Abstract
Four different techniques for 3T whole-heart coronary MRA using free-breathing 3D segmented parallel imaging and adiabatic T2-Prep were assessed. Coronary MRA at 3T is improved by shortening the acquisition window more than employing the highest spatial resolution. Double oblique whole-heart acquisitions result in better overall image quality and allow for better delineation of the LAD. It is possible to attain shorter acquisition windows and a smaller voxel size at 3T than previously reported at 1.5T.
Introduction
Whole-heart 3 dimensional (3D) coronary magnetic resonance angiography (MRA) utilizing free-breathing navigator gating is emerging as a valuable technique for the evaluation of coronary artery disease (CAD)[1–3]. Many of the more recent studies comparing x-ray angiography and coronary MRA were performed at 1.5T and utilized steady-state-free-precession (SSFP) sequences[1–4]. These SSFP sequences provide a higher signal-to-noise (SNR) and superior vascular image contrast when compared to more conventional segmented k-space gradient echo (GRE) imaging techniques[5]. The highest reported spatial resolution attained to date using these whole-heart sequences at 1.5T has been 1×1×1.5mm3 which requires approximately 13 minutes of acquisition time[2, 3] using a five-element receiver coil (parallel imaging (SENSE) factor = 2) and approximately 4 minutes [4] using 32-element coil (SENSE factor=4). This reported reduction in scan time by more than a factor of 2 is probably attributable to various factors other than increasing SENSE factor. These may include variations in temporal resolution and/or improved respiratory navigator efficiency as a result of improved diaphragm detection. The latter may have been a consequence of higher SNR achieved by use of 32-element coil at 1.5T. This may have further reduced scan time by lowering the possibility of diaphragm drift out of the respiratory navigation window, thereby, increasing and/or maintaining navigator efficiency. Imaging at higher field strength (i.e. 3T) affords higher SNR that in turn provides an opportunity for improvement of spatial resolution. Alternatively, with the implementation of parallel imaging (SENSE) at 3T, shorter scan time could be obtained or higher temporal resolution may be achieved. These advantages of scanning at 3T may be implemented to further improve whole-heart imaging. However, due to B0 and B1 field inhomogenities and specific absorption rate (SAR) restrictions at 3T, SSFP may not easily be used. Alternatively, contrast enhanced whole-heart imaging utilizing an inversion recovery technique has been shown to have promising results in patients utilizing an acquired spatial resolution of 1.3 × 1.3 × 1.3 mm3 [6].
Therefore, the development and optimization of non-contrast enhanced segmented k-space gradient echo whole-heart techniques at 3T is needed. The purpose of this study was to develop a whole-heart coronary MRA using free-breathing 3D segmented k-space technique at 3T that optimizes the large volume acquisition orientation, spatial resolution and acquisition window for coronary MRA. The evaluative end-points were the length of the longest coronary arterial segments visualized and visual image quality.
Subjects and Methods
Subjects
Ten healthy subjects provided written informed consent for participation in this Institutional Review Board approved and HIPAA-compliant study. All subjects were imaged consecutively within a span of two months. Ages ranged from 18 to 42 years old and four were males and six females. Their heart rate ranged from 60 to 90 beats per minute.
MR Angiography Acquisition
All ten subjects were imaged on a Philips 3 T Intera system (Philips Medical Systems, Best, NL) using a 6-element cardiac phased-array receiver coil, and vector electrocardiographic (VCG) gating [7]. The scanning protocol is a modification from previously described methods at 1.5T [1–3, 8–12] and 3T [13–15]. A multi-slice gradient echo (TR = 11ms; TE = 2.4ms; α = 20°) scout scan was acquired in 3 orthogonal orientations for localization of the volume for whole-heart imaging and for navigator positioning at the dome of the right hemidiaphragm. This was followed by a 3D segmented k-space gradient-echo low resolution, respiratory navigator and ECG gated whole-heart scan for localization of the coronaries. A 2D selective RF pulse with 12 revolutions in k-space and a beam radius of 15 mm was used for gating and tracking of respiratory motion [11]. The navigator beam was positioned at the dome of the right hemidiaphragm with an acceptance window of 8 mm. After the 3D scout scan, an axial ECG triggered, segmented steady-state free precession (SSFP) cine image series (TR = 3.8 ms, TE = 1.8 ms, α = 45°, and temporal resolution of 39.6 ms) at the level of the proximal-to-mid right coronary artery (RCA) was also obtained during free breathing. This was done for visual determination of the most quiescent period in the cardiac cycle which was subsequently used to set the trigger delay (TD).
