Abstract
The auditory system must be able to adapt to changing acoustic environment and still maintain accurate representation of signals. Mechanistically, this is a difficult task because the responsiveness of a large heterogeneous population of interconnected neurons must be adjusted properly and precisely. Synaptic short-term plasticity (STP) is widely regarded as a viable mechanism for adaptive processes. Although the cellular mechanism for STP is well characterized, the overall effect on information processing at the network level is poorly understood. The main challenge is that there are many cell types in auditory cortex, each of which exhibit different forms and degrees of STP. In this article, I will review the basic properties of STP in auditory cortical circuits and discuss the possible impact on signal processing.
Keywords: Auditory cortex, short-term plasticity, in vitro, circuitry
1. Introduction
A major goal in auditory neuroscience is to understand how sound is represented in the brain. Sound pressure is converted into electrical signals in the cochlea and somehow the rich acoustic space is represented as sequences of action potentials in neural networks. Equally remarkable is that this representation is preserved in a dynamic acoustic environment. The underlying processes that enable the auditory system to make the necessary adjustments to preserve information are not well understood.
A viable mechanism is short-term plasticity (STP) of postsynaptic potentials (PSPs). Throughout cortex, repetitive, brief stimulation of afferents or presynaptic neurons evoke PSPs in target neurons whose amplitude either decrease (depress) or increase (facilitate) with each stimuli. STP changes systematically with stimulus intensity and with recent history, making it an ideal neural substrate for adaptive processes. In the visual and somatosensory systems, for example, STP provides a mechanism for adjusting the magnitude of thalamic input to cortex depending on the level of activity or state of the animal (Boudreau et al., 2005; Castro-Alamancos et al., 2002). Depressing PSPs have been proposed to be a mechanism for adjusting the gain of the network so as to maintain sensitivity to input of varying intensities (Cook et al., 2003; Rothman et al., 2009). Finally, the time-dependent changes in PSP amplitudes caused by STP confer filtering capabilities to neurons (Abbott et al., 1997;Abbott et al., 2004; Dittman et al., 2000) and provide a means for processing rate and temporal signals (Markram et al., 1998b; Tsodyks et al., 1997).
These mechanisms apply generally to the nervous system but there is also evidence that links STP specifically to auditory processing (Oswald et al., 2006). The time scales of STP are well within the range of typical auditory stimuli: low spontaneous rates of 1-5 Hz and responses evoked with brief 25-50 ms tones or clicks would all be expected to produce significant changes in the amplitude of PSPs. The relatively long history dependence imparted by synaptic depression provides a good explanation for forward suppression (Bartlett et al., 2005; Brosch et al., 2000; Brosch et al., 1999; Wehr et al., 2005). STP may also underlie context dependent phenomena such as adaptive shifts in tuning properties (Malone et al., 2002). The inherent non-linearities produced by STP may underlie stimulus-dependence of spectro-temporal receptive fields (David et al., 2009) and explain the major features of temporal modulation transfer function obtained with periodic clicks (Eggermont, 1999). Finally, afferent input exhibiting different degrees of depression or facilitation could give rise to the phasic/tonic firing profiles of many neurons in auditory cortex (Oswald et al., 2006; Recanzone, 2000).
The important role for STP in the auditory system is underscored by the fact that it is highly regulated. As discussed below, the level of STP exhibited by specific cell types change with age, experience, neuromodulators, and background activity. The overall effect of these changes to the behavior of the network is likely to be very complicated. In a small patch of auditory cortex, there are thousands of excitatory and inhibitory neurons that make extensive connections with each other. Conceivably, a small change in the STP of some of these connections could have a profound effect in the way the network responds to input. It is therefore important to characterize fully the STP exhibited by the various cell types in cortex. In this review, I will summarize what is currently known about STP in the primary auditory (A1) cortical circuits and discuss its potential effect on signal processing.
2. General properties of Short-term plasticity
Before delving into STP in the auditory cortex, it is useful to briefly summarize general properties of STP, which have been more thoroughly characterized in other cortical areas ( Tsodyks et al., 1997; Markram et al., 1998a; Reyes et al., 1998; Reyes & Sakmann, 1999; Thomson et al., 2002; Feldmeyer et al., 2006). In depressing synapses, the PSP amplitudes decrease with successive stimulus pulses, reaching near steady-state levels in the range of hundreds of milliseconds (Fig. 1B, left, middle). For facilitating synapses (Fig. 1B, top right), the amplitude of PSPs increase systematically during the stimulus train, also reaching steady-state levels on the order of hundreds of milliseconds. The effects of STP is relatively long lasting and full recovery can be in the range of seconds. Mechanistically, both processes occur via presynaptic events (Neher et al., 2008; Zucker et al., 2002). Depression is generally thought to result from depletion of readily releasable pool of vesicles, which decrease with successive stimuli. In facilitating synapses, the probability that vesicles are released increases with each action potential owing to the accumulation of [Ca2+] with successive pulses (so-called residual calcium hypotheses but see Zucker et al., 2002 for alternate mechanisms). Facilitating PSPs are generally much smaller than depressing PSPs (Markram et al., 1998a; Reyes et al., 1998) consistent with the notion that because fewer vesicles are released after the first action potential, more are available for the next.
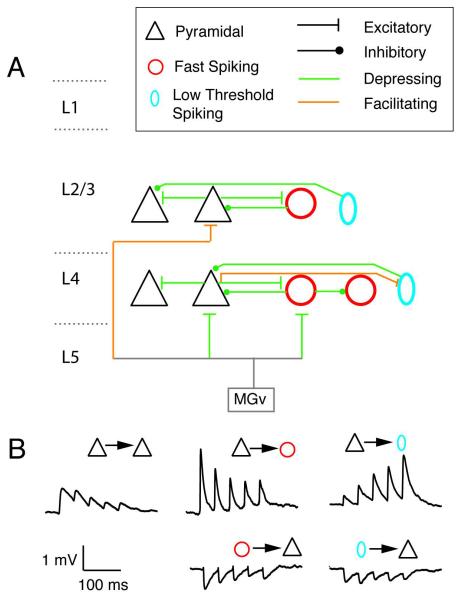
Fig. 1.
