Abstract
We examined apolipoprotein E (ApoE) genotypes in relation to Parkinson disease (PD) among 786 cases and 1,537 controls, all non-Hispanic Caucasians. Odds ratios (ORs) and 95% confidence intervals (CIs) were derived from multivariate logistic regression models, adjusting for year of birth, sex, smoking status, daily caffeine intake, and family history of PD. Compared to participants with ApoE ε33, ε4-carriers (ε34/ε44) had significantly lower odds for having PD [OR= 0.75 (95% CI: 0.59–0.94), p=0.01], whereas ε2-carriers (ε23/ ε22) did not [OR=0.95 (95% CI: 0.73–1.24), p=0.71]. Subgroup analyses showed similar results. In addition, we conducted a meta-analysis which confirmed our primary findings (ε34/ε44 vs. ε33: OR=0.90, 95% CI: 0.81–0.99, p=0.024 and ε23/ε22 vs. ε33: OR=1.10, 95% CI: 0.97–1.23, p=0.13). In PD patients, the prevalence of dementia appeared to be higher among ε4-carriers (compared with ε33, OR=1.59, 95% CI: 0.98–2.58, p=0.06), but lower among ε2-carriers (OR=0.75, 95% CI: 0.40–1.42, p=0.38), although neither test was statistically significant. Our study suggested that the ApoE ε4 allele may be associated with a lower PD risk among non-Hispanic Caucasians.
Keywords: Parkinson disease (PD), apolipoprotein E (ApoE), dementia, association
Introduction
Parkinson disease (PD) and Alzheimer’s disease (AD) share some clinical and neuropathological features (Hughes, et al., 1993). Apolipoprotein E (ApoE) is the best established susceptibility gene for late-onset AD with ε4 as the risk allele and ε2 as the protective allele (Farrer, et al., 1997). This has led to epidemiological studies to test the hypothesis that ApoE ε4 also increases PD risk, but the results have been very inconsistent. Two recent meta-analyses suggested that ε4 was not associated with higher PD occurrence (Huang, et al., 2004, Williams-Gray, et al., 2009). On the other hand, several prospective studies (de Lau, et al., 2006, Huang, et al., 2008, Simon, et al., 2007) recently showed that higher plasma cholesterol was associated with a lower risk of PD. Since ApoE plays an important role in cholesterol metabolism and transportation, it necessary to investigate further the relationship of ApoE with PD. We therefore examined ApoE genotypes in relation to PD among 786 cases and 1,537 controls, all non-Hispanic Caucasians.
1. Methods
1.1. Study population and PD case recruitment
The Parkinson’s, Genes, and Environment (PAGE) study is a case-control study within the large prospective NIH-AARP Diet and Health Study cohort (Schatzkin, et al., 2001). The cohort was established in 1995–1996 by the National Cancer Institute for cancer research, and collected comprehensive dietary and lifestyle information at baseline. Potential PD patients in this cohort were first identified from self-reports in its follow-up survey in 2004–2006, and then were confirmed either by their treating physicians or by medical record review (Chen, et al., 2010).
We began in 2007 to collect saliva samples from surviving PD patients via mail using the Oragene™ saliva collection kits (DNA Genotek Inc., Ontario Canada). In this contact, we asked patients to confirm their report of the diagnosis, and to give permission to contact their treating physicians (Chen, et al., 2010). We then contacted the patients’ treating physicians, and asked them to fill out a short diagnostic questionnaire, and also send a copy of relevant medical records. A PD case was confirmed if the diagnosis was either confirmed by the patient’s treating physician or via medical record review by a movement disorder specialist. For PD cases, we also asked for the date of first symptoms (onset) and diagnosis, presence of dementia, and family history of PD defined as having at least one first-degree blood relative with physician diagnosed PD. Only confirmed PD cases were included in this study.
