Abstract
The ratio of inorganic phosphate (Pi) to phosphocreatine (PCr) is validated marker of mitochondrial function in human muscle. The MRI rapid acquisition with relaxation enhancement (RARE) pulse sequence can acquire phosphorus-31 (31P) images with higher spatial and temporal resolution than traditional spectroscopic methods, which can then be used to create Pi:PCr ratio maps of muscle regions. While the 31P RARE method produces images that reflect the content of the 31P metabolites, it has been limited to producing an image of only one chemical shift in a scan. This increases the scan time required to acquire images of multiple chemical shifts as well as the likelihood of generating inaccurate Pi:PCr maps due to gross motion. This work is a preliminary study to demonstrate the feasibility of acquiring Pi and PCr images in a single scan by interleaving Pi and PCr chemical shift acquisitions using a chemically selective RF excitation pulse. The chemical selectivity of the excitation pulse is evaluated and Pi:PCr maps generated using the interleaved Pi and PCr acquisition method with the subject at rest and during exercise are compared to those generated using separate Pi and PCr acquisition scans. A paired t-test indicated that the resulting Pi:PCr ratios for the exercised forearm muscle regions were not significantly different between the separate Pi and PCr acquisition method (3.18 ± 1.53) (mean ± standard deviation) and the interleaved acquisition method (3.41 ± 1.66). This work demonstrates the feasibility of creating Pi:PCr ratio maps in human muscle with Pi and PCr images acquired simultaneously by interleaving between the Pi and PCr resonances in a single scan.
Keywords: phosphorus metabolism, RARE MRI, chemical shift imaging, muscle metabolism
INRODUCTION
The ratio of inorganic phosphate to phosphocreatine (Pi:PCr) is an established measure of the muscle cells’ ability to control energy production (1–7). Abnormal Pi:PCr ratios have been associated with diseases that affect the muscle tissue of the extremities (2, 3, 5, 7). The rapid acquisition with relaxation enhancement (RARE) (8) pulse sequence allows the acquisition of many spin echoes during each TR period, making it well suited to the imaging of tissues or molecules with long T1 and T2 relaxation times such as the myocellular Pi and PCr (9–12). Methods for creating PCr images using the RARE sequence have been proposed. In one study PCr images were created by acquiring the PCr signal after other 31P resonances had decayed due to T2 relaxation (9). In another study PCr signals were acquired after de-phasing unwanted 31P resonances (10). In both of these methods the signal to noise ratio (SNR) is not optimal because T2 relaxation of the PCr signal occurs before data is acquired. Furthermore, these methods can only be used to acquire images of a single 31P moiety with each scan. More recently PCr images of the human myocardium and skeletal muscle have been acquired using the RARE sequence with a chemical-shift selective excitation pulse (12). While PCr images of muscle can be used to identify regions of necrosis, they lack the sensitivity of Pi:PCr ratio maps to measure subtle changes that can occur as diseases progress (1–3, 5–7).
The chemical-shift selective RARE sequence has been used for Pi:PCr ratio mapping to assess the muscles in the feet of diabetic patients by acquiring PCr and Pi images in separate acquisitions (5). This method requires a scanning time that is twice as long as the time needed to acquire either a PCr or a Pi image. Additionally, if any motion occurs then the registration of the Pi and PCr images will be imperfect, compromising the accuracy of the measured localized Pi:PCr ratio. To address these limitations we have developed a 31P RARE imaging method that can simultaneously acquire PCr and Pi images in the same scan by interleaving chemical shift acquisitions. In this work we present preliminary results that suggest the feasibility of simultaneously acquiring Pi and PCr images in a single scan at rest and during exercise for the creation of Pi:PCr ratio maps of human muscle. An evaluation of the performance of the chemically-selective excitation pulse is also presented.