Summary of short-term plasticity in auditory cortex. (A) Schematic showing connections between pyramidal (triangles), Fast spiking interneurons (red circle), and low threshold spiking (blue oval) interneurons. MGv is the ventral division of the medial geniculate body. (B) representative synaptic potentials recorded in pairs of neurons. Simultaneous whole-cell recordings were performed from a pre- and postsynaptic cell. The presynaptic cells were stimulated with a train of suprathreshold current pulses separated by 50 ms. From Levy & Reyes, 2009.
Whether depression or facilitation occurs depends on the identities of the pre- and postsynaptic cell (Markram et al., 1998a; Reyes et al., 1998; Reyes et al., 1999). Simultaneous triple whole-cell recordings in divergent circuits (1 presynaptic cell and 2 postsynaptic cells of different types) show that stimulation of presynaptic pyramidal cells can evoke facilitating PSPs in one postsynaptic cell and depressing PSPs in the other. On the other hand, recordings in convergent circuits (2 presynaptic cells and 1 postsynaptic cell) show that the PSPs evoked in a postsynaptic tend to either both depress or both facilitate.
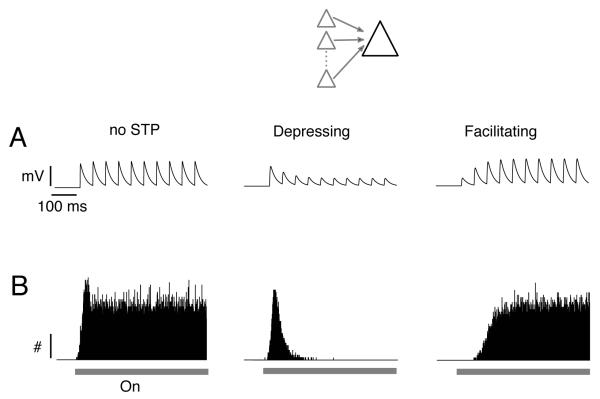
STP shapes the way neurons respond to a given input (Oswald et al., 2006; Tsodyks et al., 1997). For illustrative purposes, consider a neuron that receives sustained input from a population of presynaptic cells (Fig. 2, inset). In the presence of a stimulus (e.g. a long duration tone), the presynaptic cells start to fire, causing synaptic barrages in the postsynaptic cell. Simple simulations with leaky-integrate-and-fire (LIF) neurons show that when the presynaptic inputs have no STP (Fig. 2A, left), the neuron fires tonically for the duration of the stimulus (Fig. 2B, left). When the inputs depress (Fig. 2A, middle), the neuron fires mostly at the onset of the stimulus (Fig. 2B, middle), where the PSPs are greatest. When the inputs facilitate (Fig. 2A, right), firing occurs after a delay and then ramps up slowly to a steady level (Fig. 2B, right). These simulations, while providing useful insights into the effects of STP on network activity, are gross simplifications of auditory processing. As will be shown below, neuronal activity reflects the overall interaction of excitatory and inhibitory neurons whose synapses exhibit differing degrees of STP.
Fig 2.
Effects of short-term plasticity on neuronal firing. Simulations were performed with leaky-integrate-and-fire neurons. Inset, schematic of the simulation scenario. A postsynaptic cell receives a barrage of inputs from a population of presynaptic cells. (A) representative synaptic potential train fired by each presynaptic cell. Synaptic potentials either showed no short-term plasticity (left), synaptic depression (middle), or synaptic facilitation (right). (B) Associated poststimulus time histograms compiled from the LIF neuron over many trials. Bar indicates stimulus.
3. Characteristics of STP in primary auditory cortex
Depending on the classification scheme (Ascoli et al., 2008), there are potentially many types and subtypes of neurons in cortex. Categorizing STP for all of these defined cell types is impractical because the experimental procedures are both difficult and time-consuming. To isolate unitary PSPs and document STP, simultaneous whole-cell recordings must be performed from many pairs of excitatory and/or inhibitory neurons. Thus, as a first approximation, cell types are usually classified in 3 broad categories: excitatory pyramidal cells (P), inhibitory fast spiking (FS) interneurons, and inhibitory low threshold spiking (LTS) interneurons. These cell types can be identified based on their electrophysiological properties, morphology (Ascoli et al., 2008; Kawaguchi, 1995; McGarry et al.), and recently by using transgenic mice where specific cell types express EGFP transgene for parvalbumin (FS cells)(Chattopadhyaya et al., 2004) or somatostatin (LTS cells) (Ma et al., 2006; McGarry et al., 2010). These experiments are usually performed on an in vitro rodent slice preparation that contains the primary auditory cortex (A1), the auditory thalamus (ventral division of the medial geniculate or MGv), and the interconnecting fibers (Cruikshank et al., 2002).
The 3 cell types make extensive connections with each other. When plotted against intersomatic distance, the connection probability is approximately Gaussianly distributed. Connection probabilities depend on the cell-to cell pairings. P cells connect with low probability to each other (peak connection probability is ~0.01-0.2; Oswald et al., 2008) but connect (in both directions) with high probability (0.4-0.6) to FS and LTS interneurons (Oswald et al., 2010; Levy & Reyes, 2009). Connection probabilities are near zero for intersomatic distances of greater than 300 μm. P cells in layers 2/3 & 4 and FS cells in layer 4 have been shown to receive afferent input from MGv (Cruikshank et al., 2001; Cruikshank et al., 2002; de la Rocha et al., 2008; Hsieh et al., 2000; Metherate et al., 1999; Rose et al., 2001; Theyel et al., 2010; Viaene et al., 2011; Xu et al., 2007). Figure 1 summarizes the connections and STP of synapses that have been characterized thus far in A1. In general, these properties are similar to those found in somatosensory and visual, and motor cortices (Feldmeyer et al., 2006; Reyes & Sakmann, 1999; Thomson et al., 2002; Viaene et al., 2011).
