Abstract
Tinnitus is a phantom auditory sensation experienced by up to 14% of the United States population with a smaller percentage experiencing decreased quality of life. A compelling hypothesis is that tinnitus results from a maladaptive plastic net down-regulation of inhibitory amino acid neurotransmission in the central auditory pathway. This loss of inhibition may be a compensatory response to loss of afferent input such as that caused by acoustic insult and/or age-related hearing loss, the most common causes of tinnitus in people. Compensatory plastic changes may result in pathologic neural activity that underpin tinnitus. The neural correlates include increased spontaneous spiking, increased bursting and decreased variance of inter-spike intervals. This review will examine evidence for chronic plastic neuropathic changes in the central auditory system of animals with psychophysically-defined tinnitus. Neurochemical studies will focus on plastic tinnitus-related changes of inhibitory glycinergic neurotransmission in the adult dorsal cochlear nucleus (DCN). Electrophysiological studies will focus on functional changes in the DCN and inferior colliculus (IC). Tinnitus was associated with increased spontaneous activity and altered response properties of fusiform cells, the major output neurons of DCN. Coincident with these physiologic alterations were changes in glycine receptor (GlyR) subunit composition, its anchoring/trafficking protein, gephyrin and the number and affinity of membrane GlyRs revealed by receptor binding. In the IC, the primary afferent target of DCN fusiform cells, multi-dimensional alterations in unit-spontaneous activity (rate, burst rate, bursting pattern) were found in animals with behavioral evidence of chronic tinnitus more than 9 months following the acoustic/cochlear insult. In contrast, immediately following an intense sound exposure, acute alterations in IC spontaneous activity resembled chronic tinnitus-related changes but were not identical. This suggests that long-term neuroplastic changes responsible for chronic tinnitus are likely to be responsible for its persistance. A clear understanding of tinnitus-related plasticity in the central auditory system and its associated neurochemistry may help define unique targets for therapeutic drug development.
1. Introduction
Tinnitus is defined as a phantom auditory sensation in the absence of any external acoustic stimulus. Chronic tinnitus, defined by symptoms persisting for more than 6 months, affects up to 14% of the United States population, while 2.7 million are severely debilitated by this distressing condition (Seidmann and Jacobson, 1996; Eggermont and Roberts, 2004; Ahmad and Seidman, 2004; Roberts et al., 2010). Chronic tinnitus can affect an individual’s ability to concentrate, negatively impact sleep patterns and reduce the desire to participate in social activities, leading to depression and anxiety (Zöger et al., 2001; Marciano et al., 2003; Dobie, 2003). Most cases of tinnitus are associated with hearing loss induced by single or cumulative loud sound exposures, drug ototoxicity and may synergize with the aging process.
Although causes of tinnitus are well documented, its underlying pathophysiology is not as clearly understood. However, a growing body of evidence suggests that tinnitus emerges from partial peripheral deafferentation that produces a maladaptive down-regulation of inhibitory amino acid neurotransmitter function at multiple levels of the central auditory neuraxis. Particularly important may be initial changes seen in the dorsal cochlear nucleus (DCN). This review will consider different behavioral models of tinnitus and how they relate to electrophysiological and neurochemical changes. DCN fusiform cell spontaneous and driven activity will be described based on studies in animals with behavioral evidence of tinnitus. Parallel findings indicating that tinnitus- and age-related changes to the auditory periphery alter glycinergic inhibitory markers in DCN will be reviewed. Finally, the effects of acute sound exposure will be compared to the effects of chronic sound exposure-related changes in the inferior colliculus of the same animal model.
2. Behavioral evidence of chronic tinnitus
At least initially, most people experience tinnitus as an acute event: an apparent ringing in the ears that appears suddenly and disappears completely in a few seconds to a few minutes. The phenomenological features of acute tinnitus often closely resemble those of chronic tinnitus, except that the chronic condition persists for an indeterminate period, often the remainder of a person’s life. Despite an overlapping phenomenology, there is evidence that the underlying pathology of acute and chronic tinnitus is probably quite different. Chronic tinnitus may emerge from unique slow plastic changes in neural function not evident in acute tinnitus. Some of the most compelling evidence comes from animal models.
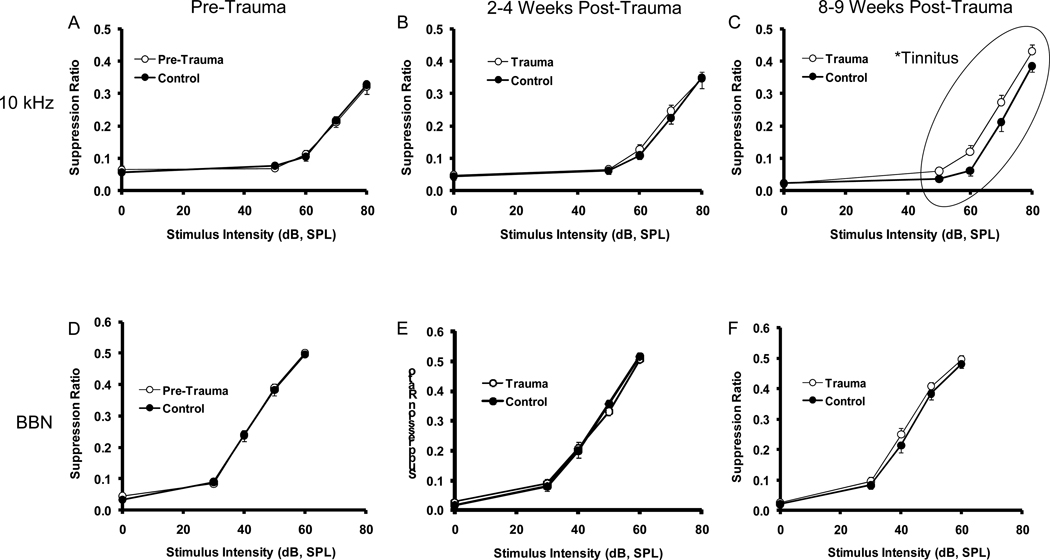
While acute changes in neural activity following sound-exposure have been shown to produce tinnitus in animals (Kaltenbach et al., 2000; Brozoski et al., 2002), often in the absence of severe hearing loss, tinnitus emerges slowly over time (Figure 1). In this study, rats were unilaterally exposed to 116 dB octave band noise centered at 17 kHz for 1 hr. Sound-exposed animals exhibited significant evidence of tinnitus emerging 3 months after exposure. The upper panels (A–C) show psychophysical evidence of a frequency-specific tinnitus, while the lower panels (D–F) show normal detection of a control test stimulus. These findings suggested that the sound-exposed rats may be experiencing chronic tonal tinnitus similar to 10 kHz (Turner et al., 2006).
Figure 1.