METHODS
MR Studies
All studies were performed on a GE 3T whole body MRI system (General Electric Medical Systems, Waukesha, WI) equipped with broadband transmit and receive channels. A double-tuned (31P/1H) low-pass quadrature birdcage radio frequency (RF) coil consisting of 8 struts with a diameter of 12 cm and a length of 12 cm was used for all imaging (13).
Study subject
One healthy 56-year-old man was recruited for this study. The subject was recreationally active but was not involved in any training programs to specifically strengthen the forearm and did not use any medications. The protocol for the study of human subject used for this work was approved by the institution’s Institutional Review Board. The subjects provided written informed consent for their participation in the study.
Chemically selective RARE pulse sequence
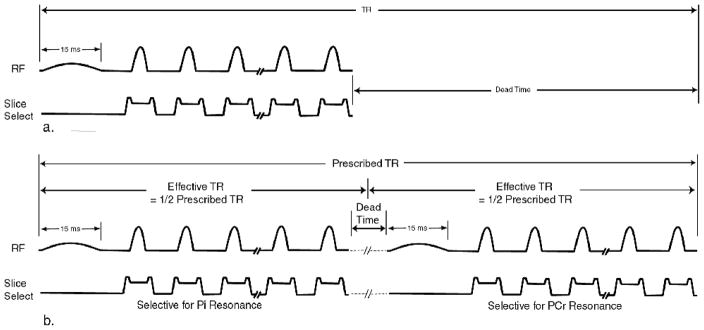
A 31P RARE sequence was implemented by modifying a GE product 2D fast spin echo (FSE) pulse sequence to allow the acquisition of 31P signal data using the scanner’s broadband transmit/receive hardware. The excitation bandwidth of the standard product radio-frequency (RF) excitation pulse was reduced by increasing its duration from the default value of 3 ms to 15 ms (14) and applying it without a slice selective gradient pulse. Slice selection was accomplished with the refocusing gradient pulses. A schematic diagram of the modified pulse sequence is shown in Figure 1a.
Figure 1.
RARE (FSE) pulse sequences with chemically selective excitation pulses. In both a. and b. the excitation pulse duration is increased to 15 ms and it is applied without a slice selection gradient pulse. In a. the excitation pulse and refocusing echo train are executed once during each prescribed TR period for the acquisition of Pi and PCr images in separate scans. In b. the excitation pulse and refocusing echo train are executed twice during each TR period for acquiring Pi and PCr images within the same TR period.
Measurement of the chemical-shift selective excitation pulse
The bandwidth of the 15 ms RF excitation pulse was determined experimentally using a 500 ml bottle containing an eighty-five percent solution of phosphoric acid (Fisher Scientific A242-500) placed inside of the 31P/1H birdcage RF coil. Axial images of the sample were acquired using the 31P RARE sequence with the following scan parameters prescribed: slice thickness = 20 mm, field-of-view (FOV) = 30 cm, acquisition matrix = 32 x 32, receiver bandwidth = 32.25 KHz, TR = 2 S and total scan time = 2 S. The scanning system was first adjusted to the resonant frequency of the sample using the scanner’s manual calibration tool. The scanner’s transmit/receive frequency was then set 300 Hz lower than the sample’s center frequency and an image was acquired. Additional images were acquired with the scanner’s frequency stepped in +10 Hz increments until the scanner’s frequency was 300 Hz higher than the sample’s resonance frequency for a total of 61 images. The images were reformatted to a 256 x 256 matrix on the scanner. The average signal intensity of an 8 x 8 pixel region of interest (ROI) was measured at the center of the phantom in each of the 61 reformatted images.
Assessment of B0 homogeneity
Unlocalized pulse-and-acquire 31P MRS was performed on the forearms of seven normal volunteers to assess the B0 homogeneity in the anatomy where the chemical-shift selective imaging was performed. The anatomy under investigation was placed at the center of the 31P/1H RF birdcage coil. Before acquiring each 31P spectrum, the scanner’s automatic linear shimming function (first order) was performed at the 3T 1H frequency to optimize the B0 homogeneity.