3.1 STP in pyramidal to pyramidal cell connections
In mature tissue (>P18, see below), the excitatory PSPs (EPSPs) evoked between P cells depress (Fig. 4A) (Atzori et al., 2001; Oswald & Reyes, 2008). In layer 2/3, the amplitudes of unitary PSPs evoked with single pulses are small (~0.6 mV; Oswald & Reyes, 2008) and exhibits relatively little depression: the average paired pulse ratio (PPR=amplitude of 2nd PSP/amplitude of 1st PSP) examined with 10 Hz stimulation of the presynaptic cell is close to 1 (0.94). Depression, however, appears to be slightly greater for connections in L4 where PPR~0.6 (Levy & Reyes, 2009). There is some evidence (Atzori et al., 2001; but see Oswald & Reyes, 2008) that there are two subclasses of connections: one where the PSP amplitudes are large and exhibit strong depression and another where the PSPs are small and, on the average, exhibit no depression.
Fig. 4.
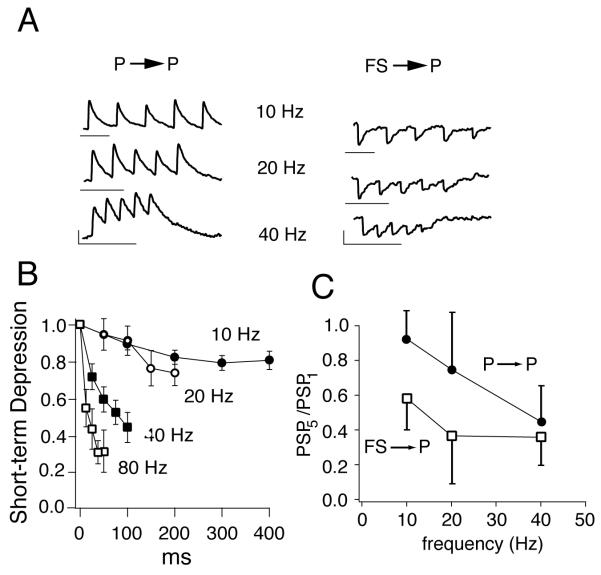
Dependence of short-term plasticity on stimulus frequency. (A) Left, representative excitatory postsynaptic potentials evoked in a fast spiking (FS) interneuron following repetitive stimulation of a presynaptic pyramidal cell (P) at different frequencies. Right, Inhibitory postsynaptic potentials evoked in P cells following stimulation of presynaptic FS cells. Vertical scale bars: 0.5 mV; horizontal: 100 ms. (B) plot of successive synaptic potential amplitudes during the train at different frequencies for P-to-P connections. Amplitudes are normalized to the amplitude of the first synaptic potential in the train. (C) Average short term synaptic depression of the 5th synaptic potential in the train relative to the 1st as a function of stimulus frequency for P to P and FS to P connections. Adapted from Oswald & Reyes, 2008, 2010.
An important feature of these and the other synapses is that synaptic depression becomes greater at higher stimulus frequencies (Fig.4A,B). Increasing the stimulus frequency from 10 to 80 Hz, for example, causes a 3-fold increase in STD, as quantified by amplitude ratio of the 5th PSP to 1st PSP (PSP5/PSP1) evoked during the train (Fig. 4B). This may provide a means for automatic regulation of excitation: during high activity regimes, the excitatory recurrent inputs from neighboring P cells decrease, thereby minimizing runaway excitation.
3.2 STP in pyramidal to FS connections
The synaptic properties of connections between pyramidal and FS cells differ considerably from those between P cells. Unitary EPSPs evoked in FS cells following single pulse stimulation of presynaptic P cells are almost 2x larger (~1.1 mV) and have a shorter time courses than those evoked between P cells (Figs. 1B & 5A, middle). The inhibitory PSPs (IPSPs; Fig. 4A right, 5A bottom) evoked from FS to P cells are comparable in amplitude (0.5 mV) to EPSPs evoked between P cells and also depress (Oswald & Reyes, 2010.; Oswald et al., 2009; Takesian et al., 2010).
Fig. 5.
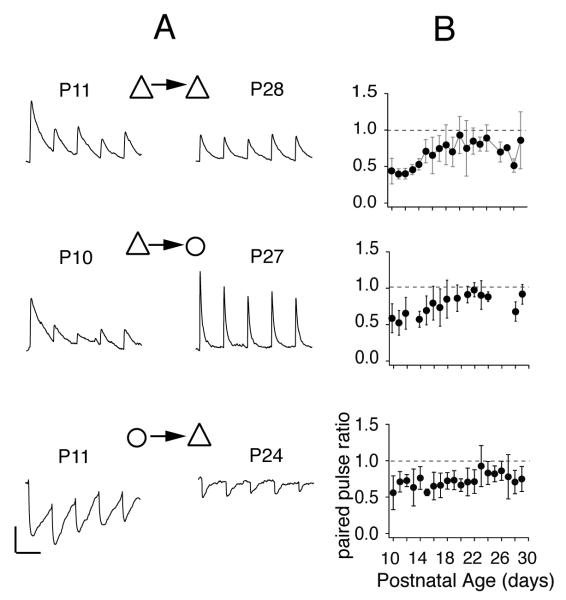
Developmental changes of short-term plasticity. (A) representative synaptic potentials evoked between pyramidal cells (top), from pyramidal cells to fast spiking interneurons (middle), and from fast spiking interneurons to pyramidal cells during 10 Hz stimulation of presynaptic cells. Left and right columns show PSPs for immature and mature synapses, respectively. Vertical scale bars: 1.0 mV; horizontal: 100 ms. (B) plot of paired pulse ratio at 10 Hz for each synapse vs. age of the animal. Adapted from Oswald & Reyes, 2008, 2010.