The slow onset of behavioral measurement of chronic tinnitus in a rat model. The upper panels (A–C) show psychophysical performance diagnostics for tinnitus across a 3-month period. Tinnitus is reflected as an up-shift in the discrimination functions. Lower panels (D–F) show performance of the same subjects to a control frequency tested in the same time frame. The tinnitus emerged in a frequency-specific 10 kHz after 3 months.
3. Neural hyperactivity and tinnitus
The DCN has been implicated as a primary initial tinnitus generator with sound-induced hyperactivity generally not observed in the auditory nerve (Liberman and Kiang, 1978) or ventral cochlear nucleus (VCN) (Salvi et al., 1978; Quirk et al., 1996; Kaltenbach et al., 1998). Numerous studies (see Table 1) have found that moderate to severe sound exposure can induce an increase in DCN spontaneous activity in diverse species, including rat (Zhang and Kaltenbach, 1998), hamster (Kaltenbach and Afman, 2000), guinea pig (Imig and Durham, 2005; Shore, et al., 2008) and chinchilla (Brozoski et al., 2002). Following the initial series of studies demonstrating that moderate sound exposure increased spontaneous activity in the DCN (for review see Kaltenbach, 2007), Brozoski and colleagues (2002) developed a chinchilla tinnitus model which linked increased DCN fusiform cell spontaneous activity and stimulus-evoked hyperactivity with behavioral tinnitus evidence.
Table 1.
Tinnitus-Related Auditory Neuronal Activity Changes Following Sound Exposure: DCN and IC
| Exposure | Structure | Species | Result/Measured | Reference |
|---|---|---|---|---|
| 10kHz, 125–130dB, 4hrs. | DCN | Hamster | Increase/Spontaneous activity (SA), multiunit. | Kaltenbach et al., 1998 |
| 10kHz, 125–130dB, 4hrs. | Rat | Increase/SA, multiunit. | Zhang and Kaltenbach, 1998 | |
| 4 kHz, 80 dB, 30–60 min. | Rat | Increase/fusiform cell SA, altered 1.0kHz rate-intensity functions | Brozoski et al., 2002 | |
| 10 kHz tone o at 125–130 dB SPL, 4hrs | Rat | Increase bursting, decrease in regular SA | Chang et al., 2002 | |
| 250 Hz band of noise, at 10 kHz 105–120 dB SPL, 4 hrs | Cat | No changes/SA for DCN neurons | Ma and Young, 2006 | |
| Broadband noise at 120 dB, 4hrs. | Guinea pig | Increase/SA single units | Shore et al., 2008 | |
| 10kHz, 115dB, 4 hrs. | Hamster | Increase/Spontaneous discharge rates of single units, multiunit activity | Finlayson and Kaltenbach, 2009 | |
| White noise, 95 or 110dB, 30sec. | IC | Mouse | Increase/post-stimulus-time Single unit activity. | Willott and Lu, 1982 |
| 2kHz, 105dB, 5 days. | Chinchilla | Increase/IC evoked potentials. | Salvi et al., 1990 | |
| 4.0kHz & 8kHz-Continuous, 104dB, 30min. | Rat | Increase/Superthreshold click evoked response. | Szczepaniak and Moller, 1995 | |
| Pure-tone, 95–115dB, 15–25min. | Chinchilla | Increase/Superthreshold responses and single unit activity. | Wang et al., 1996 | |
| 16kHz centered noise, 103db, 1 hr. | Mouse | Increase/SA in a subpopulation of IC response types. | Ma et al., 2006 | |
| Noise, 100 dB, 10mins. | Chinchilla | Increase/number of spontaneously active neurons | Bauer et al., 2008 | |
| 10-kHz pure tone at 124 dB SPL, 1 hr | Guinea pig | Increase SA/more neurons show high spontaneous firing rate | Mulders and Robertson, 2009 | |
| 10-kHz pure tone at 124 dB SPL, 1 hr | Guinea pig | Increase average spontaneous firing rate | Dong et al., 2010a |
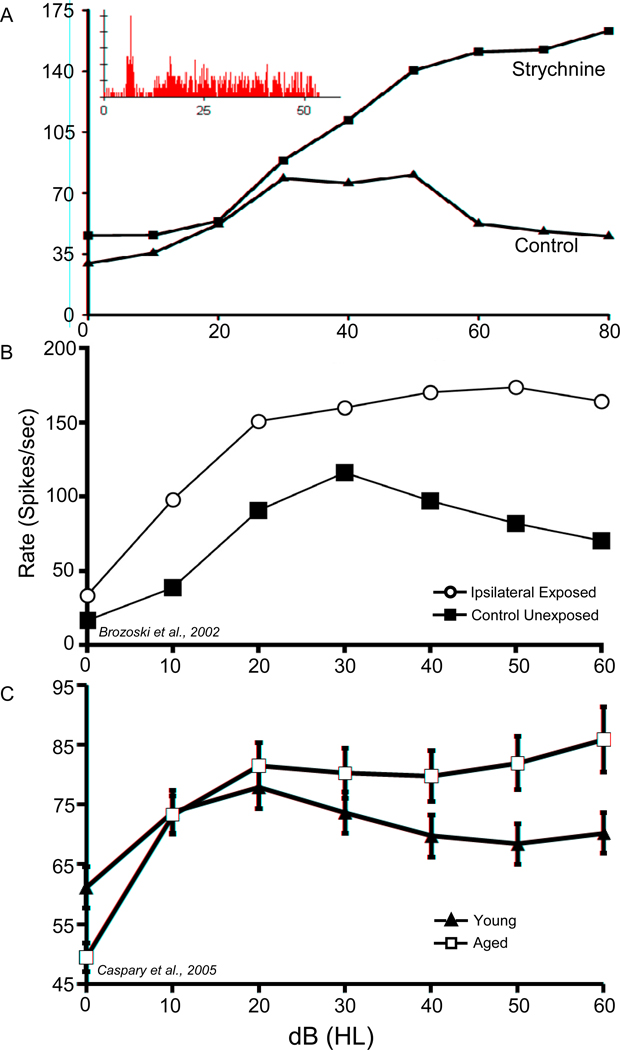
Figure 2A displays a characteristic non-monotonic rate-level function (RLF) recorded from a functionally identified DCN fusiform cell from a young control Fischer Brown Norway (FBN) rat in response to supra-threshold characteristic frequency (CF) tonal stimuli. Glycine receptor (GlyR) blockade altered this normal non-monotonic RLF, producing a more monotonic RLF. Based on this observation, abnormal monotonic RLF could reflect the loss of normal synaptic fusiform cell glycinergic inhibition seen at higher intensities likely arising from vertical or d-multipolar cells (Caspary et al., 1987; Davis and Young, 2000). Loss of normal glycinergic function in the DCN, and the attendant loss of fusiform cell non-monotonicity, suggests a mechanism for tinnitus neuropathy (Brozoski et al., 2002). Is tinnitus, at least in part, due to altered glycinergic neurotransmitter function? Consistent with the observed behavioral evidence of tinnitus, Brozoski and colleagues (2002) found that, following sound exposure, functionally identified fusiform cells showed significantly elevated spontaneous activity and greater stimulus-evoked responses to some tones and to noise. The tinnitus-related loss of inhibition, reflected by the loss of RLF non-monotonicity (Figure 2B), is similar to the age-related RLF changes subsequently obtained from a large population of functionally identified fusiform cells (Figure 2C) (Caspary et al., 2005). The age-related changes were interpreted as emerging from a loss of glycinergic inhibition that produced more monotonic RLF responses to near-CF stimuli in aged fusiform cells (Figure 2C). Finlayson and Kaltenbach (2009) recently reported similar tinnitus-related findings in the response properties of rat fusiform cells.
