Abstract
It is suspected that apart from tick-borne encephalitis virus several additional European Arboviruses such as the sandfly borne Toscana virus, sandfly fever Sicilian virus and sandfly fever Naples virus, mosquito-borne Tahyna virus, Inkoo virus, Batai virus and tick-borne Uukuniemi virus cause aseptic meningo-encephalitis or febrile disease in Europe. Currently, the microarray technology is developing rapidly and there are many efforts to apply it to infectious diseases diagnostics. In order to arrive at an assay system useful for high throughput analysis of samples from aseptic meningo-encephalitis cases the authors developed a combined multiplex ligation-dependent probe amplification and flow-through microarray assay for the detection of European Bunyaviruses. These results show that this combined assay indeed is highly sensitive, and specific for the accurate detection of multiple viruses.
Keywords: European Bunyaviruses, MLPA, Flow-through chip, Aseptic meningo-encephalitis
Introduction
While climate warming in Europe and global social changes have led to focussing attention to novel emerging infectious diseases [1, 2], there are many hardly noticed European arboviral infections causing neurological disorders like encephalitis or meningo-encephalitis [3, 4] and fevers [5, 6]. The aetiology of aseptic meningo-encephalitis in 50% of the cases in Europe for example remains unclear [3, 7]. In this regard seven Bunyaviruses, particularly, sandfly borne Toscana virus (TOSV), sandfly fever Sicilian virus (SFSV) and sandfly fever Naples-Virus (SFNV), mosquito-borne Tahyna virus (TAHV), Inkoo virus (INKV), Batai virus (BATV) and tick-borne Uukuniemi virus (UUKV), may be of greater importance than hitherto realized. The laboratory diagnosis of viral infections is generally performed by various serological assays e.g., haemagglutination-inhibition (HAI), enzyme-linked immunoassay (EIA or ELISA), viral cell culture in combination with immuno-fluorescence (IF) or electron microscope [8, 9]. These conventional methods are well established but often time-consuming, and in addition the sensitivity is often low [8]. Molecular methods have become more and more important in the diagnosis of viral infections and diseases [10, 11]. These methods are based on the direct detection of viral-specific DNA or RNA (via PCR or reverse transcription (RT)-PCR) without the need of virus cultivation. One of the most successful technologies in recent years is real-time PCR, which allows amplification of nucleic acids and simultaneous confirmation of the amplified product by specific probes [12, 13]. Although these approaches are rapid, accurate, and offer high specificity and sensitivity, they also introduce new disadvantages. As successful identification depends on appropriately chosen primer sets and probes used in separate parallel amplifications. In order to solve this disadvantage for virus detection, researchers have developed multiplex real-time PCR assays to detect many viruses in one tube [14, 15]. The disadvantage of multiplex PCR and real-time PCR is that only a limited number of targets can effectively be identified simultaneously due to restrictions caused by the spectral overlap of the fluorescent dyes used [16].
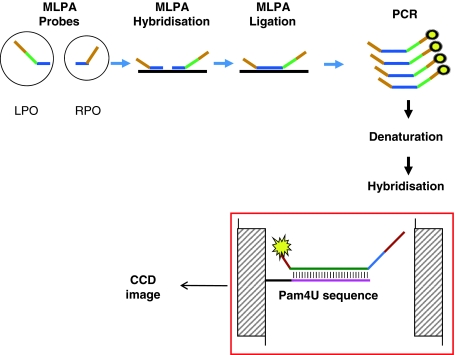
Currently, DNA microarray technology appears attractive in this regard [17, 18] for its ability of simultaneous screening for several pathogens in a single step reaction-array [19, 20]. However, the microarray technology is not yet widely used in clinical virology laboratories, because of problems with sensitivity and the requirement of large starting amounts of nucleic acids, and expense [21, 22]. After thorough analysis of the literature the authors decided to try an amplification and hybridisation approach, which amplifies a synthetic signal molecule, which is dependent on sequence-specific hybridisation rather than an amplification technique amplifying the target molecule itself. The authors have developed an approach that combines Multiplex ligation-dependent probe amplification (MLPA) with a flow-through chip hybridisation system (PamGene, Netherlands). The principle of this assay is presented and described on Fig. 1.
Fig. 1.
Outline of Flow-through microarray-based MLPA assay. MLPA probes consist of two oligonucleotides: the left probe oligonucleotide (LPO) and the right probe oligonucleotide (RPO), respectively, containing left- and right-hybridisation sequences (LHS and RHS) specific for the sequence of interest marked in blue. They also contain universal hybridisation sequences for the SALSA primers (in brown) which drive the MLPA PCR reaction. In addition, the LPO includes a unique artificial sequence (Zip-code or Cpam marked in green) which is complementary to the Pam4U sequence immobilized on the PamChip® 4U (marked in pink) for the signal detection. The fluorescent signal of the hybridized FAM tagged amplicon is read out by a CCD camera (Color figure online)
This set up allows an easy design of several MLPA systems that can be hybridised simultaneously to the microarray without the need for optimisation of hybridisation sequences and specific microarray probe printing. In Addition, this technology reduces chip costs and allows rapid hybridisation and reduced input amounts of nucleic acid samples in the comparison to classic DNA arrays. The flow-through microarray offered full process automation, including incubation, washing, hybridisation, altogether reducing the potential for cross contamination.
Taken together the author developed an assay based on the combination of RT-MLPA and flow-through 3D universal microarray for the detection of seven European Bunyaviruses, particularly (TOSV, SFSV, SFNV, TAHV, INKV, BATV and UUKV) for an expanded accurate diagnosis of aseptic meningo-encephalitis and fevers of unknown origin in Europe.
Materials and Methods
Viral Cultures
The virus-isolates (TOSV, SFSV, SFNV, TAHV, INKV, BATV and UUKV) were grown in VeroB4 cells or VeroE6 cells in Minimum essential or Dulbecco’s modified Eagle medium, respectively, supplemented with 2% foetal calf serum and penicillin/streptomycin, at 37°C in 5% CO2 atmosphere. Each strain was passaged for at least four times. The supernatants were harvested when the cytopathic effect (CPE) of the infected cells was more than 90%. Subsequently the supernatants were used for viral RNA extraction.
