Abstract
Background.
To investigate the association between hearing loss and cognitive function in a nationally representative sample of older adults.
Methods.
We analyzed data from the 1999 to 2002 cycles of the National Health and Nutritional Examination Survey during which participants aged 60–69 years (n = 605) underwent both audiometric and cognitive testing. Hearing loss was defined by a pure tone average of hearing thresholds at 0.5, 1, 2, and 4 kHz in the better hearing ear. Cognitive testing consisted of the Digit Symbol Substitution Test (DSST), a nonverbal test that assesses executive function and psychomotor processing. Data on hearing aid use, demographics, and medical history were obtained from interviews. Regression models were used to examine the association between hearing loss and cognition while adjusting for confounders. Analyses incorporated sampling weights to yield results that are generalizable to the U.S. population.
Results.
Greater hearing loss was significantly associated with lower scores on the DSST after adjustment for demographic factors and medical history (DSST score difference of −1.5 [95% confidence interval: −2.9 to −0.23] per 10 dB of hearing loss). Hearing aid use was positively associated with cognitive functioning (DSST score difference of 7.4 [95% confidence interval: −0.62 to 15.4]). The reduction in cognitive performance associated with a 25 dB hearing loss was equivalent to the reduction associated with an age difference of 7 years.
Conclusions.
Hearing loss is independently associated with lower scores on the DSST. Further research is needed to determine whether hearing loss is a modifiable risk factor or an early marker of cognitive decline.
Keywords: Hearing loss, Cognition, Sensory decline
AUDIOMETRIC hearing loss has recently been found to be independently associated with incident all-cause dementia in the Baltimore Longitudinal Study of Aging (1). Mechanistic pathways hypothesized to explain this observed association include a shared pathologic etiology, the effects of hearing loss on cognitive load and cognitive reserve, and/or mediation through social isolation and loneliness. These hypothesized pathways are not mutually exclusive, and coexistent pathways could contribute to the development of cognitive impairment.
A first step in further exploring the association of hearing loss with dementia is to investigate the association of hearing loss with cognition in other independent data sets. Declines in measures of memory (2–5) and executive function (5–9) typically precede dementia by 7 and 3 years, respectively (5,10,11). In the present study, we investigate the association between hearing loss and cognitive function in the nationally representative data set of the National Health and Nutritional Examination Survey (NHANES) using the Digit Symbol Substitution Test (DSST). The DSST is a nonverbal measure of executive function and psychomotor speed (12), and it is one of the first tests to decline prior to dementia onset (8,13). We hypothesized a priori that greater hearing loss would be associated with poorer cognitive performance on the DSST.
METHODS
Study participants
Subjects were participants (age 60–69 years) in the 1999–2002 cycles of NHANES who underwent both audiometric and cognitive testing. During this period, audiometry was administered to a half sample of all adults aged 20–69 years, and cognitive testing with the DSST was administered to all adults aged 60 years and older. The NHANES is an ongoing program of studies designed to assess the health, functional, and nutritional status of the civilian noninstitutionalized U.S. population. Each sequential cross-sectional study uses a complex sampling design to survey a sample of the population, with selective oversampling of low-income individuals, racial minorities, and older adults (14). Sampling weights allow for analyses that account for the complex sampling survey and yield results that are generalizable to the U.S. population.
Audiometric Assessment
Audiometry was performed by a trained examiner according to established NHANES protocols (15). Briefly, air conduction hearing thresholds were obtained from both ears in a dedicated sound-isolating room in the mobile examination center. Testing was conducted according to a modified Hughson–Westlake procedure using the automated testing mode of the audiometer (Interacoustics AD226) and/or manually as per the testing protocol. Quality assurance and quality control were established through daily calibration of equipment and monitoring of ambient noise levels using a sound-level meter. The audiometric test room met or exceeded ANSI S3.1-1991 guidelines for maximum permissible ambient noise levels. Air conduction stimuli were presented primarily through supra-aural earphones (TDH 39P). Insert earphones (ER3A) were reserved for cases of collapsing ear canals or for a crossover retesting protocol in cases of asymmetric hearing loss (masking was not performed). As an additional quality measure, thresholds were measured twice at 1 kHz in both ears, and audiometry was repeated if there was greater than 10 dB discrepancy between the threshold measurements.