Potential controls were randomly selected from cohort participants who did not report PD in the follow-up survey, frequency matched to self-reported cases on year of birth, sex, and ethnicity. Information on family history of PD as well as saliva samples was also collected from controls. In total, 838 of physician-confirmed PD cases and 1,703 controls were genotyped. To avoid population stratification, we excluded 30 cases and 80 controls who were not Non-Hispanic Whites or who did not report ethnicity. We further excluded 22 cases and 86 controls whose genotyping failed at either of the two ApoE loci (rs429358 / rs7412); this left a total of 786 cases and 1,537 controls for the final analyses.
1.2. Assessments of potential confounders
Information on smoking and caffeine intake was obtained from the cohort’s baseline survey in 1995–1996 (Schatzkin, et al., 2001). For most cases, this information was collected before PD diagnosis and therefore suitable for etiological research. Participants were asked whether they had ever smoked more than 100 cigarettes during their lifetime; and for ever smokers, the typical amount of smoking, current smoking status and years since last smoking (Chen, et al., 2010). Caffeine intake was derived from a food frequency survey that asked for consumption of coffee and caffeine containing drinks and foods in the past year. In addition, the baseline survey also collected information on date of birth, sex, and ethnicity.
1.3. DNA extraction and ApoE genotyping
DNA was extracted from saliva samples and genotyped by BioServe Biotechnologies, Ltd. (Beltsville, MD), using MassARRAY iPLEXTM platform. Two SNPs were genotyped: rs7412 for ApoE ε2 and rs429358 for ApoE ε4 (Grupe, et al., 2007). We randomly included 51 duplicates to which the genotyping laboratory was “blind”, and the reproducibility was 100%.
1.4. Statistical analyses
Hardy-Weinberg equilibrium was examined with chi-square statistics. Odds ratios (ORs) and 95% confidence intervals (CIs) were derived from logistic regression models, adjusting for year of birth (in four groups), sex, smoking status (never, former, or current ), daily caffeine intake (quintile), and family history of PD (yes / no). All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc, Cary, NC) combined with Plink v1.06 (Purcell, et al., 2007). Two-sided p<0.05 was considered statistically significant. The primary analyses involved three independent tests (ε4-carriers, ε2-carriers or ε24-carriers v.s. ε33). Therefore, after Bonferroni correction, p<0.017 should be considered statistical significant in the primary analyses.
To examine the robustness of our primary findings, we conducted subgroup analyses according to year of birth (≤ 1931 vs. > 1931), sex (men vs. women), smoking status (never vs. ever), caffeine intake (below or above the median) or family history of PD (yes or no). As PD patients with longer disease duration were more likely to have dementia or other health conditions that had prevented them from participating in the study, we further conducted a sensitivity analysis according to the year of diagnosis (before or after 2000) to examine potential bias from this source. As no date of diagnosis was available for controls, we used the same control group in this sensitivity analysis.
Finally, we conducted meta-analyses to pool our data with those from published studies (pdgene.org). We only included studies of Non-Hispanic Caucasians with Hardy-Weinberg Equibrilium among controls. To keep consistent with our analysis, for each study, we calculated the crude OR and 95% CI comparing ε44/ ε34 or ε22/ ε23 vs. ε33 from published genotype frequencies. Pooled estimates were quantified using the inverse variance method based on the random-effects models (DerSimonian and Laird, 1986). Heterogeneity across studies was assessed using Cochran’s Q and I2 statistics (Higgins and Thompson, 2002). Meta-analyses were performed using STATA (version 10.0, StataCorp LP, College Station, TX).
1.5. Standard protocol approvals, registrations and patient consent
The study protocol was approved by the Institutional Review Board of the National Institute of Environmental Health Sciences and all study participants provided informed written consent.
2. Results
As expected, PD cases were less likely to smoke or had lower caffeine intake than controls (Table 1, p<0.0001). Further, compared with controls, PD cases were more likely to report a family history of PD (p<0.0001). Among PD cases, the average age at onset was 65.7 ± 7.5 years and age at diagnosis was 66.7 ± 7.3 years; further, 131 (16.7%) of them had dementia as reported by their physician or documented in medical records. ApoE genotypes were in Hardy-Weinberg equilibrium in both cases and controls (p>0.05).