Figure 2.
(A) Effects of the GlyR blocker, strychnine, iontophoretically applied onto a functionally identified fusiform cell in DCN of a young-adult FBN rat. The control rate-level function (RLF) shows the prototypic nonmonotonic fusiform cell properties in response to increasing intensity CF tone-burst stimuli. The insert shows a post-stimulus time histogram displaying the classic pauser-buildup response from this fusiform cell. The strychnine trace shows the effects of GlyR blockade. The change in RLF shape suggests that the nonmonotonic/flat RLF response seen for these neurons is due to an inhibitory glycinergic input which increases with increasing intensity. (B) Representative RLFs recorded from chinchilla DCN. One recording is from a functionally identified fusiform cell from a control animal and one fusiform cell is recorded from DCN ipsilateral to an earlier tinnitus-inducing acoustic trauma. Each panel is labeled with the stimulus condition (sound-bursts, 30 dB above CF threshold, 50 msec duration, 5 msec rise–fall, 5/sec; adapted from Brozoski et al., 2002). (C) Composite/population average RLFs for young (n=91) and aged (n=88) neurons recorded from functionally identified fusiform cells (error bars indicate SEM). The greatest age-related changes occurred at the highest intensities where glycine inhibition is the greatest (adapted from Caspary et al., 2005). HL, Hearing level.
However, a previous study by Brozoski and Bauer (2005) showed bilateral dorsal DCN ablation did not significantly affect the psychophysical evidence of tinnitus in animals with established tinnitus. Those results suggested that the initial generator of tinnitus may lie centrally, perhaps in DCN, but DCN does not simply serve as a feed-forward source of peripherally-generated tinnitus in perpetuity. Ma and Young (2006) and Chang et al. (2002) in vivo and in vitro studies, respectively, did not find elevated spontaneous activity in the DCN following sound exposure. Differences in animal species, sound exposure conditions and recording timeline could explain these findings which differ from those reviewed above. In addition, both studies used DCN preparations disconnected from other central auditory structures which theoretically could be generators of the increased spontaneous activity in intact preparations. Animals in Ma and Young (2006) could still have developed tinnitus, although DCN neuronal spontaneous activity was unchanged 30 days following sound exposure in this decerebrate preparation. Understanding the exact mechanism behind these changes will require additional investigation.
Fusiform cells are the major output neurons of the DCN, projecting via the dorsal acoustic stria to the contralateral IC (Beyerl, 1978; Cant and Benson, 2003). With tinnitus-related alterations in DCN output, it would be expected that IC neurons would show altered/increased or reorganized spontaneous activity and altered response properties. For this purpose, we recorded single-neuron spontaneous activity in the IC of chinchillas with behavioral evidence of tinnitus (Bauer et al., 2008). The IC has been associated with abnormally elevated activity in both human (Melcher et al., 2000, 2009) and animal tinnitus studies (Chen and Jastreboff, 1995; Brozoski et al., 2007; Holt, et al., 2010). Immediately following an intense sound exposure, people often report tinnitus, which is considered acute tinnitus.
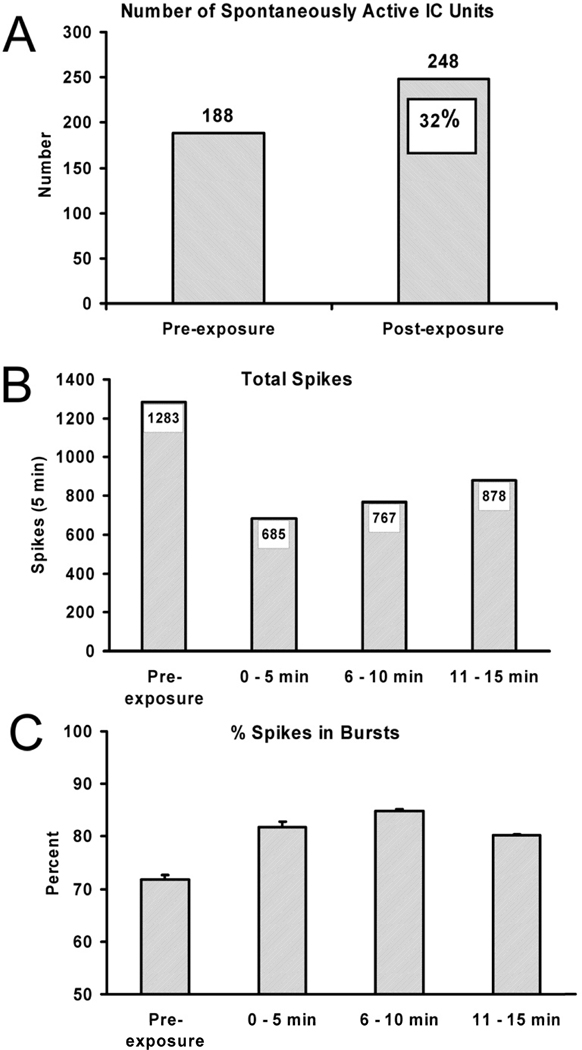
To simulate this phenomenon, we exposed chinchillas to intense noise (100 dB, SPL for 10 min) and recorded spontaneous, single-neuron activity from the IC immediately before and after exposure. In this study, normal-hearing young-adult chinchillas were anesthetized and multichannel silicon substrate electrodes were used to record activity from all lamina of the IC similar to Bauer et al. (2008). Only two significant post-exposure effects were obtained. The number of spontaneously active neurons (≥ 2 spikes in 5 min) increased by about 30 percent (Figure 3A) and the clustering of spikes into well-defined bursts (defined by the following criteria: 1. maximum allowable burst duration, 310 sec; 2. maximum ISI at burst start, 500 msec; 3. maximum within-burst ISI, 500 msec; 4. minimum interval between bursts, 1 sec; 5. minimum burst duration, 5 msec; and 6. minimum number of spikes comprising a burst, 2; (see Bauer et al., 2008)) increased (Figure 3C). In contrast, spontaneous spike rates of individually identified neurons actually decreased (Figure 3B) as did absolute number of bursts, peak discharge rates within bursts and inter-spike-interval variance (not shown). Therefore, the immediate neural consequence of loud sound exposure on spontaneous activity in the IC was threefold: 1) Neurons that were silent prior to exposure appeared to be disinhibited and became spontaneously active; 2) neurons active prior to exposure displayed a ”slope?” decrease in spontaneous activity and 3) spontaneously active neurons, after exposure, increased the clustering of spontaneous spikes into bursts (although total number of bursts decreased). This suggests that short-term mechanisms can both enlarge and reorganize the pool of spontaneously active neurons in the IC, in a species shown capable of experiencing chronic tinnitus (Brozoski et al., 2002; Bauer et al., 2008).