Phosphorus-31 imaging
Axial Pi and PCr images of the forearm of a normal volunteer were acquired in separate scans using the 31P RARE sequence with chemical-shift selective excitation (excitation pulse width = 15 ms, slice thickness = 25 mm, field-of-view (FOV) = 30 cm, acquisition matrix = 64 x 64, receiver bandwidth = 4.0 KHz, TR = 12 S, 10 averages and total scan time = 4 minutes/image). In all 31P RARE scans that were performed with human subjects, the spacing between echoes was set to 26 ms to suppress the ATP signal as previously described (11, 15). Pi and PCr images were acquired with the subjects’ forearms at rest and again during an exercise protocol. The exercise protocol consisted of squeezing a firm rubber block between the thumb and one finger to recruit a subset of muscles in the forearm. When the subject indicated fatigue a 4-minute spectrally selective RARE scan was started with the scanner’s center frequency set to the PCr resonance. The subject continued exercising at constant intensity throughout the data acquisition during the dead time between data acquisition intervals to maintain the Pi and PCr concentrations at steady levels that were different from their resting values. This resulted in a constant Pi:PCr ratio that was different than the normal resting Pi:PCr ratio to simulate abnormal metabolic function. A 4-minute Pi-selective scan was started immediately following the PCr-selective scan while the subject continued the exercise protocol. Following the exercise portion of the study, a 1H T2-weighted (T2-W) spin echo scan was performed to identify regions of edema for verification of the muscles that were recruited by the exercise, TR = 1.5 S, TE = 40 ms receiver bandwidth = 16.0 KHz, FOV = 15 x 15 cm, matrix = 2562 (16).
The 31P RARE sequence was further modified to allow the acquisition of both the Pi and PCr resonances at the same slice location within a single 4-minute scan by shifting the center frequency between the 2 resonances in an interleaved fashion, Figure 1b. Inorganic phosphate and PCr images of the same normal subject’s forearm were acquired at rest and during exercise using the same protocol described above. The time between the exercise sessions for the separate and interleaved 31P image acquisition methods was more than one week. While the excitation pulse was chemical-shift selective, the refocusing pulses were not and their bandwidth was wide enough to affect both resonances. Since both resonances were irradiated during each refocusing echo train, the effective TR was approximately equal to one-half that of the prescribed TR. The scan parameters were identical to those described for the exercise protocol above except that the receiver bandwidth was reduced to 2 Khz. The interleaving of the metabolite acquisitions effectively reduced to one-half the value of the prescribed TR (i.e the effective TR was 6 S). The T1 values of Pi and PCr at 3T are 4.1 ms and 4.3 ms, respectively (11). Reducing the effective TR to 6 S increases saturation of both the Pi and the PCr signals, resulting in a signal decrease of approximately 20%. The reduction in receiver bandwidth from 4 Khz to 2 Khz was used to compensate for the reduction in SNR caused by the reduction in effective TR.
Data Processing
To characterize the B0 homogeneity, the PCr and Pi linewidths of all of the acquired spectra were measured at the one-half maximum point of the spectral peaks.
All image processing was performed using IDL software (Research Systems, Inc., Boulder, CO). The FOV of all of the 31P images that were acquired for this work was 30 cm and the FOV of all of the 1H images was 15 cm. For visual comparison between the 31P and 1H images the 31P images were cropped from 2562 pixels to 1282 pixels about the center of the images and resized to 2562 pixels. Maps of the Pi:PCr ratio were calculated from the Pi and PCr RARE images of the human subjects. The noise in the PCr images was eliminated by setting a pixel intensity threshold value. The image pixels that had signal intensity above the threshold value were considered to represent muscle tissue that was metabolically active (i.e. viable muscle tissue). A “mask” was created using the pixels in the resting PCr images that had values above the threshold value and was used to define the total muscle region in the resting Pi image and the exercise Pi and PCr images for eliminating the background (noise) pixels. Maps of the Pi:PCr ratio were created by dividing the resulting Pi images by the PCr images.
