Abstract
Clinical reports suggest an association of distinct Mycobacterium tuberculosis strains with CNS disease. We therefore examined CNS dissemination by different laboratory strains (two M. tuberculosis H37Rv, one CDC1551) in a guinea pig aerosol infection model. Although all strains grew exponentially in lungs, with similar bacterial burdens at the time of extrapulmonary dissemination, M. tuberculosis CDC1551 disseminated to the CNS significantly more than the H37Rv strains. No CNS lesions were observed throughout the study, with only a modest cytokine response. These data suggest that M. tuberculosis may have virulence factors that promote CNS dissemination, distinct from those required for pulmonary TB.
Keywords: tuberculosis, central nervous system, meningitis, dissemination, guinea pig
INTRODUCTION
Central nervous system tuberculosis (CNS TB) is the most severe and debilitating form of TB. Primarily affecting children in developing nations, diagnosis and management of CNS TB is tremendously challenging due to non-specific clinical presentation, poor diagnostics, and limited availability of adequate clinical resources. By the time of diagnosis, the patient often exhibits progressive disease, with hydrocephalus and vasculitis leading to infarction1.
Over the course of more than a century of TB research, many models have been employed as tools for the study of TB pathogenesis and disease mechanisms2. However, the majority of model systems for CNS TB have employed direct inoculation of bacilli into the cerebrospinal fluid (CSF) or cerebrum3, 4. Although such models are useful for the study of acute and chronic meningitis, they do not simulate the natural course of disease, typified by bacillary dissemination via blood. In the current study, we describe a guinea pig model of extrapulmonary dissemination and utilize it to study CNS dissemination of three different laboratory strains of M. tuberculosis after an aerosol infection.
RESULTS
Extrapulmonary and CNS dissemination of M. tuberculosis
On day 1 after infection, log10 colony forming units (CFU) were 3.1 ± 0.1, 2.2 ± 0.3, and 3.0 ± 0.3 in animals infected with M. tuberculosis CDC1551, H37Rv JHU, and H37Rv TAMU, respectively. Pulmonary bacillary burdens reached a plateau 14 days after infection, which were not significantly different between the 3 strains (P > 0.3). Complete lung CFU data for these animals have been described previously by Ahmad, et al.5.
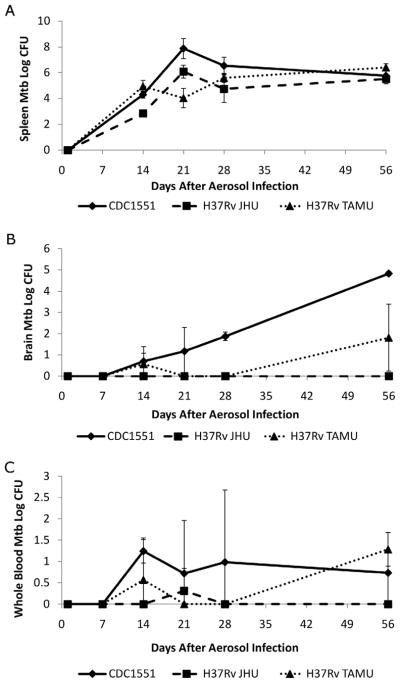
Extrapulmonary dissemination was first observed 14 days after infection for all three strains (Fig. 1A). Although M. tuberculosis H37Rv JHU demonstrated a lower spleen bacillary burden at day 14, there was no difference between CDC1551 and H37Rv TAMU at this time point. Moreover, no significant differences were found amongst the 3 strains at days 28 and 56. However, M. tuberculosis CDC1551 were detected at significantly higher levels in the brain at all time points, compared to the two H37Rv strains (P < 0.04) (Fig. 1B). No bacteria were recovered from brains of guinea pigs infected with M. tuberculosis H37Rv JHU at any of the time points examined, while bacteria were detected only in low numbers from brains of guinea pigs infected with M. tuberculosis H37Rv TAMU at days 14 and 56 after infection. Bacteria were also cultured from blood and CFU counts extrapolated to the whole blood volume (25 mL) of the guinea pig. Bacillemia (< 2.5 log10 CFU) was first detected in M. tuberculosis CDC1551-infected animals 14 days after infection and remained relatively constant over the course of the infection. Bacillemia (< 2.0 log10 CFU) was also detected intermittently in animals infected with M. tuberculosis H37Rv TAMU or H37Rv JHU strains (Fig. 1C).
