Abstract
Based on the multiple logistic regression analysis of data from a random sample of 1,023 old adults collected in Taiwan in 2000, we found that interactions between carrying the APOE4 allele and one of four life stress factors (relocated mainlander, living in a crowded household with six or more persons, living in an earthquake-damaged house, and monthly financial difficulty) significantly increased the odds ratio of poor self-reported health. Correlations between carrying the APOE4 allele and the life stress factors were ruled out by statistical tests. These life stress factors had a substantially larger adverse impact on self-reported health in APOE4 allele carriers than in noncarriers. This study provides evidence that interaction between carrying APOE4 allele and chronic life stressors has significant impacts on self-reported health while controlling for various sociodemographic and health behavior factors. Further studies with richer biomarkers are warranted for deeper understanding of the biological mechanisms.
Keywords: APOE4 allele, Life stress, Genetic–environment interactions, Self-reported health, Population aging
SELF-REPORTED health (SRH) has often been used as an indicator of underlying health status in population health research. Studies have shown that SRH is associated with mortality (1–3) and can sometimes be a better predictor of diverse aspects of well-being than clinical factors among older people (4). SRH itself is predicted by several dimensions of healthy aging—independent living, vision, hearing, activities of daily living, instrumental activities of daily living, absence of physical illness, cognition, healthy mood, social support and participation, and religious participation and spirituality (5). These relationships imply that SRH is a good indicator of health among older adults. Therefore, examination of other dimensions that influence SRH should help improve our understanding of healthy aging in human populations.
A number of social and behavioral factors are associated with SRH. Advanced age, female gender, and low socioeconomic status are important risk factors for poor SRH (6–8). Socioeconomic status at the community level may be important too as it has been reported that individuals living in poor neighborhoods in seven Dutch cities tend to have poor SRH (9). Health behaviors such as active physical exercise may reduce the likelihood of poor SRH (10). Social variables such as social network participation and support are associated with better SRH (11). A recent study demonstrated that both chronic life stress and stress from a traumatic shock are strongly associated with poor SRH, and the effects seem to be stronger in women (12). Life stress may affect health outcomes in several ways, including via activating the hypothalamic–pituitary–adrenal axis and sympathetic nervous system, which has been associated with elevated risk of poor health outcomes, such as hypertension and heart disease, if activation is excessive or prolonged (13). Stress also may affect health through changes in immune function (14).
Apolipoprotein E (APOE), one of the most studied genes in the human population, is associated with a number of chronic diseases. Studies have shown that carriers of the APOE4 allele have an increased risk for cardiovascular disease, Alzheimer’s disease, and Parkinson’s disease (15–18). The APOE4 allele is associated with a number of major health outcomes, including mortality, decline of cognitive function, decreased functional status among elderly participants without dementia (19,20), and an earlier age of onset of Alzheimer’s disease and Parkinson’s disease (21,22). A twin-based study suggested that one third of the variability in SRH can be attributed to genetic factors (23). Recently, the APOE4 allele was found to be associated with poor SRH; the association was stronger in women than in men, after controlling for demographic factors, socioeconomic status, and health behaviors (12). These findings suggest that the APOE4 allele may play a multifaceted role in exacerbating a number of disease conditions associated with human aging.
Research has shown that, in addition to direct genetic transmission, the interactions between genetic and environmental factors also play a crucial role in health and aging (24); environmental factors may regulate gene expression via DNA methylation and histone modification, which then influence health and longevity of the elderly participants (25,26). Thus, gene expressions depend on the environment surrounding the organism and gene–environment interactions. Based on intensive literature reviews and investigations/discussions, including several workshops, the Institute of Medicine Committee concluded in their widely cited report: “Few diseases or conditions are caused purely by genetic factors; most are the result of interactions between genetic and environmental factors. Therefore, to expand our knowledge of how to improve the health of individuals and populations, it becomes imperative to conduct research that explores the effects of interactions among social, behavioral, and genetic factors on health” (25).
Given the growing consensus concerning the importance of interactions between genotypes and environmental risk factors (abbreviated as “G × E” hereafter), several studies have indicated sizable impacts of interactions between the APOE4 genotype and environmental exposures. For example, the impact of a number of environmental factors on the risk of cardiovascular disorders and neurological disorders such as Parkinson’s disease appears to be exacerbated in carriers of the APOE4 allele (27). Similarly, a study on the expected benefit of physical activity in reducing risk of dementia did not observe this beneficial effect in APOE4 carriers, further demonstrating the potential adverse effect of the APOE4 allele (28).
A number of animal studies have focused on examining how stress level and APOE status interact to affect glucocorticoid levels and cognitive performance; however, only a limited number of studies in this field have been conducted in humans (29). In human studies, both stress and the APOE4 allele have been associated with chronic elevation in cortisol, which may lead to memory loss (29,30). One study found that increased life stress levels were associated with more depressive symptoms in nondemented female caregivers who are carriers of APOE4 allele, but this relationship was not significant in noncarriers of APOE4 (31). This suggests that carriers of the APOE4 allele may respond differently to psychological stress than do noncarriers of the APOE4 allele. Although suggesting that there might be a theoretical basis for important interactions between life stress factors and APOE4 status, these studies were based on small sample sizes of less than 100 participants, and potential confounders such as sociodemographic and behavioral factors were not controlled for (29–31).