Subsequently, 3D whole-heart navigator gated and 3D segmented k-space gradient echo coronary MRA were acquired using this visually identified TD. No intravenous contrast agents were used, therefore, to generate contrast, a T2Prep pulse was used that takes advantage of natural T2 differences between blood and myocardium [16]. However, enhanced B1 inhomogeneity at 3T limits the effectiveness of this pulse to generate contrast. For this reason we have developed a B1 insensitive adiabatic variant of this T2Prep (TE=50ms) that incorporates two adiabatic 180° refocusing pulses [17] and have used it for vascular image contrast generation in the present study. Spectrally selective fat saturation was also utilized for additional endogenous image contrast enhancement between the coronary blood-pool and the surrounding fat. Real-time navigator as described above [18], with a 5 mm gating acceptance window and slice tracking was used for respiratory motion suppression.
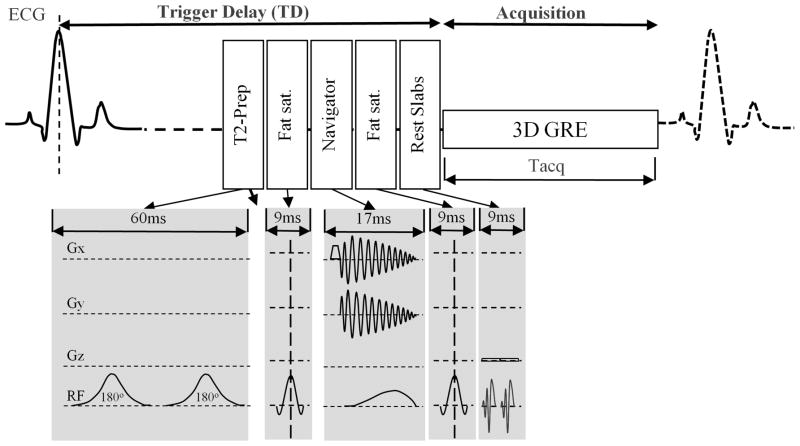
When not otherwise specified, the typical whole-heart scan parameters were: TR = 4.2– 4.6ms, TE = 1.5ms, α = 20°, pixel bandwidth = 299.5Hz, number of RF excitations for imaging per cardiac cycle (TFE factor). = 27. Parallel imaging (SENSE acceleration factor of 2)[19] was used for all whole-heart acquisitions. The pulse sequence diagram is presented in figure 1. To evaluate the relative impact of acquisition plane orientation, window-duration and acquired spatial resolution, these 3D whole-heart volumes were acquired for each subject using four different acquisition schemes with the following parameter differences:
Figure 1.
Schematic diagram for general pulse sequence used in this study. The main components are represented here on relationship to the cardiac cycle. Modifications are made to LA scans in the 3 dimensional segmented k-space gradient echo (3D GRE) acquisition but either changing the imaging orientation for OBL scans, resolution for HR scans, or acquisition time (Tacq) for SA scans.
Conventional trans-axial plane with an acquisition window of 114–124 msec and anisotropic voxel size (1 × 1 × 2 mm3)—Whole Heart Long Acquisition window (LA);
Double-oblique, short-axis plane parallel to the course of the right coronary artery (RCA) with an acquisition window of 114–124 msec and anisotropic voxel size (1 × 1 × 2 mm3)—Whole Heart Oblique (OBL);
Conventional trans-axial plane with a short acquisition window of 53 msec, (TR=3.3, TE 1.8 and pixel bandwidth = 612.7Hz, TFE factor=16) and anisotropic voxel size (1 × 1 × 2 mm3)—Whole Heart Short Acquisition window (SA);
Conventional trans-axial plane with an acquisition window of 114–124 msec and isotropic voxel size (1 × 1 × 1 mm3)—Whole Heart High Resolution (HR).