Figure 3.
The immediate acute effect of a 10 min unilateral exposure to 100 dB (SPL) noise on spontaneous neural activity in the inferior colliculus (IC) of normal-hearing young-adult chinchillas (n = 8). The sorted single-neuron data were obtained from the contralateral IC using multi-channel silicon-substrate electrodes dorso-ventrally spanning all lamina. Only two significant effects were obtained: The number of spontaneously active units increased by approximately 30 percent (A) and the clustering of spikes into bursts increased (C). However, after exposure, spontaneous activity, as indicated by total spiking (B), decreased as did the total number of bursts (not shown).
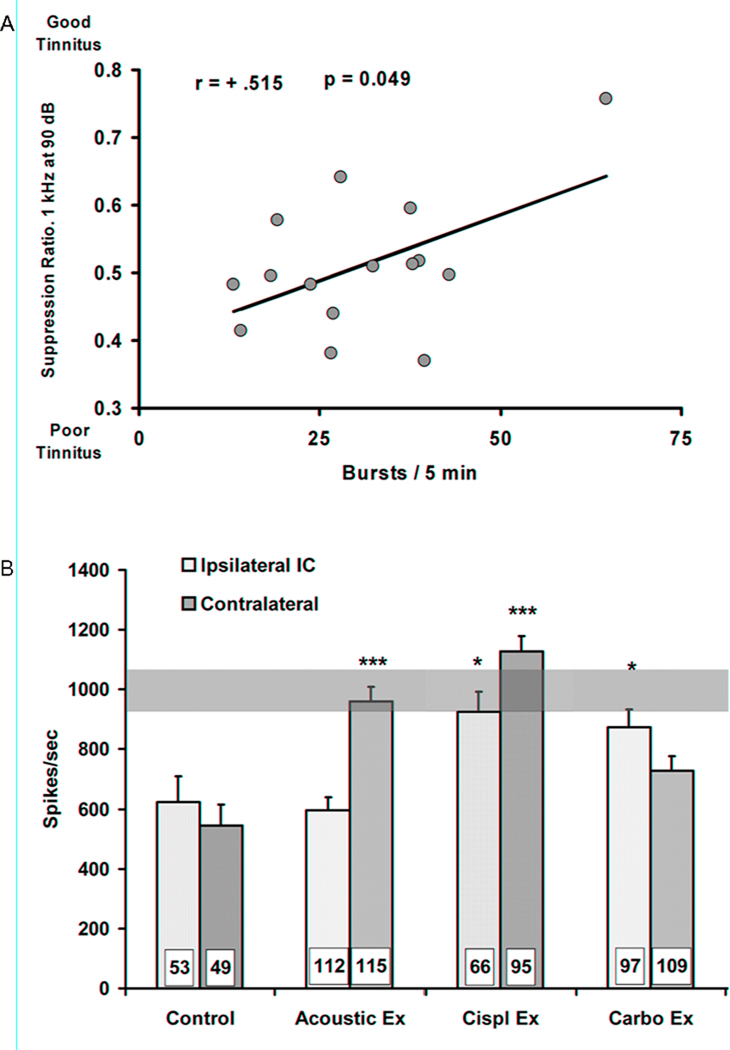
How do these acute changes contrast with neural alterations evident in the IC of animals with chronic tinnitus? Bauer et al. (2008) exposed chinchillas to three different forms of cochlear trauma and psychophysically tested for tinnitus 3 to 5 months after trauma. At the conclusion of psychophysical testing, spontaneous single-neuron activity was measured in IC. While the chronic and acute exposure stimuli were quite different: Chronic = 85 dB 4kHz for 1.0 hr.; acute = 100 dB BBN for 10min, this may be irrelevant since two chronic groups had no sound exposure at all, only ototoxic treatment. In the chronic experiment, unexposed control subjects were run parallel with the exposed tinnitus group. Increased spontaneous activity bursting of IC neurons in animals with chronic tinnitus was positively correlated with their psychophysical evidence of tinnitus (Figure 4A). The tinnitus-related spontaneous activity in IC neurons of animals showing chronic tinnitus also displayed a unique triad of bursting, increased regularity of discharge and very high peak discharge rates within bursts (Figure 4B). A similar pattern of bursting was simulated in a model neural network by Llano and Feng (2000) and was achieved by maintaining or increasing feed-forward excitation while simultaneously decreasing recurrent inhibition. If a similar mechanism underpins chronic tinnitus pathology in the IC, loss of intrinsic or extrinsic inhibition in combination with increased rostral drive from the DCN (Brozoski et al., 2002) could underpin the tinnitus signal. Since neither bursting nor spontaneous activity was enhanced in the acutely exposed animals, this suggests that plastic alterations at the level of the IC may unfold slowly to produce chronic tinnitus.
Figure 4.
The long-term chronic effect of unilateral cochlear trauma in the IC of young-adult chinchillas. Cochlear trauma was produced by either loud sound exposure, round-window application of cisplatin (destroying outer hair cells) or round-window application of carboplatin (destroying inner hair cells). All groups showed similar psychophysical evidence of tinnitus 3 to 5 months post trauma. Single neuron records of spontaneous activity in the IC were obtained 5 to 7 months post trauma, using methods similar to those used to obtain acute IC records. The electrophysiological signature common to all trauma subjects was an increased regularity of spiking (i.e., decreased inter-spike interval variance) within bursts and increased peak spike rates within bursts. IC bursting was positively correlated with psychophysical evidence of tinnitus (A), while peak spike rates within bursts were significantly higher for trauma subjects (B). Significance levels * p < 0.05, *** p < 0.001.
4. Neurochemical evidence of glycinergic/GABAergic inhibition and tinnitus
In support of the hypothesis that a partial peripheral deafferentation from diverse causes results in increased or altered neuronal activity in the DCN and IC, neurochemical studies consistently find evidence of a net down-regulation of markers of adult glycinergic inhibition in the DCN and GABAergic inhibition in the IC.