Enrichment of the Viral Particles and Viral RNA Extraction
The viral particles were enriched from supernatants using 30% Polyethylene glycol (PEG). In brief, 20 ml cell culture supernatant was centrifuged in 50 ml Falcon tubes at 4°C, 2,000 rpm for 10 min to eliminate cell debris, transferred into new 50 ml Falcon tubes, and supplemented with 1.48 ml of 5 M NaCl and 10.8 ml of PEG8000. Afterwards the supernatant was shaken carefully on a spinning-wheel at 4°C for 30 min and centrifuged at 4°C, 6,000 rpm for 1 h. The enriched virus particle pellets were processed for RNA isolation. The viral RNA genome of all investigated viruses was extracted using PeqGold TriFastTM (PeqLab, Germany) according to the manufacturer’s instructions and eluted into 40 μl of RNAase-free water. Subsequently, the RNAs were quantified using a NanoDrop spectrophotometer (PeqLab, Germany).
Generation of DNA and RNA Standards
Isolated RNAs were reverse transcribed using the Transcriptor First Strand cDNA Synthesis Kit (Roche, Germany) and the corresponding specific primers for the S-segment according to the manufacturer’s instructions. The resulting cDNAs were amplified using AccuPrime Pfx Polymerase (Invitrogen, Germany), dNTPs and specific primers designed for the UUKV S-segment forward primer: 5′-ACACAAAGACCTCCAACTTAGCTC-3′, reverse primer: 5′-ACACAAAGACCC TCCAACATTAAGC-3′) and the S-segment of SFNV, forward primer: 5′-TGCCCAACCCCCCTACAT-3′, reverse primer: 5′-ACACAAAGAATCCCGTTATT TAA-3′), and specific primers for TOSV, SFSV, TAHV, INKV and BATV published by the group [23, 24] using the following temperature profile: 30 cycles of 95°C/30 s, 60°C/30 s, 72°C/60 s. Amplificates were ligated into pCR®II using the TA-Cloning kit (Invitrogen, Germany). Ligated S-segment sequences were confirmed by sequencing (Seqlab, Germany).
In order to generate synthetic RNA standards in vitro transcription and quantification of transcribed RNA was performed as previously described [25] with an improved step for DNA digestion using the DNA-free kit (Ambion). In order to generate template targets for the establishment of the MLPA experiments the S-segments were amplified from the recombinant plasmids using specific primers and Taq DNA polymerase (5 U/μl) using the above amplification program. The amplification products were analysed on a 1% agarose gel and purified using the ZymocleanTM Gel DNA Recovery Kit (Zymo Research). The purified amplicons were quantified with a NanoDrop spectrophotometer (PeqLab, Germany).
Primers, Taqman and MLPA Probes Design
Primer and TaqMan design: sequences were downloaded from the NCBI GenBank database. Multi-alignments were performed using MegAlign and SeqMan software to generate consensus sequences used as templates for the design of specific primers and Taqman probes for new developed real-time PCR of UUKV, SFNV (reference sequences NC_005221, EF201829), and the corresponding real-time PCR assays of TOSV, SFSV, THAV, INKV and BATV were published previously by us [23, 24]. The MLPA probes of TOSV, SFSV, SFNV, TAHV, INKV, BATV and UUKV are listed in Table 1.
Table 1.
Specific viral hybridization sequences of MLPA probes
| Virus | Specific hybridization sequence of the left probe (LHS) | Specific hybridization sequence of the right probe (RHS) | Size of RT or MLPA amplicons |
|---|---|---|---|
| PAM16 Toscan-1 | GCATCCATAGTGGTCCCAGAC | ACGGGCAGGGATTCTGACAGG | 104 bp |
| PAM20 Toscan-2 | CAGGGATTCTGACAGGACACGTAG | TGCCTGA ACAGTCCACGGAAC | 107 bp |
| PAM13 SFS-1 | CCCGGCAATTAACATTGATGTGAGG | TGTCATAGACTCCTCTCCAG | 107 bp |
| PAM15 SFS-2 | TGCAACCAGGCTCTTAACTTCTC | TTGCTCCCTCTGGAGACATTCTC | 105 bp |
| PAM44 SFN S-1 | GAACCCCACCCAAATCAATGACC | AATTATTCACCCTTACCCTTCCCTCCCC | 113 bp |
| PAM45 SFN S-2 | ATTCACCCTTACCCTTCCCTC | CCCACATTGGCAGTCTACTTG | 104 bp |
| PAM84 Tahyna-1 | GAGCCTAAAGGATGTTGAGCAGC | TTAAATGGGGTAGAGGCGGCC | 106 bp |
| PAM85 Tahyna-2 | GGTGCTAGAGCAGTTCAAAGAAAATG | AAGACGCAGCTCAAAGGGAGTTG | 111 bp |
| PAM78 Inkoo-1 | TTTGGAGAGTGGCAGGTGGAGATTG | TCAATAATCATTTTCCTGGAAA + C+A + G+G* | 113 bp |
| PAM75 Inkoo-2 | GAGAGTGGCAGGTGGAGATTGTC | AATAATCATTTTCCTGGAAAACAGG | 106 bp |
| PAM100 Batai-1 | CAGATGGGGAGGAGGTTTACC | CTCTCATTCTTTCCAGGGT + C+T + G | 105 bp |
| PAM102 Batai-2 | CAGATGGGGAGGAGGTTTACC | TCTCATTCTTTCCAGGGTCTG | 104 bp |
| PAM42 Uuk S-1 | ACCATCAACGTGAAGCACCGC | GGAATGGAGGCCAAGGAGATC | 104 bp |
| PAM64 Uuk S-2 | CCCTCATCCACCTCAAGCCAAC | CCACTGACGGCATATCCACACC | 106 bp |
All sequences are given in 5′ to 3′ orientation, * nucleotides marked with + are locked nucleotides
Preliminary experiments were performed in order to determine the best sensitivity, specificity and linear range of the real-time RT-PCR assays. All reactions with the RNA standards were performed with the LightCycler RNA Master Hydrolysis Probe kit (Roche, Germany) according the manufacturer recommendations.