The rationale behind the presented choices was to acquire the best possible images while maintaining a scan time below 20 minutes. We strived to push the limits of spatial and temporal resolution within this scan time constraint. Scanning with a higher spatial or temporal resolution will increase the scan time to above 20 minutes and consequently become impractical for clinical practice. Additionally, the possibility and severity of motion artifacts and drifting of the diaphragm out of the navigator acceptance window increases with longer acquisitions; potentially causing failure of these 3D scans altogether. A double-oblique acquisition was employed to investigate whether changing scan orientation would improve image quality.
Image Post-processing and Analysis
The four sets of 3D whole-heart volume images of all 10 subjects were reviewed by an experienced (> 10 years) cardiac radiologist who was blinded to the differences in imaging techniques. A qualitative review of the images was performed by this blinded reader. For each subject, a previously described score of 1–4 [20] was assigned to the set of 3D whole-heart volume images for each technique. A score of 1 indicated poor image quality in which the coronary artery is poorly visible with markedly blurred borders or edges; 2, good image quality in which the coronary artery is visible with moderately blurred borders or edges; 3, very good image quality in which the coronary artery is visible with mildly blurred borders or edges; and 4, excellent image quality in which the coronary artery is visible with sharply defined borders or edges. Additionally, multiplanar reformatted images and length measurement of the RCA (n=40), left main and left anterior descending coronary arteries combined (LAD, n=40) and left circumflex artery (LCX, n=40) were obtained for each acquisition using commercially available image analysis software (Virtual Place Advance; AZE, Tokyo, Japan).
Statistical Evaluation
A two tailed paired student’s t-test was used to compare vessel lengths, while a Wilcoxon test was used for statistical comparison of the image quality scores. Bonferroni correction was applied to all p values to account for multiple comparisons. A p value of < 0.05 was considered statistically significant.
Results
A total of 40 whole-heart scans were successfully performed in all 10 subjects and results are shown in Table 1 and 2 for the parameters (vessel length and visual score) and statistical analysis respectively. Acquisition times were 6–10 min for LA and OBL, 12–16 minutes for SA, and 14–18 minutes for HR. These values account for a respiratory navigator efficacies on the order of 40%–60% achieved in all subjects. The measured RCA and LCX lengths were significantly improved using the SA compared to the LA techniques (p<0.05 for both vessels), but were not affected by other parameter changes. The LAD on the other hand, was significantly better visualized when using the double oblique orientation (OBL) when compared to images obtained with the same acquisition window of 114–124 msec that were oriented in the transaxial plane using similar (LA) (p<0.05) or smaller voxel size (HR) (p<0.05) (figure 2). Like the RCA (figure 3) and LCX, the LAD was also better seen on the SA than LA (p<0.05). The visual score was also significantly higher for the technique with the shorter acquisition windows (SA) in comparison to those obtained with prolonged acquisition windows whether using similar (LA) or higher spatial resolution (HR). The double oblique oriented images (OBL) scored significantly higher when compared to all other methods except the images obtained during short acquisition windows (SA). Improved spatial resolution does not seem to have any significant impact on either the image quality score or the measured vessel length.
Table 1.
Coronary lengths and reader scores.
| LA | OBL | SA | HR | |
|---|---|---|---|---|
| RCA mean length | 131.1±26.2 | 139.4±26.7 | 141.3±24.2 | 135.4±28.6 |
| LAD mean length | 75.2±24.5 | 104.9±35.7 | 111.2±40.5 | 88.2±26.8 |
| LCX mean length | 52.7±21.1 | 66.1±24.0 | 72.6±26.1 | 58.1±21.3 |
| Mean reader score | 1.3 | 3.7 | 3.1 | 1.8 |
Note: lengths (mm) ± standard deviation
Table 2.
P values from statistical analyses.
| Vessel | LA-SA | LA-HR | LA-OBL | SA-HR | SA-OBL | HR-OBL |
|---|---|---|---|---|---|---|
| RCA | 0.03 | NS | NS | NS | NS | NS |
| LAD | 0.018 | NS | 0.032 | NS | NS | 0.035 |
| LCX | <0.001 | NS | NS | NS | NS | NS |
| SCORE | <0.001 | NS | <0.001 | 0.027 | NS | <0.001 |
Significant p values are displayed in red. NS is non-significant.