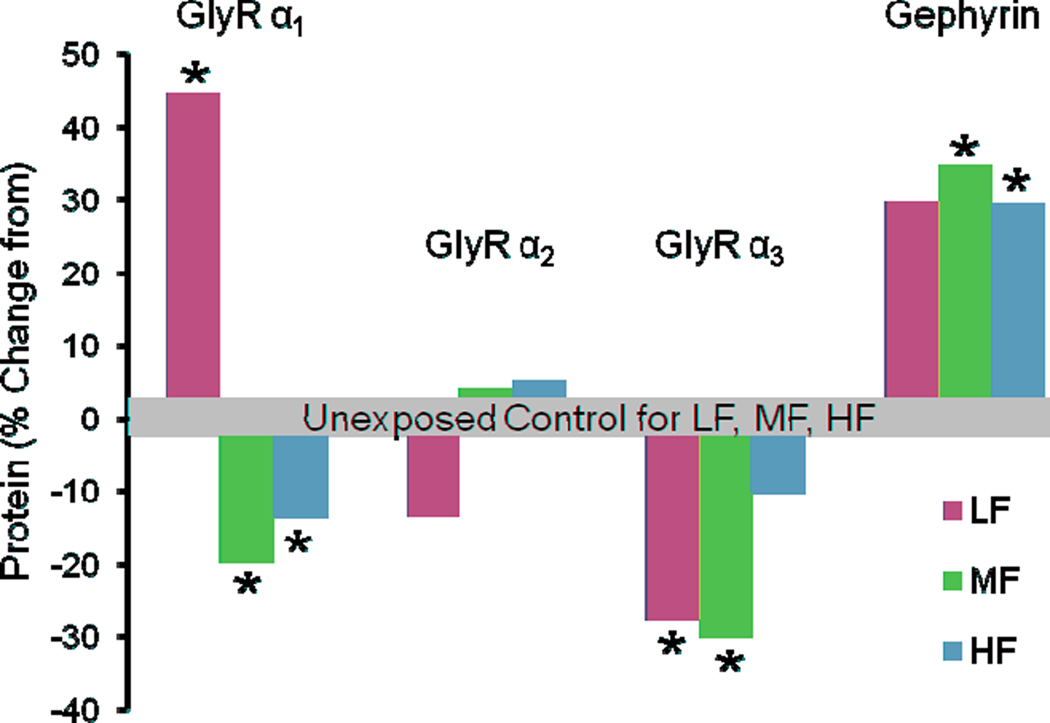
The hypothesis delineated above proposes that chronic tinnitus results, in part, from a net decline of glycinergic inhibition in DCN and/or loss of GABAergic inhibition in higher auditory structures (Brozoski et al., 2002; Dong et al., 2009, 2010a,b; Wang et al., 2009b; see also Roberts et al., 2010 for review). A series of neurochemical studies describe a persistent decline of glycinergic functional markers including glycine uptake, release and GlyR binding following peripheral auditory insults (Suneja et al., 1998a,b; Potashner et al., 2000). These studies suggest that a decrease or loss of peripheral auditory input induced by unilateral cochlear ablation or ossicular disarticulation can cause a long-term decrease of glycinergic inhibition in DCN. Naturally-occurring GlyRs are pentameric protein complexes and are composed of four possible α subunits and a β subunit (Grudzinska et al., 2005). A number of studies on aging and partial or total deafferentation find an alteration of glycine receptor function or of subunit expression in the central auditory system (Milbrandt and Caspary, 1995; Willott et al., 1997; Suneja et al., 1998a,b; Holt et al., 2005; Caspary et al., 2008). In DCN following cochlear ablation, decreases in receptor binding using tritiated strychnine, which serves as a marker for GlyR loss, and/or subunit changes to GlyRs, can last up to 3 months (Suneja et al., 1998a,b; Potashner et al., 2000). A recent study by Wang et al. (2009b) reported that protein levels of GlyR α1 and α3 subunits were significantly decreased in middle and high frequency areas of DCN eighty days following sound exposure (Figure 5). This observed decrease in certain GlyR subunit proteins was supported by a significant reduction in tritiated strychnine binding in these same frequency regions. Scatchard analysis reflected a decreased number of GlyR binding sites (Bmax) and an increase in receptor affinity as seen in the increased slope in the Scatchard plot. Altered affinity can be explained, in part, by altered subunit composition (Wang et al., 2009b). Similar decreases in GlyR binding have been seen in aged rodents who undergo slow peripheral changes with increasing age (Milbrandt and Caspary, 1995; Willott et al., 1997; Wang et al., 2009a). Collectively, these results suggest that peripheral auditory deafferentation results in long-term deficiencies in glycinergic synaptic inhibition in DCN compared with normal adults.
Figure 5.
The protein levels of glycine receptor subunits α1, α2, α3 and anchoring protein gephyrin in the DCN of sound-exposed young FBN rats. The y-axis represents subunit protein percentage difference between unexposed control and sound-exposed rat DCN ipsilateral to the exposure for low, middle and high frequency areas of DCN. Note that protein levels of glycine receptor α1 and α3 subunits significantly decreased in most areas of DCN, while protein level of gephyrin significantly increased in the whole DCN (*p < 0.05). HF, high frequency; MF, middle frequency; LF, low frequency. Modified from Wang et al. (2009b).
To support the GlyR findings delineated above, Wang et al. (2009b) examined gephyrin, a peripheral membrane protein found at the cytoplasmic face of glycinergic synapses and plays a crucial role in the postsynaptic clustering of GlyRs (Triller et al., 1985; Kirsch and Betz, 1993). A number of studies demonstrate that gephyrin is not only used for anchoring receptors to the membrane but is also involved in antero- and retrograde intracellular trafficking of GlyR complexes (Maas et al., 2006; Kneussel and Loebrich, 2007; Fritschy et al., 2008). Alterations in either GlyR subunit composition or clustering of GlyRs by a gephyrin-dependent mechanism may contribute to plasticity at inhibitory synapses. For example, external application of strychnine-induced intracellular retrograde movement of gephyrin: this suggests that blocking glycinergic inhibition may remove GlyRs from membrane sites by gephyrin-mediated transport (Maas et al., 2006). Wang et al. (2009a,b) found a significant sound-exposure and age-related increase in gephyrin message and protein levels in DCN, accompanied by decreased GlyR α1 protein levels. It is tempting to speculate that the sound-exposure and aging-induced reduction of glycinergic inhibition in the DCN is, in part, mediated by a gephyrin-related mechanism involving intracellular receptor trafficking.