MLPA Probe Design
LHS and RHS probes (note, the abbreviations are explained on Fig. 1) were designed for 2 positions of the corresponding S-segments of the investigated European viruses (see above). A total of 14 MLPA probes were designed using the DNA primer design software program (PamGene, The Netherlands) and selected using the following criteria: The MLPA probes never overlap and locate directly adjacent to each other, total probe length (LPO + RPO) 100–132 bp, PCR primer sequence 42 nucleotides (nt), unique Cpam 20 nt, max length of LHS + RHS 70 nt at a melting temperature (Tm) of between 67 and 70°C. In addition, Tm was checked using Exiqon Tm prediction (http://lna-tm.com/) and the Raw program from MRC-Holland and secondary structure was assessed using the mfold server (http://www.bioinfo.rpi.edu/applications/mfold/cgi-bin/rna-form1.cgi). The specificity of the designed probes was checked using a Blastn-analysis against the sequences in the NCBI database and against the 124 zip-codes spotted on the PamChip® 4U. The designed MLPA probes were synthesized by Biolegio (Nijmegen, The Netherlands) and are listed in Table 1, showing the size (100–113 bp) of MLPA/RT-MLPA PCR amplicons.
MLPA and RT-MLPA Reaction
MLPA and RT-MLPA reagents were obtained from MRC-Holland (Amsterdam, The Netherlands). The reactions were performed according to the manufacturer’s protocol. The purified S-segment amplicons of TOSV, SFSV, SFNV, TAHV, INKV BATV and UUKV (100–150 ng) or water as a negative control were diluted in TE buffer (pH 8) to 5 μl and heated at 98°C for 5 min. After cooling at 25°C, 1.5 μl of the corresponding MLPA probe mix and 1.5 μl of MLPA buffer were added to each sample, followed by denaturation at 95°C for 1 min and hybridization at 60°C for 12–16 h. Ligation was performed by adding 30 μl of ligation mix at 54°C for 15 min to each sample. After the inactivation of ligase at 98°C for 5 min, the MLPA PCR was carried out in a final volume of 50 μl using the SALSA forward primer 5′-GGGTTCCCTAAGGGTTGGA-3′ and reverse primer 5′-GTGCCAGCAAGATCCAATCTAGA-3′) PCR primers complementary to the primer binding sites of the LPO (5′ FAM label) and RPO probes (MRC-Holland, The Netherlands) using he following temperature profile: 35 cycles of 95°C/30 s, 60°C/30 s and 72°C/60 s, and a final elongation step at 72°C for 20 min.
For the RT-MLPA reactions RNA standards or water as negative control were transcribed to cDNA using MMLV reverse transcriptase enzyme (Promega, Germany) and specific or hexamer primers according to Promega protocol instructions. In order to increase the sensitivity of MLPA probe sets 20% formamide buffer was added to the hybridization step of MLPA probes to the target cDNAs and assayed at a temperature range of 56–60°C. In order to check the specificity of MLPA probes, MLPA/RT-MLPA negative control reactions were performed with water instead of MLPA probes.
Gel Electrophoresis by Agilent Bioanalyzer
Prior the microarray hybridization, the amplified MLPA and RT-MLPA products were analysed by gel electrophoresis using the Agilent Bioanalyzer (Agilent Technology, Germany). The short-length amplicons were analysed using the DNA 1000 LabChip® kit.
3D Microarray Hybridization and Detection System
The MLPA, RT-MLPA PCR and negative control products were subsequently analysed by flow-through microarray using a universal customized PamChip® 4U which consisted of a 4-arrays of a porous aluminium-oxide solid matrix. 124 zip-code addresses spotted as duplicate onto each array (PamGene, The Netherlands). The technology is available for DNA and RNA applications (Fig. 1). Hybridization, washing and detection was performed on the PamStation®12 which is an automated microarray processing system described elsewhere [26, 27], that also allows automated data analysis. 11 μl of each MLPA, RT-MLPA or negative control PCR reaction was denaturated at 95°C for 5 min, 5.5 μl hybridization buffer containing 5× SSPE and 0.5 N-lauroylsarcosine and 11.5 μl of water was added. The array was subjected to an initial threefold wash with 1× hybridisation buffer containing 1× SSPE and 0.1% N-lauroylsacrosine (pH 7.0). The samples (25 μl) were loaded onto the arrays and pumped back and forth through the porous membrane by air pressure during hybridisation at 50, 53, 56, 59 and 62 °C for 20 min. Positive signals of the hybridization were detected by the integrated CCD camera of the PamStation®12 at the following exposure times (ET), 100, 300, 500, 800 and 1000 ms, using the Cy3 filter set to detect the FAM labelled hybridized RT-MLPA products.
Microarray Data Analyis
The recorded images of each array in the corresponding data files were transferred to the BioNavigator software (PamGene, The Netherlands) and were converted into spot intensity values. Median signal intensities and the local background were calculated for each spot on the hybridized array. The signal of local background was subtracted and the fluorescence saturation was limited to <1% of the spots. These calculated median signals were transported as text files, and the mean of signal intensity and standard deviation (SD) were calculated by Microsoft Exel and presented on melting curve diagrams.
Results
MLPA Reaction
An outline of the MLPA reaction is shown in Fig. 1. This reaction allows the detection of up to 45 different targets in one simple reaction, using a single PCR primer pair [28, 29]. Since the MLPA reaction was originally developed for genomic DNA applications [28], the authors started MLPA reactions using 100–150 ng highly purified PCR amplicons of the S-segments of the European Bunyaviruses TOSV, SFSV, SFNV, TAHV, INKV, BATV and UUKV as target template for the MLPA hybridisation.