Our careful literature review has indicated that, although much prior research have discovered the association between APOE4 and health outcome including SRH as well as the association between environmental factors and SRH, few studies have investigated the effects of the interactions between carrying the APOE4 allele and the environmental risk factors on SRH in general; more specifically, in our literature search, we could locate no population-based studies with large sample sizes adequate for genetic analysis that have been conducted to examine the interaction between APOE4 status and life stress on SRH or other health conditions in older adults. Our present study fills this research gap. Because SRH is believed to be a summary indicator of different dimensions of healthy aging and based on the theoretical foundation that both APOE4 and prolonged stress are associated with poor health outcomes, we hypothesize that interactions between APOE4 status and life stress factors may significantly increase the odds of self-reported poor heath; namely, the negative effects of life stress on SRH may be substantially more serious in APOE4 carriers than in noncarriers. We have tested this hypothesis via multiple random-effect logistic regression analysis of data collected from a population-based study of older adults in Taiwan while controlling for demographic, socioeconomic, and health behavior factors.
DATA SOURCE, MEASUREMENTS, AND METHODS
Data Source and Study Participants
The study participants were adults 55 years of age or older from the Social Environment and Biomarkers of Aging Study conducted in 2000, which was based on a random subsample of respondents to the longitudinal Survey of Health and Living Status in Taiwan. Social Environment and Biomarkers of Aging Study randomly selected a total of 1,713 respondents from the 1999 wave of the Survey of Health and Living Status (4). Of the 1,713 selected Survey of Health and Living Status respondents, 1,497 (87.4%) participated in the Social Environment and Biomarkers of Aging Study interviews. Of the respondents who were interviewed, 1,023—590 men (58%) and 433 women (42%)—participated in the physical examination and biospecimen collection (32).
Each face-to-face in-home interview was conducted by a local public health nurse. On a scheduled day several weeks after the interview, participants collected a urine sample after fasting 12 hours overnight and had a medical exam the next morning at a nearby hospital, where medical personnel drew a blood specimen and anthropometric measurements from the participants (32).
Measurements
The health outcome measure was SRH, which was originally measured on a 5-point scale: excellent, good, average, not so good, and poor (12.0, 13.0, 47.8, 23.58, and 3.5%, respectively, in this study sample). For multiple regression analysis, a binary SRH score was recoded as 1 if an individual rated his or her health as not so good or poor and 0 if excellent, good, or average. Because we mainly focused on examining the interaction of APOE4 and life stress factors, we classified APOE4 genotype into a binary variable (APOE4 carrier status) following the dominant model. Individuals who carry one or two copies of the minor allele of APOE4 are coded as “1” (carrier), and the individuals who do not carry the minor allele are coded as “0” (noncarrier).
As discussed in the literature, life stress can be measured by perceived stress, economic difficulties, security, and safety, and it has been associated with physiological dysregulation in older adults (33,34). In this study, we use the variable of six or more persons living in the household and difficulty meeting monthly living expenses to indicated chronic living environment and financial stress. Experiencing housing damage during the 1999 earthquake is used to measure life stress due to a traumatic event. We also used another variable as an indicator of life stress: mainlander people who were forced to leave the Mainland of China for Taiwan in 1948–1949 at the end of the Chinese Civil War. Almost all of them have been isolated from the rest of their family of origin and hometowns throughout their life up to 2000 when the survey was conducted. (The forced relocation of about one million military force and governmental officers of the Kuomingtang party from the Mainland to Taiwan occurred in 1948–1949. Consequently, almost all the mainlander participants aged 55 years and older were forced to come to Taiwan either as an adult or as a child with parents and totally isolated from the rest of their family members and hometowns throughout their life up to 2000. The exchanges and communications between Taiwan and the Mainland only became increasingly available after 2000 [and accelerated in most recent years].) As a result, they and their family members may have suffered from chronic life stress.
Control variables included in the analyses were health behaviors and sociodemographic factors. Health behaviors included diet, alcohol use, smoking, and exercise. Socio demographic factors included age, gender, marital status, urban or rural residence, education, and occupation. Obesity, defined as body mass index greater than 30 weight/height (kg/m2), was included to measure physical condition. Table 1 shows the distributions of sociodemographics by gender. There were remarkable differences in age, ethnicity, education, and occupation between men and women. The nonmainlander Fujianese and Hakka people (Both Fujianese and Hakka people are the native Taiwanese.) account for 71% and 12% of the total sample, respectively, whereas mainland people account for 17%.
Table 1.