The IC is considered an important putative site for the generation, maintenance and modulation of tinnitus. It receives convergent inputs from ascending and descending auditory nuclei and from numerous brainstem cell types, including a direct input from DCN fusiform cells (Winer and Schreiner, 2005). IC neurons receive numerous GABAergic inputs derived primarily from the contralateral dorsal nucleus of the lateral lemniscus and local GABAergic interneurons (Shneiderman et al., 1988; Caspary et al., 1990; Gonzalez-Hernandez et al., 1996). As suggested above, peripheral auditory deafferentation produces elevated neuronal spontaneous activity in IC, which may be attributed to the loss of GABAergic inhibition (Szczepaniak and Møller, 1995; Wang et al., 2002; Ma et al., 2006). Milbrandt et al. (2000) found that cytosolic levels of glutamic acid decarboxylase (GAD), the critical enzyme for GABA synthesis, were significantly decreased in the IC up to 30 days following sound exposure. This sound exposure-related inhibitory deficit was consistent with findings of an age-related decrease in GABA levels and altered GABAA receptor binding in the IC (Caspary et al., 1990, 1999; Milbrandt et al., 1996, 1997; see also Caspary et al., 2008 for review; Burianova et al., 2009). Recent studies by Dong and colleagues (2010a,b) reported that following loud sound exposure, IC neuronal activity increased bilaterally while mRNA and protein levels of the α1 subunit of the GABAA receptor (GABAAR α1) were down-regulated in the IC (Dong et al., 2009, 2010a,b). These studies strongly suggest that decreases in wild-type GABAAR levels contribute to the elevation of IC spiking activity. Consistent with previous unilateral cochlear ablation study, activity-related decreases in GABAergic inhibition was greater in the contralateral IC than the ipsilateral IC (Dong et al., 2009; Vale et al., 2004). Whether changes in ipsilateral IC are directly related to ipsilateral DCN changes or reflect activity changes in commissural connections between contralateral and ipsilateral IC or descending influences from higher auditory centers will require further study.
5. Summary
The present review focused on neural plasticity in two central auditory structures in response to peripheral auditory deafferentation and its potential significance for the phantom auditory sensation of tinnitus. Behavioral, electrophysiological, and neurochemical studies, point to the DCN and IC as important sites in the development of tinnitus. A plausible neural mechanism underlying tinnitus generation could be the loss of glycinergic inhibition in DCN and/or GABAergic inhibition in IC and higher centers, which results in durable neural activity changes in the auditory neuraxis. Better understanding of these mechanisms may lead to the development of more effective therapeutics with specific functionally-related inhibitory amino acid receptor targets.
Research Highlights.
Tinnitus is a phantom sensation generally associated with hearing loss.
Tinnitus may result from maladaptive plastic down-regulation of inhibition.
Acute and chronic tinnitus models show functional changes of neural activity in DCN and IC.
DCN and IC show tinnitus-related decrease of adult inhibitory neurotransmission.
Understanding tinnitus-related plasticity may help define targets for drug treatment.
Acknowledgements
We thank Judith Bryan and Lynne Ling for helpful editing. This research is supported by NIH Grant RO1DC 00151 (DMC), RO1DC008532 (DMC), RO1DC04803 (TJB) and RO1DC009669 (TJB).
Abbreviations
- ABR
auditory brainstem response
- ANOVA
analysis of variance
- CF
characteristic frequency
- CN
cochlear nucleus
- DCN
dorsal cochlear nucleus
- GlyR
glycine receptor
- VCN
ventral cochlear nucleus
- IC
inferior colliculus
- RLF
rate-level function
- GAD
glutamic acid decarboxylase
- GABAARα1
α1 subunit of the GABAA receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahmad N, Seidman M. Tinnitus in the older adult: epidemiology, pathophysiology and treatment options. Drugs Aging. 2004;21:297–305. doi: 10.2165/00002512-200421050-00002. [DOI] [PubMed] [Google Scholar]
- Bauer CA, Turner JG, Caspary DM, Myers KS, Brozoski TJ. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res. 2008;86:2564–2578. doi: 10.1002/jnr.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyerl BD. Afferent projections to the central nucleus of the inferior colliculus in the rat. Brain Res. 1978;145:209–223. doi: 10.1016/0006-8993(78)90858-2. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci. 2002;22:2383–2390. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA. The effect of dorsal cochlear nucleus ablation on tinnitus in rats. Hear Res. 2005;206(1–2):227–236. doi: 10.1016/j.heares.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Ciobanu L, Bauer CA. Central neural activity in rats with tinnitus evaluated with manganese-enhanced magnetic resonance imaging (MEMRI) Hear Res. 2007;228:168–179. doi: 10.1016/j.heares.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Burianova J, Ouda L, Profant O, Syka J. Age-related changes in GAD levels in the central auditory system of the rat. Exp Gerontol. 2009;44:161–169. doi: 10.1016/j.exger.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Cant NB, Benson CG. Parallel auditory pathways: projection patterns of the different neuronal populations in the dorsal and ventral cochlear nuclei. Brain Res Bull. 2003;60:457–474. doi: 10.1016/s0361-9230(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Holder TM, Hughes LF, Milbrandt JC, McKernan RM, Naritoku DK. Age-related changes in GABA(A) receptor subunit composition and function in rat auditory system. Neurosci. 1999;93:307–312. doi: 10.1016/s0306-4522(99)00121-9. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol. 2008;211:1781–1791. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Pazara KE, Kossl M, Faingold CL. Strychnine alters the fusiform cell output from the dorsal cochlear nucleus. Brain Res. 1987;417:273–282. doi: 10.1016/0006-8993(87)90452-5. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Raza A, Lawhorn Armour BA, Pippin J, Arneric SP. Immunocytochemical and neurochemical evidence for age-related loss of GABA in the inferior colliculus: implications for neural presbycusis. J Neurosci. 1990;10:2363–2372. doi: 10.1523/JNEUROSCI.10-07-02363.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Schatteman TA, Hughes LF. Age-related changes in the inhibitory response properties of dorsal cochlear nucleus output neurons: role of inhibitory inputs. J Neurosci. 2005;25:10952–10959. doi: 10.1523/JNEUROSCI.2451-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Chen K, Kaltenbach JA, Zhang J, Godfrey DA. Effects of acoustic trauma on dorsal cochlear nucleus neuron activity in slices. Hear Res. 2002;164(1–2):59–68. doi: 10.1016/s0378-5955(01)00410-5. [DOI] [PubMed] [Google Scholar]