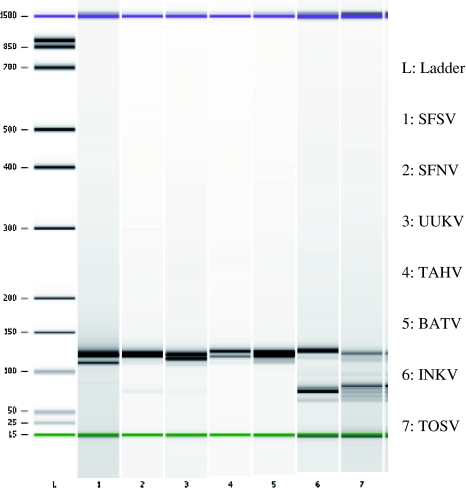
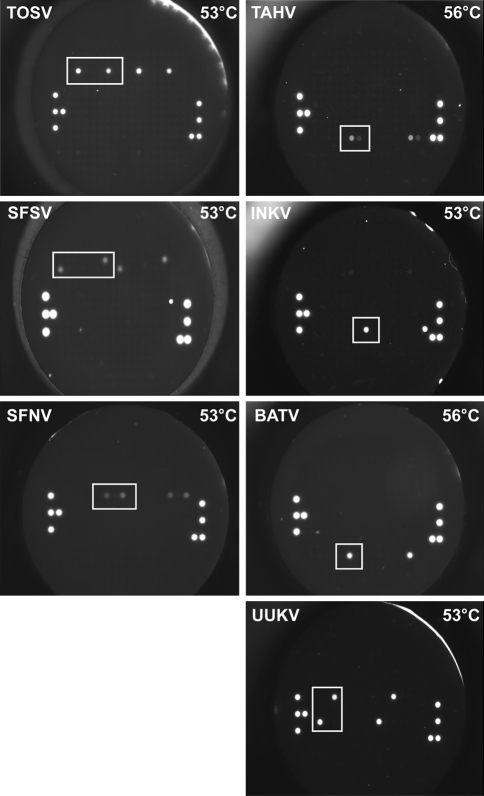
The target DNA was denaturated and corresponding MLPA probes were added and hybridized for 12–16 h at 60°C in a thermocycler with heated lid. Ligation and inactivation of ligase was followed by PCR amplification of the ligated MLPA probe system using a FAM labelled forward primer. The analysis of the MLPA amplicons using the Agilent Bioanalyzer confirmed the corresponding amplicon of all investigated MLPA systems. The amplicon bands of one virus migrated separately from each other but banded a little higher than expected. This was attributed to a combination of secondary DNA structure of the amplicons and the sizing error of the Bioanalyzer which is given as 10%. Furthermore the SFSV, INKV and TOSV showed additionally bands presenting an excess of primer dimers and unligated MLPA probe in the reaction (Fig. 2). Finally, the detection of these MLPA amplicons on the PamChip® 4U was performed using a continuous hybridisation temperature profile protocol at 50, 53, 56, 59 and 62 °C to identify the optimal hybridisation temperature of each MLPA probe set for each virus. The results showed that the authors could identify all seven European Bunyaviruses on the DNA level, however, the signal intensity of MLPA PCR amplicons of TOSV, BATV, INKV and UUKV were stronger than those of SFNV, SFSV and TAHV. The specific signals were accompanied by some background/cross-hybridisation at a hybridisation temperature of 50°C but disappeared at increased temperatures. The signals at optimal microarray hybridisation temperature are presented on Fig. 3. TOSV, SFSV, SFNV, INKV and UUKV showed optimal hybridisation temperature at 53°C, while TAHV and BATV hybridised optimal at 56°C.
Fig. 2.
Analysis of MLPA amplicons. Gel electrophoresis of MLPA PCR products generated by amplification of ligated MLPA probes for seven European Bunyaviruses. Two-three S-segment-based MLPA probe sets for each virus were used. L. Ladder, Lanes: 1. SFSV, 2. SFNV, 3. UUKV, 4. TAHV, 5. BATV, 6. INKV, 7. TOSV
Fig. 3.
MLPA hybridisation pattern on the flow-through chip. Representative images of MLPA amplicon hybridisation on flow-trough microarray for the detection of seven the European arboviruses, TOSV, SFSV, SFNV, TAHV, INKV, BATV and UUKV
RT-MLPA Reaction
After establishing the 3D microarray based MLPA assay at the DNA level, the authors introduced a RT step into the MLPA reaction for the transcription of target RNA into cDNA to adapt the method for the detection of European Bunyaviruses. Initially, the RT step was performed with specific primers but later the procedure was simplified using hexamer primers. The RT step preceded MLPA probe hybridization, MLPA probe ligation and probe amplification. Again the RT-MLPA amplicon size was examined and confirmed at the correct size by Agilent Bioanalyzer and then the amplicons were detected on the PamChip® 4U using the optimised hybridization protocol. The observed signals after the RT-MLPA amplicon microarray hybridization of all investigated viruses were similar to those of the MLPA amplicon microarray hybridisation (Figs. 3 and 4).
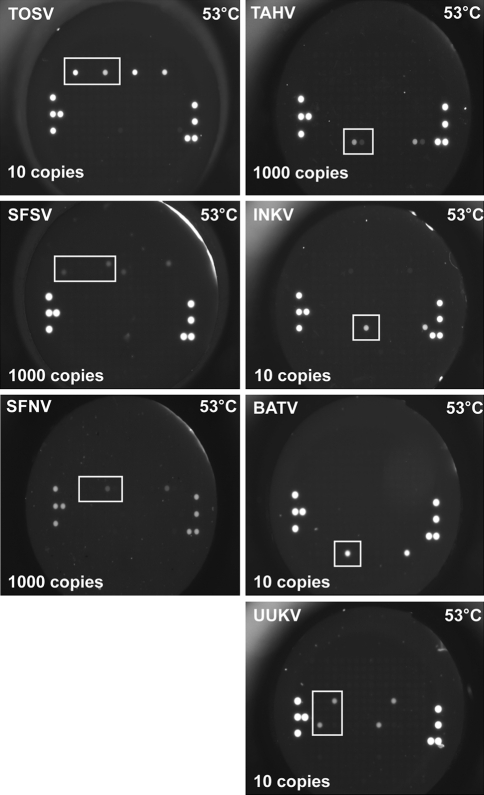
Fig. 4.