Sample Distribution (%) of Sociodemographic Characteristics of the Participants
| Variable | Categories | Total | Men | Women |
| N = 1,023 | N = 590 | N = 433 | ||
| Marital status | Married | 71 | 80 | 59 |
| Widowed | 24 | 13 | 39 | |
| Others | 5 | 7 | 2 | |
| Total | 100 | 100 | 100 | |
| Age | <60 | 20 | 20 | 22 |
| 60–69 | 32 | 29 | 36 | |
| 70–79 | 38 | 42 | 33 | |
| 80+ | 9 | 9 | 9 | |
| Total | 100 | 100 | 100 | |
| Ethnicity | Fujian | 71 | 65 | 78 |
| Hakka | 12 | 11 | 15 | |
| Mainlander | 17 | 24 | 8 | |
| Total | 100 | 100 | 100 | |
| Education | No education | 33 | 19 | 53 |
| Primary education | 40 | 45 | 33 | |
| Secondary education | 19 | 25 | 11 | |
| College and above | 7 | 11 | 2 | |
| Total | 100 | 100 | 100 | |
| Occupation | Professional | 5 | 6 | 4 |
| Clerical | 21 | 31 | 8 | |
| Blue-collar worker | 35 | 37 | 32 | |
| Farmer | 39 | 26 | 56 | |
| Total | 100 | 100 | 100 | |
| Obesity | % obese (body mass index >30) | 7 | 10 | 4 |
| Residence | % urban residents | 30 | 31 | 29 |
APOE was genotyped using the polymerase chain reaction amplification refractory mutation system (PCR_ARMS) and polymerase chain reaction restriction fragment length polymorphism (PCR_RFLP) analysis. The chi-square test was performed to test Hardy–Weinberg equilibrium in the APOE gene so that one could assess potential genotyping error and the appropriateness of sample selection (32).
Statistical Analysis
Because the Social Environment and Biomarkers of Aging Study survey design involved multistage cluster sampling, individuals within the same cluster may be correlated statistically because they share some unobserved heterogeneity. Statistically, ignoring this type of correlation may lead to overestimates of standard errors of the parameters. Therefore, multiple random-effect logistic regression was performed to correct for the intra-cluster correlation by including random effects into the model.
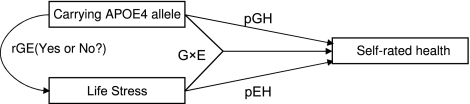
Note that statistically significant estimates of interaction terms derived from the multiple random-effect logistic regression analysis represent significant synergistic effects of the genetic–environment interactions. The synergistic effects may not exactly reflect the interaction effects of G × E as the estimates may or may not be confounded by gene–environment correlations (abbreviated as “rGE” hereafter). The rGE refers to the idea that certain genotypes have causal effects on environmental risk factors and influence health outcomes indirectly through these pathways; in other words, genes influence people’s tendencies to create their own environments (35,36). Thus, we need to do additional analysis to detect whether rGE exists. For all significant estimates of G × E interaction terms with a continuous environmental variable, we use the analysis of variance model and the multiple regression model (adjusting for the confounders) to test whether there are significant differences in the continuous environmental factors between the carriers and noncarriers of the APOE4 genotype. For all significant estimates of G × E interaction terms with a discrete environmental variable, we use the chi-squared test and the multiple regression model (adjusting for the confounders) to assess whether there are significant differences in the environmental categories between the carriers and noncarriers of the APOE4 genotype. For all significant rGE detected by analysis of variance or chi-square test and the multiple regression analysis (Note that if the chi-squared test or the analysis of variance F test [both are not adjusted for any covariates] shows a significant difference in the frequency or the mean of the environmental factor, we need to further perform the multiple regression analysis because the difference may be due to the other covariates rather than the genotype.), we need to conduct path analysis employing advanced structural equation modeling or other kinds of multivariate statistical analysis, adjusted for the potentially confounding factors, to further explore the interplay among genetic variant, environment, and health outcome. Based on the path analysis, we may estimate the direct genetic effect (pGH in Figure 1) and the genetic–environment interaction effects (G × E in Figure 1) on the health outcome; we may also decompose the total effects of the environmental exposure on the health outcome (pEH in Figure 1) into two components: (a) the genetic factor’s indirect effects that work through its impacts on environmental exposure and (b) the effects solely caused by the environmental exposure (37–39)—see Figure 1 for the theoretical framework of analysis to explore the gene–environmental interactions (G × E) and correlations (rGE).
Figure 1.
Theoretical framework of analysis to explore the gene–environmental interactions (G × E) and correlations (rGE).
All analyses were performed using STATA statistical software Release 9 (40).
RESULTS
Table 2 provides the distribution of prevalence of poor SRH by a number of life stress factors. Overall, there was little difference in prevalence of poor SRH by ethnicity. Among APOE4 carriers, mainlander people reported poor SRH more often (50%) compared with the Fujian (30.69%) and Hakka (18.75%). However, this ethnic pattern was not observed in APOE4 noncarriers. Individuals who lived in a crowded household with six or more persons, had monthly financial difficulties, or whose house was damaged during the 1999 earthquake (which occurred 1 year before the interview) were more likely to have poor SRH. This was consistent among APOE4 carrier and noncarrier groups. APOE4 allele carriers tended to have higher prevalence of poor SRH (32.87%) than APOE4 noncarriers (25.99%).
Table 2.
