Abstract
The binuclear manganese metalloenzyme human arginase I (HAI) is a potential protein drug for cancer chemotherapy, in that it is capable of depleting extracellular l-Arg levels in the microenvironment of tumor cells that require this nutrient to thrive. Substitution of the native Mn2+2 cluster with a Co2+2 cluster in the active site yields an enzyme with enhanced catalytic activity at physiological pH (~7.4) that could serve as an improved protein drug for l-Arg depletion therapy. A different catalytic mechanism is proposed for Co2+2-HAI compared with that of Mn2+2-HAI, including an unusual Nε---Co2+ coordination mode, to rationalize the lower KM value of l-Arg and the lower Ki value of l-Orn. However, we now report that no unusual metal coordination modes are observed in the cobalt-reconstituted enzyme: the X-ray crystal structures of unliganded Co2+2-HAI determined at 2.10 Å resolution (pH 7.0) and 1.97 Å resolution (pH 8.5), as well as the structures of Co2+2-HAI complexed with the reactive substrate analogue 2(S)-amino-6-boronohexanoic acid (ABH, pH 7.0) and the catalytic product l-Orn (pH 7.0) determined at 1.85 Å resolution and 1.50 Å resolution, respectively, are essentially identical to the corresponding structures of Mn2+2-HAI. Therefore, in the absence of significant structural differences between Co2+2-HAI and Mn2+2-HAI, we suggest that a higher concentration of metal-bridging hydroxide ion at physiological pH for Co2+2-HAI – a consequence of the lower pKa of a Co2+-bound water molecule compared with a Mn2+-bound water molecule – strengthens electrostatic interactions with cationic amino acids and accounts for enhanced affinity as reflected in the lower KM value of l-Arg and the lower Ki value of l-Orn.
Arginase is a binuclear manganese metalloenzyme that catalyzes the hydrolysis of l-arginine (l-Arg)1 to form l-ornithine (l-Orn) and urea (1-5). This metalloenzyme is becoming increasingly prominent in the exploration and development of new approaches to cancer chemotherapy. For example, arginase activity is upregulated in certain human colon cancer and breast cancer cell lines, resulting in decreased l-Arg levels and increased l-Orn levels; since l-Orn is a biosynthetic precursor of polyamines that facilitate tumor cell growth and proliferation, arginase inhibition decreases l-Orn levels and disrupts the l-Orn supply for polyamine biosynthesis, thereby inhibiting cell proliferation (6-9). Conversely, some cancer cells are auxotrophic for l-Arg and depend on extracellular l-Arg to thrive; such cancer cells can be targeted with l-Arg depletion therapy (10, 11). For example, hepatocellular carcinoma cells (12) and prostate carcinoma cells (10, 13) require extracellular l-Arg to thrive, in the absence of which they undergo apoptosis (14). Accordingly, arginase is a potential protein drug for cancer chemotherapy insofar that it can efficiently deplete extracellular l-Arg levels in the tumor microenvironment. To this end, the substitution of the native Mn2+2 cluster with a Co2+2 cluster in the active site of human arginase I (HAI) yields an enzyme with enhanced catalytic activity at physiological pH (~7.4) that can potentially serve as an even more effective agent for l-Arg depletion therapy (14).
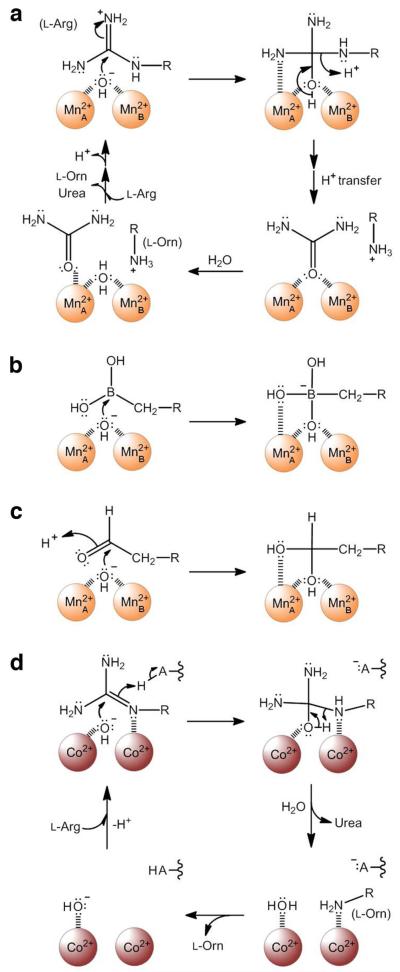
The catalytic mechanism of Mn2+2-HAI is believed to be initiated by the nucleophilic attack of a metal-bridging hydroxide ion at the guanidinium carbon of l-Arg to form a neutral tetrahedral intermediate, which subsequently collapses to form products l-Orn and urea (Figure 1a) (5, 15, 16). A key feature of this mechanistic proposal is a non-metal binding site for the guanidinium group of l-Arg in the precatalytic enzyme-substrate complex. This hypothesis is consistent with structure-activity relationships established for rat arginase I mutants in which the metal binding sites are perturbed by substitution of the metal ligands (17). These mutants exhibit nearly invariant KM values regardless of whether Mn2+A or Mn2+B binding is perturbed; a more significant effect on KM would be expected if an inner-sphere substrate-metal coordination interaction occurred in the precatalytic enzyme-substrate complex. This hypothesis is also consistent with the X-ray crystal structures of HAI as well as rat arginase I complexed with boronic acid substrate analogue inhibitors (16, 18). Specifically, the trigonal planar boronic acid moieties of substrate analogues 2(S)-amino-6-boronohexanoic acid (ABH) (19) and S-(2-boronoethyl)-l-cysteine (20) undergo nucleophilic attack to form negatively charged tetrahedral boronate anions. Each tetrahedral boronate anion coordinates to the Mn2+2 cluster in the HAI active site in much the same manner expected for the tetrahedral intermediate in catalysis (Figure 1b); a similar binding mode is also observed for 2(S)-amino-7-oxoheptanoic acid, an aldehyde amino acid analogue of l-Arg, which binds as the tetrahedral gem-diol (Figure 1c) (21). The geometry required for the binding of such boronic acid or aldehyde hydrates as analogues of the tetrahedral intermediate is not readily compatible with a substrate-metal coordination interaction in the precatalytic enzyme-substrate complex. However, coordination of the Nη2 atom of l-Arg to Mn2+A can occur during nucleophilic attack: formation of the C–O bond breaks the “Y”-shaped guanidinium π system and localizes a lone electron pair on the Nη2 atom, which can then coordinate to Mn2+A (Figure 1a) (5).