Figure 2.
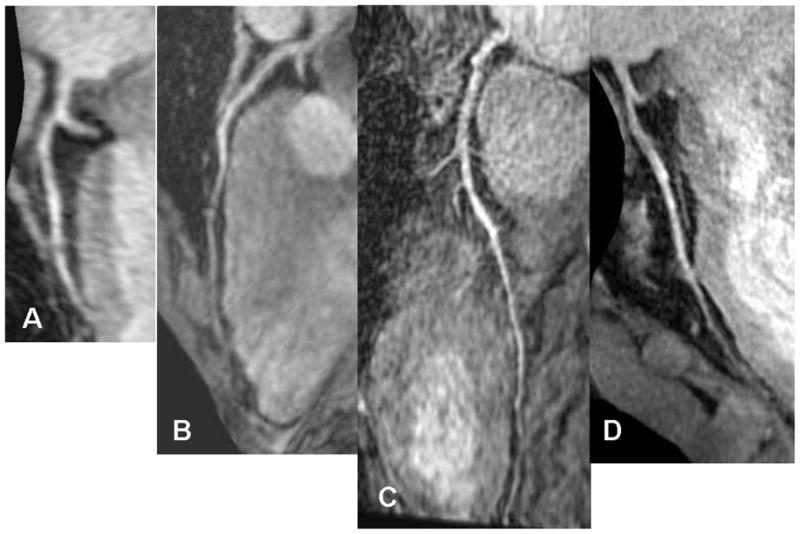
Multiplanar stretch reformatted image of the Left Anterior Descending coronary artery (LAD) of the same person A) LA, B) OBL, C) SA D) HR. Notice the difference in length of vessel visualized by the different techniques.
Figure 3.
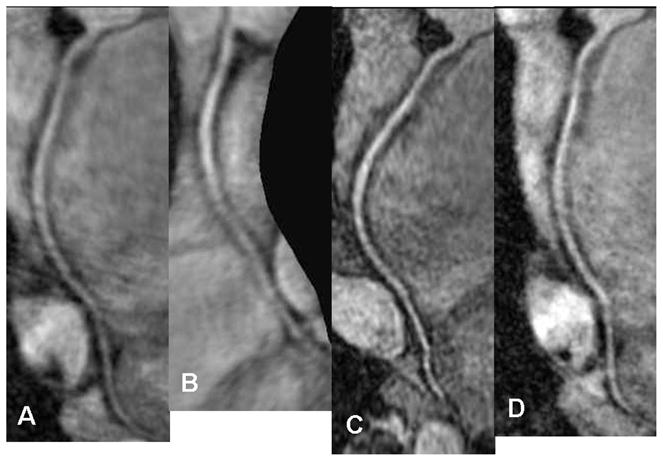
Multiplanar stretch reformatted image of the Right Coronary Artery (RCA) using the four different techniques A) LA, B) OBL, C) SA D) HR.
