Abstract
BACKGROUND
The diagnosis-specific Graded Prognostic Assessment (GPA) was published to clarify prognosis for patients with brain metastases. This study refines the existing Breast-GPA by analyzing a larger cohort and tumor subtype.
METHODS
A multi-institutional retrospective database of 400 breast cancer patients treated for newly-diagnosed brain metastases was generated. Prognostic factors significant for survival were analyzed by multivariate Cox regression (MCR) and recursive partitioning analysis (RPA). Factors were weighted by the magnitude of their regression coefficients to define the GPA index.
RESULTS
Significant prognostic factors by MCR and RPA were Karnofsky Performance Status (KPS), HER2, ER/PR status, and the interaction between ER/PR and HER2. RPA showed age was significant for patients with KPS 60–80. The median survival time (MST) overall was 13.8 months, and for GPA scores of 0–1.0, 1.5–2.0, 2.5–3.0 and 3.5–4.0 was 3.4 (n=23), 7.7 (n=104), 15.1 (n=140) and 25.3 (n=133) months, respectively (p < 0.0001). Among HER2-negative patients, being ER/PR-positive improved MST from 6.4 to 9.7 months whereas in HER2-positive patients, being ER/PR-positive improved MST from 17.9 to 20.7 months. The log-rank statistic (predictive power) was 110 for the Breast-GPA versus 55 for tumor subtype.
CONCLUSIONS
The Breast-GPA documents wide variation in prognosis and shows clear separation between subgroups of patients with breast cancer and brain metastases. This tool will aid clinical decision-making and stratification of clinical trials. These data confirm the effect of tumor subtype on survival and show the Breast-GPA offers significantly more predictive power than the tumor subtype alone.
Keywords: breast cancer, brain metastases, prognosis, radiation therapy, stereotactic radiosurgery, estrogen, progesterone, HER2, outcomes
INTRODUCTION
In 2010, over 209,000 women were diagnosed with breast cancer in the United States and over 40,000 died from the disease (1). Brain metastases occur in 10–15 percent of all women with breast cancer. (2,3,4).
Although few clinical trials have focused specifically on breast cancer patients with brain metastases, several risk factors have been identified. Overexpression of human epidermal growth factor receptor 2 (HER2) appears to be an independent risk factor for developing brain metastases (5–9), as does estrogen receptor negativity (5–7,10,11). Young age, nodal status, high tumor grade, and tumor size (> 2cm) are also associated with the development of brain metastases (5–7,10). Patients with triple negative tumors also appear to be at particularly high risk (12–14).
DNA microarray analysis has revealed that tumor subtype is a key prognostic factor in breast cancer (15,16). These subtypes can be approximated using HER2, estrogen receptor (ER) and progesterone receptor (PR) status. Multiple studies have demonstrated tumor subtype affects prognoses (16–18), however, neither the RTOG-RPA classification system nor the current Breast-Specific GPA system include tumor subtype in their models.
Many other authors have described other prognostic factors, albeit with conflicting findings (19–37). Gaspar et al. published a seminal work on a prognostic index for patients with brain metastases, the Radiation Therapy Oncology Group‘s Recursive Partitioning Analysis (RTOG-RPA) in 1997 (38). That index was validated (39) and quickly adopted for purposes of stratification in clinical trials. Two weaknesses of the RTOG-RPA are it is not diagnosis-specific and is limited to considering the variables available in the RTOG database.
The Graded Prognostic Assessment (GPA) is a prognostic index for patients with brain metastases (40). We established this prognostic index based on a database of 1,960 patients from four Radiation Therapy Oncology Group (RTOG) protocols involving patients with brain metastases (41–44). The original GPA was validated (45) then refined with diagnosis-specific prognostic indices based on a second, independent multi-institutional retrospective analysis of 4,259 other patients with brain metastases from breast carcinoma, small cell and non-small cell lung carcinoma, gastrointestinal cancers, melanoma and renal cell carcinoma (46,47). This study refines the existing breast cancer-specific GPA index by analyzing a larger sample with additional variables, including HER2 and ER/PR status.
METHODS
Patient Population
An IRB-approved retrospective database of 865 patients with breast cancer treated for brain metastases between 06/1993–01/2010 was generated from the Radiation Oncology departments at 11 institutions. Of these 865 patients, 465 were excluded for the following reasons: recurrent (not newly diagnosed) brain metastases (157), unknown or surgery-only treatment (7) or missing data on survival (12)/tumor subtype (295)/KPS (9). The analysis is based on the 400 patients with complete data for these variables; 283 patients in the current analysis were included in an earlier analysis (46).
Assignment of Tumor Subtype
Tumor subtype may be approximated as follows: Basal (triple negative or HER2/ER/PR-negative), Luminal A (HER2-negative, ER/PR-positive), Luminal B (HER2/ER/PR-positive) and HER2 (HER2-positive, ER/PR-negative).
Statistical Methods
Prognostic Factors for Survival
Survival time was measured from the time of first treatment for brain metastases to the date of death or last follow-up. Prognostic factors for survival were analyzed by two methods: multivariate Cox regression (MCR) and recursive partitioning analysis (RPA). This dual MCR-RPA methodology has been previously shown to be an effective tool in the design of prognostic indices (48). Prognostic factors found to be significant by either method were weighted relative to the magnitude of their regression coefficients to define the GPA index.