Figure 1.
(a) Proposed mechanism of Mn2+2-HAI (5). (b) The binding of a boronic acid inhibitor as the tetrahedral boronate anion mimics the tetrahedral intermediate and its flanking transition states in catalysis (16, 18, 20). (c) The binding of an aldehyde inhibitor as the tetrahedral gem-diol mimics the tetrahedral intermediate and its flanking transition states in catalysis (21). (d) Proposed mechanism of Co2+2-HAI (14).
Curiously, Co2+2-HAI is proposed to catalyze l-Arg hydrolysis through a significantly different mechanism than Mn2+2-HAI (14). Specifically, the Nε atom of l-Arg is proposed to coordinate directly to an unspecified Co2+ ion in the precatalytic enzyme-substrate complex, after which the Co2+-bound guanidinium group undergoes nucleophilic attack by a hydroxide ion bound to the adjacent Co2+ ion to form the tetrahedral intermediate; this intermediate is stabilized by coordination of the Nε and Oη atoms to the Co2+ ions and ultimately collapses to yield l-Orn and urea (Figure 1d). This proposal emanates from two observations. First, the KM of l-Arg at pH 8.5 with Co2+2-HAI is 11-fold lower than that measured with Mn2+2-HAI, which implicates the Co2+2 cluster in a stronger substrate binding interaction. Second, the inhibition constant (Ki) of l-Orn is 26-fold lower than that of l-leucine against Co2+2-HAI at pH 8.5, whereas the corresponding Ki values are relatively unchanged against Mn2+2-HAI at pH 8.5. These data are interpreted to suggest that the side chain Nε atom of l-Orn coordinates directly to a Co2+ ion (14).
To better understand structure-mechanism relationships in the cobalt-reconstituted enzyme, we now report the X-ray crystal structures of Co2+2-HAI and its complexes with a boronic acid substrate analogue as well as the catalytic product. Specifically, we describe the structures of metal-free HAI at 1.64 Å resolution, unliganded Co2+2-HAI at pH 7.0 (2.10 Å resolution) and pH 8.5 (1.97 Å resolution), Co2+2-HAI complexed with ABH at 1.85 Å resolution, and Co2+2-HAI complexed with l-Orn at 1.50 Å resolution. These structures show that Co2+ substitution does not trigger any significant structural changes in the active site of HAI. Moreover, these structures do not provide any evidence for different enzyme-substrate, enzyme-intermediate, and enzyme-product binding modes compared with corresponding structures of Mn2+2-HAI.
Experimental Procedures
Preparation of Crystalline Co2+2-HAI and Complexes
Recombinant HAI was expressed in Escherichia coli and purified as described (16), and crystallized in the unliganded state according to published procedures (22). Metal-free HAI crystals were prepared by soaking Mn2+2-HAI crystals for 7 days in 15 mM EDTA, 15 mM dipicolinic acid, 100 mM HEPES (pH 7.0), 30% (v/v) Jeffamine ED-2001. Essentially complete metal removal was confirmed by X-ray crystal structure determination, which revealed the absence of both metal ions in the active site. Crystals of metal-free HAI were reconstituted with Co2+ at pH 7.0 by soaking in a buffer solution containing 20 mM CoCl2, 100 mM HEPES (pH 7.0), 30% (v/v) Jeffamine ED-2001 for 25 hours. Metal-free HAI crystals were reconstituted with Co2+ at pH 8.5 by soaking in 20 mM CoCl2, 100 mM Bicine (pH 8.5), 32% (v/v) Jeffamine ED-2001 for 21 hours. The Co2+2-HAI-ABH complex was prepared by soaking a Co2+2-HAI crystal in 20 mM ABH, 5 mM CoCl2, 100 mM HEPES (pH 7.0), 30% (v/v) Jeffamine ED-2001 for 39 hours. The Co2+2-HAI-l-Orn complex was prepared by soaking a Co2+2-HAI crystal in 20 mM l-Orn, 5 mM CoCl2, 0.1 M HEPES (pH 7.0), 30% (v/v) Jeffamine ED-2001 for 24 hours. All crystals were flash-cooled in liquid nitrogen with their corresponding mother liquor solutions serving as cryoprotectants.
X-ray Crystal Structure Determinations
X-ray diffraction data from single crystals of metal-free HAI, Co2+2-HAI, the Co2+2-HAI-ABH complex, and the Co2+2-HAI-l-Orn complex were collected on beamline X29 (λ = 0.9795 Å) of the National Synchrotron Light Source at Brookhaven National Laboratory (Upton, NY). Diffraction data were indexed, integrated, and scaled using the HKL-2000 suite (23). These twinned arginase crystals belonged to the apparent space group P3, as reported for the Mn2+2-HAI-ABH complex (16), and their unit cell dimensions were very similar (Table 1).
