohesin from chromosomes upon entry into anaphase requires Esp1, which is essential for sister chromatid separation (Ciosk et al. 1998). Anaphase-promoting proteolysis triggers the destruction of Pds1, an inhibitor of Esp1, resulting in the activation of Esp1, which may subsequently promote disruption of the linkage between chromatids by removing Scc1. However, we have not yet found any genetic and biochemical relations between Mis4 and Cut1 (a homolog of Esp1) or between Mis4 and Cut2 (a homolog of Pds1; Kumada et al. 1998). Therefore, we consider a different mechanism for the implication of Mis4 in the onset of anaphase.
We propose a simple model in which Mis4 associates with chromosomes throughout the cell cycle and forms the connection between sister chromatids directly or indirectly through another linking protein. Disruption of the link might occur by splitting of the oligomeric Mis4 complex into halves or removal of the linking protein from chromosomes in anaphase while Mis4 remains associated with chromosomes. Post-translational modification such as protein dephosphorylation (Ohkura et al. 1989) may be important in disconnecting the linkage. Many other models may be possible, but the one described here is among the simplest. During S phase, Mis4 should be added to a newly replicated DNA. If Mis4 is inactive at the time of replication, the connection between chromatids will not be made, resulting in precocious separation of sister chromatids after replication. Once formed, sister cohesion would be maintained even if Mis4 is later inactivated, but would result in normal-looking mitosis with a cryptic irreversible defect. Identification of actual chromosome defects at the molecular level will be an important step toward understanding the regulation of sister chromatid cohesion. Both cohesin and adherin are essential for sister chromatid cohesion, and the loss of either one results in premature chromatid separation. Determination of their hierarchical relationship, if any exists, will also be a subject for future investigation.
Materials and methods
Strains and media
S. pombe haploid strains were used. Handling of temperature-sensitive strains, mis4, cut9, cdc25, and cdc17 and media used were described previously (Takahashi et al. 1994; Yamada et al. 1997). The strain used for integration of the cloned mis4+ gene onto the chromosome was h− mis4-242 ura4.
Plasmids
Procedures for gene cloning in S. pombe by transformation were described previously (Takahashi et al. 1994). An S. pombe genomic library was used for isolation of the mis4+ gene. The selection marker was the S. cerevisiae LEU2 gene. Integration plasmid pYC6 containing the ura4+ gene was used for integration of a 3-kB BamHI–BglII fragment into the chromosome by homologous recombination. The carboxyl terminus of mis4+ was tagged in frame with the 3× HA antigen. A plasmid carrying the tagged mis4+ was able to suppress the temperature-sensitive phenotype of mis4–242. The vector plasmid used was pSK248.
Molecular biological methods
The nucleotide sequence of a 7-kb long BamHI–BglII fragment in pKT221 was determined. Three of the presumed introns in the carboxyl terminus were confirmed by PCR amplification of cDNA. 12CA5, an antibody against HA, was used for immunoblotting to detect Mis4–HA. Cells were irradiated at 36°C in a warm room with temperature control. The FACScan analysis was performed according to the procedures described by Costello et al. (1986). Cells collected were washed in 70% ethanol. DNA contents were measured by a Becton-Dickinson FACScan apparatus after propidium iodide staining.
Gene disruption
The procedures described by Rothstein (1983) were followed. The BglII–PstI fragment in pKT221 was subcloned, and an internal HindIII fragment was removed and substituted with the S. pombe ura4+ gene. The resulting plasmid, pKF230, was digested with SnaBI and SpeBI and used for transformation of a diploid (5A/1D). Stable transformants for the Ura+ marker were isolated, and gene disruption was verified by genomic Southern hybridization. The resulting heterozygous diploid Δ10 was sporulated and tetrads dissected.