Multivariate Cox Regression
Multivariate survival analysis was performed using the Cox proportional hazards model. The Cox model was stratified by institution to allow for potentially different shapes of the baseline hazard function. Factors initially considered were age, KPS, number of brain metastases, whether extracranial metastases were present, HER2/ER/PR status and all possible two-way interactions. A forward selection procedure with a cutoff p-value of 0.10 was used to establish the initial model. Analysis was performed using SAS version 9.2 (SAS Institute, Cary, NC).
Recursive Partitioning Analysis
Recursive partitioning analysis was used to supplement MCR in the construction of the index. RPA splits the sample into two subgroups or nodes, choosing a splitting rule from among all possible splits over all prognostic factors. The split that maximizes the homogeneity of each subgroup with respect to survival is chosen. This procedure is performed recursively to generate a tree, which is then pruned to an optimal size (49,50). This analysis was performed using R version 2.10.1 (R Development Core Team, 2009).
Derivation of the Breast-GPA Index
Prognostic factors found to be significant by either MCR or RPA were retained in the final MCR model in order to improve its prognostic ability. The relative magnitude of the regression coefficients (i.e. log hazard ratios) from the final model were used to design and weight the Breast-GPA, an additive point-based prognostic index. A score of 4.0 correlates with the best prognosis and 0.0, the worst. The Kaplan-Meier method was used to estimate the survival curve for each prognostic group. The log-rank test was used to test whether significant survival differences were present between adjacent classes in Breast-GPA and among all classes.
RESULTS
Table 1 shows patient and treatment characteristics including a breakdown of survival as it relates to various subgroups. The overall MST was 13.8 months. At the time of data collection, 95 patients (24%) were alive. For these patients, the median follow-up time is 17.1 months (range 0.2–94.8 months). Regarding systemic therapies, 77% of HER2-positive patients received trastuzumab and 82% of ER/PR-positive patients received hormonal therapy. Chemotherapy data was not available.
Table 1.
Patient Characteristics, Frequency and Median Survival Time
| Factor | Level | N (%) | MST (95% CI) |
|---|---|---|---|
| Age (Median = 53) | <50 | 146 (37%) | 16.7 (13.6, 19.9) |
| 50–59 | 149 (37%) | 12.6 (9.7, 17.5) | |
| 60–69 | 74 (19%) | 10.8 (7.3, 18.0) | |
| ≥ 70 | 31 (8%) | 7.9 (3.4, 11.4) | |
| KPS (Median = 80) | <60 | 17 (4%) | 2.7 (0.6, 4.0) |
| 60 | 21 (5%) | 3.8 (1.6, 15.1) | |
| 70 | 70 (18%) | 12.3 (8.6, 15.0) | |
| 80 | 102 (26%) | 9.7 (7.4, 13.4) | |
| 90 | 143 (36%) | 21.9 (18.0, 26.0) | |
| 100 | 47 (12%) | 20.2 (15.2, 34.1) | |
| Genetic Subtype | Luminal B | 103 (26%) | 20.7 (16.0, 26.5) |
| HER2 | 122 (31%) | 17.9 (13.4, 22.9) | |
| Luminal A | 78 (20%) | 9.7 (6.7, 19.1) | |
| Basal | 97 (24%) | 6.4 (4.8, 9.1) | |
| Number of Brain Metastases | 1 | 117 (29%) | 17.4 (13.4, 20.0) |
| 2 | 80 (20%) | 12.9 (8.8, 20.0) | |
| 3 | 51 (13%) | 16.3 (10.0, 21.9) | |
| 4 | 56 (14%) | 11.5 (7.4, 15.2) | |
| 5 | 25 (6%) | 12.3 (4.9, 20.7) | |
| >5 | 71 (18%) | 10.3 (4.5, 15.1) | |
| Extracranial Metastases | Absent | 139 (35%) | 15.5 (10.1, 19.8) |
| Present | 261 (65%) | 12.9 (10.8, 15.3) | |
| Gender | Female | 394 (98.5%) | 13.8 (11.5, 15.7) |
| Male | 6 (1.5%) | 20.0 (4.9, 23.1) | |
| Treatment | WBRT alone | 131 (33%) | 7.4 (4.4, 9.9) |
| SRS alone | 115 (29%) | 12.8 (10.0, 18.7) | |
| WBRT + SRS | 86 (22%) | 15.5 (13.2, 21.0) | |
| SURG + WBRT | 28 (7%) | 18.3 (12.6, 32.2) | |
| SURG+SRS+WBRT | 20 (5%) | 29.5 (8.6, 34.5) | |
| SURG + SRS | 19 (5%) | 24.0 (13.6, 32.9) | |
| Observation | 1 (0.25%) | 10.0 (NA) | |
| Overall | 400 (100%) | 13.8 (11.5, 15.9) |
Basal: Triple negative (HER2/ER/PR-negative)
Luminal A: HER2-negative, ER/PR-positive
Luminal B: Triple positive (HER2/ER/PR-positive)
HER2: HER2-positive, ER/PR-negative
WBRT: Whole Brain Radiation Therapy
SRS: Stereotactic Radiosurgery
Among the different treatment groups, there were no significant differences in the distribution of patients among the prognostic factors, except that the patients treated with surgery plus stereotactic radiosurgery (SRS) had a lower median age (45 versus 53 overall) and higher median KPS (90 versus 80 overall). Any differences in MST by treatment group could be explained by selection bias in this retrospective database.