Figure 1. Guinea pig brain and spleen CFU following aerosol infection.
A. Extrapulmonary dissemination was first observed 14 days after infection for all 3 strains. M. tuberculosis H37Rv JHU demonstrated a lower bacterial burden at day 14, although there was no significant difference between CDC1551 and H37Rv TAMU at this time point or between any strains after day 28. B. M. tuberculosis CDC1551 were detected at significantly higher levels in the brain at all time points, compared to the two H37Rv strains (P < 0.04) (Fig. 1B). No bacteria were recovered from brains of guinea pigs infected with M. tuberculosis H37Rv JHU at any of the time points examined, while bacteria were detected only in low numbers from brains of guinea pigs infected with M. tuberculosis H37Rv TAMU at 14 and 56 days after infection. C. Blood CFU counts shown are extrapolated to the whole blood volume (25 ml) of the guinea pig. Bacteria were detected in the whole blood starting at day 14 after infection. Bacillemia was most consistent for strain CDC1551, but remained low in each animal (< 2.5 log10 CFU) at each time point. At least 3 animals were used for data collection at each time point.
Cytokine analysis of M. tuberculosis infected brain tissues
Lesions were not observed in the brains of any of the infected groups upon gross pathological examination at any time points during the study. Similarly, no inflammation or cellular infiltrates were observed on histological analyses of the brain tissues during the course of the study. In order to evaluate the immune response elicited in the CNS during infection with M. tuberculosis CDC1551, levels of two proinflammatory cytokines were examined by ELISA. TNF-α and IL-1β levels were elevated (relative to day 1) at days 14 and 56 (P < 0.0001) following aerosol infection (Fig. 2), but were significantly below what is observed in the lungs after infection in guinea pigs6.
Figure 2. Immune response in guinea pig brain tissue following aerosol infection with M. tuberculosis CDC1551.
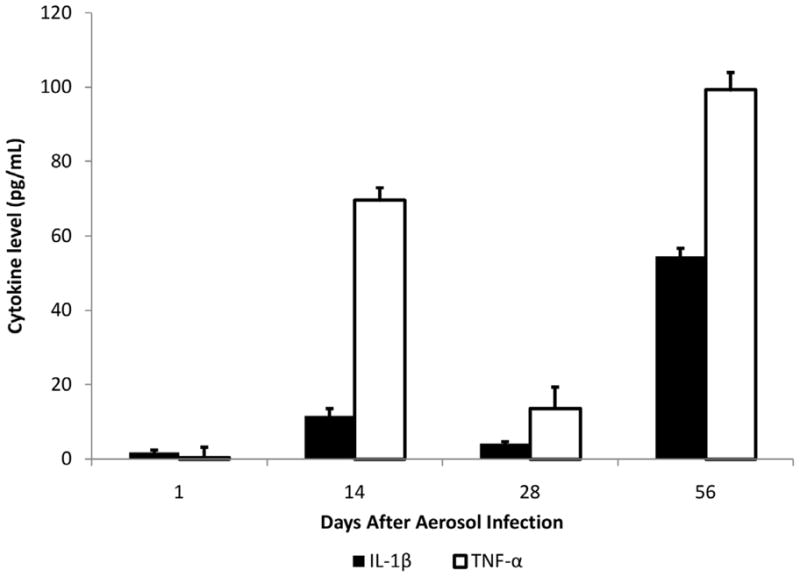
The cytokines IL-1β and TNF-α were measured at days 1, 14, 28 and 56 after infection. Both of these proinflammatory mediators were found to be modestly but significantly elevated (relative to day 1) at days 14 and 56 (P < 0.0001) following aerosol infection, although at much lower levels than is typically observed in the lungs. At day 28, TNF-α levels were only slightly elevated (P = 0.003), and no change was observed for IL-1β (P = 0.6). Three animals were used for cytokine analysis at each time point.