Table 1.
Population characteristics according to case-control status
| PD cases, n (%) | Controls, n (%) | p | |
|---|---|---|---|
| All | 786 | 1537 | |
| Year of Birth | 0.04 | ||
| 1925–1929 | 281 (35.8) | 603 (39.2) | |
| 1930–1934 | 262 (33.3) | 544 (35.4) | |
| 1935–1939 | 159 (20.2) | 253 (16.5) | |
| 1940- | 84 (10.7) | 137 (8.9) | |
| Men | 600 (76.3) | 1211 (78.8) | 0.18 |
| Smoking | <.0001 | ||
| Never | 366 (46.6) | 537 (34.9) | |
| Former | 385 (49.0) | 893 (58.1) | |
| Current | 24 (3.1) | 91 (5.9) | |
| Missing | 11(1.4) | 16(1.0) | |
| Caffeine intake (mg/day) | 295.2 ± 335.2 | 361.3 ± 361.5 | <.0001 |
| Family History | <.0001 | ||
| Yes | 111 (14.1) | 84 (5.5) | <.0001 |
| No | 668 (85.0) | 1318 (85.8) | |
| Missing | 7 (0.9) | 135 (8.8) | |
| ApoE | 0.32 | ||
| ε22 | 6 (0.8) | 12 (0.8) | |
| ε23 | 100 (12.7) | 198 (12.9) | |
| ε33 | 522 (66.4) | 952 (61.9) | |
| ε34 | 133 (16.9) | 316 (20.6) | |
| ε44 | 11 (1.4) | 25 (1.6) | |
| ε24 | 14 (1.8) | 34 (2.2) | |
| Dementia | |||
| Yes | 131 (16.7) | ||
| No | 589 (74.9) | ||
| Missing | 66 (8.4) | ||
| Year of diagnosis | |||
| Before 2000 | 360 (45.8) | ||
| 2000 and after | 426 (54.2) |
Means were compared with t-tests and proportions were compared with chi-square tests.
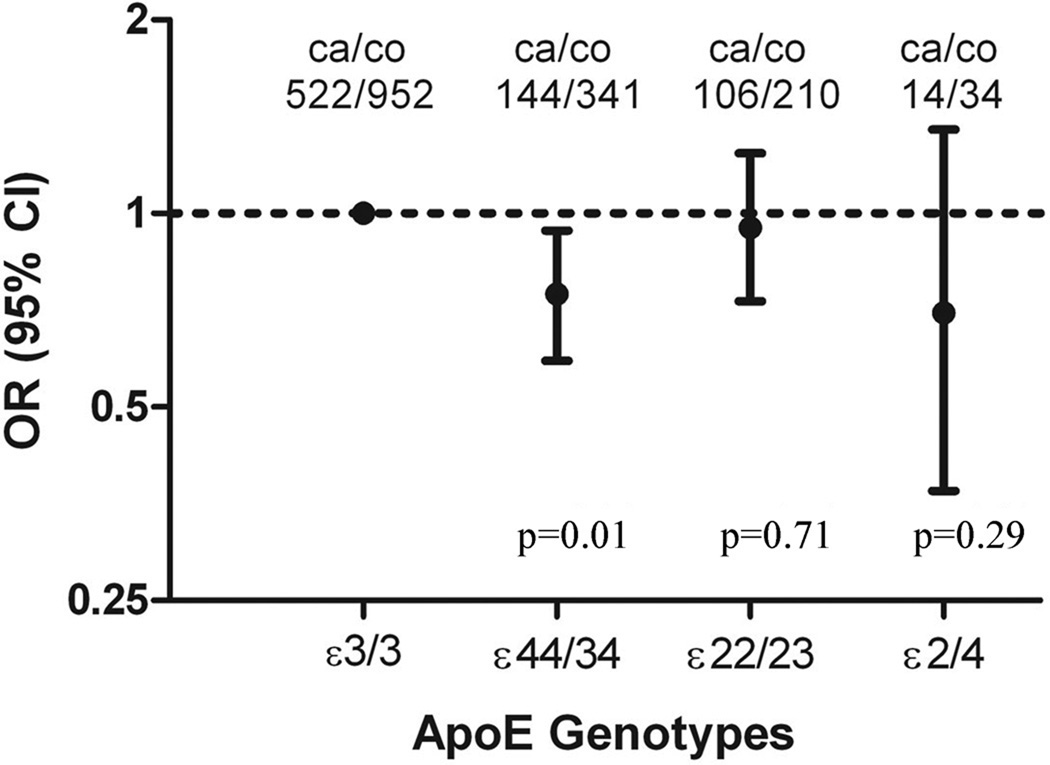
Compared with ApoE ε33, ApoE ε4-carriers (ε34 and ε44) had a significantly lower odds for PD (Figure) [OR = 0.75, 95% CI (0.59–0.94), p=0.01]. This association remained statistically significant even after Bonferroni correction (p<0.017). In contrast, PD occurrence among ε2-carriers (ε23 and ε22) was not significantly different from that of ε33 carriers [OR = 0.95, 95% CI (0.73–1.24), p=0.71]. We analyzed ε24-carriers as a separate group because ε2 and ε4 may have opposite effect on PD. Although the number was small, ε24-carriers also tended to have a lower PD risk compared with ε33-carriers [OR = 0.70, 95% CI: (0.37–1.35), p = 0.29]. Stratified analyses generally showed similar results (Table 2). In a post-hoc analysis, we further compared ε34/ε44/ε24 with ε33 and the OR was 0.75 [95% CI: (0.60–0.93), p=0.009].