Synchronous culture
The procedures described by Moreno et al. (1989) and Kinoshita et al. (1990) were followed. A Beckman elutriator rotor was used for collecting small G2 cells. Procedures for nitrogen starvation were employed to concentrate cells in G1 followed by release into complete medium (Saka and Yanagida 1993). EMM2-N lacking NH4Cl was used for starvation (2 × 107 /ml for 24 hr at 26°C). A cell concentration of 1 × 106 /ml was employed for the shift into complete medium at 36°C. Block and release experiments with the cdc25 mutant were done as described (Funabiki et al. 1996; Yamada et al. 1997).
GFP constructs
The use of GFP for tagging the carboxyl terminus of Mis4 and integrating of the construct into the chromosome was done as described (Nabeshima et al. 1995). The integration vector pYC11 was used, and the resulting plasmid pMIS4-GFP was used for transformation of the strain h− leu1 mis4-242 after linearization. The resulting Leu+ Ts+ integrants were used for microscopy. A fission yeast strain was constructed to visualize cenDNA (Nabeshima et al. 1998) A haploid S. pombe strain h− lys1 his7 was transformed simultaneously with the two plasmids pMK24A and pMK2A. pMK24A carried the GFP–LacI–NLS (Straight et al. 1996) driven by the promoter region of dis1+ (Nabeshima et al. 1995) and the his7+ gene; NLS is the nuclear location signal. The plasmid pMK2A contained the LacO repeat and the lys1+ gene, which is tightly linked to the cen1 locus (∼30 kb; Takahashi et al. 1992). The resulting Lys+ His+ transformants were isolated. Transformant MKY5A contained the integrated GFP–LacI–NLS gene at the his7 locus and also the LacO array at the lys1 locus.
Microscopy
FISH analyses and immunofluorescence microscopy with anti-tubulin antibody were done as described by Funabiki et al. (1993). Staining of DNA in living cells was done as described previously (Chikashige et al. 1994). Detailed procedures for the culture of cells for microscopy were described by Nabeshima et al. (1997). A MRC-1024 confocal microscope was used with excitation at 488 nm and the filter 522DF32.
Acknowledgments
This study was supported by grants from the Japan Science Technology Corporation, the Human Frontier Science Program Organization, and the Science and Technology Agency of Japan.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked ‘advertisement’ in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL yanagida@kozo.biophys.kyoto-u.ac.jp; FAX 81 75 753 4208.
References
- Adachi Y, Usukura J, Yanagida M. A globular complex formation by Nda1 and the other five members of the MCM protein family in fission yeast. Genes to Cells. 1997;2:467–479. doi: 10.1046/j.1365-2443.1997.1350333.x. [DOI] [PubMed] [Google Scholar]
- Birkenbihl RP, Subramani S. Cloning and characterization of rad21 an essential gene of Schizosaccharomyces pombe involved in DNA double-strand-break repair. Nucleic Acids Res. 1992;20:6605–6611. doi: 10.1093/nar/20.24.6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikashige Y, Ding D-Q, Funabiki H, Haraguchi T, Mashiko S, Yanagida M, Hiraoka Y. Telomere-led premeiotic chromosome movement in fission yeast. Science. 1994;264:270–273. doi: 10.1126/science.8146661. [DOI] [PubMed] [Google Scholar]
- Ciosk R, Zachariae W, Michaelis C, Shevchenko A, Mann M, Nasmyth K. An ESP1/PDS1 complex regulates loss of sister chromatid cohesion at the metaphase to anaphase transition in yeast. Cell. 1998;93:1067–1076. doi: 10.1016/s0092-8674(00)81211-8. [DOI] [PubMed] [Google Scholar]
- Costello G, Rodgers L, Beach D. Fission yeast enters the stationary phase G0 state from either mitotic G1 or G2. Curr Genet. 1986;11:119–125. [Google Scholar]