Sensitivity of RT-MLPA hybridisation patterns on the flow-through chip. Representative microarray images of RT-MLPA PCR products for the detection of seven European Arboviruses, showing detection limits for TOSV, SFSV, SFNV, TAHV, INKV, BATV and UUKV
Melting Curve Analysis
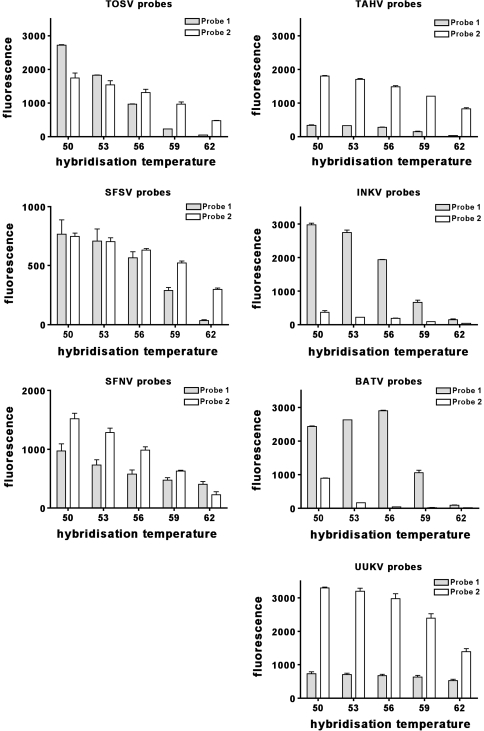
The authors generated the melting curves for each MLPA set from the continuous microarray hybridization fluorescence read out data at 50, 53, 56, 59 and 62 °C. The mean of the signal intensities of each MLPA probe set was calculated and plotted in Fig. 5, showing the signal intensity of each MLPA probe set at different hybridization temperatures. It was observed that in general the signal strength of each MLPA probe set decreased with increasing hybridisation temperature and with decreasing RNA molecule numbers as expected. The signal intensities of different MLPA probe sets for the detection of one particular virus showed distinct sensitivities. This type of analysis helped to identify highly sensitive probe sets. Remarkably, the optimal hybridisation temperature for all RT-MLPA amplicons was now observed at 53°C—ideal for a multiplex MLPA assay.
Fig. 5.
Melting analysis. Mean fluorescence values of hybridized RT-MLPA amplicons for the detection of two probe sets each for TOSV, SFSV, SFNV, TAHV, INKV, BATV and UKV. Standard RNAs (107–106), at 50, 53, 56, 59 and 62 °C
Sensitivity Analysis
In order to determine the sensitivity of the 3D microarray hybridization of RT-MLPA PCRs products on the PamChip® 4U, RNA standards for TOSV, SFSV, SFNV, TAHV, INKV, BATV and UUKV were generated (see Materials and Methods). Serial dilutions of RNA standards were prepared and subjected to by real-time PCR. Subsequently these serial dilutions of RNA standards were used for RT-MLPA reactions. The RT-MLPA hybridization step was performed with and without 20% formamide buffer to improve specific and reduce unspecific hybridisation. The RT-MLPA amplicons were detected on PamChip® 4U using the optimized microarray hybridization protocol described above.
It was observed an increased probe sensitivity using 20% formamide during the MLPA probe target hybridization reaction step in comparison to the standard procedure. Starting the procedure from the transcribed RNA standards the sensitivity of detection of the combined RT-MLPA and hybridisation on the PamChip® 4U for TOSV, BATV, UUKV and INKV was 10 copies, while SFSV, SFNV and TAHV was 1000 copies (Fig. 4).
Probe Specifity
In order to evaluate the specificity of the developed assay, all of the designed MLPA probes were tested in a monoplex and in a multiplex RT-MLPA reaction, containing the generated RNA standards of TOSV, SFSV, SFNV, TAHV, INKV, BATV and UUKV. The generated RT-MLPA PCR amplicons were purified to remove the unligated MLPA probes, analysed by gel electrophoresis and subsequently hybridized on the PamChip® 4U. The results clearly show that even in the multiplex mixture containing all investigated probe sets only specific MLPA hybridization, ligation and hybridisation to the microarray occurred. Cross detection was not observed.
In order to test for cross reactions of the microarray-based MLPA assays on the Pamgene system, all total RNAs of TOSV, SFSV, SFNV, TAHV, INKV, BATV and UUKV were extracted from the supernatants of the corresponding viral cell cultures and used as template for RT-MLPA reaction. Afterwards the RT-MLPA amplicons were examined by gel electrophoresis on their amplicon size and analysed by PamGene microarray hybridization on PamChip® 4U. The data analysis showed similar results to those with standards RNAs for all investigated European Bunyaviruses. The pattern of the specific signal for each virus was observed as expected. Again cross detection was not observed.
Comparison of RT-MLPA Microarray assays with Real-time RT-PCR assays
The capacity of both assays to detect each targeted virus was investigated by using RNA standards and RNAs from viral cell cultures. As mentioned above serial dilution of standard RNAs and RNA from cell culture for TOSV, SFSV, SFNV, TAHV, INKV, BATV and UUKV were performed and used for real-time RT-PCR, MLPA, as well as RT-MLPA flow-through microarray assays. The results are shown in Table 2, indicating that the sensitivity of each monoplex real-time PCR assay ranged from 10 to 1000 copies of target RNA per reaction, depending on the virus targeted. The sensitivity of MLPA or RT-MLPA flow-through microarray assay ranged from 10 copies for TOSV, INKV, BATV and UUKV to 1000 copies for SFSV, SFNV and TAHV. In most cases, the sensitivity of both methods was comparable.
Table 2.