Figure 1.
Odds ratios (OR) and 95% confidence intervals (CI) of Parkinson disease by ApoE genotypes, adjusted for year of birth, gender, smoking status, daily caffeine intake and family history of PD. P values were not adjusted for multiple comparison. With Bonferroni correction, P<0.017 should be considered statistically significant.
Table 2.
Subgroup analysis of ApoE genotypes and Parkinson disease
| Case /control |
ε4/4+ε3/4 vs ε3/3 |
p | Case /control |
ε2/2+ε2/3 vs ε3/3 |
p | Case /control |
ε2/4 vs ε3/3 |
p | |
|---|---|---|---|---|---|---|---|---|---|
| Year of birth | |||||||||
| ≤1931 | 329/688 | 0.76 (0.55–1.05) | 0.10 | 324/632 | 1.06 (0.75–1.51) | 0.75 | 268/529 | 0.72 (0.28–1.85) | 0.50 |
| >1931 | 337/605 | 0.73 (0.53–1.02) | 0.06 | 304/530 | 0.83 (0.55–1.26) | 0.38 | 268/457 | 0.66 (0.26–1.69) | 0.39 |
| Gender | |||||||||
| Men | 507/1026 | 0.72 (0.55–0.94) | 0.02 | 486/924 | 0.99 (0.73–1.34) | 0.96 | 415/787 | 0.86 (0.41–1.82) | 0.70 |
| Women | 159/267 | 0.81 (0.50–1.31) | 0.39 | 142/238 | 0.78 (0.44–1.40) | 0.41 | 121/199 | 0.42 (0.10–1.71) | 0.23 |
| Smoking status | |||||||||
| Never | 310/456 | 0.70 (0.49–0.99) | 0.04 | 292/397 | 1.07 (0.70–1.62) | 0.77 | 250/342 | 0.72 (0.28–1.87) | 0.50 |
| Ever | 348/821 | 0.78 (0.57–1.07) | 0.12 | 329/752 | 0.87 (0.61–1.24) | 0.44 | 277/631 | 0.54 (0.20–1.48) | 0.23 |
| Caffeine intake | |||||||||
| Below median | 365/608 | 0.82 (0.60–1.12) | 0.22 | 347/543 | 1.11 (0.77–1.60) | 0.57 | 291/462 | 0.80 (0.33–1.93) | 0.62 |
| Above median | 301/685 | 0.67 (0.48–0.95) | 0.02 | 281/619 | 0.80 (0.53–1.19) | 0.27 | 245/524 | 0.60 (0.22–1.69) | 0.33 |
| Family History | |||||||||
| Yes | 93/70 | 0.69 (0.32–1.48) | 0.34 | 86/62 | 0.94 (0.38 2.30) | 0.89 | 74/50 | 1.77 (0.16–19.1) | 0.64 |
| No | 566/1113 | 0.75 (0.59–0.96) | 0.02 | 537/997 | 0.98 (0.74–1.30) | 0.89 | 457/854 | 0.63 (0.31–1.29) | 0.21 |
Adjusted for Year of birth, gender, smoking status, daily caffeine intake and family history of PD; P values were not adjusted for multiple comparison.