We utilized hearing thresholds from 0.5 to 4 kHz, using the first threshold tested at 1 kHz and incorporating manual retest thresholds as needed. Hearing loss was defined as a speech-frequency pure tone average of thresholds at 0.5, 1, 2, and 4 kHz in the better hearing ear as per the definition of hearing loss adjudicated by the World Health Organization (16). Categories of hearing loss severity were based on American Speech-Language Hearing Association guidelines (17), but several of the categories were collapsed to simplify analyses (normal hearing ≤ 25 dB, mild loss > 25 and ≤ 4 0 dB, moderate loss > 40 and ≤ 70 dB, severe loss > 70 dB). All hearing thresholds are reported as dB HL (ANSI, 2004)
Cognitive Testing
The DSST, a component of the Wechsler Adult Intelligence Test (18), was administered as per NHANES protocol (19). Participants were asked to correctly code a series of numbers with the corresponding symbol. Testing was administered in a quiet setting with minimal distractions, and tests were scored as the number of correct responses generated in 120 seconds (maximal score 133).
Other Study Variables
Data on demographic variables and medical history were obtained from interviews. Self-reported race/ethnicity was grouped as Mexican American/other Hispanic (Hispanic), non-Hispanic white (white), non-Hispanic black (black), or other race. Education and household income were both collapsed into a four level variable. Hearing aid use was based on whether an individual reported using a hearing aid at least once a day over the preceding year. Variables related to medical history included diabetes (based on self-reported diagnosis and/or current use of insulin or other diabetic medications), smoking (current/former/never), hypertension (told by physician on two or more visits about hypertension diagnosis), and stroke (self-reported history).
Statistical Methodology
We accounted for the complex sampling design in all analyses by using sample weights according to National Center for Health Statistics guidelines (20) except for Table 1. The purpose of Table 1 was only to give descriptive statistics on the characteristics of the study cohort rather than nationally generalizable estimates, and hence, weights were not used. Locally weighted scatterplot smoothing (lowess) was used to graphically explore the association of hearing loss and age with cognitive scores and to identify nonlinear data trends. Linear regression was then used to model the association between cognitive scores and hearing loss while adjusting for age and other covariates. The β-coefficients from these regressions are interpreted as the average difference in DSST scores (+ values indicate higher cognitive scores) per 10 dB of hearing loss or with hearing aid use. As per National Center for Health Statistics guidelines, the Taylor Series Linearization method was used for variance estimation. All analyses were conducted using STATA 11.1 (StataCorp, College Station, TX), and two-sided p values < .05 were considered statistically significant.
Table 1.
Demographic Characteristics of Participants Aged 60–69 Years With Both Cognitive and Audiometric Testing, National Health and Nutritional Examination Surveys, 1999–2002
| Characteristic | Cohort (n = 605) |
| Age, mean years (SD) | 64.1 (2.9) |
| Hearing loss, mean dB HL (SD) | 20.9 (11.6) |
| Hearing loss category, n (%) | |
| Normal (≤25 dB) | 433 (71.6) |
| Mild (26–40 dB) | 128 (21.2) |
| Moderate (41–70 dB) | 43 (7.1) |
| Severe (≥71 dB) | 1 (0.2) |
| Hearing aid use, n (%) | 13 (2.2) |
| Sex, n (%) | |
| Female | 320 (52.9) |
| Race/ethnicity, n (%) | |
| White | 292 (48.3) |
| Black | 120 (19.8) |
| Hispanic | 182 (30.1) |
| Other | 11 (1.8) |
| Education | |
| <12th grade | 245 (40.5) |
| High school graduate | 133 (22.0) |
| Some college | 128 (21.2) |
| College graduate | 99 (16.4) |
| Smoking, n (%) | |
| Never | 273 (45.1) |
| Former | 240 (40.0) |
| Current | 92 (15.2) |
| Hypertension, n (%) | 257 (42.8) |
| Diabetes, n (%) | 119 (19.7) |
| Stroke, n (%) | 28 (4.6) |
RESULTS
Demographics for the study population are presented in Table 1. From 1999 to 2002, 605 participants aged 60–69 years had concurrent audiometric and cognitive testing in NHANES. Hearing loss more than 25 dB was prevalent in 29% of these participants, and among those with hearing loss, hearing aids were used in 6.7%. The vast majority of participants had hearing loss thresholds in the normal-to-mild range with only 7.3% of participants having a moderate or greater hearing loss.