The reciprocal connections between P cells and FS neurons are among the most powerful in cortex, owing to the high connection probability and large amplitude PSPs (Oswald & Reyes, 2010; Oswald et al., 2009, Levy & Reyes, 2009). However, a combination of depressing P-to-FS EPSPs, depressing FS-to-P IPSPs, and depressing thalamically evoked EPSPs (see below) means that the net inhibitory effect of FS cells to P cells is likely to be transient, with the peak occurring near the stimulus onset. Thus, FS cells may make more of a contribution to processing of short stimuli, as in tone pips or clicks. Under certain conditions, however, the reciprocal interaction between P and FS cells could also lead to oscillatory behavior in the network (Oswald et al., 2009).
3.3 STP in pyramidal to LTS connections
LTS cells are somewhat more difficult to identify than FS cells because at least 3 cell types express EGFP in the transgenic mice (McGarry et al., 2010). Preliminary paired recordings from pyramidal cells and labeled interneurons, show both depressing and facilitating EPSPs (Levy & Reyes, 2009). Based on the electrophysiological properties (McGarry et al., 2010), cells that received the facilitating EPSPs were the low threshold spiking (LTS) interneurons. In layer 4, the unitary EPSPs evoked in these cells are small (0. 4 mV) but exhibit PPR of approximately 1.4 and steady state facilitation (PSP5/PSP1) of approximately 2.3. The IPSPs evoked in P cells are small (0.3 mV) and show weak depression (PPR = ~0.7; Levy & Reyes, 2009) during low frequency (10-20 Hz) stimulation of presynaptic LTS cells in layer 4 of mice and weak depression/facilitation in gerbils (Takesian et al. , 2010)
The contribution of LTS mediated inhibition to auditory processing is not immediately clear. On one hand, long sustained input from P cells appear necessary for the facilitating EPSPs to reach steady state and generate appreciable LTS cell firing (Fig. 2B, right): brief tone pips or clicks may not be sufficient to fully recruit LTS mediated inhibition. On the other hand, prolonged LTS firing would allow sufficient time for IPSPs onto P cells to depress. In addition, the effect of LTS inhibition on P cell firing is also more subtle because the LTS axons terminate distally on the apical dendrites, unlike those of FS cells which terminate near the soma and the spike initiation region of P cells (Kawaguchi, 1995). Therefore, LTS inhibition may selectively attenuate dendritic inputs rather than to halt overall P cell firing.
3.4 STP of Thalamic PSPs in pyramidal and FS cells
With the auditory thalamocortical slice preparation, EPSPs may be evoked in both P cells and FS cells by extracellular stimulation of MGv (Cruikshank et al., 2001; Cruikshank et al., 2002; de la Rocha et al., 2008; Hsieh et al., 2000; Metherate et al., 1999; Rose et al., 2001; Theyel et al.; Viaene et al.; Xu et al., 2007). Thalamic PSPs evoked in L 4 P cells depress strongly, as do those evoked in FS cells (Lee et al., 2008; Rose et al., 2005; Xu et al., 2007). In stark contrast, PSPs evoked in L2/3 P cells facilitate strongly (Viaene et al., 2011), which is somewhat surprising given nearly all of the intracortical connections between P cells in L2/3 depress. It is currently unknown whether LTS cells in A1 receive direct thalamic inputs.
The depressing inputs to pyramidal and FS cells in L4 would produce transient firing in both (Fig. 2, middle). This is in line with the observations that cortical cell firing is much more phasic than thalamic cell firing (Creutzfeldt et al., 1980). The firing of P cells would be further shortened by the arrival of powerful inhibition from the FS cells a few milliseconds later (see below). Intracellular recordings in vivo studies often show a stereotypic excitatory-inhibitory synaptic sequence following a brief tone stimulus (Tan et al., 2009; Tan et al., 2004; Tan et al., 2007; Wehr et al., 2005; Zhang et al., 2003).
3.4 Combined effects on firing
The extensive connections between the different cell types coupled with the varying degrees of STP can generate a wide range of firing patterns. The simulations in Fig. 2 assumed that a given neuron received input from a homogeneous population of independently firing presynaptic cells. Very different firing patterns are obtained when the presynaptic cells are heterogeneous and are connected to each other.
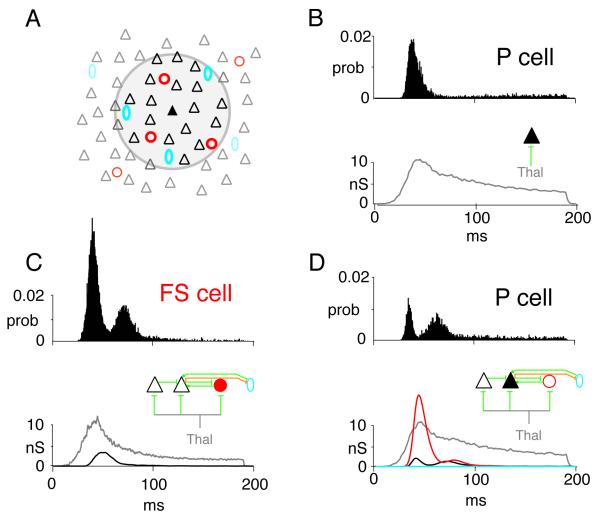
To develop some intuition about the combined effects of network activity on neuronal firing, it is useful to perform simulations with a network model of layer 4 auditory cortex (Fig. 3A). The network is a sheet of neurons (10,000 P cells, triangles; 1000 FS cells, red circles; 1000 LTS cells, cyan ovals) that incorporates the documented synaptic and intrinsic properties of P, FS, and LTS cells and connection profiles (Fig. 1; Oswald & Reyes, 2008, 2010; Levy & Reyes, 2009). The intracortical connections are (arrows denote direction of connections): P←→P, P← →FS, and P←→LTS. P and FS cells receive depressing inputs from the thalamus. For the following, a portion of the network is driven with thalamic input (gray circle, Fig. 3A) for 200 ms and the responses of P cells near the center (filled triangle) were documented.