As expected, more cases diagnosed before 2000 had dementia (21.1%) as compared with cases diagnosed 2000 and after (12.9%). ApoE ε4-carriers tended to have lower odds of having PD for both case groups: the ORs comparing ε4-carriers with ε33 were 0.83 [95% CI (0.61–1.12), p = 0.25] for cases diagnosed before 2000 and 0.69 [95% CI (0.51–0.92), p = 0.01] for cases diagnosed 2000 and after.
Among PD patients, ApoE ε4-carriers tended to have a higher prevalence of dementia as compared with ε33-carriers (OR = 1.59, 95% CI: 0.98–2.58, p = 0.06), whereas ε2-carrier tended to have a lower prevalence (OR = 0.75, 95% CI: 0.40–1.42, p = 0.38). We observed no difference in age of PD onset or diagnosis across ApoE genotypes, the average ages at PD diagnosis were 66.3 ± 7.4 years for of ε4-carriers, 67.1 ± 7.4 years for ε2-carriers, and 66.7 ± 7.3 years for ε33 carriers.
The meta-analyses confirmed our primary findings that ApoE ε 4 carriers had lower risk than ε33 carriers (OR=0.90, 95% CI: 0.81 – 0.99, p = 0.024, Supplemental Figure 1) while ε 2 carriers was not associated with PD risk (OR=1.10; 95% CI: 0.97 – 1.23, p = 0.13, Supplemental Figure 2).
3. Discussion
In this large population-based case-control study among non-Hispanic Caucasians, ApoE ε4 was associated with a lower odds of having PD, although ε4-carriers, as expected, tended to have a higher prevalence of dementia among PD cases. ApoE ε2, on the other hand, was not associated with PD occurrence. Neither ε4 nor ε2 seems to affect age of PD onset in our study population. Our primary findings were consistent with the supplemental meta-analyses.
The exact physiological and pathological roles of ApoE in the central nervous systems are not entirely clear, but ApoE protein is produced in abundance in the brain by glia, macrophages, and neurons (Elliott, et al., 2007, Pitas, et al., 1987). It is well-known that ApoE ε4 is a major genetic risk factor for late onset sporadic AD (Farrer, et al., 1997), and has also been associated with poor clinical outcome in patients with acute head trauma and stroke (Nicoll, et al., 1996, Slooter, et al., 1997). On the other hand, the ApoE ε2 allele was associated with lower risk for AD (Farrer, et al., 1997) and is over-represented among centenarians (Rea, et al., 2001, Schachter, et al., 1994).