Treatment is not a factor in the Breast-GPA. Primarily, this is because the GPA is intended to be useful in making the treatment choice rather than to evaluate outcomes after treatment. Nonetheless the impact of adding treatment to the final MCR model was analyzed; this did not significantly change the direction or magnitude of the estimated hazard ratio (HR) of the prognostic factors in Table 2.
Table 2.
Multivariate Cox Regression (MCR) Model using GPA Index Categories
| Factor | Level | Regression Coefficient a.k.a. Log Hazard Ratio (Standard Error) |
P-value | Hazard Ratio (95% CI) |
|---|---|---|---|---|
| KPS | KPS ≤ 50 | 1.00 | ||
| KPS = 60 | −0.80 (0.37) | 0.0295 | 0.45 (0.22, 0.92) | |
| KPS = 70 or 80 | −1.36 (0.30) | <.0001 | 0.26 (0.14, 0.46) | |
| KPS = 90 or 100 | −1.94 (0.31) | <.0001 | 0.14 (0.08, 0.26) | |
| Genetic1 | Basal | 1.00 | ||
| Subtype | Luminal A | −0.69 (0.18) | 0.0002 | 0.50 (0.35, 0.72) |
| HER2 | −0.96 (0.17) | <.0001 | 0.38 (0.28, 0.53) | |
| Luminal B | −1.06 (0.18) | <.0001 | 0.35 (0.25, 0.49) | |
| Age | Age ≥ 60 | 1.00 | ||
| Age < 60 | −0.26 (0.14) | 0.0640 | 0.77 (0.59, 1.02) |
The hazard ratio of HER2 positive and ER/PR positive to HER2 positive and ER/PR negative is 0.91 (95% CI 0.65, 1.28, p=0.6).
Multivariate Cox Regression
The statistically significant prognostic factors obtained by the multivariate Cox model with the forward selection procedure were KPS (p < 0.0001), ER/PR status (p = 0.0002), HER2 status (p < 0.0001), and the interaction of ER/PR and HER2 (p = 0.027). Relative to the Basal subtype, the risk of death (HR) was: 0.50 (95% CI 0.35–0.72) for the Luminal A subtype, 0.38 (95% CI 0.28–0.53) for the HER2 subtype, and 0.35 (95% CI 0.25–0.49) for the Luminal B subtype. For KPS, the HR was 0.70 (95% CI 0.63–0.78) which means the risk of death decreases 30% for each decile increase in KPS. Age, number of brain metastases, and whether extracranial metastases were present or absent were all insignificant.
Recursive Partitioning Analysis
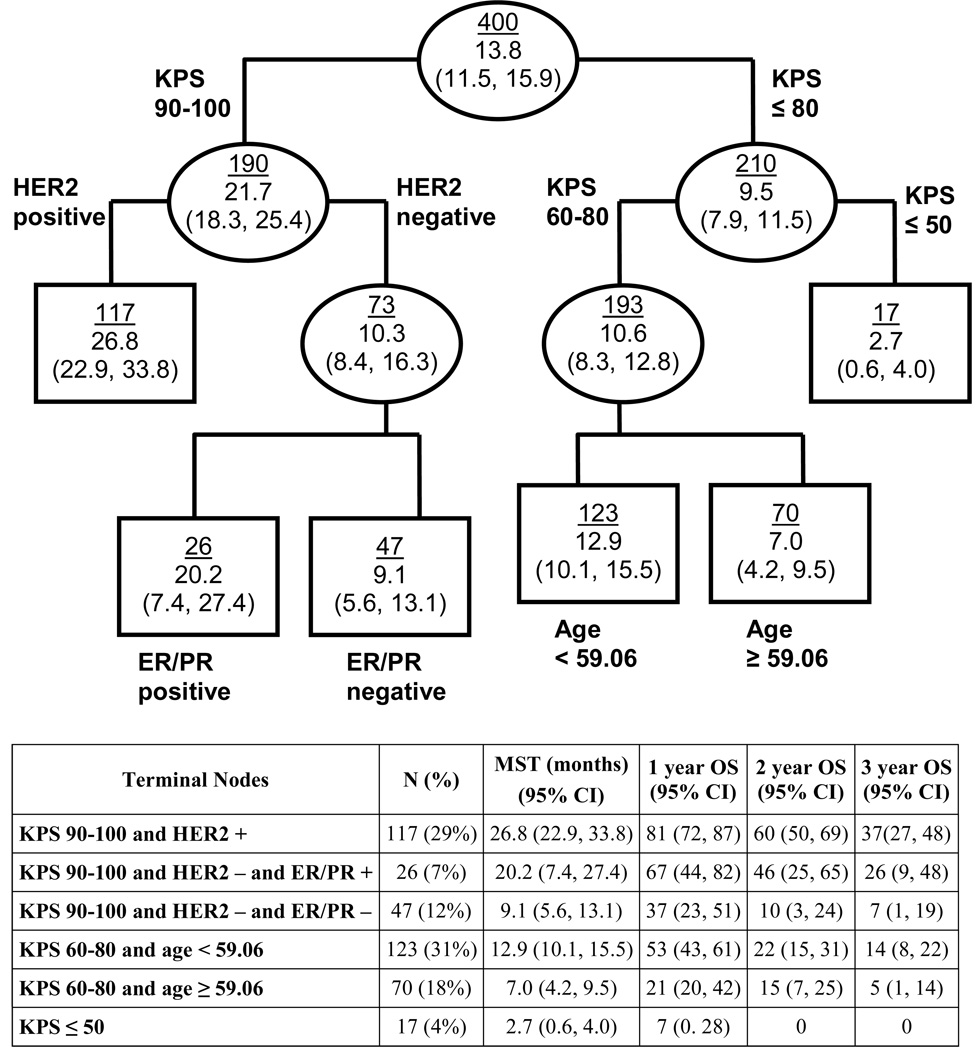
Figure 1 shows the recursive partitioning analysis. RPA results were consistent with MCR in that the same prognostic factors were identified (KPS, ER/PR, and HER2), and the interaction of ER/PR with HER2 was confirmed. Age was also found to be a significant factor for patients with KPS 60–80. We found the direct use of terminal nodes from RPA (Figure 1), and combinations thereof, as prognostic index classes did not separate patients as well as the point-based index derived from MCR, however review of the six terminal nodes confirms the same interaction between ER/PR and HER2 that was found in the MCR analysis: ER/PR positivity is more beneficial if the patient is HER2-negative (MST 20.2 for ER/PR-positive and 9.1 for ER/PR-negative), whereas ER/PR is not significant for HER2-positive patients.