Fig. 3.
Effects of short-term plasticity on network activity. (A) Schematic of network. Simulations were performed with adaptive integrate and fire neurons with parameters adjusted to reproduce firing of pyramidal (P, black triangles), fast spiking (FS, red circles), and low threshold spiking (LTS, cyan ovals) neurons (Naud et al., 2008). Network is a sheet of neurons (100×100 P; 31 × 31 FS; 31 × 31 LTS). The connections between neurons are (arrows denote direction of connections): P←→P, P← →FS, and P←→LTS. The experimentally determined probability of connections between each cell type is Gaussian distributed such that connection probability is greatest for nearby cells. The input to the network from the thalamus (gray) is Gaussian distributed (2 dimension) where the peak is the total number of inputs. (B, top) poststimulus time histogram (PSTH) of a P cell near the center with only thalamic inputs. Bottom shows averaged synaptic conductance from thalamus. (C, top) PSTH from an FS cell near the center in the fully connected network. Bottom, synaptic conductances from thalamus (gray) and neighboring P cells (black). (D, top) PSTH from a P cell in the fully connected network. Bottom, synaptic conductances from thalamus (gray), FS cells (red), neighboring P cells (black), and LTS cells (cyan). Conductance from LTS cells is nearly zero.
The simulations, though by no means complete, nevertheless provide some insights as to the complexity introduced by the neuronal interactions. In the absence of intracortical connections, the P cell PSTH has a strong phasic component (Fig. 3B, top), as expected from the depressing thalamic input (bottom). In the presence of intracortical connections (Fig. 3D, top), the initial peak narrows and is followed by a broader secondary peak. These additional features reflect the appearance of a large input from FS cells (bottom, red) and a multipeaked recurrent input from neighboring P cells (bottom, black). The PSTH of FS cells are transient and also exhibit two peaks (Fig. 3C, top) due in part to depressing thalamic inputs (Fig. 3C, bottom, gray) and the appearance of excitatory inputs from neighboring P cells (bottom, black). Under the condition of the simulations, the LTS cells do not provide substantial inhibition (not shown): the EPSPs from neighboring P cells are too small and the P cell firing too brief to generate appreciable LTS cell firing. However, further simulations and experiments are needed to determine whether there are more optimal stimuli for engaging LTS cells and whether LTS cells receive inputs from thalamus or other cortical areas.
The firing patterns are likely to change with the type of the stimulus. Because of differences in the STP of the various synapses, the relative contribution of each component may be different for pure tone, amplitude modulated, broadband auditory, or optimal vs. non-optimal stimuli. Moreover, as discussed in the next section, the STP of some synapses is strongly modulated with the state or age of the animal. The strong interdependencies between each cell type means that a change in the STP of one of the synapses could have a substantial effect on the overall network activity.
4. Regulation of STP
There is accumulating evidence that STP in the auditory cortex is not fixed but is modulated. In most cases, the change is not global but often occurs for a specific set of synapses, potentially changing qualitatively the responsiveness of the network to stimuli. This is of some consequence because it suggests that A1 might be under different operational modes at different states or stages of development.
4.1 Development
The PSPs between P cells and between P and FS cells undergo substantial changes after the onset of hearing (Oswald & Reyes, 2008; Oswald & Reyes, 2010; Takesian et al., 2010). In mice, stimulation of presynaptic P cells evoked PSPs that depress strongly in other P cells (PPR with 10 Hz stimulation = 0.68 ) and FS cells (PPR=0.4) at postnatal day age 10-11 (P10-11, Fig. 5A). As the brain matures, the PSPs become progressively less depressing (P27, P28) reaching steady PPR values (P cells = 0.94; FS cells = 0.8) by P19 (Fig. 5B, top, middle), approximately 7-9 days post hearing (Ehret, 1976). Similar developmental decreases in depression were observed with longer stimulus trains and at frequencies ranging from 10-80 Hz (Oswald & Reyes, 2008; Oswald & Reyes, 2010). The FS cell IPSPs evoked in P cells do not seem to change much with age (PPR = 0.7; Oswald et al. 2010; Takesian et al., 2010) (Fig. 5A,B, bottom). In gerbils, the LTS cell IPSPs evoked in P cells depress strongly prior to the onset of hearing (P8-11) but become weakly facilitating/depressing after P17 (Takesian et al., 2010).
Normal development of STP also depends on experience. In gerbils, hearing loss increases synaptic depression of thalamic inputs to L2/3 P cells (Xu et al., 2007), decreases facilitation of LTS inhibitory inputs to P cells, but has minimal effect on FS to pyramidal cell inhibitory PSP depression (Takesian et al., 2010).
Though STP needs to be examined in other synapses, there seems to be a general trend toward less synaptic depression as the animal matures. How this affects the overall excitability of the network is not immediately obvious since reduced depression would increase excitability of not only P cells but also those of the FS and LTS cells that provide inhibitory feedback.
4.2 Neuromodulators
Several compounds modulate STP, though relatively few studies have been performed in A1 specifically. Norepinephrine (NE) has different effects on the STP of inhibitory postsynaptic currents (IPSCs) evoked in L2/3 P cells, depending on the source of input (Salgado et al., 2010; Salgado et al., 2011). NE decreases depression of IPSCs evoked with extracellular stimulation of layer 1 but has opposite effects on IPSCs evoked with simulation of L2/3. In developing A1, activation of presynaptic GABAB receptors regulates synaptic depression in LTS- but not FS- mediated inhibitory input to P cells (Takesian et al., 2010). There is some evidence that, like in somatosensory cortex (Levy et al., 2008)), activation of muscarinic receptors with Ach agonists reduces PPR of facilitating PSPs evoked in LTS cells but has no effect on FS cells (Levy & Reyes, unpublished). In general, neuromodulators do not affect all synapses equally but seem to selectively target STP in specific synapses.