- Funabiki H, Hagan I, Uzawa S, Yanagida M. Cell cycle-dependent specific positioning and clustering of centromeres and telomeres in fission yeast. J Cell Biol. 1993;121:961–976. doi: 10.1083/jcb.121.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabiki H, Yamano H, Kumada K, Nagao K, Hunt T, Yanagida M. Cut2 proteolysis required for sister-chromatid separation in fission yeast. Nature. 1996;381:438–441. doi: 10.1038/381438a0. [DOI] [PubMed] [Google Scholar]
- Guacci V, Koshland D, Strunnikov AV. A direct link between sister chromatid cohesion and chromosome condensation revealed through the analysis of MCD1 in S. cerevisiae. Cell. 1997;91:47–57. doi: 10.1016/s0092-8674(01)80008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan IM, Yanagida M. Novel potential mitotic motor protein encoded by the fission yeast cut7+ gene. Nature. 1990;347:563–566. doi: 10.1038/347563a0. [DOI] [PubMed] [Google Scholar]
- Hirano T, Funahashi S, Uemura T, Yanagida M. Isolation and characterization of Schizosaccharomyces pombe cut mutants that block nuclear division but not cytokinesis. EMBO J. 1986;5:2973–2979. doi: 10.1002/j.1460-2075.1986.tb04594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway S, Glotzer M, King RW, Murray AW. Anaphase is initiated by proteolysis rather than by the inactivation of maturation-promoting factor. Cell. 1993;73:1393–1402. doi: 10.1016/0092-8674(93)90364-v. [DOI] [PubMed] [Google Scholar]
- Kerrebrock AW, Moore DP, Wu JS, Orr-Weaver TL. Mei-S332, a Drosophila protein required for sister-chromatid cohesion, can localize to meiotic centromere regions. Cell. 1995;83:247–256. doi: 10.1016/0092-8674(95)90166-3. [DOI] [PubMed] [Google Scholar]
- Kinoshita N, Ohkura H, Yanagida M. Distinct, essential roles of type 1 and 2A protein phosphatases in the control of the fission yeast cell division cycle. Cell. 1990;63:405–415. doi: 10.1016/0092-8674(90)90173-c. [DOI] [PubMed] [Google Scholar]
- Kitazono A, Matsumoto T. ‘Isogaba Maware’: Quality control of genome DNA by checkpoints. BioEssays. 1998;20:391–399. doi: 10.1002/(SICI)1521-1878(199805)20:5<391::AID-BIES6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Kumada K, Nakamura T, Nagao K, Funabiki H, Nakagawa T, Yanagida M. Cut1 is loaded onto the spindle by binding to Cut2 and promotes anaphase spindle movement upon Cut2 proteolysis. Curr Biol. 1998;8:633–641. doi: 10.1016/s0960-9822(98)70250-7. [DOI] [PubMed] [Google Scholar]
- Losada A, Hirano M, Hirano T. Identification of Xenopus SMC protein complexes required for sister chromatid cohesion. Genes & Dev. 1998;12:1986–1997. doi: 10.1101/gad.12.13.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis C, Ciosk R, Nasmyth K. Cohesins: Chromosome proteins that prevent premature separation of sister chromatids. Cell. 1997;91:35–45. doi: 10.1016/s0092-8674(01)80007-6. [DOI] [PubMed] [Google Scholar]
- Molnar M, Bahler J, Sipiczki M, Kohli J. The rec8 gene of Schizosaccharomyces pombe is involved in linear element formation, chromosome pairing, and sister-chromatid cohesion during meiosis. Genetics. 1995;141:61–73. doi: 10.1093/genetics/141.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DP, Page AW, Tang TT, Kerrebrock AW, Orr-Weaver TL. The cohesion protein MEI-S332 localizes to condensed meiotic and mitotic centromeres until sister chromatids separate. J Cell Biol. 1998;140:1003–1012. doi: 10.1083/jcb.140.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Hayles J, Nurse P. Regulation of p34cdc2 protein kinase during mitosis. Cell. 1989;58:361–372. doi: 10.1016/0092-8674(89)90850-7. [DOI] [PubMed] [Google Scholar]