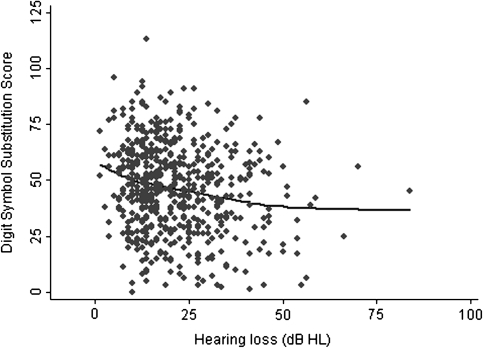
Exploratory analysis of the cross-sectional association between hearing loss and cognitive scores demonstrated that increasing hearing loss was negatively associated with DSST scores (r = −.18, p < .001; Figure 1). A similar association between age and lower DSST scores was also observed (data not shown). After adjusting for age, sex, and hearing aid use, greater hearing loss was significantly associated with lower DSST scores (DSST score difference of −3.3 per 10 dB of hearing loss, p < .001; Table 2). Further adjustment for both demographic (income, education, race) and cardiovascular risk factors (diabetes, hypertension, smoking, stroke) did not substantially affect these results. In this latter model adjusting for all covariates, a 10 dB increase in hearing loss was associated with a DSST score difference of −1.5 (95% confidence interval: −2.9 to −0.23). Restricting the analytical cohort to only those participants with hearing loss less than 40 dB (thereby excluding those with a moderate or severe hearing loss) did not affect the magnitude of our results (Table 2).
Figure 1.
Cross-sectional association of hearing loss and Digit Symbol Substitution Scores, National Health and Nutritional Examination Survey 1999–2002.
Table 2.
Stepwise Regression Models of the Association of Hearing Loss and Hearing Aid Use With Digit Symbol Substitution Scores, National Health and Nutritional Examination Survey 1999–2002
| N | Hearing Loss, β per 10 dB (95% CI) | Hearing Aid Use, β (95% CI) | |
| Base model (hearing loss, hearing aid use, age, sex) | 605 | −3.3*** (−5.0 to −1.6) | 17.1*** (8.8 to 25.3) |
| Base + demographic factors† | 605 | −2.0** (−3.4 to −0.53) | 8.8* (1.2 to 16.5) |
| Base + demographic factors + cardiovascular risk factors‡ | 600 | −1.5* (−2.9 to −0.23) | 7.4+ (−0.62 to 15.4) |
| Base + demographic factors + cardiovascular risk factors + restricted to participants with hearing loss ≤ 40 dB | 557 | −1.6* (−3.3 to −0.03) | 11.9* (0.26 to 23.6) |
Notes: β-Coefficients represent the average difference in cognitive scores associated with a 10 dB increase in hearing loss or with hearing aid use. Negative βs indicate poorer cognitive function.
Demographic factors include race/ethnicity, education, and income.
Cardiovascular risk factors include smoking status, diabetes mellitus, hypertension, and stroke.
+p< .10; *p < .05; **p < .01; ***p < .001.