Table 1. Data collection and refinement statistics.
| Co2+2-HAI | |||||
|---|---|---|---|---|---|
| metal-free HAI | pH 7.0 | pH 8.5 | Co2+2-HAI-ABH | Co2+2-HAI-l-Orn | |
| Data collection | |||||
| resolution limits (Å) | 50.0 - 1.64 | 50.0 - 2.10 | 50.0 - 1.97 | 50.0 - 1.85 | 50.0 - 1.50 |
| total/unique reflections measured |
837181/78451 | 174332/37395 | 179056/40321 | 247148/53857 | 558194/102235 |
| unit cell dimensions | |||||
| a, b, c (Å) | 90.86, 90.86, 69.88 | 90.41, 90.41, 69.74 | 87.42, 87.42, 67.25 | 90.43, 90.43, 69.43 | 90.49, 90.49, 69.69 |
| α, β, γ (deg) | 90, 90, 120 | 90, 90, 120 | 90, 90, 120 | 90, 90, 120 | 90, 90, 120 |
| R merge a,b | 0.080 (0.430) | 0.126 (0.417) | 0.097 (0.608) | 0.078 (0.586) | 0.109 (0.550) |
| I/σ(I)a | 35.18 (5.46) | 12.25 (4.18) | 14.98 (2.20) | 19.55 (2.32) | 14.63 (2.89) |
| completeness (%)a | 99.3 (95.4) | 100 (100) | 98.9 (96.3) | 99.3 (98.4) | 99.9 (100) |
| Refinement | |||||
| reflections used in refinement/test set |
70907/6432 | 34308/1833 | 36032/1913 | 46410/5045 | 94996/4852 |
| twinning fraction | 0.50 | 0.45 | 0.30 | 0.50 | 0.45 |
| R twinc c | 0.155 | 0.147 | 0.144 | 0.195 | 0.148 |
| R twin/free c | 0.197 | 0.209 | 0.193 | 0.239 | 0.178 |
| solvent moleculesd | 207 | 127 | 223 | 233 | 277 |
| ligand moleculesd | 0 | 0 | 2 | 2 | 3 |
| Co2+ ionsd | 0 | 4 | 4 | 4 | 4 |
|
Root mean square
deviations e | |||||
| bonds (Å) | 0.008 | 0.008 | 0.007 | 0.007 | 0.007 |
| angles (deg) | 1.5 | 1.5 | 1.5 | 1.5 | 1.6 |
| Average B-factors (Å2) f | |||||
| main chain | 24 | 24 | 24 | 27 | 20 |
| side chain | 26 | 25 | 26 | 28 | 21 |
| solvent | 26 | 24 | 28 | 28 | 23 |
| ligand | -- | -- | 36 | 22 | 23 |
| Co2+ ions | -- | 20 | 25 | 20 | 15 |
| Ramachandran plot (%) e | |||||
| allowed | 89.2 | 85.7 | 88.4 | 86.9 | 90.4 |
| additionally allowed | 10.2 | 13.9 | 11.2 | 12.7 | 9.2 |
| generously allowed | 0.6 | 0.0 | 0.4 | 0.2 | 0.2 |
| disallowed | 0.0 | 0.4 | 0.0 | 0.2 | 0.2 |
| PDB accession code | 3TF3 | 3TH7 | 3THE | 3THH | 3THJ |
Values in parenthesis are for the highest resolution shell.
Rmerge = Σ |I - 〈I〉|/ Σ I, where I is the observed intensity and 〈I〉 is the average intensity calculated from replicate data.
Rtwin = Σ |[|Fcalc/A|2 + |Fcalc/B| 2]1/2 - |Fobs| |/Σ |Fobs| for reflections contained in the working set. |Fobs| is the observed structure factor amplitude, and |Fcalc/A| and |Fcalc/B| are the structure factor amplitudes calculated for twin domains A and B, respectively. Rtwin underestimates the residual error in the model over the two twin-related reflections by a factor of approximately 0.7. The same expression describes Rtwin/free, calculated for test set reflections excluded from refinement.
Per asymmetric unit cell
Calculated using PROCHECK (35)
Calculated using MOLEMAN (36)
Structures were solved by molecular replacement using the program Phaser (24) as implemented in CCP4 (25), with the chain A structure of the Mn2+2-HAI-ABH complex (PDB 2AEB) (16) less inhibitor, Mn2+ ions, and solvent atoms used as the search probe for rotation and translation function calculations. Each refinement was performed with CNS (version 1.2) (26) and model building was performed with Coot (version 0.6.1) (27). Hemihedral twinning operation parameters used in the refinement were -h, -k, and l while the twinning fraction depended on the dataset (Table 1).
Crystallographic refinement of each structure against twinned intensity data was performed as previously described (16). Water molecules were included in the later stages of each refinement. For the Co2+2-HAI-ABH and Co2+2-HAI-l-Orn complexes, gradient omit maps clearly showed ligands bound to the active site of each monomer in the asymmetric unit, and ligand atoms were added and refined with full occupancy. Thermal B factors for ligands were consistent with the average B factor calculated for the entire protein (Table 1). Disordered segments M1–S5 and P320–K322 at the N- and C-termini, respectively, were excluded from all final models. Ramachandran plots revealed Q65 with a disallowed conformation in certain structures. For Co2+2-HAI (pH 7.0), this included monomers A and B; in the Co2+2-HAI-ABH and Co2+2-HAI-l-Orn complexes, this included only monomer A. Generally speaking, Q65 was characterized by well-defined electron density in these structures, so its conformation was not ambiguous. Moreover, this residue adopted a similar conformation in Mn2+2-HAI (PDB 2PHA) (22), so its unfavorable conformation was not likely to be an artifact. Data collection and refinement statistics for all structure determinations are recorded in Table 1.
Results
Metal-free HAI
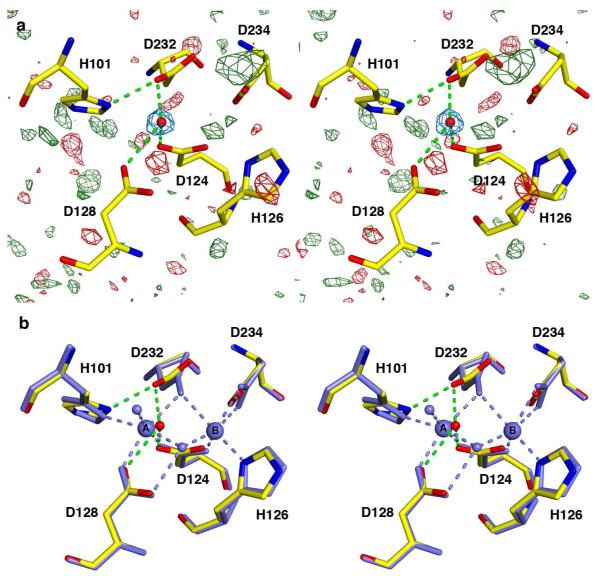
The structure of metal-free HAI is the first structure of any arginase in which both active site metal ions are absent (Figure 2a). The overall structure is comparable to that of unliganded wild-type HAI (Mn2+2-HAI, PDB 2PHA (22)) with a root-mean-square (r.m.s.) deviation of 0.24 Å for 313 Cα atoms. Metal binding residues D128, D124, H126, D232, D234 and H101 remain fully ordered despite the loss of Mn2+A and Mn2+B; however, H101 and D232 undergo slight conformational changes to form a hydrogen bond upon metal dissociation (Figure 2b). Thus, the metal binding site is nearly pre-formed with optimal geometry for metal binding, i.e., minimal conformational changes are necessary for binding the two metal ions required for catalysis.