To compare the interleaved acquisition method to the method of acquiring Pi and PCr images in separate scans, we measured the Pi:PCr ratio in 5 regions of interest (ROIs). The ROI locations were selected within muscle regions that experienced exercise during the exercise protocol as indicated by bright regions of the exercise Pi:PCr maps (Figures 3g and 4g). Five ROI measurements were also made in corresponding locations in the resting Pi:PCr maps (Figures 3c and 4c) and in the resting PCr images (Figures 3b and 4b) using both methods. The SNR at the ROI locations in the PCr images was calculated using a background noise measurement. The mean and standard deviation of the Pi:PCr ratios and SNR was calculated across all of the voxels in these five ROIs for both acquisition methods with rest and exercise. A paired, two-tailed t-test was then used to compare the two acquisition methods.
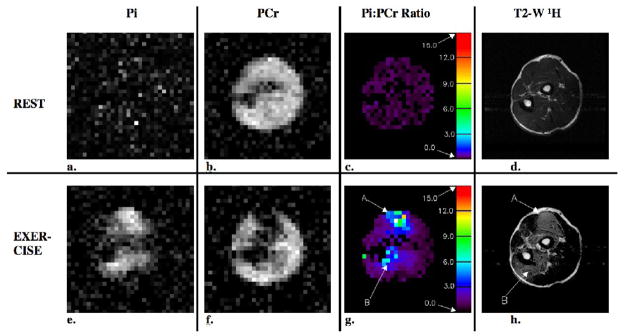
Figure 3.
Axial 31P and 1H images and Pi:PCr ratio maps from the forearm of a healthy volunteer under resting and exercise conditions. Both the resting and exercise data for these images were acquired in 2 separate 4-minute scans. In the images that were acquired under exercise conditions, there is a clear spatial correspondence between elevated levels of Pi, reduced levels of PCr, increased Pi:PCr ratio (regions A and B) and hyperintense signal in the 1H T2-W image.
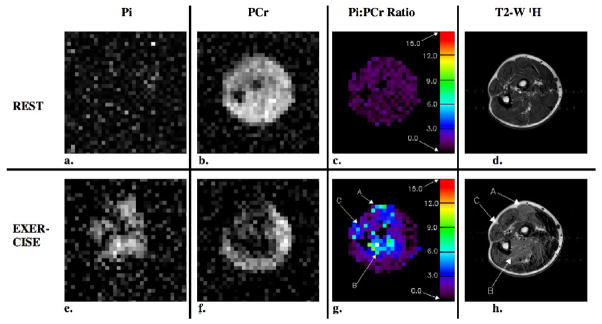
Figure 4.
Resting and exercise axial 31P and 1H images and Pi:PCr ratio maps that were acquired from the forearm of the same healthy volunteer as in Figure 3 using the interleaved single acquisition method. As in Figure 3, there is a spatial correspondence between elevated levels of Pi, reduced levels of PCr, increased Pi:PCr ratio and hyperintense signal in the 1H T2-W image. In this case, the subject also recruited muscle tissue in a new region (region C) during the exercise portion of the exam.
RESULTS
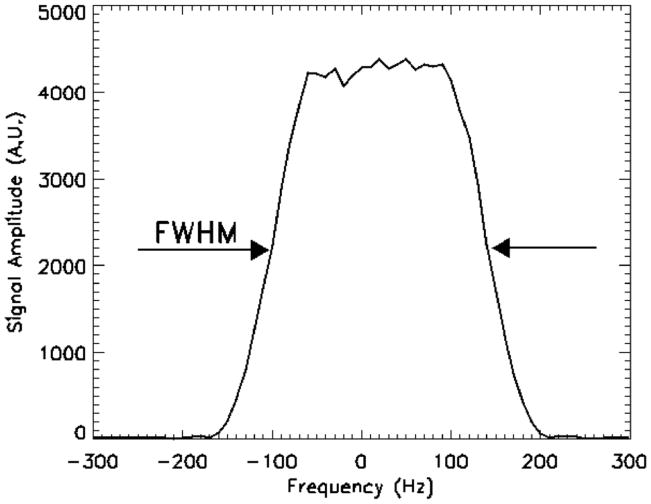
The bandwidth profile of the 15 ms duration RF excitation pulse at the 3T 31P frequency applied without a slice selection gradient is shown in Figure 2. The full width at half maximum (FWHM) was 240 Hz and the width at the base of the skirt was 380 Hz. The Pi and PCr line widths measured in the forearms of seven subjects were 48.8 ± 16.19 Hz (mean ± standard deviation) and 34.12 ± 16.12 Hz, respectively.