4.3 Background network activity
STP has been studied primarily in vitro, because the preparation is sufficiently stable to permit simultaneous recordings from pairs of cells. There is accumulating evidence that the STP documented in the quiescent slice may differ somewhat from those under the more active in vivo preparation (reviewed in Borst, 2010). In the intact brain, neurons are not silent but maintain spontaneous rates ranging from 1-20 Hz under laboratory conditions (lognormal distribution with median ~2-3 Hz; (Hromadka et al., 2008; Watkins & Barbour, 2010)) and probably more in natural environment, in the presence of ambient noise. Hence, synaptic inputs to neurons may be tonically depressed or facilitated. In the visual system, thalamocortical inputs from the LGN to cortex are depressed to saturated levels and no further depression occurs when LGN is stimulated repetitively (Boudreau et al., 2005). When the baseline activity was reduced, the evoked PSP amplitude increased transiently presumably because of partial recovery from depression. Analogous dependence of STP on activity was demonstrated in the somatosensory system (Castro-Alamancos et al., 2002) and in a slice preparation (Reig et al., 2006).
Because the change in STP with frequency varies across cell types, the overall effect of background activity on network excitability is again difficult to predict. In Fig. 4C, for example, the level of STP (quantified as the amplitude ratio of the 5th EPSP in the train over the 1st EPSP) exhibited by P-to-P EPSPs and FS-to-P IPSPs are different at 10 Hz but become comparable at 40 Hz. Consequently, the balance between excitation and inhibition is likely to shift with network activity. This is consistent with in vivo data showing that inhibition and excitation becomes unbalanced with increasing stimulus intensity, giving rise to non-monotonic rate-level functions (Tan et al., 2007). The background activity would also change the release probability prior to the arrival of the stimulus, effectively altering the neurons’ filtering capabilities (Abbott et al., 2004; Abbott et al., 1997; Dittman et al., 2000)
5. Conclusions
The presence of STP throughout cortex implies that the synaptic weights of neurons are not fixed but rather are in constant flux. The coupling strengths between neurons increase or decrease within tens of milliseconds effectively tracking rapid changes in the stimulus conditions or state of the animal. Experiments in vivo and modeling studies suggest that the network adapts to an operating regime that maintains efficient coding of stimuli.
Despite having a relatively firm understanding of the salient properties of STP, its precise role in controlling network activity in cortex is still poorly understood. Most of the existing models have focused mainly on STP function in single neurons (Markram et al., 1998b; Tsodyks et al., 1997; Varela et al., 1997). However, even the simplest auditory stimuli probably trigger activity in a fairly large network of neurons. In A1, as in other cortical areas, there are many classes and subclasses of excitatory and inhibitory cells, which make extensive connections with neighbors both within and across cortical layers. Based on the small circuit examined thus far (Fig. 1), STP and its response to modulators and activity can vary widely, depending on the identity of the pre- and postsynaptic cells. Consequently, a change in the STP of one synapse could potentially have substantial effects on the dynamics of the entire network and on the balance of excitation and inhibition. The way the network responds to stimuli may change both quantitatively and qualitatively. With the numerous feedback and recurrent excitatory and inhibitory connections, it is difficult to trace the net effect of STP even for the small circuit in Fig. 1. The challenge over the next few years will be to further characterize the circuitry in A1 and to use models like Fig. 3 to elucidate the basic principles and rules governing STP in large neural networks.
 We summarize the basic cellular properties of short-term plasticity.
We summarize the basic cellular properties of short-term plasticity. We describe short-term plasticity in auditory cortical circuits
We describe short-term plasticity in auditory cortical circuits We discuss the potential impact of short-term plasticity on signal processing in auditory cortex.
We discuss the potential impact of short-term plasticity on signal processing in auditory cortex.
Acknowledgement
Supported by NIH DC005787-01A1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott LF, Regehr WG. Synaptic computation. Nature. 2004;431:796–803. doi: 10.1038/nature03010. [DOI] [PubMed] [Google Scholar]
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–4. doi: 10.1126/science.275.5297.221. [DOI] [PubMed] [Google Scholar]
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsaki G, Cauli B, Defelipe J, Fairen A, Feldmeyer D, Fishell G, Fregnac Y, Freund TF, Gardner D, Gardner EP, Goldberg JH, Helmstaedter M, Hestrin S, Karube F, Kisvarday ZF, Lambolez B, Lewis DA, Marin O, Markram H, Munoz A, Packer A, Petersen CC, Rockland KS, Rossier J, Rudy B, Somogyi P, Staiger JF, Tamas G, Thomson AM, Toledo-Rodriguez M, Wang Y, West DC, Yuste R. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–68. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzori M, Lei S, Evans DI, Kanold PO, Phillips-Tansey E, McIntyre O, McBain CJ. Differential synaptic processing separates stationary from transient inputs to the auditory cortex. Nat Neurosci. 2001;4:1230–7. doi: 10.1038/nn760. [DOI] [PubMed] [Google Scholar]
- Bartlett EL, Wang X. Long-lasting modulation by stimulus context in primate auditory cortex. J Neurophysiol. 2005;94:83–104. doi: 10.1152/jn.01124.2004. [DOI] [PubMed] [Google Scholar]