Figure 2.
(a) Final |Fo| - |Fc| map of metal-free HAI contoured at 3.0σ (green) and −3.0σ (red). Only spurious noise peaks are observed, and none correspond to residual metal ions. A water molecule (red sphere), confirmed in a simulated annealing omit map contoured at 3.0σ (blue), is hydrogen bonded (green dashed lines) to D124, D128, and D232. Atoms are color-coded as follows: C = yellow, N = blue, O = red. (b) Superposition of chains A for metal-free HAI (pH 7.0, color-coded as in (a)) and Mn2+2-HAI (pH 7.5) (PDB 2PHA, all atoms and interactions colored light blue). Apart from small conformational changes of D232 and H101, protein residues in the apoenzyme are nearly pre-formed for ideal metal coordination interactions.
Co2+2-HAI
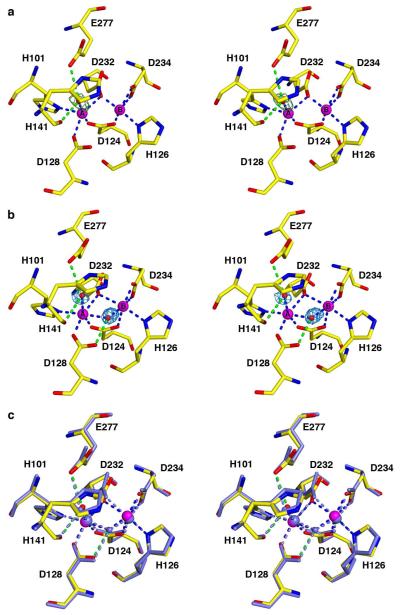
The overall fold of unliganded Co2+2-HAI at pH 7.0 and pH 8.5 is essentially identical to that of unliganded Mn2+2-HAI at pH 7.5 (PDB 2PHA (22)), with an r.m.s. deviation of 0.26 Å for 313 Cα atoms or 0.47 Å for 313 Cα atoms, respectively. Interestingly, no metal-bound solvent molecules are observed at pH 7.0, except for a single solvent molecule bound to the Co2+A ion of chain A (Figure 3a), which could imply weaker coordination or disorder. However, metal-bound solvent molecules are fully visible in the structure of Co2+2-HAI determined at pH 8.5 (Figure 3b) and are identical to those observed in the structure of unliganded Mn2+2-HAI (22) (Figure 3c). Parenthetically, we note that a bicine buffer molecule is observed to bind at the mouth of the Co2+2-HAI active site at pH 8.5 (data not shown).
Figure 3.
(a) Simulated annealing omit map (green) of the Co2+A-bound solvent molecule in chain A of Co2+2-HAI at pH 7.0, contoured at 3.5σ. Atoms are color-coded as follows: C = yellow, N = blue, O = red, Co2+ = magenta spheres, solvent = red sphere. Metal coordination and hydrogen bond interactions appear as blue and green dashed lines, respectively. (b) Simulated annealing omit map (blue) of the Co2+-bound solvent molecules in Co2+2-HAI at pH 8.5, contoured at 4.2σ. Atoms and intermolecular interactions are color-coded as in (a). (c) Superposition of chains A for Co2+2-HAI (pH 8.5) (color-coded as in (b)) and Mn2+2-HAI (pH 7.5) (PDB 2PHA, all atoms light blue).
Co2+2-HAI-ABH complex
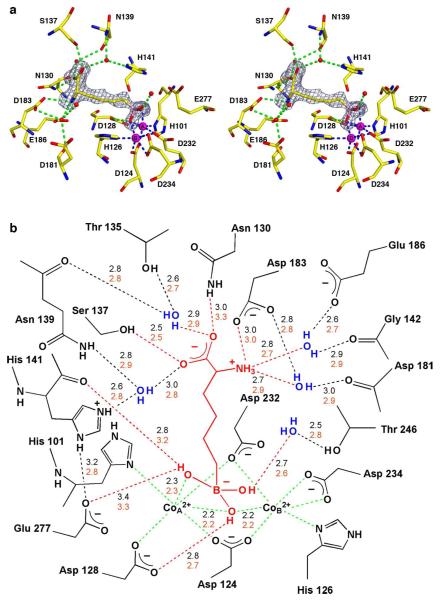
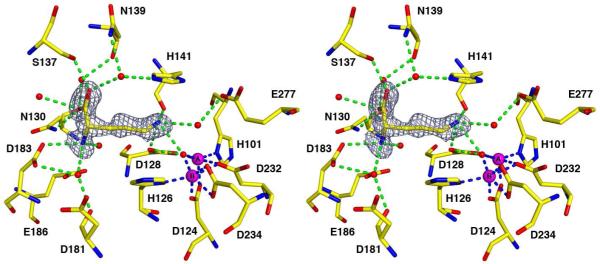
The structure of the Co2+2-HAI-ABH complex at pH 7.0 is essentially identical to the structure of the Mn2+2-HAI-ABH complex (PDB 2AEB) (16) in both the overall fold (r.m.s. deviation = 0.25 Å for 313 Cα atoms) and the intermolecular interactions of ABH in the active site. A simulated annealing omit map is shown in Figure 4a along with a superposition of the Mn2+2-HAI-ABH complex. The boronic acid side chain of ABH undergoes nucleophilic attack, probably by the metal-bridging hydroxide ion, to yield a tetrahedral boronate anion that coordinates to the Co2+A and Co2+B ions. The α-carboxylate of ABH accepts hydrogen bonds from N130, S137, and two water molecules, and the α-amino group donates hydrogen bonds to D183 and two water molecules. Water molecules hydrogen bonded with the α-carboxylate and the α-amino groups also hydrogen bond with active site protein residues as summarized in Figure 4b.