Figure 2.
The experimentally derived bandwidth profile of the 15 ms chemical-shift selective RF excitation pulse. The bandwidth of the pulse is sufficiently wide to excite all of the spins at either the Pi or PCr resonance yet narrow enough to avoid exciting the spins of the unwanted resonance.
The images and Pi:PCr ratio maps that resulted from the forearm exercise study where the Pi and PCr images were acquired separately and simultaneously in the interleaved acquisition are shown in Figures 3 and 4. The signal intensity is uniform in the PCr images acquired at rest, Figures 3b and 4b, with the exception of signal voids that occur at the location of the ulna and radius bones. Areas of bright signal intensity are visible in the exercise Pi images, Figures 3e and 4e, and correspond to muscles that were recruited by the exercise protocol verified in the post-exercise T2-W proton images, Figures 3h and 4h. Regions of reduced signal, which indicate reduced levels of PCr in response to exercise appear in the exercise PCr images, Figures 3f and 4f, and correspond to the bright signal regions in the exercise Pi images. Pseudo-colored Pi:PCr ratio maps are shown for both resting and exercise conditions, Figures 3c, 3g, 4c and 4g. The color scales on the right side of the maps indicate the value of the Pi:PCr ratio. In the resting map, the Pi:PCr ratio values are in the normal resting range (black to dark purple), whereas colors that indicate substantial increases in the Pi:PCr ratio appear in the exercise Pi:PCr ratio maps. The mean values ± the standard deviation of the Pi:PCr ratios measured in 5 ROIs within the regions that experienced exercise in the rest and exercise images for the separate and simultaneous (interleaved) acquisition methods are given in Table 1.
Table 1.
Average Pi:PCr ratio and SNR values within muscle regions that were recruited during the exercise protocol (mean ± standard deviation)
| Acquisition Type | Average Pi:PCr Ratio | SNR of At Rest PCr image | |
|---|---|---|---|
| At Rest | During Exercise | ||
| Separate | 0.23 ± 0.04 | 3.18 ± 1.53 | 6.75 ± 0.42 |
| Interleaved | 0.20 ± 0.04 | 3.41 ± 1.66 | 6.31 ± 1.00 |
T2-W 1H images acquired before and after the exercise periods for the separate and interleaved acquisitions are shown in Figures 3d 3h, 4d and 4h. Bright signal regions in the post-exercise T2-W images (Figure 3h, regions A and C; and Figure 4h, regions A, B and C) indicate regions of intracellular water accumulation resulted from exercise of the muscles in those regions (16). The bright regions in the T2-W 1H exercise images correspond to bright regions in the exercise Pi images, to dark regions in the PCr exercise images and to regions of elevated Pi:PCr ratio in the exercise Pi:PCr ratio maps.
The SNR mean and standard deviation values of the PCr images acquired at rest using a separate and interleaved acquisitions, Figure 3b and 4b, measured at the same 5 locations as in the Pi:PCr ratio maps are listed in Table 1.
The results of the paired t-test, comparing the results of the Pi:PCr ratio and SNR measurements indicated that the differences between separate Pi and PCr acquisitions and the interleaved (simultaneous Pi and PCr) acquisition were not significantly different (p > 0.05) in all cases.