The hypothesis that ApoE ε4 also increases PD risk has been investigated in over thirty epidemiological studies (www.PDgene.org) with inconsistent results. Most studies reported a null association, although few reported either a significantly lower (1997, Blazquez, et al., 2006) or a significantly higher risk for PD among ε4 carriers (Ghebremedhin, et al., 2006, Papapetropoulos, et al., 2007). Most of these studies were included in two recently published meta-analyses: first by Huang et al. (Huang, et al., 2004), and then updated by Williams-Gray et al. (Williams-Gray, et al., 2009) In both reports, ApoE ε4 was not associated with a higher risk of PD. In contrast, ApoE ε2, the allele that protects against AD, was associated with slightly higher odds of PD. While these meta-analyses are informative, conclusions must be drawn cautiously. First, previous studies were often hospital-based with heterogeneous study populations, had small sample sizes, and did not consider potential confounders. In addition, publication bias could not be easily excluded for publications on ApoE ε4 given its well-known adverse effect on AD and cardiovascular diseases (Stampfer, 2006). Nevertheless, results from previous studies appear to exclude a detrimental role of ApoE ε4 in PD.
One potential link between ApoE and PD may come from the role of ApoE in lipoprotein metabolism (Menzel, et al., 1983). The ApoE ε4 allele was associated with higher plasma cholesterol (Weisgraber and Mahley, 1996), and ApoE ε2 allele was associated with lower plasma cholesterol. Several recent prospective studies suggested that higher plasma cholesterol was associated with a lower occurrence of PD (de Lau, et al., 2006, Huang, et al., 2008, Mascitelli, et al., 2009), although the evidence is not entirely consistent (Hu, et al., 2008). It may very well be useful for future studies to include serum cholesterol in the investigation of ApoE in PD pathogenesis.
We briefly asked for the presence of dementia as part of PD diagnostic confirmation and therefore was not ideal to assess the relationship between ApoE and dementia/AD among PD patients. We nevertheless evaluated ApoE genotypes in relation to dementia among PD cases to make sure that our data are compatible with previous knowledge. Although not associated with higher PD risk, ApoE ε4 tended to be associated with about 60% higher risk of dementia among PD patients, which was consistent with the previous two meta-analyses: OR=1.6 [95% CI (1.0–2.5)] (Huang, et al., 2006) and 1.74 [95% CI (1.36–2.23), p= 0.0001] (Williams-Gray, et al., 2009).
The current study was based on a large number of participants. We controlled for several known PD risk factors in data analysis, and conducted multiple sensitivity analyses to examine the robustness of study results. Nonetheless, our report has several limitations. First, although the diagnoses were confirmed either by each patient’s treating physician or by medical record review, a few PD cases might have been misdiagnosed due to the lack of standardized clinical examinations. Second, our study included prevalent cases with various disease durations. It is conceivable that individuals with dementia might have been less likely to participate than individuals without dementia. This, however, might have affected both cases and controls. Further, because dementia is more prevalent among elderly participants and PD patients with long disease duration, we conducted stratified analysis by year of birth (Table 2) and year of PD diagnosis. Both subgroup analyses showed similar results across subgroups and therefore we expect limited impact of this bias on our analysis. In addition, ApoE genotypes tended to be associated with dementia among PD patients in the expected direction.
In summary, in this large population-based study, the presence of ApoE ε4 was associated with a lower occurrence of PD. Although this is somewhat unexpected when viewed in isolation, it is consistent with what would be predicted based on recent reports of the association between higher cholesterol and lower risk of PD.
Supplementary Material
Acknowledgements
This study was supported by the intramural research program of the National Institute of Environmental Health Sciences (Z01-ES-101986) and the National Cancer Institute (Z01 CP010196-02), R01 NS060722 (Dr. Huang), and a grant from the Pennsylvania Commonwealth Universal Research Enhancement Program (Drs. Mailman and Huang).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors disclose no conflicts of interest. All authors contributed to this study and approved its submission. The study protocol was approved by NIHES IRB with written consent from study participants.