Discussion and Conclusion
Whole-heart 3D coronary MRA performed at 1.5T has been shown to be a valuable tool for the evaluation of CAD[1–3], without the use of radiation or potentially nephrotoxic contrast agents. However, this technique at 1.5T only approaches the level of sensitivity or specificity reported for CT coronary angiography. The best reported sensitivity and specificity for whole-heart technique at 1.5T was 82% and 91% [2] which remains inferior compared to the best results using 64 slice CT scanners reaching up to 99% and 95% respectively [21]. This might be attributable to CTA’s relatively better spatial resolution (voxel 0.4mm3) [21], short temporal resolution (83msec) [22] and good respiratory motion compensation using breath holding [22] which are further complemented by the additional use of beta blockers to provide steady heart rates and nitroglycerin for improved vasodilation. With the growing availability of 3T MRI units it is important to consider whether the higher field strengths confer the anticipated benefits of higher resolution or faster speed or both. Practical optimization involves addressing a number of technical and technique related concerns. Some of these have been addressed, however, using a contrast enhanced method that utilize an inversion recovery technique [6] achieving the shortest temporal resolution of 89±8ms with a total acquisition time of 9±1.9min. This resulted in an improvement of sensitivity up to 94% from studies performed at 1.5T. These inversion recovery techniques utilize the advantage of prolonged T1 time at higher magnetic field. This prolonged T1 time may present a challenge for T1 weighted techniques such as GRE sequences used in this study. Due to the time constraints for coronary MRA where only a short time during diastole is available for imaging in addition to the prolonged T1 relaxation parameter at higher magnetic fields, expected SNR improvement at 3T would be difficult to achieve to its full extent. A longer inter-echo repetition time (TR) is needed, which was implemented in this study compared to the typical TR at 1.5T. For a potentially higher SNR gain, the full relaxation of magnetization needs to be reached, which would require more than one cardiac cycle delay between data acquisitions. However, this would have made the scanning prohibitively long and the gain in SNR will still be compromised by the additional motion artifacts due to the longer scan time, therefore was not utilized in this study. The advantage of prolonged T1 time is best harnessed in spin labeling sequences or when using intravenous contrast materials which were not applied in this study. Hence, techniques that do not need gadolinium based contrast agents similar to those used at 1.5T need to be optimized. These pulse sequences that are ordinarily satisfactory at 1.5T must be adapted to 3T due to Specific Absorption Rate (SAR) limits, susceptibility and motion artifacts and the different relaxometries of tissue and thus contrast at 3T. In addition to addressing these technical concerns, it is important to evaluate the practical technique variables of data acquisition temporal resolution, spatial resolution and even plane of acquisition orientation on the image quality. This is the first study that accomplishes these goals. These parameters have crucial effects on edge definition, which is important for coronary imaging. This study investigated these variables within a practical scan time of less than 20 minutes. Further exploration of these variables beyond the 20 minutes time limit may potentially, decrease SNR and/or respiratory navigator efficacy. Additionally, this prolonged scan time will prohibit several separate whole-heart acquisitions in the same subject in a single session, as performed in this study.
Since SAR for a given technique is proportional to the square of the frequency, doubling the field strength quadruples the power deposition and SAR. Thus SSPF, which has become the sequence of choice for bright blood/angiographic imaging at 1.5T is more challenging to utilize for whole-heart imaging at 3T given its short TR related high power deposition characteristics. This becomes further manifested when utilizing sequences involving trains of RF pulses required for cardiac imaging. Thus image quality will probably be compromised by using a longer TR and/or smaller flip angles. Adapting SSFP sequences to high-field 3D coronary MRA and utilizing T2-prep will even add more to the aforementioned SAR concerns, requiring even more compromise in imaging parameters. These complexities are further accentuated by the need for sophisticated high-order 3D shimming and flow/motion artifact correction. The use of segmented k-space gradient echo imaging avoids exceeding SAR limitations with whole-heart angiographic imaging. B1 inhomogeneity related artifact and contrast irregularities were removed using adiabatic T2-Preparation[17]. An advantage of 3T is that SENSE can be used with the gradient echo sequence due to the relatively higher SNR. This SNR also ultimately allows for shortening the acquisition window and decreasing the voxel size, as was evaluated in this study. Whole-heart images were successfully acquired in a window as short as 53msec and with a voxel size as small as 1 × 1 × 1 mm3. Both of these parameter values are improvements to those achieved with the best whole-heart techniques previously described at 1.5T[2, 3, 12] where the shortest acquisition window reported was 70msec [12] and voxel size was 1 × 1 × 1.5mm3[2, 3]. In this initial sample of healthy volunteers, we were able to visualize long segments of the three coronary arteries, including the terminal end of the RCA. On average the RCA length visualized was 14.1 cm, which compares favorably to the average length of 10.7cm reported as visualized at 1.5T [2, 3, 12]. The LAD and LCX lengths visualized on average were similar to measurements reported at 1.5T [12] which were 11.7cm and 6.9cm compared to the average lengths in our study of 11.1cm and 7.3cm respectively. Despite these improvements, the longest acquisition time for the isotropic (HR) 1 × 1 × 1 mm3 wholeheart sequence was still within the range of 13–14 minutes previously reported at 1.5T as the best average imaging time[2, 3] using similar SENSE factor of 2.