- Nabeshima K, Kurooka H, Takeuchi M, Kinoshita K, Nakaseko Y, Yanagida M. p93dis1 required for sister chromatid separation is a novel microtubule and spindle pole body associating protein phosphorylated at the Cdc2 target sites. Genes & Dev. 1995;9:1572–1585. doi: 10.1101/gad.9.13.1572. [DOI] [PubMed] [Google Scholar]
- Nabeshima K, Saitoh S, Yanagida M. Use of green fluorescent protein for intracellular localization in living fission yeast cells. Methods Enzymol. 1997;283:459–471. doi: 10.1016/s0076-6879(97)83037-6. [DOI] [PubMed] [Google Scholar]
- Nabeshima, K., Y. Nakagawa, A.F. Straight, A. Murray, Y. Chikashige, Y. Yamashita, Y. Hiraoka, and M. Yanagida. 1998. Dynamics in fission yeast Metaphase and Anaphase A: Constant spindle length period requiring Dis1 reveals distinct centromere movements. Mol. Biol. Cell 9: (in press). [DOI] [PMC free article] [PubMed]
- Ohkura H, Kinoshita N, Miyatani S, Toda T, Yanagida M. The fission yeast dis2+ gene required for chromosome disjoining encodes one of two putative type 1 protein phosphatases. Cell. 1989;57:997–1007. doi: 10.1016/0092-8674(89)90338-3. [DOI] [PubMed] [Google Scholar]
- Rothstein RJ. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Rowles A, Blow JJ. Chromatin proteins involved in the initiation of DNA replication. Curr Opin Genet Dev. 1997;7:152–157. doi: 10.1016/s0959-437x(97)80123-2. [DOI] [PubMed] [Google Scholar]
- Saitoh S, Takahashi K, Yanagida M. Mis6, a fission yeast inner centromere protein, acts during G1/S and forms specialized chromatin required for equal segregation. Cell. 1997;90:131–143. doi: 10.1016/s0092-8674(00)80320-7. [DOI] [PubMed] [Google Scholar]
- Saka Y, Yanagida M. Fission yeast cut5+ gene required for the onset of S-phase and the restraint of M-phase is identical to the radiation-damage repair gene rad4+ Cell. 1993;74:383–393. doi: 10.1016/0092-8674(93)90428-s. [DOI] [PubMed] [Google Scholar]
- Samejima I, Yanagida M. Bypassing anaphase by fission yeast cut9 mutation: Requirement cut9+ gene to initiate anaphase. J Cell Biol. 1994;127:1665–1670. doi: 10.1083/jcb.127.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz LC, Tang K, Cummings WJ, Zolan ME. The rad9 gene of Coprinus cinereus encodes a proline-rich protein required for meiotic chromosome condensation and synapsis. Genetics. 1996;142:1105–1117. doi: 10.1093/genetics/142.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight AF, Belmont AS, Robinett CC, Murray AW. GFP tagging of budding yeast chromosomes reveals that protein-protein interactions can mediate sister chromatid cohesion. Curr Biol. 1996;6:1599–1608. doi: 10.1016/s0960-9822(02)70783-5. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Murakami S, Chikashige Y, Niwa O, Funabiki H, Yanagida M. A low copy number central sequence with strict symmetry and unusual chromatin structure in the fission yeast centromere. Mol Biol Cell. 1992;3:819–835. doi: 10.1091/mbc.3.7.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamada H, Yanagida M. Fission yeast minichromosome loss mutants mis cause lethal aneuploidy and replication abnormality. Mol Biol Cell. 1994;5:1145–1158. doi: 10.1091/mbc.5.10.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzawa S, Yanagida M. Visualization of centromeric and nucleolar DNA in fission yeast by fluorescence in situ hybridization. J Cell Sci. 1992;101:267–275. doi: 10.1242/jcs.101.2.267. [DOI] [PubMed] [Google Scholar]
- Yamada H, Kumada K, Yanagida M. Distinct subunit functions and cell cycle regulated phosphorylation of 20S APC/cyclosome required for anaphase in fission yeast. J Cell Sci. 1997;110:1793–1804. doi: 10.1242/jcs.110.15.1793. [DOI] [PubMed] [Google Scholar]