Figure 1. Recursive Partitioning Analysis Model.
Ellipses represent internal nodes; rectangles represent terminal nodes (nodes for which no additional splitting can improve the model). The top number within each node is the number of patients in that node; the bottom is the median survival time and its 95% CI.
Final MCR Model
The final MCR model with prognostic factors from both MCR and RPA was fit and presented in Table 2, where KPS and age were discretized based on the RPA result and a thorough investigation of univariate regression models. Age was retained in the final MCR model because it improved the predictive power (overall log-rank statistic was 110 with age [model shown in Table 3], 94 without age [model not shown], and the p-values for adjacent comparisons improve) in addition to age being significant in RPA.
Table 3.
Graded Prognostic Assessment (GPA) Index for Women with Breast Cancer and Brain Metastases
| Factor | 0.0 | 0.5 | 1.0 | 1.5 | 2.0 |
|---|---|---|---|---|---|
| KPS | ≤ 50 | 60 | 70–80 | 90–100 | - |
| Genetic Subtype | Basal | - | Luminal A | HER2 | Luminal B |
| Age | ≥ 60 | < 60 | - | - | - |
Comparison to Tumor Subtype Alone
Breast-GPA was also compared to an index using tumor subtype alone, which used the four combinations of ER/PR/HER2 shown in Table 1, without KPS or age. Our final MCR model had significantly better goodness-of-fit than the tumor subtype model, measured by the partial likelihood ratio test (p<0.0001). The log-rank statistic was 55 for the tumor subtype model. Breast-GPA has an overall log-rank statistic twice as large (110), indicating substantially better separation of prognostic classes than tumor subtype alone.
The Breast-GPA Index
Table 3 shows the definition of the Breast-GPA index for women with breast cancer and brain metastases. The sum of the relevant values defines the total Breast-GPA score for a given patient. Age, being the least significant factor, was given the lowest weight in the point system. The interaction effect was modeled by placing ER/PR and HER2 in the same row. Being HER2-positive is given 1.5 points, and being ER/PR-positive is given 1 point, since the effect size (in log hazard ratio) is 1.45 times larger for HER2 (−0.96 for HER2 vs. −0.69 for ER/PR, see Table 2). However, being both ER/PR and HER2-positive (Luminal B) is worth 2 points total instead of 2.5, since the benefits of both together are not as large as the combination of their individual benefits (−1.06 for both positive vs. −1.65 = −0.69–0.96, see Table 2). In HER2-negative patients, the MST for ER/PR-positive patients improves from 6.4 to 9.7 months (52%) relative to ER/PR-negative patients. In HER2-positive patients, being ER/PR-positive only improves the MST from 17.9 to 20.7 months (16%) relative to ER/PR-negative, (Table 1). Being ER/PR-positive provides a similar absolute benefit (about 3 months) but is of greater relative benefit if the patient is HER2-negative than HER2-positive. The same finding is reflected in the risk of death (Table 2).