Analytical sensitivities of various assay types during the MLPA-microarray development given as molecules detected
| Virus assay | RT-PCR | MLPA | MLPA-microarray |
|---|---|---|---|
| TOSV | 101 | 101 | 101 |
| SFSV | 102 | 102 | 103 |
| SFNV | 102 | 102 | 103 |
| TAHV | 102 | 102 | 103 |
| INKV | 103 | 101 | 101 |
| BATV | 101 | 101 | 101 |
| UUKV | 101 | 101 | 101 |
Discussion
The authors have developed a combined MLPA and universal flow-through microarray detection assay for European Bunyaviruses, particularly, TOSV, SFSV, SFNV, TAHV, INKV, BATV and UUKV. The combined assay design used the MLPA technique the amplicons of which were hybridized to the 3D universal flow-through microarray (PamChip® 4U). MLPA probes (LPO/RPO) were designed for two conserved regions of the Bunyavirus S-segments. After MLPA or RT-MLPA amplification using a FAM labelled driving primer the amplicon samples were actively pumped back and forth through the porous structure of the array membrane of the PamChip® 4U by air pressure, and hybridised to the zip-code addresses which are immobilized on the array membrane.
The aluminium-oxide matrix significantly improves the signal capture, as compared with spotted arrays or in situ synthesised arrays on glass surfaces [30]. The signal intensities observed for the hybridisation results were indeed very strong and specific for all analyzed viruses, indicating high efficiency and fast hybridisation on the array during their identification.
The procedure of the hybridisation on the chip at the temperature range used (50, 53, 56, 59 and 62 °C) took only about 2 h. This is an advantage over longer (overnight) hybridisation periods used in many glass slide based microarray systems [27, 31]. The MLPA probe hybridisation, however, is still very long and at 12–18 h almost negates the advantages of the rapid microarray hybridisation.
Although the Cpam sequences conveying the hybridisation onto the microarray zip- code (Pam4U sequence) probes, have all been designed to meet a narrow hybridisation reaction window, the authors observed differing hybridisation characteristics of the various RT-MLPA-PCR amplicons to the respective microarray probes (Fig. 5). This may be explained by the variant sequences flanking the Cpam hybridisation sequence (especially the virus-specific MLPA hybridisation sequences RHS/LHS) and the influence they elicit on the TM of the Cpam sequence and on the multiple hybridisation states that can occur between hybridisation partners as described by Santa Lucia [32, 33].
Quan et al. [21] reported that randomly primed amplification of DNA microarray assays allow the detection of a large number of pathogens. The sensitivity of such a generic approach is, however, much reduced in comparison to pathogen-specific priming [34, 35] and is the main reason why microarray diagnostics have not yet arrived in the clinical diagnostic laboratory [36].
In order to compare the sensitivity of Taqman probe realtime RT-PCR assays and RT-MLPA assays, the respective amplicons and MLPA probes were designed in the same target region. The comparative analysis showed that the analytical sensitivity of the real-time PCR and MLPA assays was almost identical (Table 2 columns 2 and 3) and only a little loss of sensitivity was observed for the detection of SFSV, SFNV and TAHV viruses when the RT-MLPA amplicons were hybridised to the microarray (Table 2 columns 2 and 4). Remarkably, the sensitivity of INKV virus MLPA amplicon hybridisation on the chip was higher than real-time PCR. These results confirm that an amplification step is necessary to achieve a high sensitivity in microarray hybridisation assays. It should be noted that in the case of the MLPA the detection signal and not the target sequence is amplified, which significantly reduces the problem of target molecule carry over contamination in a diagnostic setting.
The data analysis was easy to perform thus simplifying the microarray data management. The obtained data were highly reproducible, as the standard deviation and the variance of the calculated mean for the individual spot signal intensity was negligible (data not shown). Altogether the results support other reports on MLPA assays combined with flow through microarrays describing high sensitivity, specificity, speed, and reproducibility [37, 38].
Although real-time RT-PCR is fast and sensitive, it is typically designed for detecting one to three targets per reaction. For the detection of seven Bunyaviruses, the samples would need to be divided into multiple reactions. This may be an issue when the amount of sample (e.g., cerebrospinal fluid) is small. In contrast, the MLPA method combined with the narrow reaction window hybridisation (zip-codes) on a 3D flow-through array allows the highly complex analysis of up to 45 targets and the authors believe that the assay format is easily extensible to include more viruses.
For the evaluation of the specificity of the monoplex and the multiplex RT-MLPA for the mentioned viruses, the authors used the corresponding standard RNAs or viral RNAs extracted from cell cultures as target molecules for RT-MLPA reaction. The results showed that MLPA probes were specific and did not cross-hybridize to any of the other tested European Bunyaviruses or to the cellular background. The MLPA products showed specific hybridisation to the microarray.
In order to optimize the multiplex assay it was therefore intend to extend the MLPA probe design to M- and L-segments of these viruses to create a higher specificity since amplification probes of any kind suffer from sequence variation mismatch.
Currently, there is no report on the use of a microarray for the detection of Bunyaviruses, but similar assays have been reported for the detection of respiratory viruses [39, 40], West Nile virus and Dengue virus in clinical materials [38, 41].
A multiplex MLPA assay using an RT-PCR pre-amplification step developed for the simultaneous detection of six DNA viruses causing infections of the central nervous system termed MeningoFinder [42] was evaluated using extracts from cerebrospinal fluid (CSF) samples. Similarly the RespiFinder RT-MLPA system was used for the simultaneous detection of fifteen respiratory RNA viruses in clinical samples. It included a pre-amplification step by RT-PCR protocol to reduce the hybridisation time of the MLPA reaction from 12 to 1 h. The improved hybridisation time of the RespiFinder, however, is achieved at the cost of another contamination risk during pre-amplification [39].
In another approach for the detection of respiratory viruses a multiplex PCR followed by primer extension and microarray hybridization was also used and showed good agreement with real-time RT-PCR when using clinical samples [40]. The most comparable study is the development of a multiplex reverse transcriptase PCR-ligase detection assay (RT-LDR) which hybridised to a universal microarray for the detection of several West Nile virus genes [38] and a similar setup for the four Dengue viruses which was successfully tested using clinical samples [41]. It therefore seems highly likely that the RT-MLPA combined with flow-through universal microarray assay presented here, which also uses a ligation step without pre-amplification of the target sequence could be as sensitive in clinical samples as it was shown using synthetic RNA standards and viral cell cultures.