It may be useful to note that while the longest vessel length was obtained with the shortest acquisition window of 53msec, this came at the expense of scanning time. The double oblique plane technique, however, allowed for the visualization of similar LAD segment lengths without sacrificing scan time. This is probably due to the cross sectional orientation of the LAD in this imaging plane, which may protect it from the anisotropic pixel volume with the asymmetric length of the voxel along the length of the LAD. It may, however, be affected by volume averaging as it approaches the cardiac apex. An additional advantage of this orientation is that the RCA and LCX lie in a plane where they are visualized with the higher in-plane spatial resolution. This may contribute to the significantly higher subjective score assigned to this technique.
The lack of SNR measurement is a relative limitation of this study. However, this may have been a misleading measurement because of the use of SENSE which results in a high variability of noise across the field of view specially when using different imaging orientations as in our study. Since SENSE was used for all the acquisitions, it was thought that vessel length and subjective image quality score would be good comparative indicators to identify the better techniques.
We conclude from this study that non-contrast enhanced whole-heart imaging using segmented gradient echo free-breathing technique at 3T is improved by shortening the acquisition window more than resorting to higher spatial resolution. In addition, double oblique whole-heart acquisitions result in better overall image quality and allow for better delineation of the LAD. It is more reader friendly without prolonging the scan time. Finally, it is possible to attain shorter acquisition windows and a smaller voxel size for whole-heart 3D coronary MRAs using free-breathing techniques than previously reported at 1.5T. Further improvements in coronary MRA will likely depend most critically on advancing methods for avoiding or compensation for physiologic motion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jahnke C, Paetsch I, Nehrke K, et al. Rapid and complete coronary arterial tree visualization with magnetic resonance imaging: feasibility and diagnostic performance. Eur Heart J. 2005 doi: 10.1093/eurheartj/ehi391. [DOI] [PubMed] [Google Scholar]
- 2.Sakuma H, Ichikawa Y, Suzawa N, et al. Assessment of coronary arteries with total study time of less than 30 minutes by using whole-heart coronary MR angiography. Radiology. 2005;237:316–321. doi: 10.1148/radiol.2371040830. [DOI] [PubMed] [Google Scholar]
- 3.Sakuma H, Ichikawa Y, Chino S, Hirano T, Makino K, Takeda K. Detection of coronary artery stenosis with whole-heart coronary magnetic resonance angiography. J Am Coll Cardiol. 2006;48:1946–1950. doi: 10.1016/j.jacc.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 4.Nehrke K, Bornert P, Mazurkewitz P, Winkelmann R, Grasslin I. Free-breathing whole-heart coronary MR angiography on a clinical scanner in four minutes. J Magn Reson Imaging. 2006;23:752–756. doi: 10.1002/jmri.20559. [DOI] [PubMed] [Google Scholar]
- 5.Spuentrup E, Buecker A, Stuber M, et al. Navigator-gated coronary magnetic resonance angiography using steady-state-free-precession: comparison to standard T2-prepared gradient-echo and spiral imaging. Invest Radiol. 2003;38:263–268. doi: 10.1097/01.RLI.0000064341.68310.c0. [DOI] [PubMed] [Google Scholar]