Prevalence Rate (%) of Self-reported Poor Health by Life Stress Factors Among APOE4 Allele Carriers* and Noncarriers
| All Participants |
APOE4 Allele Noncarriers |
APOE4 Allele Carriers |
|||||
| Categories | N | % Poor SRH | N | % Poor SRH | N | % Poor SRH | |
| Ethnicity | Fujian | 712 | 27.39 | 611 | 26.84 | 101 | 30.69 |
| Hakka | 122 | 24.59 | 106 | 25.47 | 16 | 18.75 | |
| Mainlander | 171 | 26.9 | 145 | 22.76 | 26 | 50.00 | |
| Household size | Living alone | 75 | 22.67 | 70 | 22.86 | 5 | 20 |
| 2–5 | 454 | 22.91 | 386 | 22.02 | 68 | 27.94 | |
| ≥ 6 | 473 | 31.29 | 403 | 30.02 | 70 | 38.57 | |
| Monthly financial difficulty | No | 753 | 23.64 | 649 | 22.65 | 104 | 29.81 |
| Yes | 249 | 37.35 | 211 | 36.49 | 38 | 42.11 | |
| 1999 earthquake-damaged house | No | 882 | 25.74 | 756 | 25 | 126 | 30.16 |
| Yes | 123 | 35.77 | 106 | 33.02 | 17 | 52.94 | |
| Living in bad neighborhood | No | 952 | 26.58 | 815 | 25.28 | 137 | 34.31 |
| Yes | 52 | 34.62 | 46 | 39.13 | 6 | 0 | |
| APOE4 allele carriers | No | 862 | 25.99 | ||||
| Yes | 143 | 32.87 | |||||
Notes: SRH = self-reported health.
Carrying one or two copies of the APOE4 minor allele, following the dominant model.
Table 3 presents APOE carrier status frequency by age group and gender. The proportion carrying at least one copy of the APOE4 allele was slightly different between women (16.2%) and men (13.1%). It appeared that the frequency of at least one copy of the APOE4 allele was higher in both younger (age <70 years) and older (age >80 years) women than in men. The overall APOE4 allele frequency distributions in this sample from Taiwan were similar to those reported in other populations of Asian origin (41).
Table 3.
Percent of APOE4 Allele Carriers by Age and Gender
| Age Group (years) | Total |
Men |
Women |
|||
| N | % Carrier | N | % Carrier | N | % Carrier | |
| <60 | 209 | 13.88 | 115 | 12.17 | 94 | 15.96 |
| 60–69 | 330 | 16.67 | 173 | 15.03 | 157 | 18.47 |
| 70–79 | 392 | 13.01 | 250 | 13.20 | 142 | 12.68 |
| 80+ | 92 | 13.04 | 52 | 7.69 | 40 | 20.00 |
| Total | 1,023 | 14.37 | 590 | 13.05 | 433 | 16.17 |
Panel A of Table 4 presents multiple logistic regression estimates of effects (measured by odds ratio, abbreviated as “OR” hereafter) of life stress factors and APOE4 allele carrier status on SRH, after adjusting for demographic, social, and behavioral factors listed in Table 1. Compared with Fujian people (native Taiwan Chinese), the relocated mainland people were more likely to have poor SRH (OR = 1.55, p = .08, 95% CI: 0.95–2.52). Individuals living in crowded household with six or more persons had significantly higher risk of reporting poor SRH (OR = 2.05, p = .04, 95% CI: 1.03–4.05) compared with those living alone. Individuals who experienced housing damage in the 1999 earthquake were significantly more likely to have poor SRH (OR = 1.69, p = .017, 95% CI: 1.10–2.60). Monthly financial difficulty significantly increased risk of poor SRH (OR = 1.74, p = .002, 95% CI: 1.23–2.47).
Table 4.
Multiple Random-Effect Logistic Regression Estimates of Effects (OR) of Life Stress Factors, APOE4 Allele Carrier Status and Their G × E Interactions on Self-reported Poor Health, Adjusted for Various Confounding Factors
| OR | p | 95% Wald Test Confidence Interval | |
| (A) Estimates of effects of life stress factors and APOE4 allele carrier status on SRH | |||
| APOE4 allele carrier (noncarrier) | 1.50 | .053 | 0.99–2.25 |
| Ethnicity (Fujian) | |||
| Hakka | 1.04 | .868 | 0.64–1.71 |
| Mainlander | 1.55 | .080 | 0.95–2.52 |
| Household size (living alone) | |||
| 2–5 | 1.46 | .288 | 0.73–2.95 |
| ≥6 | 2.05 | .040 | 1.03–4.05 |
| Living in earthquake-damaged house (no) | 1.69 | .017 | 1.10–2.60 |
| Monthly financial difficulty (no) | 1.74 | .002 | 1.23–2.47 |
| Living in a poor neighborhood (no) | 1.57 | .172 | 0.82–3.02 |
| APOE4 allele carrier (noncarrier) | 1.50 | .053 | 0.99–2.25 |
| (B) Interactions between the APOE4 allele carrier status and life stress factors | |||
| APOE4 interaction with Hakka | 0.74 | .661 | 0.20–2.79 |
| APOE4 interaction with relocated mainlander | 3.73 | .014 | 1.30–10.68 |
| APOE4 interaction with living with 2–5 persons | 2.02 | .119 | 0.83–4.88 |
| APOE4 interaction with living with ≥6 persons | 3.08 | .009 | 1.32–7.15 |
| APOE4 interaction with Living in earthquake-damaged house | 3.45 | .018 | 1.23–9.63 |
| APOE interacts with monthly financial difficulty | 2.57 | .011 | 1.24–5.33 |
Notes: Category in the parentheses is the reference group. OR = odds ratio.