Figure 4.
(a) Simulated annealing omit map (grey) of the inhibitor ABH bound in the active site of Co2+2-HAI, contoured at 4.0σ. The boronic acid moiety of ABH binds as a tetrahedral boronate anion that mimics the tetrahedral intermediate and its flanking transition states in the reaction catalyzed by Co2+2-HAI. Atoms are color-coded as follows: C = yellow, N = blue, O = red, B = pink, Co2+ = magenta spheres, solvent = red spheres; metal coordination and hydrogen bond interactions are indicated by blue and green dashed lines, respectively. (b) Scheme illustrating average distances (Å) of intermolecular interactions in the Co2+2-HAI-ABH complex (black numbers) and Mn2+2-HAI-ABH complex (PDB 2AEB) (orange numbers).
Co2+-HAI-l-Orn complex
The structure of the Co2+2-HAI-l-Orn complex determined at pH 7.0 has an overall fold similar to that of the Mn2+2-HAI-l-Orn complex determined at pH 6.5 (28), and the r.m.s. deviation for 314 Cα atoms is 0.17 Å. A simulated annealing omit map is shown in Figure 5. The terminal amino group of l-Orn donates hydrogen bonds to D128, the backbone carbonyl of H141, and two solvent molecules, one of which is the metal-bridging hydroxide ion. The α-amino and α-carboxylate groups of l-Orn make interactions similar to those observed for ABH in the Co2+2-HAI-ABH complex (Figure 4) and the Mn2+2-HAI-ABH complex (16). Parenthetically, we note that in the Co2+2-HAI-l-Orn complex, an additional l-Orn molecule binds to the surface of monomer A in the asymmetric unit, where the backbone carbonyl groups of T134 and T135 accept hydrogen bonds from the α-amino group and the ε-amino group of l-Orn, respectively (data not shown). This second l-Orn molecule is characterized by weak electron density consistent with reduced occupancy; since Stone and colleagues do not report any nonlinearity in product inhibition (14), l-Orn binding to this second site does not appear to significantly affect catalysis.
Figure 5.
Simulated annealing omit map (grey) of the catalytic product l-Orn bound in the active site of Co2+2-HAI at pH 7.0, contoured at 3.0σ. Atoms are color-coded as follows: C = yellow, N = blue, O = red, Co2+ = magenta spheres, solvent = red spheres; metal coordination and hydrogen bond interactions are indicated by blue and green dashed lines, respectively.
Discussion
The first successful preparation of a crystalline metal-free arginase described in this work allows for the substitution of metal ions other than the native Mn2+ ions in the active site. Previous studies with rat arginase I show that only one Mn2+ ion is readily extracted from the Mn2+2 cluster (29), so the human enzyme offers a distinct advantage for the preparation of the metal-free apoenzyme. Significantly, Co2+2-HAI is said to have ideal functional properties for use in the treatment of l-Arg auxotrophic tumors, e.g., as assayed against human melanoma and hepatocellular carcinoma cell lines: a decreased pKa for metal-bound water, a decreased KM for substrate l-Arg, and a decreased Ki for product l-Orn (14).
Functional studies of Co2+2-HAI (14) are interpreted to reflect the proposed mechanism in Figure 1d, which includes the following chemical steps: (1) deprotonation of l-Arg, (2) tautomerization of the neutral guanidinium group and coordination of the Nε atom to a Co2+ ion, (3) nucleophilic attack at the guanidinium carbon by a Co2+-bound hydroxide ion, (4) formation of the tetrahedral intermediate in which the Oη and Nε atoms are coordinated to separate Co2+ ions, (5) collapse of the tetrahedral intermediate to yield product l-Orn with its Nε atom coordinated to one Co2+ ion, and a water molecule that displaces urea to coordinate to the other Co2+ ion, and (6) ionization of Co2+-bound water to regenerate the nucleophilic Co2+-bound hydroxide ion.
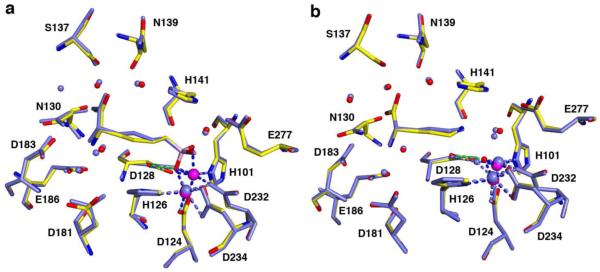
X-ray crystal structures of Co2+2-HAI allow us to evaluate certain aspects of this mechanistic proposal. First, it is intriguing to consider the possibility that the Nε atom of l-Arg or l-Orn coordinates to an active site metal ion. While it is unusual to consider the inner-sphere coordination of the guanidinium Nε or Nη atom of l-Arg to a metal ion, such interactions are occasionally observed (30). However, since the Nε atom of l-Orn does not coordinate to metal ions in the active site of Co2+2-HAI (Figure 5) or Mn2+2-HAI (28), the observed binding mode is at odds with the Nε---Co2+ coordination mode proposed for l-Orn, and by inference the Nε---Co2+ coordination mode proposed for l-Arg, in Figure 1d (14). Since l-Orn adopts an identical binding mode in the active sites of Mn2+2-HAI and Co2+2-HAI (Figure 6), the lower Ki value of l-Orn for binding to Co2+2-HAI must therefore arise from an indirect effect of the metal ions, e.g., the hydrogen bond between the l-Orn side chain and the metal-bridging solvent molecule (Figure 5). The concentration of metal-bridging hydroxide ion is greater for Co2+2 than for Mn2+2 at physiological pH (14), which in turn would strengthen its hydrogen bond and electrostatic interactions with the positively charged side chain of l-Orn.
