Abstract
Ligand-induced down-regulation of two growth factor receptors, EGF receptor (ErbB-1) and ErbB-3, correlates with differential ability to recruit c-Cbl, whose invertebrate orthologs are negative regulators of ErbB. We report that ligand-induced degradation of internalized ErbB-1, but not ErbB-3, is mediated by transient mobilization of a minor fraction of c-Cbl into ErbB-1-containing endosomes. This recruitment depends on the receptor’s tyrosine kinase activity and an intact carboxy-terminal region. The alternative fate is recycling of internalized ErbBs to the cell surface. Cbl-mediated receptor sorting involves covalent attachment of ubiquitin molecules, and subsequent lysosomal and proteasomal degradation. The oncogenic viral form of Cbl inhibits down-regulation by shunting endocytosed receptors to the recycling pathway. These results reveal an endosomal sorting machinery capable of controlling the fate, and, hence, signaling potency, of growth factor receptors.
Keywords: Endocytosis, ErbB/HER, protein degradation, signal transduction, tyrosine kinase
The ErbB family of growth factor receptors carrying an intrinsic tyrosine kinase activity constitutes a layered signaling network that includes a large family of EGF- like ligands that bind to four transmembrane receptors capable of forming ten homo- and heterodimeric combinations (for review, see Alroy and Yarden 1997). Whereas the EGF receptor (ErbB-1) binds several growth factors whose prototype is EGF, both ErbB-3 and ErbB-4 bind all isoforms of the Neu differentiation factor (NDF). The third layer of the network includes a large group of signaling molecules sharing one of several types of phosphotyrosine-binding (PTB) domains (e.g., an SH2 domain). Although nonidentical sets of signaling proteins are recruited, all ErbB receptors, like their invertebrate orthologs (Perrimon and Perkins 1997), funnel their signals into the mitogen-activated protein kinase (MAPK) pathway. The kinetics of MAPK activation and its relative potency, however, display remarkable differences that correlate with the rate of ligand-induced endocytosis of receptors, termed down-regulation (Pinkas-Kramarski et al. 1996), and recruitment of c-Cbl by the activated receptor (Levkowitz et al. 1996).
c-Cbl is the mammalian ortholog of the Sli-1 protein of Caenorhabditis elegans (Yoon et al. 1995). Genetic evidence indicated that Sli-1 negatively regulates signaling downstream of the single nematode ErbB protein (Jongeward et al. 1995). c-Cbl is a major cellular substrate of tyrosine phosphorylation: It undergoes increased phosphorylation in response to ligand-induced stimulation of a variety of surface receptors, including the EGF receptor, lymphokine receptors, immunoglobulin receptors, antigen receptors, and integrin receptors (Thien and Langdon 1997, and references therein). Overexpression of c-Cbl attenuates signaling down-stream of the immunoglobulin E receptor (Ota and Samelson 1997) and the T-cell receptor (Boussiotis et al. 1997), yet the mechanism of Cbl action remains unknown. Here we report that c-Cbl can increase the rate of degradation of ErbB-1, but not ErbB-3. The underlying mechanism involves transient physical associations between c-Cbl and ErbB-1 in endosomes, and subsequent ubiquitination of the degradation-destined receptors. An oncogenic viral Cbl appears to interfere with the sorting function of c-Cbl, thereby directing incoming receptors to the recycling pathway.
Results
c-Cbl mediates selective degradation of ligand-stimulated ErbB-1, but not ErbB-3
To investigate the possibility that EGF-driven ErbB-1 is destined to lysosomal degradation because ErbB-1 can interact with c-Cbl (Levkowitz et al. 1996), whereas ErbB-3 is shunted to the recycling pathway (Waterman et al. 1998) because it cannot recruit c-Cbl, we transiently overexpressed c-cbl and erbB-1 in Chinese hamster ovary (CHO) cells. In addition to c-Cbl, we used two deletion mutants that are schematically presented in Figure 1A. These are a peptide-tagged amino-terminal portion of c-Cbl analogous to the murine viral form, v-Cbl, and the complementary deletion mutant, Cbl-C (Fig. 1A). Cells were briefly stimulated with EGF and tyrosine phosphorylation of the receptor analyzed by immunoblotting with anti-phosphotyrosine antibodies. The results of this experiment indicated that ErbB-1 underwent enhanced tyrosine phosphorylation in the presence of its ligand (∼10-fold), but c-Cbl overexpression almost abolished this effect (Fig. 1B). Neither v-Cbl nor Cbl-C were active, implying that the combination of amino- and carboxy-terminal sequences is essential for the effect of c-Cbl on receptor phosphorylation.
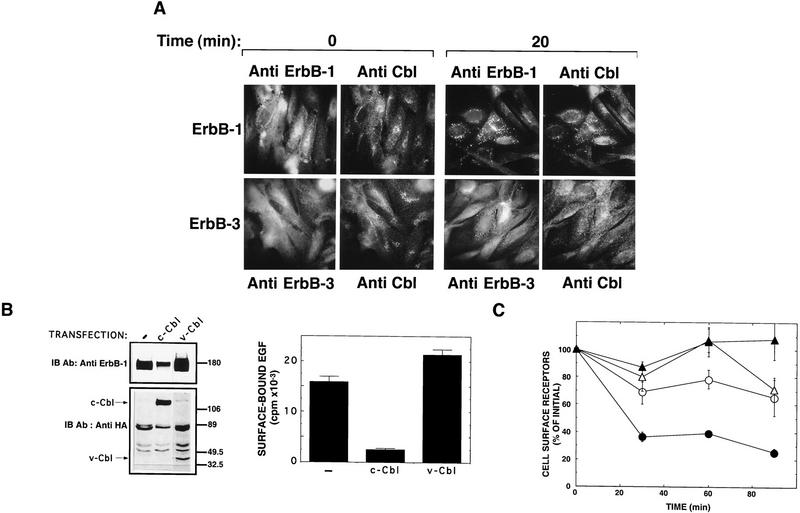
Figure 1.
c-Cbl, but not v-Cbl, increases degradation of ErbB-1 by its removal from the cell surface. (A) Shown are the domain structures of c-Cbl and three derivative proteins. The following structural motifs are represented: A 7 residue-long histidine stretch (7 His), a positively charged basic domain (Basic), a ring-finger domain (RF), a proline-rich domain (Pro-Rich), and a leucine-zipper (LZ). An influenza virus hemagglutinin (HA) epitope tag was added to the amino-terminal end of each protein. cDNAs corresponding to the three natural forms of Cbl were transiently transfected into CHO cells. Forty-eight hours after transfection, cells were lysed and whole cell lysates subjected to immunoblotting (IB) with an anti-HA antibody. (B) ErbB-1 (left) or ErbB-3 (right) were transiently expressed in CHO cells by cotransfection with plasmids encoding the indicated Cbl proteins, or with a control empty plasmid (Cont.). Cells were incubated for 45 min at 37°C with EGF or NDF (each at 100 ng/ml). Thereafter, whole cell lysates were subjected to immunoprecipitation (IP) and immunoblotting (IB) with the indicated antibodies. (C) CHO cells were co-transfected with an ErbB-1 expression vector together with a vector directing expression of c-Cbl or a control empty vector (Cont.) Cell surface-exposed proteins were covalently labeled with biotin at 4°C. Soluble biotin was then removed and cells incubated at 37°C for 45 min in the presence or absence of EGF. Cell lysates were subjected to immunoprecipitation (IP) with an anti ErbB-1 antibody and the electrophoretically resolved proteins were probed either with horseradish peroxidase-conjugated Streptavidin, or with an anti-Shc antibody. The locations of molecular mass markers are indicated in kilodaltons. Note that the three Shc isoforms (p66, p52, and p46) associate with ErbB-1.