- Borst JG. The low synaptic release probability in vivo. Trends Neurosci. 2010;33:259–66. doi: 10.1016/j.tins.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Boudreau CE, Ferster D. Short-term depression in thalamocortical synapses of cat primary visual cortex. J Neurosci. 2005;25:7179–90. doi: 10.1523/JNEUROSCI.1445-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosch M, Schreiner CE. Sequence sensitivity of neurons in cat primary auditory cortex. Cereb Cortex. 2000;10:1155–67. doi: 10.1093/cercor/10.12.1155. [DOI] [PubMed] [Google Scholar]
- Brosch M, Schulz A, Scheich H. Processing of sound sequences in macaque auditory cortex: response enhancement. J Neurophysiol. 1999;82:1542–59. doi: 10.1152/jn.1999.82.3.1542. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Oldford E. Cortical sensory suppression during arousal is due to the activity-dependent depression of thalamocortical synapses. J Physiol. 2002;541:319–31. doi: 10.1113/jphysiol.2002.016857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24:9598–611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DL, Schwindt PC, Grande LA, Spain WJ. Synaptic depression in the localization of sound. Nature. 2003;421:66–70. doi: 10.1038/nature01248. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt O, Hellweg FC, Schreiner C. Thalamocortical transformation of responses to complex auditory stimuli. Exp Brain Res. 1980;39:87–104. doi: 10.1007/BF00237072. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Killackey HP, Metherate R. Parvalbumin and calbindin are differentially distributed within primary and secondary subregions of the mouse auditory forebrain. Neuroscience. 2001;105:553–69. doi: 10.1016/s0306-4522(01)00226-3. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Rose HJ, Metherate R. Auditory thalamocortical synaptic transmission in vitro. J Neurophysiol. 2002;87:361–84. doi: 10.1152/jn.00549.2001. [DOI] [PubMed] [Google Scholar]
- David SV, Mesgarani N, Fritz JB, Shamma SA. Rapid synaptic depression explains nonlinear modulation of spectro-temporal tuning in primary auditory cortex by natural stimuli. J Neurosci. 2009;29:3374–86. doi: 10.1523/JNEUROSCI.5249-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Rocha J, Marchetti C, Schiff M, Reyes AD. Linking the response properties of cells in auditory cortex with network architecture: cotuning versus lateral inhibition. J Neurosci. 2008;28:9151–63. doi: 10.1523/JNEUROSCI.1789-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittman JS, Kreitzer AC, Regehr WG. Interplay between facilitation, depression, and residual calcium at three presynaptic terminals. J Neurosci. 2000;20:1374–85. doi: 10.1523/JNEUROSCI.20-04-01374.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont JJ. The magnitude and phase of temporal modulation transfer functions in cat auditory cortex. J Neurosci. 1999;19:2780–8. doi: 10.1523/JNEUROSCI.19-07-02780.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret G. Development of absolute auditory thresholds in the house mouse (Mus musculus) J Am Audiol Soc. 1976;1:179–84. [PubMed] [Google Scholar]
- Feldmeyer D, Lubke J, Sakmann B. Efficacy and connectivity of intracortical pairs of layer 2/3 pyramidal cells in the barrel cortex of juvenile rats. J Physiol. 2006;575:583–602. doi: 10.1113/jphysiol.2006.105106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hromadka T, Deweese MR, Zador AM. Sparse representation of sounds in the unanesthetized auditory cortex. PLoS Biol. 2008;6:e16. doi: 10.1371/journal.pbio.0060016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CY, Cruikshank SJ, Metherate R. Differential modulation of auditory thalamocortical and intracortical synaptic transmission by cholinergic agonist. Brain Res. 2000;880:51–64. doi: 10.1016/s0006-8993(00)02766-9. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological subgroups of nonpyramidal cells with specific morphological characteristics in layer II/III of rat frontal cortex. J Neurosci. 1995;15:2638–55. doi: 10.1523/JNEUROSCI.15-04-02638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Synaptic properties of thalamic and intracortical inputs to layer 4 of the first- and higher-order cortical areas in the auditory and somatosensory systems. J Neurophysiol. 2008;100:317–26. doi: 10.1152/jn.90391.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy RB, Reyes AD, Aoki C. Cholinergic modulation of local pyramid-interneuron synapses exhibiting divergent short-term dynamics in rat sensory cortex. Brain Res. 2008;1215:97–104. doi: 10.1016/j.brainres.2008.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Hu H, Berrebi AS, Mathers PH, Agmon A. Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J Neurosci. 2006;26:5069–82. doi: 10.1523/JNEUROSCI.0661-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone BJ, Scott BH, Semple MN. Context-dependent adaptive coding of interaural phase disparity in the auditory cortex of awake macaques. J Neurosci. 2002;22:4625–38. doi: 10.1523/JNEUROSCI.22-11-04625.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Wang Y, Tsodyks M. Differential signaling via the same axon of neocortical pyramidal neurons. Proc Natl Acad Sci U S A. 1998a;95:5323–8. doi: 10.1073/pnas.95.9.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Gupta A, Uziel A, Wang Y, Tsodyks M. Information processing with frequency-dependent synaptic connections. Neurobiol Learn Mem. 1998b;70:101–12. doi: 10.1006/nlme.1998.3841. [DOI] [PubMed] [Google Scholar]
- McGarry LM, Packer AM, Fino E, Nikolenko V, Sippy T, Yuste R. Quantitative classification of somatostatin-positive neocortical interneurons identifies three interneuron subtypes. Front Neural Circuits. 2010;4:12. doi: 10.3389/fncir.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metherate R, Cruikshank SJ. Thalamocortical inputs trigger a propagating envelope of gamma-band activity in auditory cortex in vitro. Exp Brain Res. 1999;126:160–74. doi: 10.1007/s002210050726. [DOI] [PubMed] [Google Scholar]
- Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59:861–72. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Naud R, Marcille N, Clopath C, Gerstner W. Firing patterns in the adaptive exponential integrate-and-fire model. Biol Cyber. 2008;99:335–47. doi: 10.1007/s00422-008-0264-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald AM, Doiron B, Rinzel J, Reyes AD. Spatial profile and differential recruitment of GABAB modulate oscillatory activity in auditory cortex. J Neurosci. 2009;29:10321–34. doi: 10.1523/JNEUROSCI.1703-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald AM, Reyes AD. Maturation of intrinsic and synaptic properties of layer 2/3 pyramidal neurons in mouse auditory cortex. J Neurophysiol. 2008;99:2998–3008. doi: 10.1152/jn.01160.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald AM, Reyes AD. Development of Inhibitory Timescales in Auditory Cortex. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald AM, Schiff ML, Reyes AD. Synaptic mechanisms underlying auditory processing. Curr Opin Neurobiol. 2006;16:371–6. doi: 10.1016/j.conb.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Recanzone GH. Response profiles of auditory cortical neurons to tones and noise in behaving macaque monkeys. Hear Res. 2000;150:104–18. doi: 10.1016/s0378-5955(00)00194-5. [DOI] [PubMed] [Google Scholar]
- Reig R, Gallego R, Nowak LG, Sanchez-Vives MV. Impact of cortical network activity on short-term synaptic depression. Cereb Cortex. 2006;16:688–95. doi: 10.1093/cercor/bhj014. [DOI] [PubMed] [Google Scholar]
- Reyes A, Sakmann B. Developmental switch in the short-term modification of unitary EPSPs evoked in layer 2/3 and layer 5 pyramidal neurons of rat neocortex. J Neurosci. 1999;19:3827–35. doi: 10.1523/JNEUROSCI.19-10-03827.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nat Neurosci. 1998;1:279–85. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- Rose HJ, Metherate R. Thalamic stimulation largely elicits orthodromic, rather than antidromic, cortical activation in an auditory thalamocortical slice. Neuroscience. 2001;106:331–40. doi: 10.1016/s0306-4522(01)00282-2. [DOI] [PubMed] [Google Scholar]
- Rose HJ, Metherate R. Auditory thalamocortical transmission is reliable and temporally precise. J Neurophysiol. 2005;94:2019–30. doi: 10.1152/jn.00860.2004. [DOI] [PubMed] [Google Scholar]
- Rothman JS, Cathala L, Steuber V, Silver RA. Synaptic depression enables neuronal gain control. Nature. 2009;457:1015–8. doi: 10.1038/nature07604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado H, Garcia-Oscos F, Dinh L, Atzori M. Dynamic modulation of short-term synaptic plasticity in the auditory cortex: the role of norepinephrine. Hear Res. 2011;271:26–36. doi: 10.1016/j.heares.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado H, Garcia-Oscos F, Patel A, Martinolich L, Nichols JA, Dinh L, Roychowdhury S, Tseng KY, Atzori M. Layer-specific noradrenergic modulation of inhibition in cortical layer II/III. Cereb Cortex. 2010;21:212–21. doi: 10.1093/cercor/bhq081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesian AE, Kotak VC, Sanes DH. Presynaptic GABA(B) receptors regulate experience-dependent development of inhibitory short-term plasticity. J Neurosci. 2010;30:2716–27. doi: 10.1523/JNEUROSCI.3903-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AY, Wehr M. Balanced tone-evoked synaptic excitation and inhibition in mouse auditory cortex. Neuroscience. 2009;163:1302–15. doi: 10.1016/j.neuroscience.2009.07.032. [DOI] [PubMed] [Google Scholar]
- Tan AY, Zhang LI, Merzenich MM, Schreiner CE. Tone-evoked excitatory and inhibitory synaptic conductances of primary auditory cortex neurons. J Neurophysiol. 2004;92:630–43. doi: 10.1152/jn.01020.2003. [DOI] [PubMed] [Google Scholar]
- Tan AY, Atencio CA, Polley DB, Merzenich MM, Schreiner CE. Unbalanced synaptic inhibition can create intensity-tuned auditory cortex neurons. Neuroscience. 2007;146:449–62. doi: 10.1016/j.neuroscience.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Theyel BB, Lee CC, Sherman SM. Specific and nonspecific thalamocortical connectivity in the auditory and somatosensory thalamocortical slices. Neuroreport. 2010;21:861–4. doi: 10.1097/WNR.0b013e32833d7cec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A, West DC, Wang Y, Bannister AP. Synaptiac connections and small circuits involving excitatory and inhibitory neurons in Layers 2-5 of adult rat and cat neocortex. Cereb Cortex. 2002;12:936–53. doi: 10.1093/cercor/12.9.936. [DOI] [PubMed] [Google Scholar]
- Tsodyks MV, Markram H. The neural code between neocortical pyramidal neurons depends on neurotransmitter release probability. Proc Natl Acad Sci U S A. 1997;94:719–23. doi: 10.1073/pnas.94.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela JA, Sen K, Gibson J, Fost J, Abbott LF, Nelson SB. A quantitative description of short-term plasticity at excitatory synapses in layer 2/3 of rat primary visual cortex. J Neurosci. 1997;17:7926–40. doi: 10.1523/JNEUROSCI.17-20-07926.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaene AN, Petrof I, Sherman SM. Synaptic properties of thalamic input to layers 2/3 and 4 of primary somatosensory and auditory cortices. J Neurophysiol. 2011;105:279–92. doi: 10.1152/jn.00747.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins PV, Barbour DL. Rate-level responses in awake marmoset auditory cortex. Hear Res. 2010 doi: 10.1016/j.heares.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Synaptic mechanisms of forward suppression in rat auditory cortex. Neuron. 2005;47:437–45. doi: 10.1016/j.neuron.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Xu H, Kotak VC, Sanes DH. Conductive hearing loss disrupts synaptic and spike adaptation in developing auditory cortex. J Neurosci. 2007;27:9417–26. doi: 10.1523/JNEUROSCI.1992-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LI, Tan AY, Schreiner CE, Merzenich MM. Topography and synaptic shaping of direction selectivity in primary auditory cortex. Nature. 2003;424:201–5. doi: 10.1038/nature01796. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]