Figure 6.
(a) Superposition of the Co2+2-HAI-ABH complex (color-coded as in Figure 4a) and the Mn2+2-HAI-ABH complex at pH 7.5 (PDB 2AEB, all atoms and metal coordination interactions light blue). Apart from a 0.5 Å shift of the side chain Cγ atom of ABH, the structures of these complexes are essentially identical. (b) Superposition of the Co2+2-HAI-l-Orn complex (color-coded as in Figure 5) and the Mn2+2-HAI-l-Orn complex (PDB 3GMZ, all atoms and interactions light blue).
The binding of ABH as the tetrahedral boronate anion likely mimics the binding of the tetrahedral intermediate and its flanking transition states in catalysis by Mn2+2-rat arginase I (18), Mn2+2-HAI (16), Co2+2-HAI (this work), and Mn2+2-arginase from Plasmodium falciparum (31). The binding conformation of ABH to these enzymes is essentially identical (as illustrated in Figure 6 for Co2+2-HAI and Mn2+2-HAI), such that the amino acid side chain is extended into the active site with all C-C bonds adopting trans or nearly trans conformations. Identical binding modes for ABH to these enzymes suggest identical binding modes for the corresponding tetrahedral intermediate and its flanking transition states, i.e., identical catalytic mechanisms.
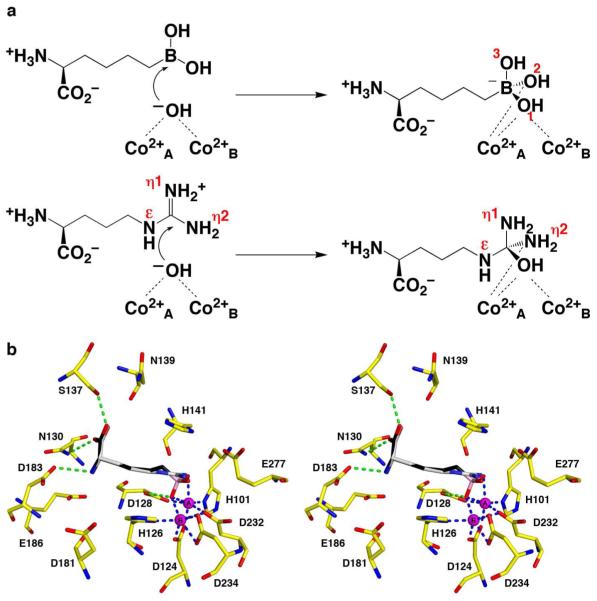
Further analysis of the binding mode of ABH to Co2+2-HAI suggests that the boronate anion hydroxyl groups O2 and O3 correspond to the two hydroxyl groups of the native boronic acid, whereas boronate anion hydroxyl group O1 corresponds to the metal-bridging hydroxide ion of the native enzyme. This binding mode is most consistent with a mechanism in which the trigonal planar boronic acid moiety of ABH (or the trigonal planar guanidinium group of the substrate) enters the active site of Co2+2-HAI and subsequently undergoes nucleophilic attack by a metal-bridging hydroxide ion to yield the tetrahedral boronate anion (or tetrahedral intermediate), with a metal-bridging hydroxyl group (i.e., the former metal-bridging hydroxide ion) and the terminal O2 hydroxyl group (or Nη2 amino group) coordinated to Co2+A (Figure 7a). This mechanistic model is identical to that outlined for Mn2+2-HAI (5) and derives from that initially proposed by Kanyo and colleagues for Mn2+2-rat arginase I (15), which satisfies the principle of least nuclear motion (Figure 7b) (32). Alternative mechanistic models, such as that initially proposed for Co2+2-HAI (14), involve additional chemical or conformational steps that would add seemingly unnecessary nuclear motions to the mechanistic sequence.
Figure 7.
(a) Attack of the nucleophilic metal-bridging hydroxide ion at the boronic acid moiety of the inhibitor ABH results in the formation of a tetrahedral boronate anion that mimics the formation of the tetrahedral intermediate in catalysis. While this chemistry is illustrated for Co2+2-HAI, it is identical to that of Mn2+2-HAI. Selected atoms discussed in the text are indicated with red labels. (b) Model of l-Arg superimposed on the experimentally determined structure of ABH bound in the active site of Co2+2-HAI, which mimics the binding of the tetrahedral intermediate and its flanking transition states in catalysis. Atoms are color-coded as follows: C = yellow (protein), black (ABH), or grey (l-Arg), N = blue, O = red, Co2+ = magenta spheres. Solvent molecules are omitted for clarity; metal coordination and hydrogen bond interactions for ABH are indicated by blue and green dashed lines, respectively. The side chain Nη1 and Nη2 atoms of l-Arg correspond to the boronate O3 and O2 atoms (atom labels are shown in (a)). Nucleophilic attack of a metal-bridging hydroxide ion (which would correspond to the position of the boronate O1 atom) at the planar guanidinium group of l-Arg satisfies the principle of least nuclear motion, and simply requires the pyramidalization of the guanidinium carbon (or the boron atom of the boronic acid) as it transitions from sp2 to sp3 hybridization. This conclusion is valid for both Co2+2-HAI and Mn2+2-HAI.
The observed binding mode of the tetrahedral boronate anion form of ABH to Co2+2-HAI is not consistent with the proposed binding mode of the tetrahedral intermediate that involves metal coordination by the Nε atom of l-Arg (Figure 1d) (14). A key structural feature that prevents a closer approach of the Nε atom of l-Arg or l-Orn to the binuclear metal cluster is the extensive array of hydrogen bond interactions that anchor the α-amino and α-carboxylate groups of amino acids bound in the arginase active site (e.g., see the scheme summarizing the binding mode of ABH in Figure 4b). This hydrogen bond array ensures the precise molecular recognition of l-amino acids in the arginase active site. Modification or deletion of these enzyme-substrate hydrogen bonds significantly compromises catalysis, either by mutagenesis in the enzyme active site (33) or by modification of substrate structure or stereochemistry (34).