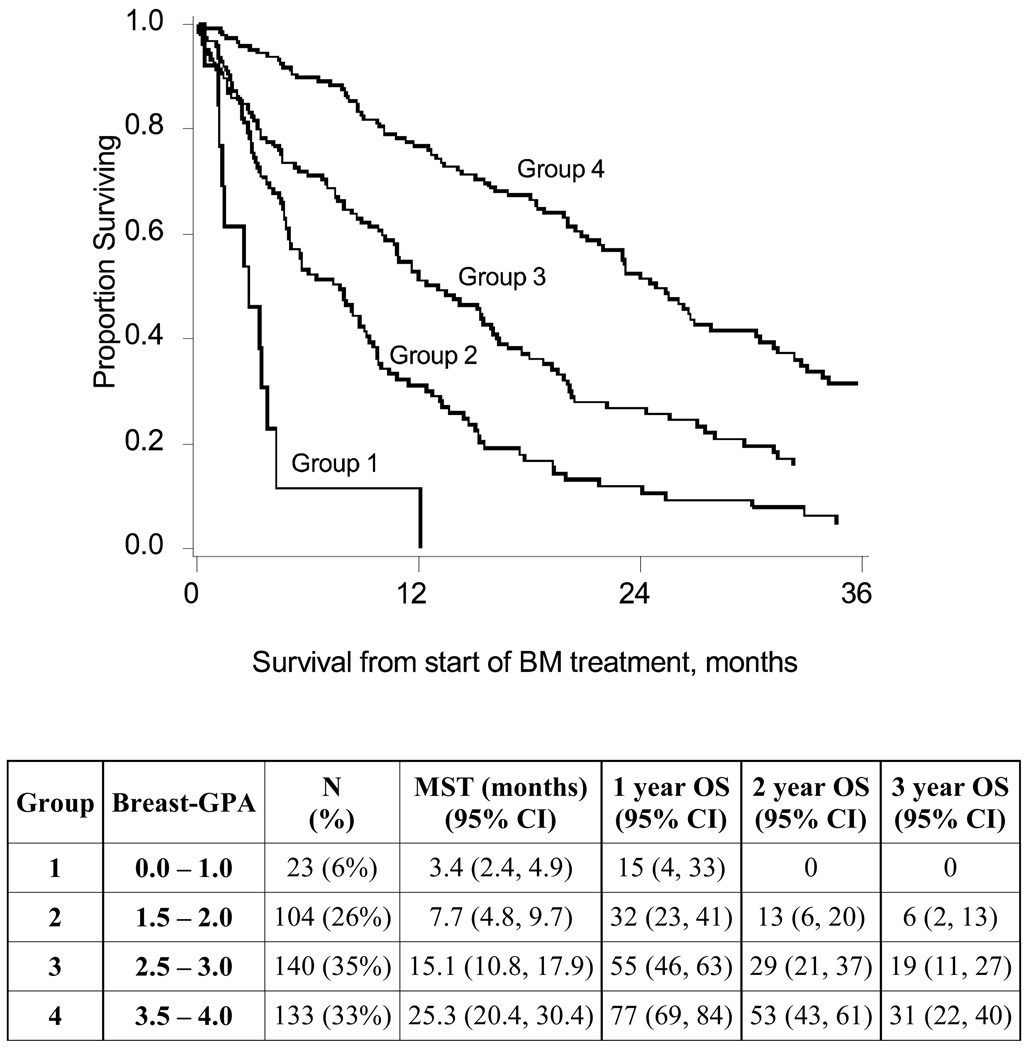
The MST of patients with total GPA scores of 0.5–1.0, 1.5–2.0, 2.5–3.0 and 3.5–4.0 was 3.4 (n=23), 7.7 (n=104), 15.1 (n=140) and 25.3 (n=133) months, respectively (p-value < 0.0001, Figure 2). The three pairwise comparisons of adjacent groups were: GPA subgroup 0.0–1.0 vs. 1.5–2.0 (p-value = 0.0006); GPA subgroup 1.5–2.0 vs. 2.5–3.0 (p-value <0.0001); GPA subgroup 2.5–3.0 vs. 3.5–4.0 (p-value < 0.0001). The overall log-rank test statistic for this index was larger than that of the previous GPA classification (43) applied to the same patients (log-rank statistic 110 vs. 94), indicating better separation between classes. The comparison between GPA groups 2.5–3.0 and 3.5–4.0 was also more significant for the new index (p < 0.0001) than for the previous GPA (p=0.03).
Figure 2. Kaplan-Meier Survival Curves for Breast-GPA Groups.
Group 1: GPA 0.0–1.0, MST 3.4 months (n = 23)
Group 2: GPA 1.5–2.0, MST 7.7 months (n = 104)
Group 3: GPA 2.5–3.0, MST 15.1 months (n = 140)
Group 4: GPA 3.5–4.0, MST 25.3 months (n = 133)
DISCUSSION
We revised the original Breast-GPA because we were able to build a larger sample size and because the HER2 and ER/PR status of many patients included in the original Breast-GPA model were not available at the time of the publication of the diagnosis-specific GPA (43,44). This study shows a dramatic range in survival for women with breast cancer and brain metastases. The MST for patients with Breast-GPA of ≤ 1.0 is 3.4 months compared to 25.3 months for patients with Breast-GPA 3.5–4.0. Interestingly, the number of brain metastases and whether extracranial metastases were present or absent were not significant prognostic factors. The finding that extracranial metastasis is not an important prognostic factor is in contrast to the RTOG-RPA, and may be due to the success of systemic treatment. The lack of prognostic value of the number of brain metastases may be important in considering patients for stereotactic radiosurgery (SRS).
A multitude of groups have reported on prognostic factors for survival among breast cancer patients with brain metastases. Although there have been conflicting findings, numerous variables have been shown to have some prognostic value including the following: Basal subtype (20,21,51); whole brain radiation dose of greater than or equal to 30 Gy (20,22); KPS (19,22–30); presence/degree of extracranial metastases (21–23,27,30,31); primary tumor control (24,32); size of primary tumor (22); interval from first cancer diagnosis to brain metastases (23); number of brain metastases (22,23,32,33); solitary metastasis (22,34); age (19,21,24,30,34); ER status (21,24,26); HER2 expression (22,24,29,30,32,35); systemic chemotherapy (28); lymphopenia (29,36); and surgical resection (28,31).