We investigated the association of hearing aid use with cognitive function. Hearing aid use was significantly associated with higher cognitive scores on the DSST after adjustment for hearing loss severity, age, sex, race, education, and income (DSST score difference of 8.8, p = .03). After further adjustment for cardiovascular risk factors, hearing aid use remained associated with higher cognitive scores but with a wider confidence interval (DSST score difference of 7.4, 95% confidence interval: −0.6 to 15.4, p = .07). However, these results are based on a small number of participants who reported hearing aid use (n = 13).
To assess the magnitude of the reduction in cognitive performance associated with hearing loss, we estimated the difference in chronological age that would be equivalent to the cross-sectional association of a 25 dB increase in hearing thresholds (analogous to shifting from normal hearing to a mild hearing loss) with cognitive scores. In a fully adjusted model accounting for age, sex, race, education, income, diabetes, smoking, hypertension, stroke, and hearing aid use, a 1-year difference in age was associated with a DSST score difference of −0.55 (95% confidence interval: −0.92 to −0.18, p = .005) and a 25 dB hearing loss was associated with a DSST score difference of −3.86 (95% confidence interval: −7.15 to −0.56, p = .02). Therefore, the difference in age equivalent to the cognitive reduction associated with a 25 dB increase in hearing loss is 7 years.
CONCLUSIONS
In this nationally representative study of 60- to 69-year-old adults, greater hearing loss was independently associated with poorer cognitive functioning using a nonverbal test of executive function and psychomotor speed. These results were robust to analyses accounting for multiple confounders and excluding participants with moderate or severe hearing loss. The magnitude of the reduction in cognitive performance associated with hearing loss is clinically significant with the reduction associated with a 25 dB hearing loss being equivalent to an age difference of 7 years. Hearing aid use was associated with higher cognitive scores, but these results were based on a small number of individuals using hearing aids.
Our results contribute to the literature examining the association between hearing loss and cognition. Our findings are consistent with prior research, demonstrating significant associations between greater hearing loss and poorer cognitive function on both verbal (21–29) and nonverbal cognitive tests (23,25,28) and in both cross-sectional and prospective studies (23,30). In contrast, other studies have not found similar associations (31,32). One key limitation across these prior studies is the variability in how hearing loss was measured and how audiometric data were analyzed (eg, choice of pure tone thresholds used to define hearing loss). Most studies utilized portable or screening audiometers (23,27,28,32) or tested participants under varying environmental conditions (eg, home-based testing) (28), whereas some did not adequately describe their audiometric testing protocol (24,26,31). The effect of biased or imprecise assessments of hearing thresholds would likely decrease sensitivity to detect associations due to increased variance. These prior studies have also generally been conducted in nonrepresentative study populations in which the observed results may not be generalizable. Strengths of our current study are that our results are based on a nationally representative sample, and a standardized audiometric testing protocol using a definition of hearing loss adjudicated by the World Health Organization (16) was applied to all individuals.
A number of mechanisms could explain the observed association between hearing loss and cognition. Poor verbal communication associated with hearing loss may confound cognitive testing or vice-versa there may be an overdiagnosis of hearing loss in individuals with subclinical cognitive impairment. Confounding by poor verbal communication is unlikely since the DSST does not rely heavily on the presentation of verbal information, and mild–moderate hearing loss minimally impairs face-to-face communication in quiet environments (ie, during cognitive testing) (33) particularly in the setting of testing by experienced examiners who are accustomed to working with older adults. We also conducted a sensitivity analysis excluding individuals with moderate or severe hearing loss, and a previous study has demonstrated that artificially induced hearing loss (through the use of occlusive headphones) did not acutely affect the results of neurocognitive testing using both verbal and nonverbal cognitive tests (34). An overdiagnosis of hearing loss in those with preexisting cognitive impairment is also a possibility but unlikely given that reliable audiometric thresholds have been obtained even in patients with early dementia (22), and pure tone audiometry is routinely performed even in children as young as 4 years.