In closing, while the proposed mechanism of Co2+2-HAI summarized in Figure 1d (14) is inconsistent with the X-ray crystal structures of Co2+2-HAI reported herein, the fact remains that functional studies clearly indicate a decreased KM value for substrate l-Arg and a decreased Ki value for product l-Orn (14). If functional differences between Co2+2-HAI and Mn2+2-HAI do not arise from structural differences in the binding of substrate, tetrahedral intermediate, and product, from what do they arise? Given that the metal-bridging solvent molecule exhibits a lower pKa in Co2+2-HAI, there would be a higher concentration of the negatively charged metal-bridging hydroxide ion at physiological pH in comparison with Mn2+2-HAI (14). As previously mentioned, this would enhance the hydrogen bond and electrostatic interactions with the positively charged side chain of l-Orn and thereby enhance affinity, which would account for the lower Ki value measured against Co2+2-HAI. Similarly, we suggest that the lower KM value measured for l-Arg with Co2+2-HAI results from an enhanced electrostatic interaction (but not a hydrogen bond interaction) between the positively charged guanidinium side chain of l-Arg and the negatively charged metal-bound hydroxide ion in the precatalytic Michaelis complex. Thus, in the absence of significant structural differences between Mn2+2-HAI and Co2+2-HAI complexes, we suggest that a simple electrostatic interaction dependent upon the predominant ionization state of a metal-bound water molecule – not a direct metal coordination interaction – could explain the functional differences between Co2+2-HAI and Mn2+2-HAI.
Acknowledgments
We thank the National Synchrotron Light Source at Brookhaven National Laboratory (beamline X29) for access to X-ray crystallographic data collection facilities. Additionally, we thank Drs. Kathryn Cole, Mustafa Köksal, and Patrick Lombardi for helpful discussions.
Footnotes
This work was supported by National Institutes of Health grant GM49758
The atomic coordinates and structure factors of metal-free human arginase I, Co2+2-human arginase I at pH 7.0 and pH 8.5, and Co2+2-human arginase I complexed with 2(S)-amino-6-boronohexanoic acid and l-ornithine have been deposited in the Protein Data Bank (www.rcsb.org) with accession codes 3TF3, 3TH7, 3THE, 3THH, and 3THJ, respectively.
Abbreviations: Mn2+2-HAI, native human arginase I; Co2+2-HAI, cobalt-reconstituted human arginase I; l-Arg, l-arginine; l-Orn, l-ornithine; ABH, 2(S)-amino-6-boronohexanoic acid; HEPES, N-(2-hydroxyethyl)piperazine-N’-(2-ethanesulfonic acid).
References
- 1.Christianson DW, Cox JD. Catalysis by metal-activated hydroxide in zinc and manganese metalloenzymes. Annu. Rev. Biochem. 1999;68:33–57. doi: 10.1146/annurev.biochem.68.1.33. [DOI] [PubMed] [Google Scholar]
- 2.Ash DE, Cox JD, Christianson DW. Arginase: a binuclear manganese metalloenzyme. In: Sigel A, Sigel H, editors. Manganese and Its Role in Biological Processes, Vol. 37 of Metal Ions in Biological Systems. M. Dekker; New York: 1999. pp. 407–428. [PubMed] [Google Scholar]
- 3.Morris SM., Jr. Regulation of enzymes of the urea cycle and arginine metabolism. Annu. Rev. Nutr. 2002;22:87–105. doi: 10.1146/annurev.nutr.22.110801.140547. [DOI] [PubMed] [Google Scholar]
- 4.Ash DE. Structure and function of arginases. J. Nutr. 2004;134:2760S–2764S. doi: 10.1093/jn/134.10.2760S. [DOI] [PubMed] [Google Scholar]
- 5.Christianson DW. Arginase: structure, mechanism, and physiological role in male and female sexual arousal. Acc. Chem. Res. 2005;38:191–201. doi: 10.1021/ar040183k. [DOI] [PubMed] [Google Scholar]
- 6.Buga GM, Wei LH, Bauer PM, Fukuto JM, Ignarro LJ. NG-hydroxy-l-arginine and nitric oxide inhibit Caco-2 tumor cell proliferation by distinct mechanisms. Am. J. Physiol. 1998;275:R1256–R1264. doi: 10.1152/ajpregu.1998.275.4.R1256. [DOI] [PubMed] [Google Scholar]
- 7.Singh R, Pervin S, Karimi A, Cederbaum S, Chaudhuri G. Arginase activity in human breast cancer cell lines: Nω-hydroxy-l-arginine selectivity inhibits cell proliferation and induces apoptosis in MDA-MB-468 cells. Cancer Res. 2000;60:3305–3312. [PubMed] [Google Scholar]
- 8.Ochoa AC, Zea AH, Hernandez C, Rodriguez PC. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin. Cancer Res. 2007;13:721s–726s. doi: 10.1158/1078-0432.CCR-06-2197. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109:1568–1573. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim RH, Coates JM, Bowles TL, McNerney GP, Sutcliffe J, Jung JU, Gandour-Edwards R, Chuang FYS, Bold RJ, Kung H. Arginine deiminase as a novel therapy for prostate cancer induces autophagy and caspase-independent apoptosis. Cancer Res. 2009;69:700–708. doi: 10.1158/0008-5472.CAN-08-3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delage B, Fennell DA, Nicholson L, McNeish I, Lemoine NR, Crook T, Szlosarek PW. Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. Int. J. Cancer. 2010;126:2762–2772. doi: 10.1002/ijc.25202. [DOI] [PubMed] [Google Scholar]
- 12.Cheng PNM, Leung YC, Lo WH, Tsui SM, Lam KC. Remission of hepatocellular carcinoma with arginine depletion induced by systemic release of endogenous hepatic arginase due to transhepatic arterial embolisation, augmented by high-dose insulin: arginase as a potential drug candidate for hepatocellular carcinoma. Cancer Lett. 2005;224:67–80. doi: 10.1016/j.canlet.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 13.Dillon BJ, Prieto VG, Curley SA, Ensor CM, Holtsberg FW, Bomalaski JS, Clark MA. Incidence and distribution of argininosuccinate synthetase deficiency in human cancers: a method for identifying cancers sensitive to arginine deprivation. Cancer. 2004;100:826–833. doi: 10.1002/cncr.20057. [DOI] [PubMed] [Google Scholar]