References
- The French Parkinson's Disease Genetics Study Group. Apolipoprotein E genotype in familial Parkinson's disease. Journal of neurology, neurosurgery, and psychiatry. 1997;63(3):394–395. [PMC free article] [PubMed] [Google Scholar]
- Blazquez L, Otaegui D, Saenz A, Paisan-Ruiz C, Emparanza JI, Ruiz-Martinez J, Moreno F, Marti-Masso JF, Lopez de Munain A. Apolipoprotein E epsilon4 allele in familial and sporadic Parkinson's disease. Neuroscience letters. 2006;406(3):235–239. doi: 10.1016/j.neulet.2006.07.037. [DOI] [PubMed] [Google Scholar]
- Chen H, Huang X, Guo X, Mailman RB, Park Y, Kamel F, Umbach DM, Xu Q, Hollenbeck A, Schatzkin A, Blair A. Smoking duration, intensity, and risk of Parkinson disease. Neurology. 2010;74(11):878–884. doi: 10.1212/WNL.0b013e3181d55f38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum cholesterol levels and the risk of Parkinson's disease. American journal of epidemiology. 2006;164(10):998–1002. doi: 10.1093/aje/kwj283. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Elliott DA, Kim WS, Jans DA, Garner B. Apoptosis induces neuronal apolipoprotein-E synthesis and localization in apoptotic bodies. Neuro sci Lett. 2007;416(2):206–210. doi: 10.1016/j.neulet.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. Jama. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- Ghebremedhin E, Del Tredici K, Vuksic M, Rub U, Thal DR, Burbach GJ, Rosenberger A, Bickeboller H, Deller T, de Vos RA, Jansen Steur EN, Braak H. Relationship of apolipoprotein E and age at onset to Parkinson disease neuropathology. Journal of neuropathology and experimental neurology. 2006;65(2):116–123. doi: 10.1097/01.jnen.0000199572.96472.1c. [DOI] [PubMed] [Google Scholar]
- Grupe A, Abraham R, Li Y, Rowland C, Hollingworth P, Morgan A, Jehu L, Segurado R, Stone D, Schadt E, Karnoub M, Nowotny P, Tacey K, Catanese J, Sninsky J, Brayne C, Rubinsztein D, Gill M, Lawlor B, Lovestone S, Holmans P, O'Donovan M, Morris JC, Thal L, Goate A, Owen MJ, Williams J. Evidence for novel susceptibility genes for late-onset Alzheimer's disease from a genome-wide association study of putative functional variants. Human molecular genetics. 2007;16(8):865–873. doi: 10.1093/hmg/ddm031. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in medicine. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Hu G, Antikainen R, Jousilahti P, Kivipelto M, Tuomilehto J. Total cholesterol and the risk of Parkinson disease. Neurology. 2008;70(21):1972–1979. doi: 10.1212/01.wnl.0000312511.62699.a8. [DOI] [PubMed] [Google Scholar]
- Huang X, Abbott RD, Petrovitch H, Mailman RB, Ross GW. Low LDL cholesterol and increased risk of Parkinson's disease: prospective results from Honolulu-Asia Aging Study. Mov Disord. 2008;23(7):1013–1018. doi: 10.1002/mds.22013. [DOI] [PubMed] [Google Scholar]
- Huang X, Chen P, Kaufer DI, Troster AI, Poole C. Apolipoprotein E and dementia in Parkinson disease: a meta-analysis. Archives of neurology. 2006;63(2):189–193. doi: 10.1001/archneur.63.2.189. [DOI] [PubMed] [Google Scholar]
- Huang X, Chen PC, Poole C. APOE-[epsilon]2 allele associated with higher prevalence of sporadic Parkinson disease. Neurology. 2004;62(12):2198–2202. doi: 10.1212/01.wnl.0000130159.28215.6a. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Lees AJ. The clinical features of Parkinson's disease in 100 histologically proven cases. Advances in neurology. 1993;60:595–599. [PubMed] [Google Scholar]