*p < .1; **p < .05; ***p < .01.
Panel B of Table 4 presents the ORs for the interaction terms (the relative excess risk due to the G × E interaction [RERI]) between carrying APOE4 allele and the life stress factors, adjusted for potential confounding factors. ORs for the interaction between APOE4 and relocated mainlander (OR = 3.73; p = .014), between APOE4 and living with six or more persons (OR = 3.08; p = .009), between APOE4 and living in an earthquake-damaged house (OR = 3.45; p = .018), and between APOE4 and monthly financial difficulty (OR = 2.57; p = .011) are statistically significant; estimates of the other interaction terms are not statistically significant.
Table 5 presents the estimates of the chi-squared test to assess the genotype frequency difference across the categories of the life stress factors of which presented statistically significant interactions with APOE4 carrier status in Table 4 (relocated mainlander, living in a household of six or more persons, living in earthquake-damaged house, and monthly financial difficulty). Clearly, the possibility of rGE correlation confounding G × E interaction effects is ruled out based on the nonsignificant estimates of chi-square tests (see Table 5). Thus, there is no need to do any further path analysis to separate the confounding rGE correlation from the G × E interaction. The estimates presented in Tables 4 and 5 suggest that the interactions between APOE4 carrier status and one of these four life stress factors (being a relocated mainlander, living with six or more persons, living in earthquake-damaged house, and monthly financial difficulty) significantly increase the likelihood of self-reporting poor health.
Table 5.
Chi-squared Test to Assess the APOE4 Allele Carrier Status Frequency Difference Across the Categories of the Life Stress Factors, Whose Interactions Terms With APOE4 Allele Carrier Status Is Statistically Significant
| Life Stress Factor | Category | Number of Participants | % of APOE4 Allele Carriers | p of the Chi-squared Test |
| Being a relocated mainlander | No | 834 | 14.15 | .48 |
| Yes | 171 | 15.20 | ||
| Living in crowded household with ≥6 persons | No | 529 | 13.80 | .59 |
| Yes | 473 | 14.80 | ||
| Living in earthquake-damaged house | No | 882 | 14.29 | .737 |
| Yes | 123 | 13.82 | ||
| Having monthly financial difficulty | No | 753 | 13.81 | .57 |
| Yes | 249 | 15.26 |
The gene–environment interaction (G × E) is conventionally defined as ‘‘a different effect of an environmental exposure on disease and health risk in persons with different genotypes (25).” Such a standard definition of the G × E interaction leads to an intuitive way to explore the G × E interaction effects by comparing the relative risks of health outcomes between those who are exposed or not exposed to the environmental factor within the group of carriers of the genotype and within the group of noncarriers. As shown in Table 6, among the noncarriers of APOE4 minor allele, being a relocated mainlander, living in a crowded household with six or more persons, living in an earthquake-damaged house, and having monthly financial difficulty increased the risk of poor SRH by 55%, 105%, 69%, and 74%, respectively. However, these four life stress factors dramatically increased the risk of poor SRH by 478%, 531%, 483%, and 347%, respectively, among carriers of the APOE4 minor allele. Clearly, the negative impacts of the life stress factors are much stronger among the APOE4 allele carriers than that among the noncarriers.
Table 6.
Estimates of the ORs of Poor SRH Across Groups (OREG) With Different Status of Carrying APOE4 Minor Allele (G = 0 or G = 1) and Life Stress Factors (E = 0 or E = 1) and the Relative Excess Risk Due to the G × E Interaction Between Carrying the APOE4 Minor Allele and the Life Stress Factors
| Status of Carrying APOE4 Minor Allele | E: Being a Relocated Mainlander |
E: Living in Crowded Household |
||||
| No (E = 0) | Yes (E = 1) | % Difference in OREG (E = 1 vs E = 0) | No (E = 0) | Yes (E = 1) | % Difference in OREG (E = 1 vs E = 0) | |
| APOE4 (G = 0) | 1.0 | 1.55* | 55.0 | 1.0 | 2.05** | 105.0 |
| APOE4 (G = 1) | 1.50* | 8.67** | 478.2 | 1.50* | 9.47** | 531.4 |
| RERI: relative excess risk due to G × E interaction | 3.73** (95% CI: 1.30–10.68) | 3.08*** (95% CI: 1.32–7.15) | ||||
| E: Living in Earthquake-Damaged House | E: Having Monthly Financial Difficulty | |||||
| APOE4 (G = 0) | 1.0 | 1.69** | 69.0 | 1.0 | 1.74*** | 74.0 |
| APOE4 (G = 1) | 1.50* | 8.75** | 483.1 | 1.50* | 6.71** | 347.2 |
| RERI: relative excess risk due to G × E interaction | 3.45** (95% CI: 1.23–9.63) | 2.57** (95% CI: 1.24–5.33) | ||||
Notes: OREG is the relative risk of poor SRH for individuals with life stress factor exposure status E and APOE carrier status G using individuals who are not exposed to the life stress factor (E = 0) and do not carry the APOE allele (G = 0) as the reference group.