Other Indices
Some authors have attempted to evaluate existing prognostic systems which utilize many of the aforementioned prognostic factors specifically in patients with brain metastases from breast cancer. Nieder and colleagues (23) assessed the ability of the RTOG-RPA, Basic Score for Brain Metastases (BSBM), Score Index for Radiosurgery in brain metastases (SIR), GPA, and the score developed by Rades et al. (27) in a cohort of 83 of their patients with brain metastases from primary breast cancer (23). They confirmed the prognostic value of the RPA classes and SIR in terms of survival, but could not do so for the BSBM, original GPA and Rades score. They also defined prognostic factors including KPS, presence of extracranial metastases, interval from first cancer diagnosis to brain metastasis, and number of brain metastases and used these four factors to design their own prognostic system which performed slightly better than the aforementioned systems. However, they acknowledge the shortcomings of their model and state, “Without doubt, the definitive prognostic score can only be created from a very large database.” Other groups have also confirmed the prognostic value of the RPA system (20,29,31,37) and SIR in terms of survival (19,26). Le Scodan created a prognostic system based on the variables found to be statistically significant in their analysis however this was a small study (n=117) from a single institution, treated with WBRT alone (29).
Potential Applications of the Breast-GPA Index
The proposed GPA has the potential to alter clinical management. An example that demonstrates both the difference between the Breast-GPA and the RTOG-RPA and how that difference could affect clinical decision-making is a 59 year-old woman with Luminal B breast cancer and asymptomatic bone and brain metastases. Such a patient would have a GPA of 4.0 (MST 25.3 months) but would be RPA Class II (MST 4.2 months). The difference in the prognosis could easily lead a patient and family to make different decisions about treatment and how they wish to spend their time and would affect the physician’s clinical decision making.
Regarding the comparison of the Breast-GPA versus tumor subtype alone, it is important to acknowledge the recent progress in our understanding of the significance of tumor subtype on prognosis (13,15,16–18,21,30,32,51–55). This analysis (specifically the log rank statistic) shows the Breast-GPA is significantly more predictive than the tumor subtype alone in distinguishing those patients with extremely good or poor prognosis and this may affect clinical decision-making.
The interaction between HER2 and ER/PR found in this study is based on a statistical correlation, not a biological interaction. The interaction described here simply means the magnitude of one factor’s effect on survival is different with or without the presence of the other factor.
Conclusion
In summary, these data confirm the effect of tumor subtype on survival and show the Breast-GPA offers significantly more predictive power than the tumor subtype alone. The Breast-GPA index may be useful in several different ways: 1) in individualized clinical decision-making; 2) in comparing trials; 3) in re-analyzing prior trials; 4) in stratifying patients enrolled in future prospective trials; 5) in guiding clinical trial development, and; 6) in designing treatment guidelines. The Breast-GPA will assist the physician in deciding whether to recommend aggressive treatment, hospice or something in between. It will guide the patient/family in deciding how they wish to spend their time and which treatment is right for them.
Further research is needed to develop ever more robust, more predictive prognostic indices, and to modify them as new data becomes available. If we do not do so, scarce resources could be wasted on clinical trials that lack adequate stratification, resulting in erroneous conclusions.
Acknowledgments
Grant Support: This research was supported in part by Grant W81XWH-062-0033 from the U.S. Department of Defense Breast Cancer Research Program, to RJW, and by NIH Grant P30-CA77598 utilizing the services of the Biostatistics and Bioinformatics Core, Masonic Cancer Center, University of Minnesota shared resource.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Dr. Mehta has served as a consultant to Adnexus, Bayer, Genentech, Merck, Schering Plough, and Tomotherapy; he serves on the Board of Directors of Pharmacyclics, and as an advisor to Stemina, and is on the DSMB for Apogenix. He holds stock options in Pharmacyclics and Tomotherapy.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973–2001) in the metropolitan Detroit cancer surveillance system. J Clin Onc. 2004;22(14):2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 3.Zimm S, Wampler GL, Stablein D, et al. Intracerebral metastases in solid-tumor patients: Natural history and results of treatment. Cancer. 1981;48:384–394. doi: 10.1002/1097-0142(19810715)48:2<384::aid-cncr2820480227>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 4.Posner JB. Neurologic Complications of Cancer. Philadelphia: FA Davies; 1995. [Google Scholar]
- 5.Bendell JC, Domchek SM, Burnstein HJ, Harris L, Younger J, Kuter I, Bunnell C, Rue M, Gelman R, Winer E. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97:2972–2977. doi: 10.1002/cncr.11436. [DOI] [PubMed] [Google Scholar]