A shared neuropathologic etiology underlying both hearing loss and cognitive function may explain our results, but the neuropathologic mechanism is unknown. Pure tone audiometry is considered to be a measure of the auditory periphery because detection of pure tones relies on cochlear transduction and neuronal afferents to brainstem nuclei without requiring significant higher auditory cortical processing (35). Neuropathology associated with Alzheimer’s disease has not been found in the peripheral auditory pathways (36,37). The likelihood of another neurobiological process such as microvascular disease causing both hearing loss and dementia is a possibility, but known cardiovascular risk factors were adjusted for in our models.
Finally, hearing loss may be associated with cognitive decline through a causal pathway, possibly mediated by social isolation or cognitive load, or through a direct neurobiologic mechanism. Communication impairments caused by hearing loss can lead to social isolation in older adults (38,39), and epidemiologic (40,41) and neuroanatomic studies (42) have demonstrated associations between poor social networks and cognitive decline and dementia. The effect of hearing loss on cognitive load is suggested by studies demonstrating that under conditions where auditory perception is difficult (ie, hearing loss), greater cognitive resources are dedicated to auditory perceptual processing to the detriment of other cognitive processes such as working memory (43–45). Finally, previous animal studies have also demonstrated a possible direct neurobiological link between hearing loss and/or environmental enrichment (possibly analogous in humans to having access to auditory and environmental stimuli) with hippocampal neurogenesis and cognitive functioning (46,47).
In the current study, self-reported hearing aid use was associated with higher scores on the DSST, but these results must be interpreted with caution because of the small number (n = 13) of participants using hearing aids. The direction of the observed association also cannot be established in this cross-sectional study. For example, although hearing aids could plausibly improve cognitive functioning through decreased social isolation or reduced cognitive load, individuals with better cognitive function may also be more likely to obtain hearing aids. Ultimately, investigating causality between hearing aid use and improved cognitive functioning will require a randomized control trial. Interestingly, one small randomized study of hearing aids performed in older military veterans has been performed, and this study demonstrated improved cognition in veterans using hearing aids (48). However, these results have not been subsequently studied or confirmed in larger and more representative cohorts.
A key limitation of our study is that our results are based on cross-sectional data rather than on longitudinal trajectories of hearing loss and cognitive function over time. Therefore, our estimates of the expected change in cognitive scores associated with hearing loss and age may be subject to bias by cohort effects or obscured by interindividual heterogeneity in participant characteristics. However, the restricted age range of our study population (60- to 69-year-olds) may help limit potential biases introduced by cohort effects. In addition, interindividual heterogeneity in participant characteristics would tend to bias any results toward the null hypothesis, whereas our results demonstrated a robust association between hearing loss and cognitive scores.
Residual confounding by other medical or environmental factors is also possible, but known risk factors for hearing loss and cognitive decline were adjusted for in our models.
If our results are confirmed longitudinally and in other independent studies, our findings potentially have significant implications for public health. Hearing loss is highly prevalent (49,50), and hearing loss may be both potentially preventable and treatable with rehabilitative devices and strategies that remain grossly underutilized (50,51). Further research into whether such interventions could impact cognition and dementia are critically needed.
FUNDING
This work was supported by the National Institute on Deafness and Other Communication Disorders (1K23DC011279) and a National Institute on Aging Pepper Older Americans Independence Center Research Career Development Award.
Conflict of Interest
F.R.L. reports no financial or personal conflicts of interest.