Reblotting with anti-ErbB-1 antibodies revealed that c-Cbl overexpression led to degradation, rather than catalytic inactivation, of ErbB-1 (Fig. 1B, bottom): primarily the hyperphosphorylated form of ErbB-1, whose electrophoretic mobility is retarded, was diminished (by 80%). This observation suggested that tyrosine phosphorylation of the surface-localized ErbB-1 molecules selectively targets ErbB-1 to degradation through c-Cbl action. Labeling of cell surface-exposed ErbB-1 molecules by surface biotinylation confirmed this scenario (Fig. 1C): Overexpression of c-Cbl enhanced disappearance of the hyperphosphorylated form of ErbB-1. Concomitant with selective removal from the cell surface, signaling downstream of ErbB-1 was down-regulated by c-Cbl. This was exemplified by the ability of the ligand-stimulated ErbB-1 to recruit one of its major substrates, the Shc protein (Fig. 1C, bottom). In contrast with the EGF receptor, the NDF-receptor (ErbB-3) was not affected by c-Cbl (Fig. 1B). In conclusion, c-Cbl can down-regulate ErbB-1 signaling, apparently by selective degradation of a hyperphosphorylated form of the receptor.
Additional support for this model was provided by analyses of ligand-induced redistribution of c-Cbl. Consistent with the observation that EGF can induce physical association of its receptor with c-Cbl in living cells (Bowtell and Langdon 1995; Galisteo et al. 1995; Meisner et al. 1995; Tanaka et al. 1995), EGF treatment of cells co-overexpressing ErbB-1 and ErbB-3 led to rapid changes in the pattern of c-Cbl subcellular localization (Fig. 2A). Whereas in untreated cells c-Cbl exhibited reticular/vesicular distribution, it assumed a more punctate, vesicular-like, pattern in EGF-treated cells. In fact, some, but not all, c-Cbl-bearing structures contained endocytosed molecules of ErbB-1. No such redistribution of c-Cbl, or ErbB-3, was noted on treatment with NDF (Fig. 2A). This observation led us to compare the effects of c-Cbl with the abilities of the two ligands to down-regulate their binding sites. To this end, we stably expressed c-Cbl, or v-Cbl, in cells already overexpressing ErbB-1. Although the introduced Cbl proteins were detectably expressed (Fig. 2B, bottom), c-Cbl- overexpressing cells displayed a significantly lower amount of the ErbB-1 protein (Fig. 2B, left) and a six-fold decreased number of EGF-binding sites relative to control- or v-cbl-transfected cells (Fig. 2B, right). This basal down-regulation of ErbB-1 was displayed by several independently selected clones, and severely reduced the sensitivity of receptor down-regulation assays. Therefore, we used transient coexpression of a receptor and c-Cbl. Because of a high level of receptor expression, EGF treatment of ErbB-1-expressing cells led to only a moderate down-regulation effect (Fig. 2C). The extent of ligand-induced down-regulation displayed variation that apparently correlates with receptor expression, but in all experiments receptor disappearance was significantly enhanced on overexpression of c-Cbl (Fig. 2C). This observation indicates that the amount of c-Cbl limits down-regulation when ErbB-1 is overexpressed. In contrast, NDF treatment of ErbB-3-expressing cells did not result in receptor down-regulation, and c-Cbl exerted no significant effect on the status of NDF-binding sites (Fig. 2C). In conclusion, c-Cbl can enhance down-regulation of the EGF-receptor, but not the NDF receptor, and this involves redistribution of c-Cbl into endocytic ErbB-1-containing vesicles.
Figure 2.
c-Cbl colocalizes with ErbB-1, but not with ErbB-3, and accelerates its down-regulation. (A) CHO cells that stably co-overexpress ErbB-1 and ErbB-3 (Tzahar et al. 1996) were plated on cover-slips and treated at 37°C with EGF (top) or NDF (bottom) for the indicated periods of time. After fixation and permeabilization, cover slips were simultaneously stained with a polyclonal anti-Cbl antibody and a mAb to ErbB-1 or to ErbB-3, as indicated. Antibody detection by immunofluorescence was performed as described (Materials and Methods). (B) Stable CHO transfections expressing c-Cbl or v-Cbl were established by cotransfection of Cbl constructs together with the pBABE/puro plasmid into CHO cells overexpressing ErbB-1 (Tzahar et al. 1996). Individual clones were screened by immunoblotting with antibodies to ErbB-1 and to the HA tag of Cbl proteins (left, the locations of c-Cbl and v-Cbl protein bands are indicated by arrows). To assess cell surface expression of ErbB-1, clones expressing c-Cbl or v-Cbl, as well as the parental ErbB-1 overexpressing cell line (−), were incubated for 90 min at 4°C with radiolabeled EGF (10 ng/ml). Cell-bound radioactivity is shown as the average and range (bars) of duplicate determinations. (C) CHO cells were cotransfected with pairs of two plasmids: an ErbB-expression vector and either a control empty pcDNA3 plasmid (○; ErbB-1; ▵, ErbB-3), or a c-Cbl expression vector (•, ErbB-1; ▴, ErbB-3). Cell monolayers were subjected to a down-regulation assay 48 hr post-transfection. The results are expressed as the average fraction (and range, bars) of original binding sites that remained on the cell surface after exposure to the nonlabeled ligand at 37°C.