- 6.Gabos Z, Sinha R, Hanson J, Chauhan N, Hugh J, Mackey JR, et al. Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J Clin Onc. 2006;24:5658–5663. doi: 10.1200/JCO.2006.07.0250. [DOI] [PubMed] [Google Scholar]
- 7.Tham YL, Sexton K, Kramer R, Hilsenbeck S, Elledge R. Primary breast cancer phenotypes associated with propensity for central nervous system metastases. Cancer. 2006;107:696–704. doi: 10.1002/cncr.22041. [DOI] [PubMed] [Google Scholar]
- 8.Chang J, Clark GM, Allred DC, Mohsin S, Chamness G, Elledge RM. Survival of patients with metastatic breast cancer: importance of prognostic markers of the primary tumor. Cancer. 2003;97:545–553. doi: 10.1002/cncr.11083. [DOI] [PubMed] [Google Scholar]
- 9.Pestalozzi BC, Zahrieh D, Price KN, Holmberg SB, Lindtner J, Collins J, et al. Identifying breast cancer patients at risk for central nervous system (CNS) metastases in trials of the International Breast cancer Study Group (IBCSG) Ann Onc. 2006;17:935–944. doi: 10.1093/annonc/mdl064. [DOI] [PubMed] [Google Scholar]
- 10.Hicks DG, Short SM, Prescott NL, et al. Breast cancers with brain metastases are more likely to be estrogen receptor negative, express the basal cytokeratin CK5/6, and overexpress HER2 or EGFR. Am J Surg Path. 2006;30:1097–1104. doi: 10.1097/01.pas.0000213306.05811.b9. [DOI] [PubMed] [Google Scholar]
- 11.Stemmler HJ, Kahlert S, Siekiera W, Untch M, Heinrich B, Heineman V. Characteristics of patients with brain metastases receiving trastuzumab for HER2 overexpressing metastatic breast cancer. Breast. 2006;15:219–225. doi: 10.1016/j.breast.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 12.Kennecke H, Yerushaimi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Onc. 2010 doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 13.Lin NU, Claus E, Sohl J, et al. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer. Cancer. 2008;113:2638–2645. doi: 10.1002/cncr.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heitz F, Harter P, Lueck HJ, et al. Triple-negative and HER2-overexpressing breast cancers exhibit an elevated risk and an earlier occurrence of cerebral metastases. Eur J Cancer. 2009;45:2792–2798. doi: 10.1016/j.ejca.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 15.Carey LA, Perou CM. Gene arrays, prognosis and therapeutic interventions. In: Harris JR, Lippman ME, Morrow M, Osborne CK, editors. Diseases of the Breast. 4th ed. Philadelphia, PA: Wolters Kluwer/ Lippincott Williams & Wilkins; 2009. pp. 458–472. [Google Scholar]
- 16.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 17.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen PL, Taghian AG, Katz MS, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER2 is associated with local and distant recurrence after breast conserving therapy. J Clin Onc. 2008;26:2373–2378. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 19.Goyal S, et al. Gamma knife surgery for the treatment of intracranial metastases from breast cancer. J Neurosurg. 2005;103(2):218–223. doi: 10.3171/jns.2005.103.2.0218. [DOI] [PubMed] [Google Scholar]
- 20.Dawood S, et al. Prognostic factors of survival in the trastuzumab era among women with breast cancer and brain metastases who receive whole brain radiotherapy: a single-institution review. Cancer. 2010;116(13):3084–3092. doi: 10.1002/cncr.25115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nam BH, et al. Breast cancer subtypes and survival in patients with brain metastases. Breast Cancer Res. 2008;10(1):R20. doi: 10.1186/bcr1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lentzsch S, et al. Brain metastases in breast cancer: prognostic factors and management. Eur J Cancer. 1999;35(4):580–585. doi: 10.1016/s0959-8049(98)00421-3. [DOI] [PubMed] [Google Scholar]
- 23.Nieder C, et al. Prognostic scores in brain metastases from breast cancer. BMC Cancer. 2009;9:105. doi: 10.1186/1471-2407-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kased N, et al. Gamma Knife radiosurgery for brain metastases from primary breast cancer. Int J Radiat Oncol Biol Phys. 2009;75(4):1132–1140. doi: 10.1016/j.ijrobp.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 25.Muacevic A, et al. Stereotactic radiosurgery for multiple brain metastases from breast carcinoma. Cancer. 2004;100(8):1705–1711. doi: 10.1002/cncr.20167. [DOI] [PubMed] [Google Scholar]
- 26.Akyurek S, et al. Stereotactic radiosurgical treatment of cerebral metastases arising from breast cancer. Am J Clin Oncol. 2007;30(3):310–314. doi: 10.1097/01.coc.0000258365.50975.f6. [DOI] [PubMed] [Google Scholar]
- 27.Rades D, et al. Evaluation of 2 whole-brain radiotherapy schedules and prognostic factors for brain metastases in breast cancer patients. Cancer. 2007;110(11):2587–2592. doi: 10.1002/cncr.23082. [DOI] [PubMed] [Google Scholar]
- 28.Ogawa K, et al. Treatment and prognosis of brain metastases from breast cancer. J Neurooncol. 2008;86(2):231–238. doi: 10.1007/s11060-007-9469-1. [DOI] [PubMed] [Google Scholar]
- 29.Le Scodan R, et al. Brain metastases from breast carcinoma: validation of the radiation therapy oncology group recursive partitioning analysis classification and proposition of a new prognostic score. Int J Radiat Oncol Biol Phys. 2007;69(3):839–845. doi: 10.1016/j.ijrobp.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 30.Melisko ME, et al. Brain metastases in breast cancer: clinical and pathologic characteristics associated with improvements in survival. J Neurooncol. 2008;88(3):359–365. doi: 10.1007/s11060-008-9578-5. [DOI] [PubMed] [Google Scholar]