- Mascitelli L, Pezzetta F, Goldstein MR. Total cholesterol and the risk of Parkinson disease. Neurology. 2009;72(9):860. doi: 10.1212/01.wnl.0000339395.41343.b3. author reply 860-861. [DOI] [PubMed] [Google Scholar]
- Menzel HJ, Kladetzky RG, Assmann G. Apolipoprotein E polymorphism and coronary artery disease. Arteriosclerosis. 1983;3(4):310–315. doi: 10.1161/01.atv.3.4.310. [DOI] [PubMed] [Google Scholar]
- Nicoll JA, Roberts GW, Graham DI. Amyloid beta-protein, APOE genotype and head injury. Ann NY Acad Sci. 1996;777:271–275. doi: 10.1111/j.1749-6632.1996.tb34431.x. [DOI] [PubMed] [Google Scholar]
- Papapetropoulos S, Farrer MJ, Stone JT, Milkovic NM, Ross OA, Calvo L, McQuorquodale D, Mash DC. Phenotypic associations of tau and ApoE in Parkinson's disease. Neuroscience letters. 2007;414(2):141–144. doi: 10.1016/j.neulet.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Pitas RE, Boyles JK, Lee SH, Foss D, Mahley RW. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim Biophys Acta. 1987;917(1):148–161. doi: 10.1016/0005-2760(87)90295-5. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. American journal of human genetics. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea IM, Mc DI, McMaster D, Smye M, Stout R, Evans A. Apolipoprotein E alleles in nonagenarian subjects in the Belfast Elderly Longitudinal Free-living Ageing Study (BELFAST) Mech Ageing Dev. 2001;122(13):1367–1372. doi: 10.1016/s0047-6374(01)00278-0. [DOI] [PubMed] [Google Scholar]
- Schachter F, Faure-Delanef L, Guenot F, Rouger H, Froguel P, Lesueur-Ginot L, Cohen D. Genetic associations with human longevity at the APOE and ACE loci. Nat Genet. 1994;6(1):29–32. doi: 10.1038/ng0194-29. [DOI] [PubMed] [Google Scholar]
- Schatzkin A, Subar AF, Thompson FE, Harlan LC, Tangrea J, Hollenbeck AR, Hurwitz PE, Coyle L, Schussler N, Michaud DS, Freedman LS, Brown CC, Midthune D, Kipnis V. Design and serendipity in establishing a large cohort with wide dietary intake distributions : the National Institutes of Health-American Association of Retired Persons Diet and Health Study. American journal of epidemiology. 2001;154(12):1119–1125. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- Simon KC, Chen H, Schwarzschild M, Ascherio A. Hypertension, hypercholesterolemia, diabetes, and risk of Parkinson disease. Neurology. 2007;69(17):1688–1695. doi: 10.1212/01.wnl.0000271883.45010.8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slooter AJ, Tang MX, van Duijn CM, Stern Y, Ott A, Bell K, Breteler MM, Van Broeckhoven C, Tatemichi TK, Tycko B, Hofman A, Mayeux R. Apolipoprotein E epsilon4 and the risk of dementia with stroke. A population-based investigation. JAMA. 1997;277(10):818–821. doi: 10.1001/jama.277.10.818. [DOI] [PubMed] [Google Scholar]
- Stampfer MJ. Cardiovascular disease and Alzheimer's disease: common links. Journal of internal medicine. 2006;260(3):211–223. doi: 10.1111/j.1365-2796.2006.01687.x. [DOI] [PubMed] [Google Scholar]
- Weisgraber KH, Mahley RW. Human apolipoprotein E: the Alzheimer's disease connection. Faseb J. 1996;10(13):1485–1494. doi: 10.1096/fasebj.10.13.8940294. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Goris A, Saiki M, Foltynie T, Compston DA, Sawcer SJ, Barker RA. Apolipoprotein E genotype as a risk factor for susceptibility to and dementia in Parkinson's disease. Journal of neurology. 2009;256(3):493–498. doi: 10.1007/s00415-009-0119-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.