, which is the OR estimate of G × E interaction terms, namely the relative excess risk due to the G × E interaction (33,40), and thus . The estimates of OR10, OR01, OR11, and RERI are parts of the multiple random-effect logistic regression output produced by STATA.
*p < .1; **p < .05; ***p < .01.
DISCUSSION
Although previous studies have indicated that both the APOE4 allele and the life stress may negatively affect health outcomes (42), there has been little research examining the effects of interactions between APOE4 and life stress factors on SRH. Remarkably, our study shows that the interactions between carrying the APOE4 allele and a number of life stress factors (being a relocated mainlander, living with six or more persons in the household, incurring 1999 earthquake damage to one's home, and suffering monthly financial difficulty) have significant effects on self-reported poor health. We also provide important evidence that these life stress factors have substantially more serious adverse impacts on SRH in carriers of the APOE4 allele than in noncarriers.
Life stress can affect health outcomes in several ways; one pathway is through the hypothalamic–pituitary–adrenal axis and sympathetic nervous system activity. Chronic stress can cause activation of the hypothalamic–pituitary–adrenal axis and sympathetic nervous system, which has been associated with elevated risk of poor health outcomes such as hypertension and heart disease if activation is excessive or prolonged (43). Stress also may affect cognitive function through changes in immune function (44).
A recent study (with less than 100 participants) found a significant interaction between carrying the APOE4 allele and life stress on an elevated level of cortisol, which may lead to memory loss, cognitive decline, and possibly other poor health outcomes in humans (27). Although the mechanisms indicating the pathways through which APOE4 and life stress interact to affect health outcomes in humans are not yet clear, animal model studies have shown that APOE in mice may cause an age-dependent increase in adrenal corticosterone content at baseline and abnormally increased plasma corticosterone levels after restraint stress. This may suggest a key role for APOE in the tonic inhibition of steroidogenesis and adrenal cortical activity (45). Research also shows that exposure to life stress factors may be involved in early development of neurodegeneration in mice with APOE4 (46). These previous studies may provide possible elicitation on biological explanations for the findings reported in this article—the interaction between life stress factors and the APOE4 allele may elevate level of cortisol and cause memory loss, which adversely affect SRH in old adults. Our findings may have important implications for public health. By reducing stressful life events or teaching coping skills to APOE4 carriers, we may be able to reduce the risk of poor SRH. Completion of the Human Genome Project and HapMap Project have provided important tools for the genetic study of complex human disorders (45). Meanwhile, advances in molecular technology over the past decade have led to more genetic research on risk factors associated with health conditions or diseases related to human aging. Therefore, studying gene–environment interactions may allow us to take an active role in lowering the risk of disease and poor health by targeting interventions based on genetic characteristics and environmental factors.
We are also aware of the important limitations in present study, such as the not-large-enough total sample size (N = 1,023, with 209 persons aged 55–59, 722 persons aged 60–79, and only 52 and 40 oldest old male and female participants aged 80+ years). The very small subsample size of the oldest old has resulted in that the proportion of APOE4 carriers abnormally fluctuated from 12.7% at ages 70–79 to 20% at ages 80+ years for women. Concentration of the limited sample on young–old aged 55–79 years also prevented us from employing cortisol or cognitive function as a health indicator. Clearly, more in-depth research, with larger samples including more oldest old, more expansive biomarker collection, and more detailed analyses, is warranted to reconfirm these findings and to provide deeper understanding of the underlying biological mechanisms.
FUNDING
This research was partially supported by the Research Triangle Institute Professional Development Fund awarded to F.Z. and by National Institute on Aging/National Institutes of Health (R01 AG023627) grant and National Natural Science Foundation of China (70533010) awarded to Y.Z.
CONFLICT OF INTEREST
The authors have declared that no competing interests exist.
Acknowledgments
We appreciate very much the helpful comments from Dan Blazer and Jessica M. Sautter and the research assistance provided by Lingguo Cheng, Yunqing Ren, and Huashuai Chen. Author contributions: Y.Z. and F.Z. designed the research, performed data analysis, and drafted/revised the paper; C.H., M.L., and J.X.L. analyzed data and suggested revisions for the paper. Human participation protection: The Research Ethics Committees of Georgetown University, Princeton University, and the Bureau of Health Promotion of the Department of Health in Taiwan of China granted approvals for the protection of human subjects for the Social Environment and Biomarkers of Aging Study, which is the data source of this study. The survey respondents gave informed consent before participating.