References
- 1.Lin FR, Metter EJ, O’Brien RJ, et al. Hearing loss and incident dementia. Arch Neurol. 2011;68:214–220. doi: 10.1001/archneurol.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elias MF, Beiser A, Wolf PA, et al. The preclinical phase of Alzheimer disease: a 22-year prospective study of the Framingham Cohort. Arch Neurol. 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- 3.Linn RT, Wolf PA, Bachman DL, et al. The ‘preclinical phase’ of probable Alzheimer's disease. A 13-year prospective study of the Framingham cohort. Arch Neurol. 1995;52:485–490. doi: 10.1001/archneur.1995.00540290075020. [DOI] [PubMed] [Google Scholar]
- 4.Rubin EH, Storandt M, Miller JP, et al. A prospective study of cognitive function and onset of dementia in cognitively healthy elders. Arch Neurol. 1998;55:395–401. doi: 10.1001/archneur.55.3.395. [DOI] [PubMed] [Google Scholar]
- 5.Grober E, Hall CB, Lipton RB, et al. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer's disease. J Int Neuropsychol Soc. 2008;14:266–278. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen P, Ratcliff G, Belle SH, et al. Patterns of cognitive decline in presymptomatic Alzheimer disease: a prospective community study. Arch Gen Psychiatry. 2001;58:853–858. doi: 10.1001/archpsyc.58.9.853. [DOI] [PubMed] [Google Scholar]
- 7.Fabrigoule C, Rouch I, Taberly A, et al. Cognitive process in preclinical phase of dementia. Brain. 1998;121(Pt 1):135–141. doi: 10.1093/brain/121.1.135. [DOI] [PubMed] [Google Scholar]
- 8.Rapp MA, Reischies FM. Attention and executive control predict Alzheimer disease in late life: results from the Berlin Aging Study (BASE) Am J Geriatr Psychiatry. 2005;13:134–141. doi: 10.1176/appi.ajgp.13.2.134. [DOI] [PubMed] [Google Scholar]
- 9.Royall DR, Chiodo LK, Polk MJ. Misclassification is likely in the assessment of mild cognitive impairment. Neuroepidemiology. 2004;23:185–191. doi: 10.1159/000078504. [DOI] [PubMed] [Google Scholar]
- 10.Hall CB, Lipton RB, Sliwinski M, et al. A change point model for estimating the onset of cognitive decline in preclinical Alzheimer's disease. Stat Med. 2000;19:1555–1566. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1555::aid-sim445>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Hall CB, Ying J, Kuo L, et al. Estimation of bivariate measurements having different change points, with application to cognitive ageing. Stat Med. 2001;20:3695–3714. doi: 10.1002/sim.1113. [DOI] [PubMed] [Google Scholar]
- 12.Wechsler D. Technical manual for the Wechsler Adult Intelligence and Memory Scale-Third Edition. New York: The Psychological Corporation; 1997. [Google Scholar]
- 13.Tabert MH, Manly JJ, Liu X, et al. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch Gen Psychiatry. 2006;63:916–924. doi: 10.1001/archpsyc.63.8.916. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2010. http://www.cdc.gov/nchs/nhanes.htm/. Accessed May 1, 2011. [Google Scholar]
- 15.Centers for Disease Control. Audiometry/Tympanometry Procedures Manual. 2001 http://www.cdc.gov/nchs/data/nhanes/au.pdf. Accessed May 1, 2011. [Google Scholar]
- 16.World Health Organization Prevention of Blindness and Deafness (PBD) Program. Prevention of Deafness and Hearing Impaired Grades of Hearing Impairment. 2011 http://www.who.int/pbd/deafness/hearing_impairment_grades/en/index.html. Accessed May 1, 2011. [Google Scholar]
- 17.Clark JG. Uses and abuses of hearing loss classification. ASHA. 1981;23:493–500. [PubMed] [Google Scholar]