DISCUSSION
The average linewidths of the forearm Pi and PCr peaks are less that 21% of the bandwidth of the 15 ms RF excitation pulse at the FWHM point. These values were measured following automatic linear shimming, which is standard on most commercial clinical MR scanning systems. These results suggest that the all of the spins of the metabolite on which the transmit frequency is centered (either the Pi or the PCr peak) will be excited by the chemical-selective excitation pulse. While a proton B0 field map would provide a visual representation of local variations in the B0 field across the anatomy, it is only important for the purpose of chemical selective imaging to measure the maximum variations in the static field. This is more easily done with spectral linewidths. Furthermore, 31P spectra provide widths of both the PCr and Pi resonances.
An exercise protocol was used in this study as an experimental method to demonstrate the ability of the interleaved acquisition method to measure Pi:PCr ratios. During intensive exercise pH values may drop to levels that would bring the distance between the Pi and PCr peaks close enough so that the spectrally selective excitation pulse cannot be effectively used to create pure Pi and PCr images. The minimum pH value where this method can reliably create separate Pi and PCr images can be determined using (4, 17).
| (1) |
where δ is the chemical shift in parts per million between the Pi and PCr peaks. Based on the maximum measured Pi and PCr linewidths and the width of the RF pulse bandwidth at the base of the skirt as reported in the Results section and Figure 1 the method described here will not reliably create separate Pi and PCr images for pH values below 6.51. The intended application of this method is the evaluation of disease states under resting conditions where such low pH values are not likely to occur.
Spectrally selective excitation would be more difficult at 1.5T and lower static field strengths where the frequency spacing between the Pi and PCr peaks for normal resting muscle is 125 Hz or less (4.88 ppm, corresponding to a normal of pH = 7.07) (17). The low RF excitation pulse bandwidth requirement to select only Pi or PCr and the lower intrinsic SNR would make the use of the spectrally selective RARE sequence described here for 31P metabolic mapping at 1.5T or lower field strengths substantially more challenging than it is at 3 T.
This work concerns a method for acquiring Pi and PCr images using a RARE pulse sequence with a chemical-shift selective RF excitation pulse. Difficulties arise when acquiring MR data from the ATP molecule. J-couplings among the three resonances of the ATP molecule result in phase and amplitude modulations of the ATP signals following excitation (18). To avoid phase and amplitude errors in the ATP signal when using multi-echo pulse sequences the spacing between echoes must be set to integer multiples of 52 ms (15). This long echo time would increase the total acquisition time and result in an increased point spread function. This is a limitation of using any multi-echo sequence for ATP acquisition. In spite of this limitation, alterations in the muscle metabolism can be detected using the Pi and PCr information and many MRS studies that describe the acquisition of 31P spectra and the correlation of the Pi:PCr ratio with diseases that affect muscle metabolism have been published (1–4, 6, 7, 17).
The results presented in Figure 4 demonstrate that the acquisition time can be reduced to one half by interleaving the acquisition of the Pi and PCr images in a single scan rather than acquiring them separately. This effectively reduces the TR to one-half of its prescribed value, resulting in a reduction in SNR. In this work, the SNR of the images that resulted from the interleaved acquisition was reduced by 6.5% percent of that which resulted using separate acquisitions. Reducing the effective TR from 12 S to 6 S theoretically reduces the PCr and Pi signals by 20% and decreasing the receive bandwidth from 4 kHz to 2 kHz should increase the SNR by 41%. The reason that the separate acquisition and interleaved acquisition methods result in a reduction in SNR may be that the reduced receive bandwidth requires longer echo spacing, resulting in greater T2 decay during early echoes with the interleaved method as prescribed for this work. Using a 2 kHz receive bandwidth when acquiring Pi and PCr images separately will likely result in a higher SNR for that method. The tradeoff between the reduced acquisition time and potential reduction in motion-related artifacts of the interleaved acquisition and the resulting reduced SNR should be considered before applying this variation.