Transient ligand-induced colocalization of c-Cbl and ErbB-1 in endosomes
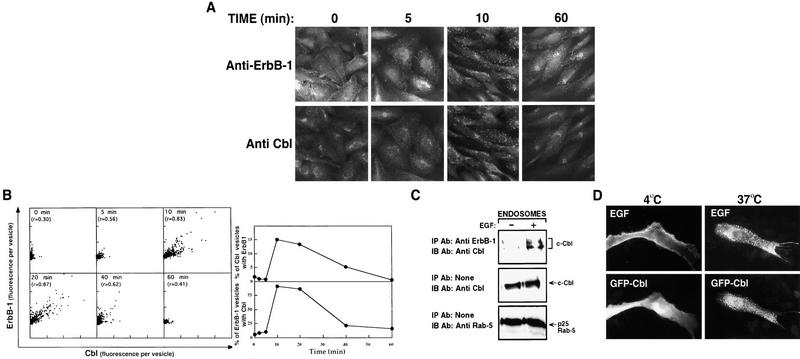
Because partial colocalization of ErbB-1 and c-Cbl was observed on a 20-min-long incubation of cells with EGF (Fig. 2A), we analyzed the kinetics of the phenomenon. The results presented in Figure 3A demonstrate redistribution of ErbB-1 and c-Cbl from their initial sites (membranal and reticular, respectively), and partial colocalization that peaks at 10–20 min. Concomitant with redistribution, the double-stained vesicular structures exhibited an apparent increase in fluorescence intensity. This process was further analyzed by use of computerized analysis of digital images. First, we defined the borders of each c-Cbl- containing particle, and then separately determined the fluorescence intensity of c-Cbl and ErbB-1 within the framed two-dimensional structure. When this was repeatedly performed at different time intervals, we obtained the results presented in Figure 3B, in which each dot represents one vesicular structure. In untreated cells, labeling for c-Cbl in individual particles was very low, but the cells contained relatively large numbers of small particles. Following 10 min of incubation, a coordinated increase in ErbB-1 and c-Cbl labeling became evident and it displayed a linear correlation coefficient of 0.83. Colocalization was maximal after 20 min of incubation (r = 0.87) and then gradually declined. Analysis of the total number of intracellular vesicles/particles per cell (data not shown), as well as the extent of colocalization (Fig. 3B, right) revealed a 25% decrease in the average density of c-Cbl-positive particles at 10 and 20 min. The number of ErbB-1 vesicles gradually increased and reached a maximal level at 10 min. Importantly, the maximal number of ErbB-1-positive vesicles was much lower than the number of c-Cbl-positive vesicles, suggesting that only a small subpopulation of c-Cbl-rich vesicles contained endocytosed ErbB-1 molecules. This was confirmed by direct evaluation of the extent of colocalization: Approximately 15% of c-Cbl-positive vesicles also contained ErbB-1, implying that nearly all of the ErbB-1-containing vesicles became associated with c-Cbl.
Figure 3.
Time dependence of EGF-induced ErbB-1 and c-Cbl co-localization in endosomes. (A) CHO cells that coexpress ErbB-1 and ErbB-3 were either fixed (0 min) or first incubated with EGF for 5 min at 37°C. Thereafter, EGF was removed and incubation continued for the indicated time intervals. Double staining of ErbB-1 and c-Cbl was performed and visualized as described in Materials and Methods. (B, left) For each time point, digital images of three representative cells were segmented according to the labeling of Cbl. The fluorescence of c-Cbl and ErbB-1 labeling was calculated for each segmented vesicle. The scatter plots (arbitrary units) present ErbB-1 fluorescence vs. the c-Cbl fluorescence in each segmented vesicle. The correlation coefficient (r) indicates the strength of a linear correlation between ErbB-1 and c-Cbl fluorescence. (B, right) Cbl-segmented vesicles, which showed ErbB-1 positivity above a threshold, were considered Cbl-vesicles with ErbB1. (Top) Percentage of those vesicles from the total number of Cbl-segmented vesicles at each time point. (Bottom) Percentage of ErbB-1 vesicles with Cbl from the total number of ErbB1-segmented vesicles. (C) CHO cells were cotransfected with ErbB-1 and c-Cbl expression vectors and cells were incubated for 15 min at 37°C with EGF. Control monolayers were mock stimulated (−). Endosomes were prepared as described in Materials and Methods and solubilized (1% Triton X-100) for 30 min at 4°C. Cell lysates were cleared and subjected to immunoprecipitation (IP) and immunoblotting (IB) with the indicated antibodies. Both the endosomal marker protein Rab-5 and c-Cbl were significantly enriched in the isolated fraction relative to other fractions that were collected (bottom; data not shown). (D) CHO cells were cotransfected with ErbB-1 and an expression vector encoding c-Cbl fused in frame to a green fluorescence protein (GFP-Cbl). Forty-eight hours after transfection, cells were incubated with Texas-red-labeled EGF (0.5 μg/ml) for 30 min at 4°C and then either transferred to 37°C (right), or left at 4°C (left), for an additional incubation of 15 min.
Biochemical analyses that involved labeling of surface ErbB-1 molecules with biotin, or selective immunoprecipitation of the membranal EGF receptors, indicated that only the endocytosed receptor fraction physically recruited c-Cbl (data not shown), consistent with the endosomal site of interaction implied by the immunofluorescence results. Indeed, separation of the endosomal fraction from CHO cells by use of the method described previously (Wada et al. 1992) and Rab-5 as an endosome marker revealed that c-Cbl is a resident protein of the endosome (Fig. 3C). In addition, physical interaction between c-Cbl and ErbB-1 was detectable in the endosomal fraction only if cells were treated with EGF prior to lysis and subcellular fractionation. c-Cbl and ErbB-1 redistribution was independently supported by use of a fluorescently labeled EGF, and c-Cbl fused to a GFP. The results presented in Figure 3D confirmed that the ligand-occupied receptor molecules colocalize with Cbl-containing vesicles.