In conclusion, the authors have shown that combined multiplex MLPA and 3D universal microarray assay is sufficiently sensitive and specific for the detection of European Bunyaviruses and show good correlation to monoplex real-RT-PCR, therefore the authors expect that this method can be developed into a diagnostic tool for the rapid analysis of infections due to European Arboviruses.
Acknowledgments
The authors thank gratefully Monique Mommersteeg and Faris Naji (PamGene company, Netherlands) for the technical assistance by the establishment of this method and data analysis, respectively. The study was supported by the Federal Ministry of Education and Research (BMBF), grant number 01KI0710, “Research on Zoonotic Infectious Diseases” programme, Emerging arthropode-borne viral infections in Germany: Pathogenesis, diagnostics and surveillance.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s12033-011-9397-3
References
- 1.Semenza JCM, Menne B. Climate change and infectious diseases in Europe. The Lancet Infectious Diseases. 2009;9(6):365–375. doi: 10.1016/S1473-3099(09)70104-5. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed J, Bouloy M, Ergonul O, Fooks A, Paweska J, Chevalier V, et al. International network for capacity building for the control of emerging viral vector-borne zoonotic diseases: ARBO-ZOONET. Eurosurveillance. 2009;14(12):19160. [PubMed] [Google Scholar]
- 3.Rudolf I, Hubalek Z, Sikutova S, Svec P. Neglected arthropod-borne viral infections in the Czech Republic. Epidemiol Mikrobiol Imunol. 2008;57(3):80–89. [PubMed] [Google Scholar]
- 4.Sanchez-Seco MP, Navarro JM. Infections due to Toscana virus, West Nile virus, and other arboviruses of interest in Europe. Enfermedades Infecciosas Y Microbiologia Clinic. 2005;23(9):560–568. doi: 10.1157/13080267. [DOI] [PubMed] [Google Scholar]
- 5.Lundstrom JO. Mosquito-borne viruses in Western Europe: a review. Journal of Vector Ecology. 1999;24(1):1–39. [PubMed] [Google Scholar]
- 6.Hubalek Z. Biogeography of tick-borne bhanja virus (bunyaviridae) in europe. Interdisciplinary Perspectives on Infectious Diseases. 2009;2009:372691. doi: 10.1155/2009/372691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rockx B, Van Asten L, Van Den Wijngaard C, Godeke GJ, Goehring L, Vennema H, et al. Syndromic surveillance in the Netherlands for the early detection of West Nile virus epidemics. Vector-Borne and Zoonotic Diseases. 2006;6(2):161–169. doi: 10.1089/vbz.2006.6.161. [DOI] [PubMed] [Google Scholar]
- 8.Lennette EH, Smith TF. Laboratory diagnosis of viral infections. 3. New York: Marcel Dekker inc.; 1999. [Google Scholar]
- 9.Storch GA. Essentials of diagnostic virology. Clinical laboratory science. New York: Churchill Livingstone; 2001. [Google Scholar]
- 10.Read SJ, Burnett D, Fink CG. Molecular techniques for clinical diagnostic virology. Journal of Clinical Pathology. 2000;53(7):502–506. doi: 10.1136/jcp.53.7.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahony JB. Detection of respiratory viruses by molecular methods. Clinical Microbiology Reviews. 2008;21(4):716–747. doi: 10.1128/CMR.00037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackay IM, Arden KE, Nitsche A. Real-time PCR in virology. Nucleic Acids Research. 2002;30(6):1292–1305. doi: 10.1093/nar/30.6.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watzinger F, Ebner K, Lion T. Detection and monitoring of virus infections by real-time PCR. Molecular Aspects of Medicine. 2006;27(2–3):254–298. doi: 10.1016/j.mam.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Templeton KE, Scheltinga SA, Beersma MFC, Kroes ACM, Claas ECJ. Rapid and sensitive method using multiplex real-time PCR for diagnosis of infections by influenza B virus, respiratory syncytial virus, and parainfluenza viruses 1, 2, 3, and 4. Journal Of Clinical Microbiology. 2004;42(4):1564–1569. doi: 10.1128/JCM.42.4.1564-1569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grondahl B, Puppe W, Hoppe A, Kuhne I, Weigl JA, Schmitt HJ. Rapid identification of nine microorganisms causing acute respiratory tract infections by single-tube multiplex reverse transcription-PCR: feasibility study. Journal of Clinical Microbiology. 1999;37(1):1–7. doi: 10.1128/jcm.37.1.1-7.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weidmann M, Armbruster K, Hufert FT. Challenges in designing a Taqman-based multiplex assay for the simultaneous detection of Herpes simplex virus types 1 and 2 and Varicella-zoster virus. Journal of Clinical Virology . 2008;42(4):326–334. doi: 10.1016/j.jcv.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Wang D, Coscoy L, Zylberberg M, Avila PC, Boushey HA, Ganem D, et al. Microarray-based detection and genotyping of viral pathogens. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(24):15687–15692. doi: 10.1073/pnas.242579699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D, Urisman A, Liu YT, Springer M, Ksiazek TG, Erdman DD, et al. Viral discovery and sequence recovery using DNA microarrays. PLoS Biology. 2003;1(2):E2. doi: 10.1371/journal.pbio.0000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nordstrom H, Falk KI, Lindegren G, Mouzavi-Jazi M, Walden A, Elgh F, et al. DNA microarray technique for detection and identification of seven flaviviruses pathogenic for man. Journal of Medical Virology. 2005;77(4):528–540. doi: 10.1002/jmv.20489. [DOI] [PubMed] [Google Scholar]