DISCUSSION
We developed a guinea pig model of extrapulmonary dissemination and utilized it to study CNS dissemination of three different laboratory strains of M. tuberculosis after an aerosol infection. Although all three strains evaluated in this study grew exponentially in the lungs and had similar bacterial burdens at the time of extrapulmonary dissemination (day 14), M. tuberculosis CDC1551 disseminated to the CNS significantly more than the two H37Rv strains. Brain CFU counts were higher than CFU in the whole blood volume of the animal at every time point after day 14, precluding the possibility of brain tissue contamination by extraneous bacteria from the blood. This increased dissemination to the CNS may be explained partially by the higher bacterial burdens noted in the spleens of guinea pigs infected with M. tuberculosis CDC1551 at day 14, although animals infected with H37Rv TAMU had comparable bacterial burdens but significantly lower dissemination to the CNS. Moreover, CNS invasion by M. tuberculosis H37Rv strains continued to be significantly lower than CDC1551 at later time points (days 28 and 56), while spleen CFUs were not different amongst the 3 strains.
Kaplan and colleagues have examined the virulence of M. tuberculosis strains in a rabbit model of TB meningitis. In contrast to our results, they demonstrated that rabbits infected with the M. tuberculosis H37Rv strain had higher bacterial burdens in the cerebrospinal fluid (CSF) and brain, with increased dissemination to other organs compared with those infected with CDC15513. However, these studies utilized direct intracisternal inoculation of bacteria into the CSF and evaluated subsequent dissemination from the CNS to the lungs or liver. This model therefore does not reflect CNS TB as it occurs in humans, where bacteria disseminate to the CNS via blood. Palanisamy et al. have also examined extrapulmonary dissemination of selected M. tuberculosis strains in the guinea pig aerosol model. Although dissemination to the CNS was not included in their studies, M. tuberculosis CDC1551 strain was found to be more virulent than the H37Rv strain7. Further, dissemination to the spleen was no different between the CDC1551 and H37Rv strains (evaluated 30 days after infection), which is consistent with our data.
As an increasing number of clinically-derived strains are genotyped, it is becoming apparent that the profile of TB disease is likely to be influenced by the infecting strain. Multiple reports have shown the association of different M. tuberculosis strains with extrapulmonary dissemination. Garcia de Viedma et al. have shown that TB patients may be concurrently infected with distinct M. tuberculosis strains that inhabit pulmonary and extra-pulmonary sites8, 9. A recent study by Hesseling et al. demonstrates that children infected with the M. tuberculosis Beijing or S genotypes were more likely to have extra-pulmonary TB compared with children infected with the LAM genotype10. Similarly, a report by Caws et al. demonstrates that the Euro-American lineage of M. tuberculosis is less capable of CNS dissemination than other M. tuberculosis strains11. Another recent report, utilizing an experimental mouse model, demonstrates that clinical strains isolated from patients with TB meningitis, but not pulmonary TB, disseminate extensively to the CNS12. These data highlight the association of specific M. tuberculosis strains with extrapulmonary dissemination and CNS TB, and suggest that M. tuberculosis may have virulence factors that promote CNS dissemination, distinct from those required for pulmonary TB. This hypothesis is consistent with our prior findings in a murine model, suggesting that distinct M. tuberculosis genes promote CNS dissemination which are not required for survival in lung tissue13. It should be noted that while we have previously shown that animal passaging of M. tuberculosis does not correlate with virulence in vivo5, 14, M. tuberculosis H37Rv JHU used in this study was extensively passaged in animals. We do not currently have access to genome data for M. tuberculosis strains H37Rv JHU and TAMU, but this and other studies will be the focus of our future work to better understand and identify the microbial determinants of CNS invasion.
Seeding of bacilli in the CNS did not yield a robust inflammatory response. Moreover, the levels of inflammatory cytokines were far below what is typically observed in the lungs at a similar stage of infection6. General extrapulmonary dissemination is first observed at day 14, which may account for the spike in an inflammatory response in the CNS at this time point, which subsided over the following two weeks. The increase at the final day 56 time point is likely due to more extensive bacillary replication. It should be noted that mouse TNF-α demonstrates > 92% homology with guinea pig protein, and is thus likely to be cross-reactive. However, the mouse TNF-α ELISA used in this study has not been validated in guinea pigs, and represents a limitation of our data. Observed levels of TNF-α may not, therefore, be linearly quantitative, but instead indicate a qualitative increase in the presence of this inflammatory mediator. Future studies in this model will be further informed by cytokine profiling via RT-PCR analysis15.