- 6.Yang Q, Li K, Liu X, et al. Contrast-enhanced whole-heart coronary magnetic resonance angiography at 3. 0-T: a comparative study with X-ray angiography in a single center. J Am Coll Cardiol. 2009;54:69–76. doi: 10.1016/j.jacc.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer SE, Wickline SA, Lorenz CH. Novel real-time R-wave detection algorithm based on the vectorcardiogram for accurate gated magnetic resonance acquisitions. Magn Reson Med. 1999;42:361–370. doi: 10.1002/(sici)1522-2594(199908)42:2<361::aid-mrm18>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Botnar RM, Stuber M, Danias PG, Kissinger KV, Manning WJ. Improved coronary artery definition with T2-weighted, free-breathing, three-dimensional coronary MRA. Circulation. 1999;99:3139–3148. doi: 10.1161/01.cir.99.24.3139. [DOI] [PubMed] [Google Scholar]
- 9.Casolo G, Del Meglio J, Rega L, et al. Detection and assessment of coronary artery anomalies by three-dimensional magnetic resonance coronary angiography. International Journal of Cardiology. 2005;103:317. doi: 10.1016/j.ijcard.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Kim WY, Danias PG, Stuber M, et al. Coronary magnetic resonance angiography for the detection of coronary stenoses. N Engl J Med. 2001;345:1863–1869. doi: 10.1056/NEJMoa010866. [DOI] [PubMed] [Google Scholar]
- 11.Stuber M, Botnar RM, Danias PG, et al. Double-oblique free-breathing high resolution three-dimensional coronary magnetic resonance angiography. J Am Coll Cardiol. 1999;34:524–531. doi: 10.1016/s0735-1097(99)00223-5. [DOI] [PubMed] [Google Scholar]
- 12.Weber OM, Martin AJ, Higgins CB. Whole-heart steady-state free precession coronary artery magnetic resonance angiography. Magn Reson Med. 2003;50:1223–1228. doi: 10.1002/mrm.10653. [DOI] [PubMed] [Google Scholar]
- 13.Sommer T, Hackenbroch M, Hofer U, et al. Coronary MR angiography at 3.0 T versus that at 1. 5 T: initial results in patients suspected of having coronary artery disease. Radiology. 2005;234:718–725. doi: 10.1148/radiol.2343031784. [DOI] [PubMed] [Google Scholar]
- 14.Huber ME, Kozerke S, Pruessmann KP, Smink J, Boesiger P. Sensitivity-encoded coronary MRA at 3T. Magn Reson Med. 2004;52:221–227. doi: 10.1002/mrm.20062. [DOI] [PubMed] [Google Scholar]
- 15.Stuber M, Botnar RM, Fischer SE, et al. Preliminary report on in vivo coronary MRA at 3 Tesla in humans. Magn Reson Med. 2002;48:425–429. doi: 10.1002/mrm.10240. [DOI] [PubMed] [Google Scholar]
- 16.Brittain JH, Hu BS, Wright GA, Meyer CH, Macovski A, Nishimura DG. Coronary angiography with magnetization-prepared T2 contrast. Magnetic Resonance in Medicine. 1995;33:689–696. doi: 10.1002/mrm.1910330515. [DOI] [PubMed] [Google Scholar]
- 17.Nezafat R, Stuber M, Ouwerkerk R, Gharib AM, Desai MY, Pettigrew RI. B1-insensitive T2 preparation for improved coronary magnetic resonance angiography at 3 T. Magn Reson Med. 2006;55:858–864. doi: 10.1002/mrm.20835. [DOI] [PubMed] [Google Scholar]
- 18.Stuber M, Botnar RM, Danias PG, Kissinger KV, Manning WJ. Submillimeter three-dimensional coronary MR angiography with real-time navigator correction: comparison of navigator locations. Radiology. 1999;212:579–587. doi: 10.1148/radiology.212.2.r99au50579. [DOI] [PubMed] [Google Scholar]
- 19.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42:952–962. [PubMed] [Google Scholar]
- 20.McConnell MV, Khasgiwala VC, Savord BJ, et al. Comparison of respiratory suppression methods and navigator locations for MR coronary angiography. AJR Am J Roentgenol. 1997;168:1369–1375. doi: 10.2214/ajr.168.5.9129447. [DOI] [PubMed] [Google Scholar]
- 21.Mollet NR, Cademartiri F, van Mieghem CA, et al. High-resolution spiral computed tomography coronary angiography in patients referred for diagnostic conventional coronary angiography. Circulation. 2005;112:2318–2323. doi: 10.1161/CIRCULATIONAHA.105.533471. [DOI] [PubMed] [Google Scholar]
- 22.Achenbach S. Computed tomography coronary angiography. J Am Coll Cardiol. 2006;48:1919–1928. doi: 10.1016/j.jacc.2006.08.012. [DOI] [PubMed] [Google Scholar]