- 20.Palacios G, Quan PL, Jabado OJ, Conlan S, Hirschberg DL, Liu Y, et al. Panmicrobial oligonucleotide array for diagnosis of infectious diseases. Emerging Infectious Diseases. 2007;13(1):73–81. doi: 10.3201/eid1301.060837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quan PL, Palacios G, Jabado OJ, Conlan S, Hirschberg DL, Pozo F, et al. Detection of respiratory viruses and subtype identification of influenza A viruses by GreeneChipResp oligonucleotide microarray. Journal of Clinical Microbiology. 2007;45(8):2359–2364. doi: 10.1128/JCM.00737-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin B, Blaney KM, Malanoski AP, Ligler AG, Schnur JM, Metzgar D, et al. Using a resequencing microarray as a multiple respiratory pathogen detection assay. Journal of Clinical Microbiology. 2007;45(2):443–452. doi: 10.1128/JCM.01870-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weidmann M, Rudaz V, Nunes MR, Vasconcelos PF, Hufert FT. Rapid detection of human pathogenic orthobunyaviruses. Journal of Clinical Microbiology. 2003;41(7):3299–3305. doi: 10.1128/JCM.41.7.3299-3305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weidmann M, Sanchez-Seco MP, Sall AA, Ly PO, Thiongane Y, Lo MM, et al. Rapid detection of important human pathogenic Phleboviruses. Journal of Clinical Virology. 2008;41(2):138–142. doi: 10.1016/j.jcv.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Weidmann M, Muhlberger E, Hufert FT. Rapid detection protocol for filoviruses. Journal of Clinical Virology. 2004;30(1):94–99. doi: 10.1016/j.jcv.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Wu Y, De Kievit P, Vahlkamp L, Pijnenburg D, Smit M, Dankers M, et al. Quantitative assessment of a novel flow-through porous microarray for the rapid analysis of gene expression profiles. Nucleic Acids Research. 2004;32(15):e123. doi: 10.1093/nar/gnh118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hokaiwado N, Asamoto M, Tsujimura K, Hirota T, Ichihara T, Satoh T, et al. Rapid analysis of gene expression changes caused by liver carcinogens and chemopreventive agents using a newly developed three-dimensional microarray system. Cancer Science. 2004;95(2):123–130. doi: 10.1111/j.1349-7006.2004.tb03192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schouten JP, Mcelgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Research. 2002;30(12):e57. doi: 10.1093/nar/gnf056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng F, Ren ZR, Huang SZ, Kalf M, Mommersteeg M, Smit M, et al. Array-MLPA: Comprehensive detection of deletions and duplications and its application to DMD patients. Human Mutation. 2008;29(1):190–197. doi: 10.1002/humu.20613. [DOI] [PubMed] [Google Scholar]
- 30.Kim J, Miles A, Gale BK. Improved biomolecule microarrays by printing on nanoporous aluminum oxide using a continuous-flow microspotter. Small. 2010;6(13):1415–1421. doi: 10.1002/smll.200902406. [DOI] [PubMed] [Google Scholar]
- 31.van Moorsel CH, an Wijngaarden EE, Fokkema IF, Den Dunnen JT, Roos D, Van Zwieten R, et al. beta-Globin mutation detection by tagged single-base extension and hybridization to universal glass and flow-through microarrays. European Journal of Human Genetics. 2004;12(7):567–573. doi: 10.1038/sj.ejhg.5201192. [DOI] [PubMed] [Google Scholar]
- 32.SantaLucia J, Jr, Hicks D. The thermodynamics of DNA structural motifs. Annual Review of Biophysics and Biomolecular Structure. 2004;33:415–440. doi: 10.1146/annurev.biophys.32.110601.141800. [DOI] [PubMed] [Google Scholar]
- 33.SantaLucia J., Jr Physical principles and visual-OMP software for optimal PCR design. Methods in Molecular Biology. 2007;402:3–34. doi: 10.1007/978-1-59745-528-2_1. [DOI] [PubMed] [Google Scholar]
- 34.Sengupta S, Onodera K, Lai A, Melcher U. Molecular detection and identification of influenza viruses by oligonucleotide microarray hybridization. Journal of Clinical Microbiology. 2003;41(10):4542–4550. doi: 10.1128/JCM.41.10.4542-4550.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kessler N, Ferraris O, Palmer K, Marsh W, Steel A. Use of the DNA flow-thru chip, a three-dimensional biochip, for typing and subtyping of influenza viruses. Journal of Clinical Microbiology. 2004;42(5):2173–2185. doi: 10.1128/JCM.42.5.2173-2185.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vernet G. Molecular diagnostics in virology. Journal of Clinical Virology. 2004;31(4):239–247. doi: 10.1016/j.jcv.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerry NP, Witowski NE, Day J, Hammer RP, Barany G, Barany F. Universal DNA microarray method for multiplex detection of low abundance point mutations. Journal of Molecular Biology. 1999;292(2):251–262. doi: 10.1006/jmbi.1999.3063. [DOI] [PubMed] [Google Scholar]
- 38.Rondini S, Pingle MR, Das S, Tesh R, Rundell MS, Hom J, et al. Development of multiplex PCR-ligase detection reaction assay for detection of West Nile virus. Journal of Clinical Microbiology. 2008;46(7):2269–2279. doi: 10.1128/JCM.02335-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reijans M, Dingemans G, Klaassen CH, Meis JF, Keijdener J, Mulders B, et al. RespiFinder: A new multiparameter test to differentially identify fifteen respiratory viruses. Journal of Clinical Microbiology. 2008;46(4):1232–1240. doi: 10.1128/JCM.02294-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raymond F, Carbonneau J, Boucher N, Robitaille L, Boisvert S, Wu WK, et al. Comparison of automated microarray detection with real-time PCR assays for detection of respiratory viruses in specimens obtained from children. Journal of Clinical Microbiology. 2009;47(3):743–750. doi: 10.1128/JCM.01297-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Das S, Pingle MR, Munoz-Jordan J, Rundell MS, Rondini S, Granger K, et al. Detection and serotyping of dengue virus in serum samples by multiplex reverse transcriptase PCR-ligase detection reaction assay. Journal of Clinical Microbiology. 2008;46(10):3276–3284. doi: 10.1128/JCM.00163-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolffs PF, Vink C, Keijdner J, Habek B, Reijans M, et al. Evaluation of MeningoFinder, a novel multiplex ligation-dependent probe amplification assay for simultaneous detection of six virus species causing central nervous system infections. Journal of Clinical Microbiology. 2009;47(8):2620–2622. doi: 10.1128/JCM.02436-08. [DOI] [PMC free article] [PubMed] [Google Scholar]