The limited immune response observed is not surprising, as the CNS displays selective and modified immunity1 and are consistent with our prior studies in the murine model of CNS TB13. The stage of infection observed in this model represents the intial seeding of bacilli in the meninges and parenchyma. At this early phase antigen presentation and lymphocytic immune surveillance is highly limited in the brain parenchyma16, 17. As was observed in the seminal studies by Arnold Rich in guinea pigs and rabbits, small foci of bacteria can exist for long periods of time without the onset of inflammatory disease18. Studies have shown that there is an absence of T-cell responses to PPD testing following intracranial injection of heat-killed Bacillus Calmette-Guérin (BCG) in rats, indicating that BCG escapes immune recognition within the parenchyma19. Studies have also shown that subsequent peripheral sensitization of the immune system results in the development of an immune-mediated delayed-type hypersensitivity (DTH) response and inflammatory lesions surrounding the heat killed BCG within the CNS20. These experimental data are in concordance with the clinical observation of delayed “paradoxical” intracranial tuberculomas, which develop in patients several weeks to months following anti-TB therapy21. Further, it should be noted that though the brain parenchyma has limited immune surveillance, the CSF has a much more robust immune response to foreign antigens16. This is consistent with the observation that rupture of the “Rich foci” into the CSF-containing subarachnoid space causes diffuse, inflammatory meningitis. We therefore believe the animals in this study to represent a pre-meningitic state. As such, the model is primarily one of dissemination and invasion, and not immune activation.
The guinea pig model of CNS infection described in this study represents an important tool for the future study of dissemination and CNS invasion. The guinea pig provides a number of physiological advantages over the mouse model of CNS dissemination, including higher whole brain bacillary burden, more temporally reliable dissemination events, and an observable, albeit modest, inflammatory response13. Further use of this model will be useful in distinguishing the capacity of clinical strains to cause CNS disease and in the identification of molecular determinants of bacterial invasion.
In summary, we have demonstrated strain-dependent CNS dissemination of M. tuberculosis in guinea pigs. These data suggest that M. tuberculosis may have virulence factors that promote CNS dissemination, distinct from those required for pulmonary TB. Future studies focusing on the identification of microbial virulence factors that promote CNS dissemination would therefore be essential to a better understanding of the pathogenesis of CNS TB.
MATERIALS AND METHODS
M. tuberculosis strains and media
Three strains were used in this study: a CDC1551 strain which was twice-passaged through mice, an H37Rv strain also twice-passaged in mice (H37Rv JHU – obtained from Johns Hopkins University), and an H37Rv strain, which was not animal-passaged (H37RV TAMU – obtained from Dr. David McMurray, Texas A&M University). All cultures were grown at 37°C in 7H9 liquid broth (Difco) supplemented with oleic acid albumin dextrose catalase (BD), 0.5% glycerol, and 0.05% Tween 80.
Aerosol infection and processing of guinea pigs
Outbred Hartley guinea pigs (Charles River Laboratories) were aerosol-infected with each strain of logarithmically-growing M. tuberculosis using an inhalation exposure system (Glas-col) calibrated to implant approximately 1000 bacilli per animal as part of the study by Ahmad, et al 5. At least three animals were sacrificed for each group and at each time point, at days 1, 7, 14, 21, 28, and 56 after infection. For each animal, 5 mL blood was obtained by intracardiac puncture following opening of the thoracic cavity. Whole blood was diluted 1:2 in phosphate-buffered saline (PBS), and the entire blood sample was cultured across ten 7H11 selective plates (BD). CFU counts were extrapolated to the whole blood volume of the guinea pig. Given the approximate 25 mL whole blood volume22, our limit of detection for circulating bacteria was 5 CFU.
Whole brains, lungs, and spleens were harvested under direct visualization and homogenized separately for each animal. Brains were homogenized in 7 mL PBS using glass Ten Broeck tissue grinders. 10 mL PBS was then added to the homogenate, and 1 mL of the suspension plated onto each of 10 plates, resulting in 50% of the brain being cultured. Spleen and lungs were homogenized in 10 mL PBS using a Kinematica Polytron Homogenizer with a 12-mm generator (Brinkman) within a Biosafety Level 3 Glovebox Cabinet (Germfree). Serial dilutions were performed in PBS, and 1 mL of diluted suspensions (1:103 – 1:106) was cultured for each organ on 7H11 selective plates. All protocols were approved by the Johns Hopkins Animal Care and Use Committee.