- 14.Stone EM, Glazer ES, Chantranupong L, Cherukuri P, Breece RM, Tierney DL, Curley SA, Iverson BL, Georgiou G. Replacing Mn2+ with Co2+ in human arginase I enhances cytotoxicity toward l-arginine auxotrophic cancer cell lines. ACS Chem. Biol. 2010;5:333–342. doi: 10.1021/cb900267j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanyo ZF, Scolnick LR, Ash DE, Christianson DW. Structure of a unique binuclear manganese cluster in arginase. Nature. 1996;383:554–557. doi: 10.1038/383554a0. [DOI] [PubMed] [Google Scholar]
- 16.Di Costanzo L, Sabio G, Mora A, Rodriguez PC, Ochoa AC, Centeno F, Christianson DW. Crystal structure of human arginase I at 1.29-Å resolution and exploration of inhibition in the immune response. Proc. Natl. Acad. Sci. U. S. A. 2005;102:13058–13063. doi: 10.1073/pnas.0504027102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cama E, Emig FA, Ash DE, Christianson DW. Structural and functional importance of first-shell metal ligands in the binuclear manganese cluster of arginase I. Biochemistry. 2003;42:7748–7758. doi: 10.1021/bi030074y. [DOI] [PubMed] [Google Scholar]
- 18.Cox JD, Kim NN, Traish AM, Christianson DW. Arginase-boronic acid complex highlights a physiological role in erectile function. Nat. Struct. Biol. 1999;6:1043–1047. doi: 10.1038/14929. [DOI] [PubMed] [Google Scholar]
- 19.Baggio R, Elbaum D, Kanyo ZF, Carroll PJ, Cavalli RC, Ash DE, Christianson DW. Inhibition of Mn2+2-arginase by borate leads to the design of a transition state analogue inhibitor, 2(S)-amino-6-boronohexanoic acid. J. Am. Chem. Soc. 1997;119:8107–8108. [Google Scholar]
- 20.Kim NN, Cox JD, Baggio RF, Emig FA, Mistry SK, Harper SL, Speicher DW, Morris SM, Ash DE, Traish A, Christianson DW. Probing erectile function: S-(2-boronoethyl)-l-cysteine binds to arginase as a transition state analogue and enhances smooth muscle relaxation in human penile corpus cavernosum. Biochemistry. 2001;40:2678–2688. doi: 10.1021/bi002317h. [DOI] [PubMed] [Google Scholar]
- 21.Shin H, Cama E, Christianson DW. Design of amino acid aldehydes as transition-state analogue inhibitors of arginase. J. Am. Chem. Soc. 2004;126:10278–10284. doi: 10.1021/ja047788w. [DOI] [PubMed] [Google Scholar]
- 22.Di Costanzo L, Pique ME, Christianson DW. Crystal structure of human arginase I complexed with thiosemicarbazide reveals an unusual thiocarbonyl μ-sulfide ligand in the binuclear manganese cluster. J. Am. Chem. Soc. 2007;129:6388–6389. doi: 10.1021/ja071567j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 24.McCoy AJ, Grosse-Kunstleve RW, Storoni LC, Read RJ. Likelihood-enhanced fast translation functions. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2005;61:458–464. doi: 10.1107/S0907444905001617. [DOI] [PubMed] [Google Scholar]
- 25.Collaborative Computational Project, No. 4 The CCP4 suite: programs for protein crystallography. Acta Crystallogr., Sect. D: Biol. Crystallogr. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 26.Brünger AT, Adams PD, Clore GM, Delano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges N, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL. Crystallography and NMR system (CNS): a new software system for macromolecular structure determination. Acta Crystallogr., Sect. D: Biol. Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 27.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 28.Ilies M, Di Costanzo L, Dowling DP, Thorn KJ, Christianson DW. Binding of α,α-disubstituted amino acids to arginase suggests new avenues for inhibitor design. J. Med. Chem. 2011;54:5432–5443. doi: 10.1021/jm200443b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scolnick LR, Kanyo ZF, Cavalli RC, Ash DE, Christianson DW. Altering the binuclear manganese cluster of arginase diminishes thermostability and catalytic function. Biochemistry. 1997;36:10558–10565. doi: 10.1021/bi970800v. [DOI] [PubMed] [Google Scholar]
- 30.Di Costanzo L, Flores LV, Christianson DW. Stereochemistry of guanidine-metal interactions: implications for l-arginine-metal interactions in protein structure and function. Proteins: Struct., Funct., Bioinf. 2006;65:637–642. doi: 10.1002/prot.21127. [DOI] [PubMed] [Google Scholar]
- 31.Dowling DP, Ilies M, Olszewski KL, Portugal S, Mota MM, Llinás M, Christianson DW. Crystal structure of arginase from Plasmodium falciparum and implications for l-arginine depletion in malarial infection. Biochemistry. 2010;49:5600–5608. doi: 10.1021/bi100390z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hine J. The principle of least nuclear motion. Adv. Phys. Org. Chem. 1978;15:1–61. [Google Scholar]
- 33.Shishova EY, Di Costanzo L, Emig FA, Ash DE, Christianson DW. Probing the specificity determinants of amino acid recognition by arginase. Biochemistry. 2009;48:121–131. doi: 10.1021/bi801911v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reczkowski RS, Ash DE. Rat liver arginase: kinetic mechanism, alternate substrates, and inhibitors. Arch. Biochem. Biophys. 1994;312:31–37. doi: 10.1006/abbi.1994.1276. [DOI] [PubMed] [Google Scholar]
- 35.Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993;26:283–291. [Google Scholar]
- 36.Kleywegt GJ, Zou JY, Kjeldgaard M, Jones TA. In: Around O, in International Tables for Crystallography. Rossmann MG, Arnold E, editors. Kluwer Academic Publishers; Dordrecht (Netherlands): 2001. pp. 353–356.pp. 366–367. [Google Scholar]