- 31.Viani GA, et al. Whole brain radiotherapy for brain metastases from breast cancer: estimation of survival using two stratification systems. BMC Cancer. 2007;7:53. doi: 10.1186/1471-2407-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eichler AF, et al. Survival in patients with brain metastases from breast cancer: the importance of HER-2 status. Cancer. 2008;112(11):2359–2367. doi: 10.1002/cncr.23468. [DOI] [PubMed] [Google Scholar]
- 33.Lederman G, Wronski M, Fine M. Fractionated radiosurgery for brain metastases in 43 patients with breast carcinoma. Breast Cancer Res Treat. 2001;65(2):145–154. doi: 10.1023/a:1006490200335. [DOI] [PubMed] [Google Scholar]
- 34.Firlik KS, et al. Stereotactic radiosurgery for brain metastases from breast cancer. Ann Surg Oncol. 2000;7(5):333–338. doi: 10.1007/s10434-000-0333-1. [DOI] [PubMed] [Google Scholar]
- 35.Park BB, et al. Prognostic factor analysis in patients with brain metastases from breast cancer: how can we improve the treatment outcomes? Cancer Chemother Pharmacol. 2009;63(4):627–633. doi: 10.1007/s00280-008-0779-6. [DOI] [PubMed] [Google Scholar]
- 36.Claude L, et al. Lymphopenia: a new independent prognostic factor for survival in patients treated with whole brain radiotherapy for brain metastases from breast carcinoma. Radiother Oncol. 2005;76(3):334–339. doi: 10.1016/j.radonc.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Mahmoud-Ahmed AS, et al. Results of whole brain radiotherapy in patients with brain metastases from breast cancer: a retrospective study. Int J Radiat Oncol Biol Phys. 2002;54(3):810–817. doi: 10.1016/s0360-3016(02)02967-x. [DOI] [PubMed] [Google Scholar]
- 38.Gaspar LE, Scott C, Rotman, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 39.Gaspar LE, Scott C, Murray K, et al. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys. 2000;47:1001–1006. doi: 10.1016/s0360-3016(00)00547-2. [DOI] [PubMed] [Google Scholar]
- 40.Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70:510–514. doi: 10.1016/j.ijrobp.2007.06.074. [DOI] [PubMed] [Google Scholar]
- 41.Komarnicky LT, Phillips TL, Martz K, et al. A randomized phase III protocol for the evaluation of misonidazole combined with radiation in the treatment of patients with brain metastases (RTOG79-16) Int J Radiat Oncol Biol Phys. 1991;20:53–58. doi: 10.1016/0360-3016(91)90137-s. [DOI] [PubMed] [Google Scholar]
- 42.Sause WT, Scott C, Kirsch R, et al. Phase I/II trial of accelerated fractionation in brain metastases, RTOG 85-28. Int J Radiat Oncol Biol Phys. 1993;26:653–657. doi: 10.1016/0360-3016(93)90284-3. [DOI] [PubMed] [Google Scholar]
- 43.Phillips TL, Scott CB, Liebel S, et al. Results of a randomized comparison of radiotherapy and bromodeoxyuridine to radiotherapy alone for brain metastases: Report of RTOG trial 89-05. Int J Radiat Oncol Biol Phys. 1995;33:339–348. doi: 10.1016/0360-3016(95)00168-X. [DOI] [PubMed] [Google Scholar]
- 44.Murray KJ, Scott C, Greenberg HM, et al. A randomized phase III study of accelerated hyperfractionation versus standard in patients with unresected brain metastases: a report of RTOG 9104. Int J Radiat Oncol Biol Phys. 1997;39:571–574. doi: 10.1016/s0360-3016(97)00341-6. [DOI] [PubMed] [Google Scholar]
- 45.Sperduto CM, Watanabe Y, Mullan J, et al. A validation study of a new prognostic index for patients with brain metastases: the graded prognostic assessment. J Neurosurg. 2008;109:87–89. doi: 10.3171/JNS/2008/109/12/S14. [DOI] [PubMed] [Google Scholar]
- 46.Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77:655–661. doi: 10.1016/j.ijrobp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 47.Sperduto PW. What is your patient’s GPA and why does it matter? Managing brain metastases and the cost of hope. Int J Radiat Oncol Biol Phys. 2010;77:643–644. doi: 10.1016/j.ijrobp.2010.02.038. [DOI] [PubMed] [Google Scholar]
- 48.van Dijk MR, Steyerberg EW, Stenning SP, Dusseldorp E, Habbema JDF. Survival of patients with nonseminomatous germ cell cancer: a review of the IGCC classification by Cox regression and recursive partitioning. Br J Ca. 2004;90:1176–1183. doi: 10.1038/sj.bjc.6601665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification and Regression Trees. Wadsworth. 1984 [Google Scholar]
- 50.Therneau TM, Atkinson EJ. Section of Biostatistics. Rochester: Mayo Clinic; 1997. An introduction to recursive partitioning using the rpart routine. Technical Report 61. [Google Scholar]
- 51.Niwinska A, Murawska M, Pogoda K. Breast cancer subtypes and response to systemic treatment after whole-brain radiotherapy in patients with brain metastases. Cancer. 2010;116:4238–4247. doi: 10.1002/cncr.25391. [DOI] [PubMed] [Google Scholar]
- 52.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 53.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 54.Schiff R, Osborne CK, Fuqua SAW. Clinical aspects of estrogen and progesterone receptors. In: Harris JR, Lippman ME, Morrow M, Osborne CK, editors. Diseases of the Breast. 4th ed. Philadelphia, PA: Wolters Kluwer/ Lippincott Williams & Wilkins; 2009. pp. 408–430. [Google Scholar]
- 55.Epstein M, Ma Y, Press MF. ERBB2 Testing: Assessment of status for targeted therapies. In: Harris JR, Lippman ME, Morrow M, Osborne CK, editors. Diseases of the Breast. 4th ed. Philadelphia, PA: Wolters Kluwer/ Lippincott Williams & Wilkins; 2009. pp. 431–442. [Google Scholar]