While the exercise that was performed for both the separate and interleaved acquisition methods primarily involved the extensor carpi radialis muscles and the flexor digitorum profundus muscles (Figures 3h and 4h, regions A and B) the exercise that was performed during the interleaved acquisition method also involved the extensor digitorum and extensor carpi unlaris muscles (Figure 4h, region C). This illustrates a limitation of this work. The exercise protocol does not rigorously control for the specific muscles that are recruited by squeezing the rubber block allowing the subject to recruit additional muscles during the exercise that was performed during the interleaved acquisition. However, the high intensity regions of the post-exercise T2-W images (Figures 3h and 4h) corresponded to regions of changes in the Pi and PCr signal intensities that occurred due to the exercise that was performed as well as the corresponding Pi:PCr ratio map, verifying the accuracy of the 31P RARE methods. An exercise protocol that uses specially designed exercise equipment would result in the same muscles being recruited consistently among subjects.
While 31P MRS has been used to study disease in the muscles of the extremities in studies that have involved limited numbers of subjects for over two decades (1–4, 6, 7, 17), it has not come into widespread clinical use. This may be because the spatial resolution has been poor and the acquisition times for spatially localized acquisitions are often too long for patient comfort. Single voxel techniques require manual placement of the voxels, making it difficult to acquire high-resolution information from the entire anatomical region of interest. Chemical shift imaging (CSI) divides a larger FOV into a grid of voxels to achieve a global assessment but high spatial resolution requires excessively long scan times. The chemical-shift selective 31P RARE imaging results presented here demonstrate that images of the Pi and PCr metabolites can be simultaneously acquired in human muscle on a 3 T clinical MR scanner. This work demonstrates that it is feasible to obtain spatially localized Pi:PCr ratio information over a large area of anatomy in relatively short examination times with spatial resolution that allows the identification of focal regions of altered metabolic activity using a 31P RARE sequence. To address the limitations of this feasibility study, a larger study involving an adequate number of subjects to provide an appropriate statistical power should be conducted with a carefully controlled exercise protocol and properly designed exercise apparatus to thoroughly validate these methods.
Acknowledgments
This work was supported by National Institutes of Health Grants R01DK071569 and R21DK58651 and by the Society for Academic Emergency Medicine Scholarly Sabbatical Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chance B, Leigh JS, Jr, Clark BJ, Maris J, Kent J, Nioka S, Smith D. Control of oxidative metabolism and oxygen delivery in human skeletal muscle: a steady-state analysis of the work/energy cost transfer function. Proc Natl Acad Sci U S A. 1985;82:8384–8. doi: 10.1073/pnas.82.24.8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hands L, Bore P, Galloway G, Morris P, Radda G. Muscle metabolism in patients with peripheral vascular disease investigated by 31P nuclear magnetic resonance spectroscopy. Clin Sci. 1986;71:283–290. doi: 10.1042/cs0710283. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki E, Kashiwag A, Hidaka H, Maegawa H, Nishio Y, Kojima H, Haneda M, Yasuda H, Morikawa S, Inubushi T, Kikkawa R. 1H and 31P-magnetic resonance spectroscopy and imaging as a new diagnostic tool to evaluate neuropathic foot ulcers in type II diabetes. Diabetologia. 2000;43:165–172. doi: 10.1007/s001250050025. [DOI] [PubMed] [Google Scholar]