- 18.Wechsler D. Manual for the Wechsler Adult Intelligence Scale-Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- 19.Centers for Disease Control. NHANES Digit Symbol Substitution Exercise: Interviewer Instructions. 2005 http://www.cdc.gov/nchs/nhanes/nhanes1999-2000/CFQ.htm. Accessed March 22, 2011. [Google Scholar]
- 20.Centers for Disease Control. NHANES Analytic and Reporting Guidelines. www.cdc.gov/nchs/data/nhanes/nhanes_03_04/nhanes_analytic_guidelines_dec_2005.pdf. Accessed May 1, 2011. [Google Scholar]
- 21.Helzner EP, Cauley JA, Pratt SR, et al. Race and sex differences in age-related hearing loss: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:2119–2127. doi: 10.1111/j.1532-5415.2005.00525.x. [DOI] [PubMed] [Google Scholar]
- 22.Uhlmann RF, Larson EB, Rees TS, et al. Relationship of hearing impairment to dementia and cognitive dysfunction in older adults. JAMA. 1989;261:1916–1919. [PubMed] [Google Scholar]
- 23.Valentijn SA, van Boxtel MP, van Hooren SA, et al. Change in sensory functioning predicts change in cognitive functioning: results from a 6-year follow-up in the Maastricht aging study. J Am Geriatr Soc. 2005;53:374–380. doi: 10.1111/j.1532-5415.2005.53152.x. [DOI] [PubMed] [Google Scholar]
- 24.Ohta RJ, Carlin MF, Harmon BM. Auditory acuity and performance on the mental status questionnaire in the elderly. J Am Geriatr Soc. 1981;29:476–478. doi: 10.1111/j.1532-5415.1981.tb01753.x. [DOI] [PubMed] [Google Scholar]
- 25.Granick S, Kleban MH, Weiss AD. Relationships between hearing loss and cognition in normally hearing aged persons. J Gerontol. 1976;31:434–440. doi: 10.1093/geronj/31.4.434. [DOI] [PubMed] [Google Scholar]
- 26.Thomas PD, Hunt WC, Garry PJ, et al. Hearing acuity in a healthy elderly population: effects on emotional, cognitive, and social status. J Gerontol. 1983;38:321–325. doi: 10.1093/geronj/38.3.321. [DOI] [PubMed] [Google Scholar]
- 27.Gussekloo J, de Craen AJ, Oduber C, et al. Sensory impairment and cognitive functioning in oldest-old subjects: the Leiden 85+ Study. Am J Geriatr Psychiatry. 2005;13:781–786. doi: 10.1176/appi.ajgp.13.9.781. [DOI] [PubMed] [Google Scholar]
- 28.Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: a strong connection. Psychol Aging. 1994;9:339–355. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- 29.Tay T, Wang JJ, Kifley A, et al. Sensory and cognitive association in older persons: findings from an older Australian population. Gerontology. 2006;52:386–394. doi: 10.1159/000095129. [DOI] [PubMed] [Google Scholar]
- 30.Peters CA, Potter JF, Scholer SG. Hearing impairment as a predictor of cognitive decline in dementia. J Am Geriatr Soc. 1988;36:981–986. doi: 10.1111/j.1532-5415.1988.tb04363.x. [DOI] [PubMed] [Google Scholar]
- 31.Gennis V, Garry PJ, Haaland KY, et al. Hearing and cognition in the elderly. New findings and a review of the literature. Arch Intern Med. 1991;151:2259–2264. [PubMed] [Google Scholar]
- 32.Anstey KJ, Luszcz MA, Sanchez L. Two-year decline in vision but not hearing is associated with memory decline in very old adults in a population-based sample. Gerontology. 2001;47:289–293. doi: 10.1159/000052814. [DOI] [PubMed] [Google Scholar]
- 33.Gordon-Salant S. Hearing loss and aging: new research findings and clinical implications. J Rehabil Res Dev. 2005;42:9–24. doi: 10.1682/jrrd.2005.01.0006. [DOI] [PubMed] [Google Scholar]
- 34.Lindenberger U, Scherer H, Baltes PB. The strong connection between sensory and cognitive performance in old age: not due to sensory acuity reductions operating during cognitive assessment. Psychol Aging. 2001;16:196–205. doi: 10.1037//0882-7974.16.2.196. [DOI] [PubMed] [Google Scholar]
- 35.Pickles JO. An Introduction to the Physiology of Hearing. Bingley, UK: Emerald Group Publishing; 2008. [Google Scholar]