References
- 1.Mossey JM, Shapiro E. Self-rated health: a predictor of mortality among the elderly. Am J Public Health. 1982;72(8):800–808. doi: 10.2105/ajph.72.8.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav. 1997;38(1):21–37. [PubMed] [Google Scholar]
- 3.Singh-Manoux A, Gueguen A, Martikainen P, Ferrie J, Marmot M, Shipley M. Self-rated health and mortality: short- and long-term associations in the Whitehall II study. Psychosom Med. 2007;69(2):138–143. doi: 10.1097/PSY.0b013e318030483a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldman N, Lin IF, Weinstein M, Lin YH. Evaluating the quality of self-reports of hypertension and diabetes. J Clin Epidemiol. 2003;56(2):148–154. doi: 10.1016/s0895-4356(02)00580-2. [DOI] [PubMed] [Google Scholar]
- 5.Ostbye T, Krause KM, Norton M. Ten dimensions of health and their relationships with overall self-reported health and survival in a predominately religiously active elderly population: the cache county memory study. J Am Geriatr Soc. 2006;54(2):199–209. doi: 10.1111/j.1532-5415.2005.00583.x. [DOI] [PubMed] [Google Scholar]
- 6.House JS, Kessler RC, Herzog AR, et al. Age, socioeconomic status, and health. Milbank Q. 1990;68(3):383–411. [PubMed] [Google Scholar]
- 7.Dunn JR, Walker JD, Graham J, Weiss CB. Gender differences in the relationship between housing, socioeconomic status, and self-reported health status. Rev Environ Health. 2004;19(3–4):177–195. [PubMed] [Google Scholar]
- 8.Andersen FK, Christensen K, Frederiksen H. Self-rated health and age: a cross-sectional and longitudinal study of 11,000 Danes aged 45-102. Scand J Public Health. 2007;35(2):164–171. doi: 10.1080/14034940600975674. [DOI] [PubMed] [Google Scholar]
- 9.Reijneveld SA. Neighbourhood socioeconomic context and self reported health and smoking: a secondary analysis of data on seven cities. J Epidemiol Community Health. 2002;56(12):935–942. doi: 10.1136/jech.56.12.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Litwin H. Social networks and self-rated health: a cross-cultural examination among older Israelis. J Aging Health. 2006;18(3):335–358. doi: 10.1177/0898264305280982. [DOI] [PubMed] [Google Scholar]
- 11.Abu-Omar K, Rütten A, Robine JM. Self-rated health and physical activity in the European Union. Soc Prev Med. 2004;49(4):235–242. doi: 10.1007/s00038-004-3107-x. [DOI] [PubMed] [Google Scholar]
- 12.Zhang F, Lewis M, Yang G, Iriondo-Perez J, Zeng Y, Liu J. Apolipoprotein E polymorphism, life stress, and self-reported health among older adults. J Epidemiol Community Health. 2008;62(4):e3–e3. doi: 10.1136/jech.2007.063917. [DOI] [PubMed] [Google Scholar]
- 13.Menzel HJ, Kladetzky RG, Assmann G. Apolipoprotein E polymorphism and coronary artery disease. Arteriosclerosis. 1983;3(4):310–315. doi: 10.1161/01.atv.3.4.310. [DOI] [PubMed] [Google Scholar]
- 14.McGregor BA, Antoni MH, Boyersb A, Alferi SM, Blomberg BB, Carver CS. Cognitive–behavioral stress management increases benefit finding and immune function among women with early-stage breast cancer. J Psychosom Res. 2004;56(1):1–8. doi: 10.1016/S0022-3999(03)00036-9. [DOI] [PubMed] [Google Scholar]
- 15.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 16.Stengard JH, Zerba KE, Pekkanen J, Ehnholm C, Nissinen A, Sing CF. Apolipoprotein E polymorphism predicts death from coronary heart disease in a longitudinal study of elderly Finnish men. Circulation. 1995;91(2):265–269. doi: 10.1161/01.cir.91.2.265. [DOI] [PubMed] [Google Scholar]
- 17.Harhangi BS, de Rijk MC, van Duijn CM, Broeckhoven CV, Hofman A, Breteler MMB. APOE and the risk of PD with or without dementia in a population-based study. Neurology. 2000;54(6):1272–1276. doi: 10.1212/wnl.54.6.1272. [DOI] [PubMed] [Google Scholar]
- 18.Albert SM, Gurland B, Maestre G, Jacobs DM, Stern Y, Mayeux R. APOE genotype influences functional status among elderly without dementia. Am J Med Genet. 1995;60(6):583–587. doi: 10.1002/ajmg.1320600621. [DOI] [PubMed] [Google Scholar]
- 19.Blazer DG, Fillenbaum G, Burchett B. The APOE-E4 allele and the risk of functional decline in a community sample of African American and white older adults. J Gerontol A Med Sci. 2001;56(12):M785–M789. doi: 10.1093/gerona/56.12.m785. [DOI] [PubMed] [Google Scholar]
- 20.Ghebremedhin E, Del Tredici K, Vuksic M, et al. Relationship of apolipoprotein E and age at onset to Parkinson disease neuropathology. J Neuropathol Exp Neurol. 2006;65(2):116–123. doi: 10.1097/01.jnen.0000199572.96472.1c. [DOI] [PubMed] [Google Scholar]
- 21.Davidson Y, Gibbons L, Pritchard A, et al. Apolipoprotein E epsilon4 allele frequency and age at onset of Alzheimer's disease. Dement Geriatr Cogn Disord. 2007;23(1):60–66. doi: 10.1159/000097038. [DOI] [PubMed] [Google Scholar]
- 22.Romeis JC, Scherrer JF, Xian H, et al. Heritability of self-reported health. Health Serv Res. 2000;35(5 Pt 1):995–1010. [PMC free article] [PubMed] [Google Scholar]