c-Cbl and v-Cbl differently affect sorting of endocytosed ErbB-1 molecules
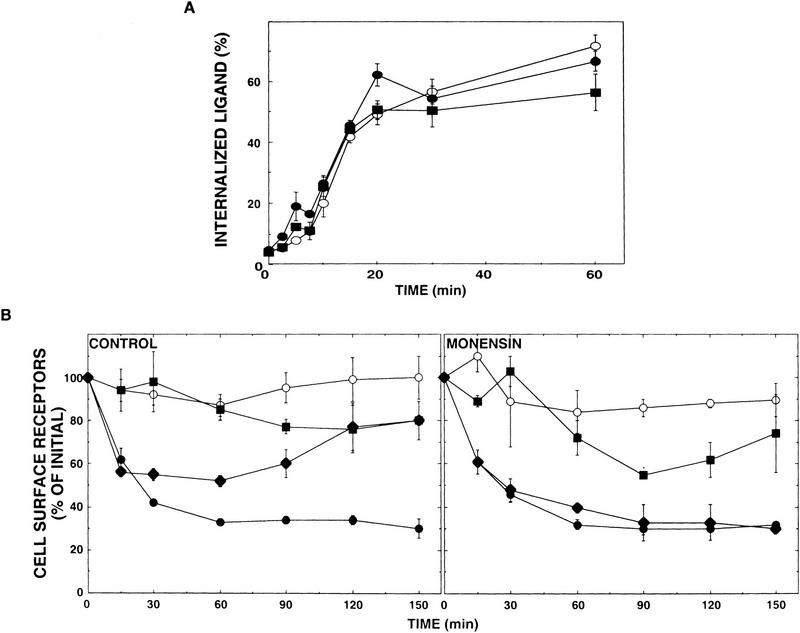
Determination of the rate of intracellular accumulation of EGF revealed that neither c-Cbl nor v-Cbl affected the rate of ligand uptake (Fig. 4A), implying that Cbl acts at a step distal to receptor binding and coated pit-mediated internalization. This conclusion was also supported by short time (1–5 min) ligand uptake experiments, and analyses of the rate of EGF degradation, which was not affected by c-Cbl or v-Cbl (data not shown). On the other hand, examination of the receptor’s fate, by use of a down-regulation assay indicated that c-Cbl, unlike v-Cbl, significantly accelerated the rate of receptor down-regulation (Fig. 4B). A surprising effect of v-Cbl, however, was revealed on the background of the extensive c-Cbl-induced down-regulation (Fig. 4B). Following a rapid phase of partial ligand-induced down-regulation, v-Cbl caused an almost complete recovery of ErbB-1 to its original surface level. This effect of v-Cbl was sensitive to monensin (Fig. 4B, right), a carboxylic ionophore that exerts diverse intracellular effects, including disruption of recycling of EGF-occupied ErbB-1 molecules after their endocytosis (Basu et al. 1981). Conceivably, whereas c-Cbl can direct ErbB-1-loaded endosomes to degradation, v-Cbl antagonizes this sorting function, and directs the receptors to the alternative recycling pathway.
Figure 4.
v-Cbl promotes receptor recycling, whereas c-Cbl induces receptor down-regulation. (A) Ligand internalization analyses. CHO cells were cotransfected with an ErbB-1 vector along with one of the following plasmids: pcDNA3 (control, ○), c-Cbl expression vector (•), or a plasmid directing v-Cbl expression (█). Cell monolayers were treated for 2 hr at 4°C with 125I-labeled EGF (at 10 ng/ml) and then transferred to 37°C for the indicated periods of time. The fraction of internalized ligand was determined by use of a low-pH wash. Each data point represents the average ± s.e. (bars) of triplicate measurements. (B) CHO cells were cotransfected with an ErbB-1-encoding plasmid along with an expression vector encoding v-Cbl (█), c-Cbl (•), both v- and c-Cbl (♦), or with an empty vector (control, ○). Cells were rinsed and incubated at 37°C for the indicated periods of time with EGF (at 250 ng/ml). Sister cultures were similarly treated, except that monensin (100 μm) was added to the medium. Down-regulation assays were performed as described in Materials and Methods.
Kinase activity and an intact carboxy-terminal region are obligatory for Cbl-induced down-regulation of ErbB-1
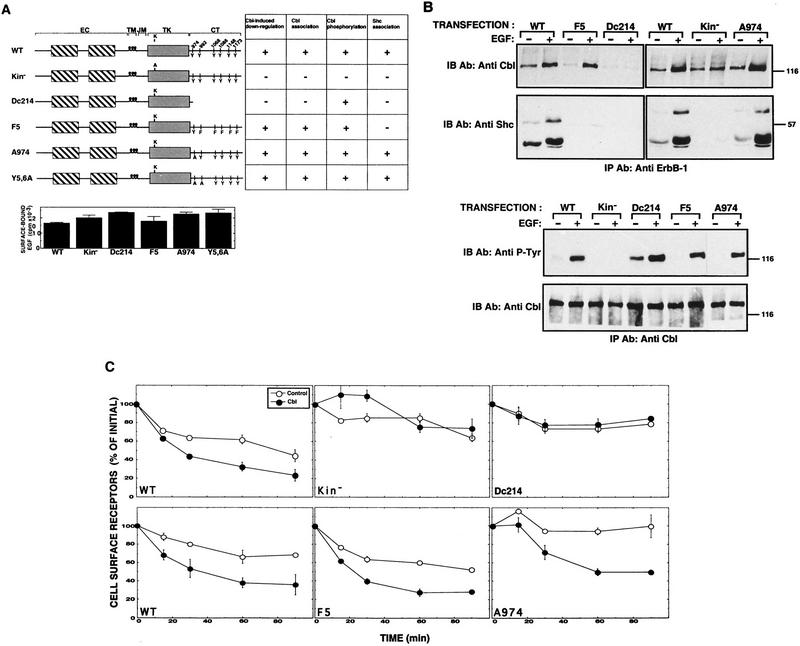
If c-Cbl plays a causative role in receptor down-regulation, as implied by our results, then its interaction with ErbB-1 may depend on kinase activity and autophosphorylation sites. This question was addressed by coexpressing a series of ErbB-1 mutant proteins, together with c-Cbl, in CHO cells. The mutant proteins, along with a wild-type receptor, are schematically depicted in Figure 5A. They include a kinase-defective form (Kin−), a deletion mutant lacking 214 amino acids of the carboxy-terminal region (denoted Dc-214), and a protein whose five major tyrosine autophosphorylation sites were replaced by phenylalanines (denoted F5). In addition, we analyzed a receptor mutated at tyrosine 974 (denoted A974) because this site has been implicated in the interactions of ErbB-1 with the AP-2 adaptor of clathrin-coated pits (Sorkin et al. 1996). Likewise, a receptor mutated at both tyrosine residues 974 and 992 (denoted Y5,6A) was analyzed because these sites are homologous to the reported Cbl-docking site of the ZAP-70 tyrosine kinase (Lupher et al. 1997). Transient expression of the mutated proteins in CHO cells resulted in receptor levels similar to that of the wild-type ErbB-1 (Fig. 5A). The following aspects of Cbl interaction with ErbB-1 were analyzed by use of this cellular system: ligand-induced physical association, as well as tyrosine phosphorylation, of c-Cbl, recruitment of Shc, and receptor down-regulation. The results of these assays are summarized in a table (Fig. 5A), and presented in Figure 5, B and C. The results obtained with Y5,6A are presented only in the table.
Figure 5.