- 36.Sinha UK, Hollen KM, Rodriguez R, et al. Auditory system degeneration in Alzheimer's disease. Neurology. 1993;43:779–785. doi: 10.1212/wnl.43.4.779. [DOI] [PubMed] [Google Scholar]
- 37.Baloyannis SJ, Mauroudis I, Manolides SL, et al. Synaptic alterations in the medial geniculate bodies and the inferior colliculi in Alzheimer's disease: a Golgi and electron microscope study. Acta Otolaryngol. 2009;129:416–418. doi: 10.1080/00016480802579074. [DOI] [PubMed] [Google Scholar]
- 38.Strawbridge WJ, Wallhagen MI, Shema SJ, et al. Negative consequences of hearing impairment in old age: a longitudinal analysis. Gerontologist. 2000;40:320–326. doi: 10.1093/geront/40.3.320. [DOI] [PubMed] [Google Scholar]
- 39.Weinstein BE, Ventry IM. Hearing impairment and social isolation in the elderly. J Speech Hear Res. 1982;25:593–599. doi: 10.1044/jshr.2504.593. [DOI] [PubMed] [Google Scholar]
- 40.Fratiglioni L, Wang HX, Ericsson K, et al. Influence of social network on occurrence of dementia: a community-based longitudinal study. Lancet. 2000;355:1315–1319. doi: 10.1016/S0140-6736(00)02113-9. [DOI] [PubMed] [Google Scholar]
- 41.Barnes LL, Mendes de Leon CF, Wilson RS, et al. Social resources and cognitive decline in a population of older African Americans and whites. Neurology. 2004;63:2322–2326. doi: 10.1212/01.wnl.0000147473.04043.b3. [DOI] [PubMed] [Google Scholar]
- 42.Bennett DA, Schneider JA, Tang Y, et al. The effect of social networks on the relation between Alzheimer's disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol. 2006;5:406–412. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- 43.Tun PA, McCoy S, Wingfield A. Aging, hearing acuity, and the attentional costs of effortful listening. Psychol Aging. 2009;24:761–766. doi: 10.1037/a0014802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pichora-Fuller MK, Schneider BA, Daneman M. How young and old adults listen to and remember speech in noise. J Acoust Soc Am. 1995;97:593–608. doi: 10.1121/1.412282. [DOI] [PubMed] [Google Scholar]
- 45.Wingfield A, Grossman M. Language and the aging brain: patterns of neural compensation revealed by functional brain imaging. J Neurophysiol. 2006;96:2830–2839. doi: 10.1152/jn.00628.2006. [DOI] [PubMed] [Google Scholar]
- 46.Kraus KS, Mitra S, Jimenez Z, et al. Noise trauma impairs neurogenesis in the rat hippocampus. Neuroscience. 2010;167:1216–1226. doi: 10.1016/j.neuroscience.2010.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenzweig MR, Bennett EL. Psychobiology of plasticity: effects of training and experience on brain and behavior. Behav Brain Res. 1996;78:57–65. doi: 10.1016/0166-4328(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 48.Mulrow CD, Aguilar C, Endicott JE, et al. Quality-of-life changes and hearing impairment. A randomized trial. Ann Intern Med. 1990;113:188–194. doi: 10.7326/0003-4819-113-3-188. [DOI] [PubMed] [Google Scholar]
- 49.Nash SD, Cruickshanks KJ, Klein R, et al. The prevalence of hearing impairment and associated risk factors: the Beaver Dam Offspring Study. Arch Otolaryngol Head Neck Surg. 2011;137(5):432–439. doi: 10.1001/archoto.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin FR, Thorpe R, Gordon-Salant S, et al. Hearing loss prevalence and risk factors among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2011;66(5):582–590. doi: 10.1093/gerona/glr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhan W, Cruickshanks KJ, Klein BE, et al. Generational differences in the prevalence of hearing impairment in older adults. Am J Epidemiol. 2010;171:260–266. doi: 10.1093/aje/kwp370. [DOI] [PMC free article] [PubMed] [Google Scholar]