- Chen GD, Jastreboff PJ. Salicylate-induced abnormal activity in the inferior colliculus of rats. Hear Res. 1995;82:158–178. doi: 10.1016/0378-5955(94)00174-o. [DOI] [PubMed] [Google Scholar]
- Davis KA, Young ED. Pharmacological evidence of inhibitory and disinhibitory neuronal circuits in dorsal cochlear nucleus. J Neurophysiol. 2000;83:926–940. doi: 10.1152/jn.2000.83.2.926. [DOI] [PubMed] [Google Scholar]
- Dobie RA. Depression and tinnitus. Otolaryngol Clin North Am. 2003;36:383–388. doi: 10.1016/s0030-6665(02)00168-8. [DOI] [PubMed] [Google Scholar]
- Dong S, Mulders WH, Rodger J, Robertson D. Changes in neuronal activity and gene expression in guinea-pig auditory brainstem after unilateral partial hearing loss. Neurosci. 2009;159:1164–1174. doi: 10.1016/j.neuroscience.2009.01.043. [DOI] [PubMed] [Google Scholar]
- Dong S, Mulders WH, Rodger J, Woo S, Robertson D. Acoustic trauma evokes hyperactivity and changes in gene expression in guinea-pig auditory brainstem. Eur J eurosci. 2010a;31:1616–1628. doi: 10.1111/j.1460-9568.2010.07183.x. [DOI] [PubMed] [Google Scholar]
- Dong S, Rodger J, Mulders WH, Robertson D. Tonotopic changes in GABA receptor expression in guinea pig inferior colliculus after partial unilateral hearing loss. Brain Res. 2010b;1342:24–32. doi: 10.1016/j.brainres.2010.04.067. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004;27:676–682. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Finlayson PG, Kaltenbach JA. Alterations in the spontaneous discharge patterns of single units in the dorsal cochlear nucleus following intense sound exposure. Hear Res. 2009;256:104–117. doi: 10.1016/j.heares.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Harvey RJ, Schwarz G. Gephyrin: where do we stand, where do we go? Trends Neurosci. 2008;31:257–264. doi: 10.1016/j.tins.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Hernandez T, Mantolan-Sarmiento B, Gonzalez-Gonzalez B, Perez-Gonzalez H. Sources of GABAergic input to the inferior colliculus of the rat. J Comp Neurol. 1996;372:309–326. doi: 10.1002/(SICI)1096-9861(19960819)372:2<309::AID-CNE11>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Grudzinska J, Schemm R, Haeger S, Nicke A, Schmalzing G, Betz H, Laube B. The beta subunit determines the ligand binding properties of synaptic glycine receptors. Neuron. 2005;45:727–739. doi: 10.1016/j.neuron.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Holt AG, Asako M, Lomax CA, MacDonald JW, Tong L, Lomax MI, Altschuler RA. Deafness-related plasticity in the inferior colliculus: gene expression profiling following removal of peripheral activity. J Neurochem. 2005;93:1069–1086. doi: 10.1111/j.1471-4159.2005.03090.x. [DOI] [PubMed] [Google Scholar]
- Holt AG, Bissig D, Mirza N, Rajah G, Berkowitz B. Evidence of key tinnitus-related brain regions documented by a unique combination of manganese-enhanced MRI and acoustic startle reflex testing. PLoS One. 2010;5(12):e14260. doi: 10.1371/journal.pone.0014260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imig TJ, Durham D. Effect of unilateral noise exposure on the tonotopic distribution of spontaneous activity in the cochlear nucleus and inferior colliculus in the cortically intact and decorticate rat. J Comp Neurol. 2005;490:391–413. doi: 10.1002/cne.20674. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA. The dorsal cochlear nucleus as a contributor to tinnitus: mechanisms underlying the induction of hyperactivity. Prog Brain Res. 2007;166:89–106. doi: 10.1016/S0079-6123(07)66009-9. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Afman CE. Hyperactivity in the dorsal cochlear nucleus after intense sound exposure and its resemblance to tone-evoked activity: a physiological model for tinnitus. Hear Res. 2000;140:165–172. doi: 10.1016/s0378-5955(99)00197-5. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Godfrey DA, Neumann JB, McCaslin DL, Afman CE, Zhang J. Changes in spontaneous neural activity in the dorsal cochlear nucleus following exposure to intense sound: relation to threshold shift. Hear Res. 1998;124:78–84. doi: 10.1016/s0378-5955(98)00119-1. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zhang J, Afman CE. Plasticity of spontaneous neural activity in the dorsal cochlear nucleus after intense sound exposure. Hear Res. 2000;147:282–292. doi: 10.1016/s0378-5955(00)00138-6. [DOI] [PubMed] [Google Scholar]
- Kirsch J, Betz H. Widespread expression of gephyrin, a putative glycine receptor-tubulin linker protein, in rat brain. Brain Res. 1993;621:301–310. doi: 10.1016/0006-8993(93)90120-c. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Loebrich S. Trafficking and synaptic anchoring of ionotropic inhibitory neurotransmitter receptors. Biol Cell. 2007;99:297–309. doi: 10.1042/BC20060120. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Kiang NY. Acoustic trauma in cats. Cochlear pathology and auditory-nerve activity. Acta Otolaryngol Suppl. 1978;358:1–63. [PubMed] [Google Scholar]
- Llano DA, Feng AS. Computational models of temporal processing in the auditory thalamus. Biological Cybernetics. 2000;83:419–433. doi: 10.1007/s004220000174. [DOI] [PubMed] [Google Scholar]
- Ma WL, Hidaka H, May BJ. Spontaneous activity in the inferior colliculus of CBA/J mice after manipulations that induce tinnitus. Hear Res. 2006;212:9–21. doi: 10.1016/j.heares.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Ma WL, Young ED. Dorsal cochlear nucleus response properties following acoustic trauma: response maps and spontaneous activity. Hear Res. 2006;216–217:176–188. doi: 10.1016/j.heares.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas C, Tagnaouti N, Loebrich S, Behrend B, Lappe-Siefke C, Kneussel M. Neuronal cotransport of glycine receptor and the scaffold protein gephyrin. J Cell Biol. 2006;172:441–451. doi: 10.1083/jcb.200506066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciano E, Carrabba L, Giannini P. Psychiatric comorbidity in a population of outpatients affected by tinnitus. Int J Audiol. 2003;42:4–9. doi: 10.3109/14992020309056079. [DOI] [PubMed] [Google Scholar]