Structural determinants of ErbB-1 that are essential for functional interactions with c-Cbl. (A) Schematic representation of ErbB-1 mutants and their interactions with c-Cbl. The domain structure of ErbB-1 is shown by boxes that correspond to the double cysteine-rich domain of the extracellular (EC) region, the transmembrane domain (TM), the juxtamembrane domain (JM), the tyrosine kinase (TK) domain, and the carboxy-terminal tail (CT). The five major tyrosine autophosphorylation sites, along with the α-adaptin tyrosine-based internalization signal [Y974; (Sorkin et al. 1996)] are indicated. The ATP-binding lysine residue (K721) was mutated to an alanine residue in the kinase-defective mutant (Kin−). A carboxy-terminal deletion mutant (Dc214) lacking 214 carboxy-terminal amino acids, and an ErbB-1 mutant in which the five major tyrosine phosphorylation sites were mutated to phenylalanine (F5) have been described previously (Sorkin et al. 1996). A double tyrosine to alanine mutant (Y5,6) is also shown. A summary of the results shown in B and C is presented in the table. The histogram presents the results of an assay that determined the binding of radioactive EGF to the surface of cells transiently expressing the indicated mutants. (B) Monolayers of CHO cells were separately cotransfected with plasmids encoding the indicated ErbB-1 mutants together with a c-Cbl-encoding vector. Sister plates were incubated for 15 min at 37°C with or without EGF (at 100 ng/ml). Thereafter, whole cell lysates were subjected to immunoprecipitation (IP) and immunoblotting (IB) with the indicated antibodies. (C) The indicated ErbB-1 mutants were introduced into CHO cells by cotransfection with a control vector (○) or a c-Cbl plasmid (•). EGF-induced down-regulation assay was then performed.
Evidently, two features of ErbB-1 are essential for productive interaction with c-Cbl. These are the intrinsic tyrosine kinase activity, and the presence of an intact carboxy-terminal region. The five autophosphorylation sites of ErbB-1 are not crucial for the interaction between c-Cbl and ErbB-1 (Fig. 5), although they were essential for physical association with another ErbB-1 substrate, namely Shc (Fig. 5B). In accordance with their inability to interact with c-Cbl, the Kin− and the Dc214 mutants displayed markedly reduced ligand internalization and down-regulation (Sorkin et al. 1992; Chang et al. 1993). We note that in some experiments basal association between c-Cbl and a kinase-defective mutant of ErbB-1 was detectable (cf. Figs. 5B and 7B), but in no case was it ligand dependent. Interestingly, the carboxy-terminally deleted mutant of ErbB-1 mediated both prolonged activation of the MAPK pathway (data not shown) and enhanced tyrosine phosphorylation of c-Cbl (Fig. 5B, bottom). Nevertheless, it was refractory to the c-Cbl-induced down-regulation effect (Fig. 5C), implying that c-Cbl phosphorylation is insufficient for degradation of ErbB-1. On the other hand, complex formation between c-Cbl and ErbB-1 correlated with down-regulation (Fig. 5A), suggesting that stable physical association of c-Cbl with the receptor is critical for directing ErbB-1 to degradation.
Figure 7.
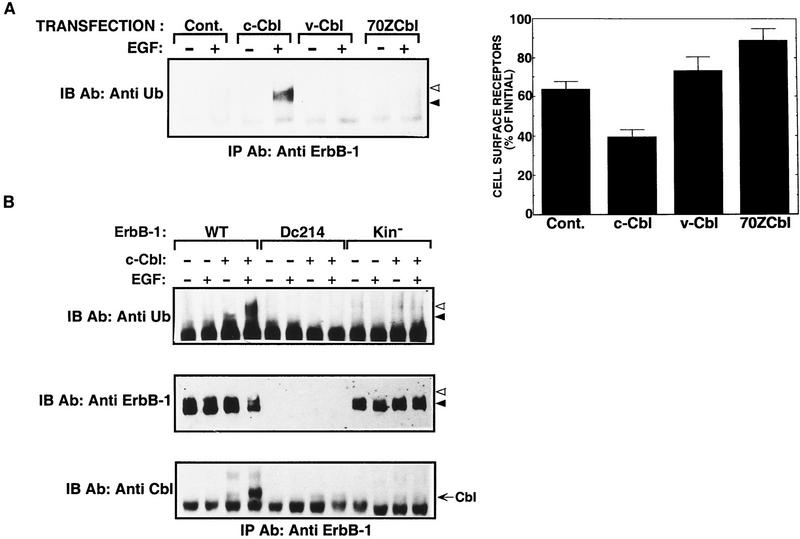
Effect of Cbl proteins on ubiquitination of ErbB-1 and its mutants. (A) CHO cells were cotransfected with a plasmid encoding ErbB-1 together with vectors directing the expression of the indicated Cbl proteins. An empty vector was used for control (Cont.). Cell monolayers were treated for 15 min at 37°C with EGF (100 ng/ml). Thereafter, we either analyzed cell lysates by immunoprecipitation (IP) and immunoblotting (IB) with the indicated antibodies (left, arrowheads are as in Fig. 6) or performed a ligand-binding assay as described in the legend to Fig. 2B. (B) Monolayers of CHO cells were transfected with a plasmid expressing c-cbl or a control empty vector (−) together with vectors encoding the wild-type form (WT) of ErbB-1, or the indicated mutants. Cell monolayers were treated with EGF as in A and their whole lysates subjected to immunoprecipitation (IP) with an antibody directed to the extracellular portion of ErbB-1. Immunoblotting (IB) was performed with an antiserum to ubiquitin, or with an antibody directed to the most carboxy-terminal 14 amino acids of ErbB-1. To confirm expression of the Dc214 mutant of ErbB-1, which was not recognized by the immunoblotting antibody, we performed a ligand-binding assay on living cells (data not shown).