- 23.Guo G, Roettger ME, Cai T. The integration of genetic propensities into social-control models of delinquency and violence among male youths. Am Sociol Rev. 2008;73(4):543–568. [Google Scholar]
- 24.IOM (Institute of Medicine, Academy of Sciences) Committee Genes, Behavior, and the Social Environment: Moving Beyond the Nature/Nurture Debate. In: Hernandez LM, Blazer DG, editors. Washington, DC: National Academia of Sciences; 2006. [Google Scholar]
- 25.Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8(May):355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 26.Talmud PJ, Lewis SJ, Hawe E, et al. No APOEepsilon4 effect on coronary heart disease risk in a cohort with low smoking prevalence: the Whitehall II study. Atherosclerosis. 2004;177(1):105–112. doi: 10.1016/j.atherosclerosis.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Podewils LJ, Guallar E, Kuller LH, Fried LP, Lopez OL, Carlson M, Lyketsos CG. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161(7):639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 28.Peavy GM, Lange KL, Salmon DP, et al. The effects of prolonged stress and APOE genotype on memory and cortisol in older adults. Biol Psychiatry. 2007;62(5):472–478. doi: 10.1016/j.biopsych.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sauro MD, Jorgensen RS, Pillow CT. Stress, glucocorticoids, and memory: a meta-analytic review. Stress. 2003;6(4):235–245. doi: 10.1080/10253890310001616482. [DOI] [PubMed] [Google Scholar]
- 30.Gallagher-Thompson D, O’Hara R, Simmons A, Kraemer HC, Murphy GM. Apolipoprotein E epsilon4 allele affects the relationship between stress and depression in caregivers of patients with Alzheimer's disease. J Geriatr Psychiatry Neurol. 2001;14(3):115–119. doi: 10.1177/089198870101400303. [DOI] [PubMed] [Google Scholar]
- 31.Weinstein M, Goldman N. Social Environment and Biomarkers of Aging Study (SEBAS) in Taiwan, 2000 [ICPSR Study No. 3792] Ann Arbor, MI: Inter-university Consortium for Political and Social Research; 2003. [Google Scholar]
- 32.Shalowitz MU, Berry CA, Rasinski KA, Dannhausen-Brun CA. A new measure of contemporary life stress: development, validation, and reliability of the CRISYS. Health Serv Res. 1998;33(5 Pt 1):1381–1402. [PMC free article] [PubMed] [Google Scholar]
- 33.Rothman KJ. Epidemiology: An Introduction. New York: Oxford University Press; 2002. [Google Scholar]
- 34.Plomin R, DeFries JC, Roberts MK. Assortative mating by unwed biological parents of adopted children. Science. 1977;196(4288):449–450. doi: 10.1126/science.850790. [DOI] [PubMed] [Google Scholar]
- 35.Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. Sociol Method. 1987;13:290–312. [Google Scholar]
- 36.Bollen KA. Total, direct, and indirect effects in structural equation models. Sociol Method. 1987;17:37–69. [Google Scholar]
- 37.Zeng Yi, Cheng L, Zhao L. Carrying ADRB2 Minor Allele and Its Interactions With Environmental Factors Significantly Affect Mental Health at Advanced Ages in Han Chinese. Paper presented at The 1st International Biodemography Network Meeting, organized by Duke University Population Research Institute, May 3–4, 2011; Durham, NC. [Google Scholar]
- 38.StataCorp. Stata Statistical Software Release 9. College Station, TX: StataCorp LP; 2005. [Google Scholar]
- 39.Evans AE, Zhang W, Moreel JFR, et al. Polymorphisms of the apolipoprotein B and E genes and their relationship to plasma lipid variables in healthy Chinese men. Hum Genet. 1993;92(2):191–197. doi: 10.1007/BF00219691. [DOI] [PubMed] [Google Scholar]
- 40.Rothman KJ. Epidemiology: An Introduction. New York: Oxford University Press; 2002. pp. 168–180. [Google Scholar]
- 41.Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3:452–456. doi: 10.1097/00001648-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 42.Crimmins E, Seeman T. Integrating biology into demographic research on health and aging (with a focus on the MacArthur Study of Successful Aging) In: Finch CE, Vaupel JW, Kinsella K, editors. Cell and Surveys: Should Biological Measures Be Included in Social Science Research? Washington, DC: National Academies Press; 2000. pp. 9–41. [PubMed] [Google Scholar]
- 43.Stress Putting the Brain Back Into Medicine. New York: Canyon Ranch Series; 2006. ILC-Report. [Google Scholar]
- 44.Raber J, Akana SF, Bhatnagar S, Dallman MF, Wong D, Mucke L. Hypothalamic-pituitary-adrenal dysfunction in Apoe(-/-) mice: possible role in behavioral and metabolic alterations. J Neurosci. 2000;20(5):2064–2071. doi: 10.1523/JNEUROSCI.20-05-02064.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou Y, Elkins PD, Howell LA, Ryan DH, Harris RHS. Apolipoprotein-E deficiency results in an altered stress responsiveness in addition to an impaired spatial memory in young mice. Brain Res. 1998;788(1–2):151–159. doi: 10.1016/s0006-8993(97)01533-3. [DOI] [PubMed] [Google Scholar]
- 46.Risch NJ. Searching for genetic determinants in the new millennium. Nature. 2000;405(6788):847–856. doi: 10.1038/35015718. [DOI] [PubMed] [Google Scholar]