- Melcher JR, Sigalovsky IS, Guinan JJ, Jr, Levine RA. Lateralized tinnitus studied with functional magnetic resonance imaging: abnormal inferior colliculus activation. J Neurophysiol. 2000;83:1058–1072. doi: 10.1152/jn.2000.83.2.1058. [DOI] [PubMed] [Google Scholar]
- Melcher JR, Levine RA, Bergevin C, Norris B. The auditory midbrain of people with tinnitus: abnormal sound-evoked activity revisited. Hear Res. 2009;257:63–74. doi: 10.1016/j.heares.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrandt JC, Caspary DM. Age-related reduction of [3H]strychnine binding sites in the cochlear nucleus of the Fischer 344 rat. Neurosci. 1995;67:713–719. doi: 10.1016/0306-4522(95)00082-t. [DOI] [PubMed] [Google Scholar]
- Milbrandt JC, Albin RL, Turgeon SM, Caspary DM. GABAA receptor binding in the aging rat inferior colliculus. Neurosci. 1996;73:449–458. doi: 10.1016/0306-4522(96)00050-4. [DOI] [PubMed] [Google Scholar]
- Milbrandt JC, Holder TM, Wilson MC, Salvi RJ, Caspary DM. GAD levels and muscimol binding in rat inferior colliculus following acoustic trauma. Hear Res. 2000;147:251–260. doi: 10.1016/s0378-5955(00)00135-0. [DOI] [PubMed] [Google Scholar]
- Milbrandt JC, Hunter C, Caspary DM. Alterations of GABAA receptor subunit mRNA levels in the aging Fischer 344 rat inferior colliculus. J Comp Neurol. 1997;379:455–465. doi: 10.1002/(sici)1096-9861(19970317)379:3<455::aid-cne10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Mulders WH, Robertson D. Hyperactivity in the auditory midbrain after acoustic trauma, dependence on cochlear activity. Neurosci. 2009;164:733–746. doi: 10.1016/j.neuroscience.2009.08.036. [DOI] [PubMed] [Google Scholar]
- Potashner SJ, Suneja SK, Benson CG. Altered glycinergic synaptic activities in guinea pig brain stem auditory nuclei after unilateral cochlear ablation. Hear Res. 2000;147:125–136. doi: 10.1016/s0378-5955(00)00126-x. [DOI] [PubMed] [Google Scholar]
- Quirk WS, Goldwyn BG, Meleca RJ, Kaltenbach JA. Dorsal cochlear nucleus blood flow during acoustic stimulation. Otolaryngol Head Neck Surg. 1996;114:613–619. doi: 10.1016/S0194-59989670255-3. [DOI] [PubMed] [Google Scholar]
- Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA. Ringing Ears: The Neuroscience of Tinnitus. J Neurosci. 2010;30:14972–14979. doi: 10.1523/JNEUROSCI.4028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi RJ, Hamernik RP, Henderson D. Discharge patterns in the cochlear nucleus of the chinchilla following noise induced asymptotic threshold shift. Exp Brain Res. 1978;32:301–320. doi: 10.1007/BF00238704. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Saunders SS, Gratton MA. Enhanced evoked response amplitudes in the inferior colliculus of the chinchilla following acoustic trauma. Hear Res. 1990;50:245–257. doi: 10.1016/0378-5955(90)90049-u. [DOI] [PubMed] [Google Scholar]
- Seidmann MD, Jacobson GP. Update on tinnitus. Otolaryngol Clin North Am. 1996;29:455–465. [PubMed] [Google Scholar]
- Shneiderman A, Oliver DL, Henkel CK. Connections of the dorsal nucleus of the lateral lemniscus: an inhibitory parallel pathway in the ascending auditory system. J Comp Neurol. 1988;276:188–208. doi: 10.1002/cne.902760204. [DOI] [PubMed] [Google Scholar]
- Shore SE, Koehler S, Oldakowski M, Hughes LF, Syed S. Dorsal cochlear nucleus responses to somatosensory stimulation are enhanced after noise-induced hearing loss. Eur Neurosci. 2008;27:155–168. doi: 10.1111/j.1460-9568.2007.05983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suneja SK, Potashner SJ, Benson CG. Plastic changes in glycine and GABA release and uptake in adult brain stem auditory nuclei after unilateral middle ear ossicle removal and cochlear ablation. Exp Neurol. 1998a;151:273–288. doi: 10.1006/exnr.1998.6812. [DOI] [PubMed] [Google Scholar]
- Suneja SK, Benson CG, Potashner SJ. Glycine receptors in adult guinea pig brain stem auditory nuclei: regulation after unilateral cochlear ablation. Exp Neurol. 1998b;154:473–488. doi: 10.1006/exnr.1998.6946. [DOI] [PubMed] [Google Scholar]
- Szczepaniak WS, Møller AR. Evidence of decreased GABAergic influence on temporal integration in the inferior colliculus following acute noise exposure: a study of evoked potentials in the rat. Neurosci. Lett. 1995;196:77–80. doi: 10.1016/0304-3940(95)11851-m. [DOI] [PubMed] [Google Scholar]
- Triller A, Cluzeaud F, Pfeiffer F, Betz H, Korn H. Distribution of glycine receptors at central synapses: an immunoelectron microscopy study. J Cell Biol. 1985;101:683–688. doi: 10.1083/jcb.101.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, Caspary DM. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci. 2006;120:188–195. doi: 10.1037/0735-7044.120.1.188. [DOI] [PubMed] [Google Scholar]
- Vale C, Juiz JM, Moore DR, Sanes DH. Unilateral cochlear ablation produces greater loss of inhibition in the contralateral inferior colliculus. Eur J Neurosci. 2004;20:2133–2140. doi: 10.1111/j.1460-9568.2004.03679.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM. Age-related changes in glycine receptor subunit composition and binding in dorsal cochlear nucleus. Neurosci. 2009a;160:227–239. doi: 10.1016/j.neuroscience.2009.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM. Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. Neurosci. 2009b;164:747–759. doi: 10.1016/j.neuroscience.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ding D, Salvi RJ. Functional reorganization in chinchilla inferior colliculus associated with chronic and acute cochlear damage. Hear Res. 2002;168:238–249. doi: 10.1016/s0378-5955(02)00360-x. [DOI] [PubMed] [Google Scholar]
- Willott JF, Milbrandt JC, Bross LS, Caspary DM. Glycine immunoreactivity and receptor binding in the cochlear nucleus of C57BL/6J and CBA/CaJ mice: effects of cochlear impairment and aging. J Comp Neurol. 1997;385:405–414. [PubMed] [Google Scholar]
- Winer JA, Schreiner CE. The Inferior Colliculus. New York, N.Y.: Springer; 2005. [Google Scholar]
- Zhang JS, Kaltenbach JA. Increases in spontaneous activity in the dorsal cochlear nucleus of the rat following exposure to high-intensity sound. Neurosci Lett. 1998;250:197–200. doi: 10.1016/s0304-3940(98)00482-0. Erratum in: Neurosci Lett.1998, 252, 668. [DOI] [PubMed] [Google Scholar]
- Zöger S, Svedlund J, Holgers KM. Psychiatric disorders in tinnitus patients without severe hearing impairment: 24 months follow-up of patients at an audiological clinic. Audiology. 2001;40:133–140. doi: 10.3109/00206090109073108. [DOI] [PubMed] [Google Scholar]