- 4.Taylor DJ, Bore PJ, Styles P, Gadian DG, Radda GK. Bioenergetics of intact human muscle. A 31P nuclear magnetic resonance study. Mol Biol Med. 1983;1:77–94. [PubMed] [Google Scholar]
- 5.Greenman RL, Panasyuk S, Wang X, Lyons TE, Dinh T, Longoria L, Giurini JM, Freeman J, Khaodhiar L, Veves A. Early changes in the skin microcirculation and muscle metabolism of the diabetic foot. Lancet. 2005;366:1711–7. doi: 10.1016/S0140-6736(05)67696-9. [DOI] [PubMed] [Google Scholar]
- 6.Wiener D, Maris J, Chance B, Wilson J. Detection of skeletal muscle hypoperfusion during exercise using phosphorus-31 nuclear magnetic resonance Spectroscopy. J Am Coll Cardiol. 1986;7:793–799. doi: 10.1016/s0735-1097(86)80338-2. [DOI] [PubMed] [Google Scholar]
- 7.Zochodne DW, Thompson RT, Driedger AA, Strong MJ, Gravelle D, Bolton CF. Metabolic changes in human muscle denervation: topical 31P NMR spectroscopy studies. Magn Reson Med. 1988;7:373–83. doi: 10.1002/mrm.1910070402. [DOI] [PubMed] [Google Scholar]
- 8.Hennig J, Nauerth A, Friedburg H. RARE imaging: a fast method for clinical MR. Magn Reson Med. 1986;3:823–833. doi: 10.1002/mrm.1910030602. [DOI] [PubMed] [Google Scholar]
- 9.Chao H, Bowers JL, Holtzman D, Mulkern RV. RARE imaging of PCr in human forearm muscles. J Magn Reson Imaging. 1997;7:1048–1055. doi: 10.1002/jmri.1880070617. [DOI] [PubMed] [Google Scholar]
- 10.Greenman RL, Elliott MA, Vandenborne K, Schnall MD, Lenkinski RE. Fast imaging of phosphocreatine using a RARE pulse sequence. Magn Reson Med. 1998;39:851–854. doi: 10.1002/mrm.1910390523. [DOI] [PubMed] [Google Scholar]
- 11.Greenman RL. Quantification of the 31P metabolite concentration in human skeletal muscle from RARE signal intensity. Magn Reson Med. 2004;52:1036–1042. doi: 10.1002/mrm.20258. [DOI] [PubMed] [Google Scholar]
- 12.Greenman R, Axel L, Ferrari V, Lenkinski R. Fast imaging of phosphocreatine in the normal human myocardium using a three-dimensional RARE pulse sequence at 4 Tesla. J Magn Reson Imaging. 2002;15:467–472. doi: 10.1002/jmri.10081. [DOI] [PubMed] [Google Scholar]
- 13.Greenman RL, Rakow-Penner R. Evaluation of the RF field uniformity of a double-tuned 31P/1H birdcage RF coil for spin-echo MRI/MRS of the diabetic foot. J Magn Reson Imaging. 2005;22:427–32. doi: 10.1002/jmri.20372. [DOI] [PubMed] [Google Scholar]
- 14.Freeman R. Selective excitation in high-resolution NMR. Chem Rev. 1991;91:1397–1412. [Google Scholar]
- 15.Chao MS, Bowers JL, Holtzman D, Mulkern RV. Multi-echo 31P spectroscopic imaging of ATP: A scan time reduction strategy. J Magn Reson Imaging. 1997;7:425–433. doi: 10.1002/jmri.1880070229. [DOI] [PubMed] [Google Scholar]
- 16.Fisher MJ, Meyer RA, Adams RA, Foley JM, Potchen EJ. Direct Relationship Between Proton T2 and Exercise Intensity in Skeletal Muscle MR Images. Invest Radiol. 1990;25:480–485. doi: 10.1097/00004424-199005000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Martin P, Gibson H, Edwards R. MRS of Muscle. In: Young I, Charles H, editors. MR Spectroscopy - Clinical Applications and Techniques. Martin Dunitz, Ltd; 1996. pp. 55–73. [Google Scholar]
- 18.Jung WI, Widmaier S, Bunse M, Seeger U, Straubinger K, Schick F, Kuper K, Dietze G, Lutz O. 31P transverse relaxation times of ATP in human brain In vivo. Magn Reson Med. 1993;30:741–743. doi: 10.1002/mrm.1910300612. [DOI] [PubMed] [Google Scholar]