At each time point following infection, a section of the right hemisphere of the brain was fixed in 10% buffered formalin for 24 hours. Tissue samples were stained with hematoxylin and eosin and for acid fast bacteria and examined for pathology. Five slide sections were examined for each brain.
Cytokine analysis
Guinea pig organ homogenates were cleared by centrifugation, and the supernatant sterilized by filtration. Cytokine levels in each sample were quantified using commercially available anti-mouse ELISA kits (R&D) according to the manufacturer’s suggestions. Primary antibodies in this IL-1β ELISA kit have been shown empirically to cross-react effectively with guinea pig protein23. Additionally, mouse TNF-α demonstrates > 92% homology with guinea pig protein, and is thus likely to be cross-reactive. At least three guinea pigs were used for each time point in each experiment, with ELISA analysis for each sample being performed in triplicate.
Statistical analysis
Statistical comparison between groups was performed using Student’s t test, and multiple comparisons were performed with the ANOVA single factor F test using Microsoft Excel 2007.
Acknowledgments
Financial support was provided by Bill and Melinda Gates Foundation #48793 and #42851, CFAR Pilot Developmental Program (Johns Hopkins), and NIH contract and grant N01 30036 and AI79590 respectively. Financial sponsors had no role in the study design, collection, analysis, or interpretation of data.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors do not have a commercial or other association that might pose a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Be NA, Kim KS, Bishai WR, Jain SK. Pathogenesis of central nervous system tuberculosis. Curr Mol Med. 2009;9:94–9. doi: 10.2174/156652409787581655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Toole R. Experimental models used to study human tuberculosis. Adv Appl Microbiol. 2010;71:75–89. doi: 10.1016/S0065-2164(10)71003-0. [DOI] [PubMed] [Google Scholar]
- 3.Tsenova L, Ellison E, Harbacheuski R, Moreira AL, Kurepina N, Reed MB, Mathema B, Barry CE, 3rd, Kaplan G. Virulence of Selected Mycobacterium tuberculosis Clinical Isolates in the Rabbit Model of Meningitis Is Dependent on Phenolic Glycolipid Produced by the Bacilli. J Infect Dis. 2005;192:98–106. doi: 10.1086/430614. [DOI] [PubMed] [Google Scholar]
- 4.van Well GTJ, Wieland CW, Florquin S, Roord JJ, van der Poll T, van Furth AM. A New Murine Model to Study the Pathogenesis of Tuberculous Meningitis. J Infect Dis. 2007;195:694–7. doi: 10.1086/511273. [DOI] [PubMed] [Google Scholar]
- 5.Ahmad Z, Klinkenberg LG, Pinn ML, Fraig MM, Peloquin CA, Bishai WR, Nuermberger EL, Grosset JH, Karakousis PC. Biphasic kill curve of isoniazid reveals the presence of drug-tolerant, not drug-resistant, Mycobacterium tuberculosis in the guinea pig. J Infect Dis. 2009;200:1136–43. doi: 10.1086/605605. [DOI] [PubMed] [Google Scholar]
- 6.McMurray DN, Allen SS, Jeevan A, Lasco T, Cho H, Skwor T, Yamamoto T, McFarland C, Yoshimura T. Vaccine-induced cytokine responses in a guinea pig model of pulmonary tuberculosis. Tuberculosis (Edinb) 2005;85:295–301. doi: 10.1016/j.tube.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Palanisamy GS, Smith EE, Shanley CA, Ordway DJ, Orme IM, Basaraba RJ. Disseminated disease severity as a measure of virulence of Mycobacterium tuberculosis in the guinea pig model. Tuberculosis (Edinb) 2008;88:295–306. doi: 10.1016/j.tube.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia de Viedma D, Marin M, Ruiz Serrano MJ, Alcala L, Bouza E. Polyclonal and compartmentalized infection by Mycobacterium tuberculosis in patients with both respiratory and extrarespiratory involvement. J Infect Dis. 2003;187:695–9. doi: 10.1086/368368. [DOI] [PubMed] [Google Scholar]