c-Cbl increases ligand-induced ubiquitination of ErbB-1
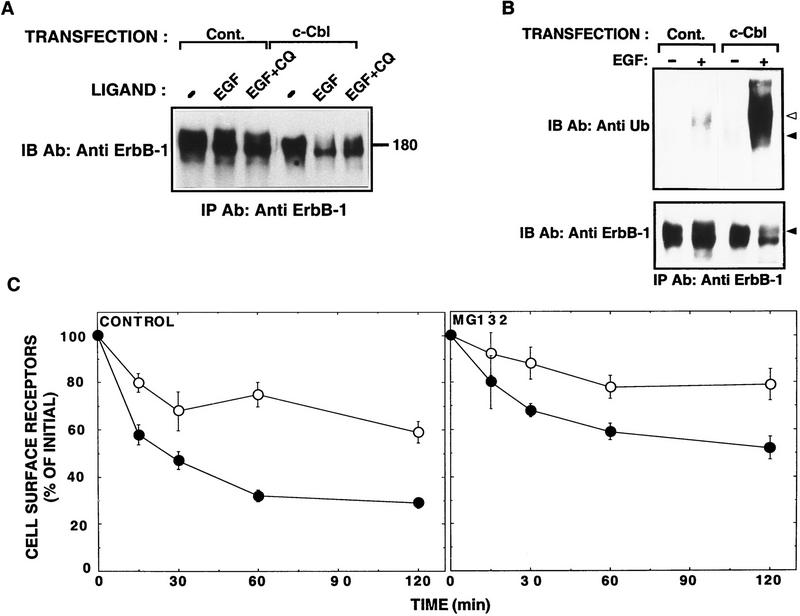
Next, we addressed the effect of c-Cbl on receptor degradation by using chloroquine, an inhibitor of prelysosomal/lysosomal proteolysis. Chloroquine exerted no effect on the limited ability of EGF to induce degradation of an overexpressed ErbB-1 in CHO cells (∼20% of ErbB-1 molecules underwent degradation following EGF treatment, regardless of chloroquine presence, Fig. 6A). Co-overexpression of c-Cbl significantly enhanced ligand-induced degradation of ErbB-1 (80% of ErbB-1 molecules underwent degradation), but chloroquine was able to partly attenuate this Cbl-mediated enhanced degradation (only 44% of ErbB-1 molecules underwent degradation). Conceivably, c-Cbl affects receptor processing upstream to the chloroquine-sensitive late endosomal step. Because ligand-induced ubiquitination of ErbB-1 precedes its intracellular degradation, and it depends on endocytosis of the ligand-receptor complexes (Galcheva-Gargova et al. 1995), we analyzed the ability of c-Cbl to affect receptor ubiquitination. In line with previous reports, we detected a ligand-induced increase in ErbB-1 ubiquitination (Fig. 6B). By overlapping anti-ErbB-1 immunoblots with the ubiquitin signals, we learned that the ubiquitinated fraction of ErbB-1 was minor (<5%, open arrow in Fig. 6B). Overexpression of c-Cbl dramatically increased the amount of ubiquitin that underwent covalent attachment to ErbB-1 (Fig. 6B), implying that the cellular level of Cbl critically controls the extent of receptor ubiquitination.
Figure 6.
c-Cbl-induced down-regulation involves an increase in ErbB-1 ubiquitination. (A) Chloroquine sensitivity. The wild-type form of ErbB-1 was expressed in CHO cells by cotransfection of an erbB-1-encoding plasmid together with either a c-Cbl-expression vector or an empty vector (Cont.). Cells were incubated for 45 min at 37°C in the absence or presence of EGF (at 100 ng/ml) and chloroquine (CQ, 0.1 mm). Cell lysates were prepared and subjected to immunoprecipitation (IP) and immunoblotting (IB) with anti-ErbB-1 antibodies. (B) CHO cells were transfected and treated as in A. Cell lysates were subjected to immunoprecipitation (IP) with antibodies to ErbB-1 and immunoblotting (IB) with antibodies to either ubiquitin (Ub) or ErbB-1. (Closed arrowheads) The major band of ErbB-1; (open arrowheads) the minor fraction that underwent ubiquitination. (C) ErbB-1 was transiently expressed in CHO cells by cotransfection with either an empty expression vector (control, ○) or a c-Cbl expression vector (•). EGF-induced down-regulation of ErbB-1 was determined in the presence or absence of the proteasomal inhibitor MG132 (10 μm).
In experiments that are not presented, we found that treatment of cells with an inhibitor of proteasomal activity, MG132, increased the extent of receptor ubiquitination in cells overexpressing c-Cbl. Therefore, we analyzed the effect of MG132 on the fraction of surface-exposed receptors by using a down-regulation assay. The presence of MG132 decreased not only the basal EGF-induced disappearance of ligand binding sites, but also the accelerated down-regulation that was promoted by an overexpressed c-Cbl (Fig. 6C). Because inhibition of either c-Cbl (Fig. 4B) or proteasomes (Fig. 6C) can enhance recycling of ErbB-1, but blocking lysosomal hydrolases allows no recycling (data not shown), it is likely that c-Cbl acts upstream to the proteasomal and lysosomal degradation processes.
Ligand-dependent ubiquitination corresponds to the down-regulation activity of mutant Cbl and ErbB-1 proteins
The contention that c-Cbl can accelerate the degradation rate of receptor molecules by increasing their ubiquitination predicts that mutant Cbl proteins, which are unable to down-regulate ErbB-1, will be defective in inducing receptor ubiquitination. The two oncogenic forms of c-Cbl, v-Cbl, and 70Z-Cbl, a deletion mutant lacking 17 internal amino acids (Langdon et al. 1989), were either unable to enhance receptor down-regulation and ubiquitination or elevated receptor expression (Fig. 7A). Likewise, mutant ErbB-1 proteins that cannot stably recruit c-Cbl displayed no ubiquitination. These are a kinase-defective receptor, and a carboxy-terminally deleted ErbB-1 (Fig. 7B). However, a receptor whose five major autophosphorylation sites were mutated (F5) retained enhanced ubiquitination (data not shown), in line with its ability to recruit c-Cbl (Fig. 5). Because the defective mutants were unable to associate with c-Cbl and accelerate receptor down-regulation and degradation (Figs. 5 and 7B), we concluded that functional interaction with c-Cbl is necessary for receptor degradation, down-regulation, and ubiquitination. The relative order of these processes and their presumed compartmentalized organization are discussed below and summarized in a model (Fig. 8).
Figure 8.
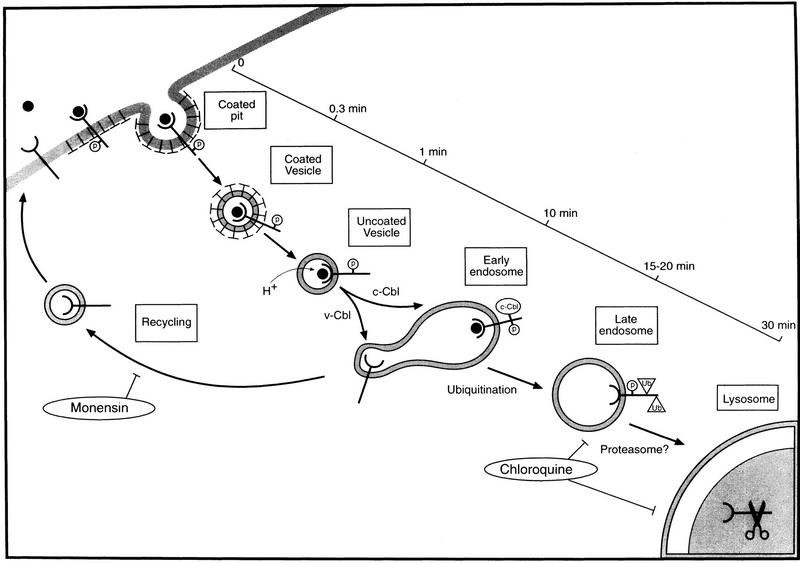
Proposed model of ligand-induced endocytosis of ErbB-1. The model summarizes the major steps of receptor endocytosis and indicates their presumed time scale. Ligand binding to ErbB-1 molecules, probably by elevating autophosphorylation (encircled P), induces their interactions with clathrin-coated areas of the plasma membrane, which rapidly invaginate to form coated pits. c-Cbl may not affect excision of the pit to form a coated vesicle and the subsequent rapid uncoating process. c-Cbl recruitment to endosome-located ErbB-1 molecules tags them for ubiquitination (Ub) and subsequent degradation through the combined action of prelysosomal/lysosomal acid hydrolases, as well as by proteasomal proteinases. v-Cbl shunts receptors to the default pathway, which involves recycling of vesicles back to the cell surface. This step is inhibitable by monensin.