- 9.Garcia de Viedma D, Marin M, Andres S, Lorenzo G, Ruiz-Serrano MJ, Bouza E. Complex clonal features in an mycobacterium tuberculosis infection in a two-year-old child. Pediatr Infect Dis J. 2006;25:457–9. doi: 10.1097/01.inf.0000217473.90673.00. [DOI] [PubMed] [Google Scholar]
- 10.Hesseling AC, Marais BJ, Kirchner HL, Mandalakas AM, Brittle W, Victor TC, Warren RM, Schaaf HS. Mycobacterial genotype is associated with disease phenotype in children. Int J Tuberc Lung Dis. 2010;14:1252–8. [PubMed] [Google Scholar]
- 11.Caws M, Thwaites G, Dunstan S, Hawn TR, Lan NT, Thuong NT, Stepniewska K, Huyen MN, Bang ND, Loc TH, Gagneux S, van Soolingen D, Kremer K, van der Sande M, Small P, Anh PT, Chinh NT, Quy HT, Duyen NT, Tho DQ, Hieu NT, Torok E, Hien TT, Dung NH, Nhu NT, Duy PM, van Vinh Chau N, Farrar J. The influence of host and bacterial genotype on the development of disseminated disease with Mycobacterium tuberculosis. PLoS Pathog. 2008;4:e1000034. doi: 10.1371/journal.ppat.1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernandez Pando R, Aguilar D, Cohen I, Guerrero M, Ribon W, Acosta P, Orozco H, Marquina B, Salinas C, Rembao D, Espitia C. Specific bacterial genotypes of Mycobacterium tuberculosis cause extensive dissemination and brain infection in an experimental model. Tuberculosis (Edinb) 2010;90:268–77. doi: 10.1016/j.tube.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Be N, Lamichhane G, Grosset J, Tyagi S, Cheng Q, Kim KS, Bishai WR, Jain SK. Murine model to study Invasion and Survival of Mycobacterium tuberculosis in the Central Nervous System. J Infect Dis. 2008;198:1520–8. doi: 10.1086/592447. [DOI] [PubMed] [Google Scholar]
- 14.Converse PJ, Eisenach KD, Theus SA, Nuermberger EL, Tyagi S, Ly LH, Geiman DE, Guo H, Nolan ST, Akar NC, Klinkenberg LG, Gupta R, Lun S, Karakousis PC, Lamichhane G, McMurray DN, Grosset JH, Bishai WR. The impact of mouse passaging of Mycobacterium tuberculosis strains prior to virulence testing in the mouse and guinea pig aerosol models. PLoS One. 2010;5:e10289. doi: 10.1371/journal.pone.0010289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen SS, McMurray DN. Coordinate cytokine gene expression in vivo following induction of tuberculous pleurisy in guinea pigs. Infect Immun. 2003;71:4271–7. doi: 10.1128/IAI.71.8.4271-4277.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–81. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 17.Ford AL, Foulcher E, Lemckert FA, Sedgwick JD. Microglia induce CD4 T lymphocyte final effector function and death. J Exp Med. 1996;184:1737–45. doi: 10.1084/jem.184.5.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rich AR, McCordock HA. The pathogenesis of tuberculous meningitis. Bull Johns Hopkins Hosp. 1933;52:5–37. [Google Scholar]
- 19.Matyszak MK, Perry VH. Bacillus Calmette-Guérin sequestered in the brain parenchyma escapes immune recognition. J Neuroimmunol. 1998;82:73–80. doi: 10.1016/S0165-5728(97)00190-2. [DOI] [PubMed] [Google Scholar]
- 20.Matyszak MK, Perry VH. Demyelination in the central nervous system following a delayed-type hypersensitivity response to bacillus Calmette-Guérin. Neuroscience. 1995;64:967–77. doi: 10.1016/0306-4522(94)00448-e. [DOI] [PubMed] [Google Scholar]
- 21.Jain SK, Kwon P, Moss WJ. Management and outcomes of intracranial tuberculomas developing during antituberculous therapy: case report and review. Clin Pediatr (Phila) 2005;44:443–50. doi: 10.1177/000992280504400510. [DOI] [PubMed] [Google Scholar]
- 22.Ancill RJ. The blood volume of the normal guinea-pig. J Physiol. 1956;132:469–75. doi: 10.1113/jphysiol.1956.sp005539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hennessy MB, Deak T, Schiml-Webb PA, Wilson SE, Greenlee TM, McCall E. Responses of guinea pig pups during isolation in a novel environment may represent stress-induced sickness behaviors. Physiol Behav. 2004;81:5–13. doi: 10.1016/j.physbeh.2003.11.008. [DOI] [PubMed] [Google Scholar]