Discussion
Endocytosis of the ErbB-1 is one of the best-characterized routes of induced internalization of ligand-receptor complexes (for review, see Sorkin and Waters 1993; Trowbridge et al. 1993). The most rapid endocytic pathway, which utilizes clathrin-coated pits and vesicles, is characterized by saturability: The rate of internalization decreases with increasing receptor occupancy (Lund et al. 1990). Another limiting step is the sorting of internalized receptors to lysosomal degradation (French et al. 1994). On transient overexpression of ErbB-1 in CHO cells (∼2–6 × 105 receptors/cell) the rapid endocytic pathway is practically saturated and very low receptor down-regulation occurs (Fig. 2C). Overexpression of c-Cbl on this cellular background revealed a Cbl-dependent limiting step that can regulate receptor degradation (Figs. 2C and 4B). Similarly, the negative regulation by c-Cbl of another tyrosine kinase, Syk, was detectable only on Cbl overexpression with recombinant vaccinia constructs (Ota and Samelson 1997).
Down-regulation is the net result of receptor degradation and recycling. Because the rate of EGF internalization was not detectably affected by c-Cbl overexpression (Fig. 4A), but receptor down-regulation was accelerated by c-Cbl (Fig. 4B), we propose that Cbl acts at a postcoated pit step. In agreement with this difference, a kinase-defective ErbB-1 mutant, which cannot interact with Cbl, retained the ability to internalize EGF molecules and escaped degradation (Felder et al. 1990). The observation that a co-overexpressed v-Cbl can reverse c-Cbl’s action by shunting internalized receptors to a monensin-sensitive pathway (Fig. 4B) led us to the proposition that the recycling endosome (Trowbridge et al. 1993) is the site of Cbl’s action. In support of this possibility, c-Cbl was localized to a vesicular compartment (Figs. 2A and 3A) and it was fractionated with an endosomal fraction (Fig. 3C). Because receptor ubiquitination was enhanced by c-Cbl (Fig. 6), and its structural requirements appear to reflect c-Cbl recruitment (cf. Figs. 7 and 5A), it is conceivable that the sorting function of c-Cbl involves receptor ubiquitination. The scheme presented in Figure 8 incorporates these conclusions into a model that integrates data from the present and previous studies. According to this model, c-Cbl is not involved in the entrapment of EGF receptors by coated regions of the plasma membrane, and in the subsequent rapid invagination and scission that form coated pits and coated vesicles, respectively. However, ∼1–2 min after binding of EGF to the cell surface, c-Cbl becomes associated with ErbB-1-containing vesicles (Fig. 3). On the basis of quantification of fluorescent images, we assume that all mature ErbB-1-containing vesicles associate with c-Cbl, but this recruitment engages only a small fraction of the cellular pool of Cbl (Fig. 3B).
How exactly c-Cbl is recruited by ErbB-1 remains unclear. One possiblity that we have not addressed is the involvement of several lysosome targeting motifs located at the carboxy-terminal region of ErbB-1 (Opresko et al. 1995). Although none of five tyrosine autophosphorylation sites located at this region appears essential for Cbl-ErbB-1 interactions, we cannot exclude the possibility that tyrosine autophosphorylation of ErbB-1 also plays a role in c-Cbl recruitment. For example, phosphorylation of tyrosine residues we have not mutated may allow ErbB-1 to bind to c-Cbl either directly or indirectly. Because c-Cbl is found in living cells complexed to several proteins, like Crk, phosphatidylinositol 3′-kinase, and the Grb2 adaptor protein (Meisner et al. 1995), it is possible that c-Cbl/Grb2, or other complexes, associate with the carboxy-terminal region of ErbB-1 through compensatory tyrosine phosphorylation sites.
Biochemical and morphological studies that utilized a kinase-defective mutant of ErbB-1 revealed causal relationships between receptor occupancy, kinase activity, and the rate of internalization (Honegger et al. 1987; Chen et al. 1989; Hopkins et al. 1990). However, the role played by the catalytic activity of ErbB-1 in lysosomal targeting is currently unclear. According to one possibility, targeting is independent of kinase activity (Wiley et al. 1991), but an alternative model implies that the intrinsic kinase can interrupt receptor recycling (Felder et al. 1990). According to this scenario, the early endocytic pathways of wild-type and kinase-impaired receptors are identical, but after 10–20 min the pathways diverge at the multivesicular body (MVB): Wild-type ErbB-1, destined for degradation, localizes to internal vesicles, whereas kinase-defective ErbB-1, destined for recycling, localizes to surface membranes of the MVBs. The coincidental peak of c-Cbl recruitment by ErbB-1-containing endosomes (Fig. 3), together with the ability of c-Cbl to promote down-regulation of wild type, but not a kinase-defective mutant of ErbB-1 (Fig. 5), strongly support the identification of c-Cbl as the protein substrate whose recruitment by internalized receptors allows translocation into internal vesicles of the MVB. Conceivably, additional endosomal molecules are involved in the sorting mechanism. Examples of candidate sorting molecules include annexin I (Futter et al. 1993), SNX1, a homolog of a yeast vacuolar sorting protein that accelerates down-regulation of ErbB-1 (Kurten et al. 1996), phosphatidylinositol 3′-kinase, (Joly et al. 1995), and Grb-2 (Wang and Moran 1996).
The relationships between proteasomal and lysosomal degradation processes is unclear at present. However, it appears likely that ubiquitination occurs already in a prelysosomal compartment. In accordance with this scenario, we were unable to detect ubiquitinated ErbB-1 molecules on the surface of CHO cells by using biotin labeling (data not shown). This observation is consistent with reports that blocking endocytosis of either the growth hormone receptor (Govers et al. 1997) or the EGF receptor (Galcheva-Gargova et al. 1995), can prevent ubiquitination. The situation is clearly different in yeast cells: Ubiquitination of the Ste2p receptor, a G protein-coupled receptor for the α factor, is necessary for receptor endocytosis (Hicke and Riezman 1996). In fact, transfer of ErbB-1 to the lysosome may involve proteasomal activity because inhibition at this step still allows some receptor recycling (Fig. 6C). We note that degradation of several other growth factor receptors such as the insulin-like growth factor-1 receptor (Sepp Lorenzino et al. 1995), the PDGF-receptor (Mori et al. 1995), and the Met receptor for the scatter factor (Jeffers et al. 1997), also depend on proteasomal activity. Thus, complete degradation of internalized ErbB-1 molecules probably involves simultaneous proteolysis by hydrolases and proteasomal proteinases at the late endosome and/or at the lysosome.
Genetic analyses of mutant worms and flies indicated that c-Cbl functions as a major negative regulator of intercellular inductive processes controlling vulva (Jongeward et al. 1995; Yoon et al. 1995) and eye (Meisner et al. 1997) development, respectively. In both examples growth factor signaling is funneled into the Ras-MAPK pathway (for review, see Perrimon and Perkins 1997). The analogous mammalian signaling machinery, which is mediated by the ErbB network, also feeds into the Ras-MAPK pathway (for review, see Alroy and Yarden 1997). However, unlike ErbB-1, which is strongly coupled to c-Cbl, the two NDF receptors do not interact with c-Cbl (Levkowitz et al. 1996). Consequently, ErbB-3 and ErbB-4 undergo no down-regulation following stimulation with NDF (Figs. 1B and 2C; data not shown), and no translocation of c-Cbl into receptor-containing endosomal vesicles occurs in response to NDF binding to ErbB-3 (Fig 2A). These differences indicate that ErbB-1 is subject to negative regulation by c-Cbl, whereas ErbB-3 and ErbB-4 escape Cbl-mediated attenuation. Consequently, the mitogenic signal elicited by EGF is significantly less potent than that induced by NDF binding to ErbB-3 (Pinkas-Kramarski et al. 1996).
Our finding with ErbB-1 and ErbB-3 may be relevant to the many other growth factor receptors that interact with c-Cbl. Thus, it will be interesting to test the prediction that most c-Cbl-coupled receptors will undergo ligand-induced ubiquitination. In addition to ErbB-1, the receptors for CSF-1 (Wang et al. 1996), PDGF (Mori et al. 1995), and the scatter factor (Jeffers et al. 1997) are also candidates for c-Cbl-mediated down-regulation through a ubiquitin-dependent process. Moreover, c-Cbl may control also nonreceptor tyrosine kinases, such as Syk (Ota and Samelson 1997), and Src (Tanaka et al. 1996). If verified, this function may account for the ubiquitous expression of c-Cbl, and for its general involvement in apparently unrelated signaling pathways.
Materials and methods
Materials and antibodies
A recombinant form of NDF-β1177–246 was provided by Amgen (Thousand Oaks, CA). Texas red-labeled EGF was from Molecular probes (Eugene, OR). Radioactive materials were purchased from Amersham (Buckinghamshire, UK). Iodogen was from Pierce. Biotin-X-NHS, MG-123, and lactacystin were from Calbiochem. Rabbit anti-c-Cbl (C-15) antibodies, as well as anti-ErbB-3 antibodies, and a monoclonal antibody (mAb) to phosphotyrosine antibody were from Santa-Cruz Biotechnology. Murine mAbs to human ErbB-1 and ErbB-3 were from NeoMarkers (Fremont, CA). For immunoblot analysis of ErbB-1, Rab-5, and Shc, we used antibodies from Transduction Laboratories (Lexington, KY). Anti-ubiquitin antibody was kindly provided by Drs. S. Yokota (Yamanashi Medical University, Japan) and A. Amsterdam (Weizmann Institute, Rehovot, Israel). The anti-hemagglutinin (HA) mAb was purchased from Boehringer Mannheim.
Construction and transfection of expression vectors
To subclone c-cbl and 70Z-cbl into the pcDNA3 expression vector (Invitrogen) containing the HA sequence tag (a gift from Dr. Y. Haupt, Hebrew University, Jerusalem, Israel), we inserted the cDNAs into the BamHI and XbaI or BamHI and XhoI sites of pcDNA3, respectively. To generate the v-Cbl and Cbl-C truncation mutants, we similarly subcloned cDNA fragments corresponding to amino acids 1–357 and 358–906, respectively. A GFP–Cbl expression vector was generated by replacement of Cbl’s stop codon with a SmaI site and insertion into the KpnI and SmaI sites of pEGFP-N1 (Clontech). Expression vectors (pcDNA3) containing the full-length cDNA of human ErbB-1 and ErbB-3 were described previously (Tzahar et al. 1996). The ErbB-1 deletion mutant lacking 214 carboxy-terminal amino acids [Dc-214 (Sorkin et al. 1992)], as well as the F5 mutant in which the five major tyrosine autophosphorylation sites were mutated to phenylalanine residues (Soler et al. 1993), and the A974 in which tyrosine 974 was replaced by an alanine (Sorkin et al. 1996), have been described. All three mutants were subcloned into pcDNA3. To generate a kinase-defective ErbB-1 (lysine 721 mutated to alanine), and the double tyrosine to alanine mutant Y5,6A (tyrosine residues 974 and 992 mutated to alanines), we used oligonucleotide-directed mutagenesis with T7-DNA polymerase. Expression vectors were introduced to CHO cells by the Lipofectamine transfection method (GIBCO-BRL). The total amount of DNA in each transfection was normalized with pcDNA3 plasmid. Twenty-four hours following transfection, cells were split and assayed 24 hr later.
Ligand binding, internalization analyses, and endosome purification
Recombinant human ligands were labeled with Iodogen, and their binding to cell monolayers and internalization were performed as described (Waterman et al. 1998). An endosomal fraction was prepared as described (Wada et al. 1992) with the following modifications: monolayers of CHO cells were scraped, washed with PBS and resuspended in homogenization buffer (250 mm sucrose, 3 mm imidazole, 1 mm EDTA) containing protease and phosphatase inhibitors. Cells were homogenized (20 strokes, pestle B in a Dounce homogenizer) and centrifuged for 10 min at 1500g. The sucrose concentration in the postnuclear supernatant was adjusted to 1.15 m, overlaid with 1.00 and 0.25 m sucrose cushions and centrifuged at 200,000g for 1.5 hr (Beckman SW41-Ti rotor). Endosomes were collected at the 0.25–1.00 m sucrose interface. The endosomal marker protein Rab-5 was significantly enriched in this fraction relative to other fractions of the sucrose gradient.
Receptor down-regulation and surface biotinylation assays
Ligand-induced receptor down-regulation was measured as follows: cells grown in 24-well plates were incubated at 37°C for up to 2.5 hr with or without 250 ng/ml EGF or NDF in binding buffer, and then rinsed with ice-cold binding buffer. Surface-bound ligand molecules were removed by use of a low pH wash (Pinkas-Kramarski et al. 1996). The number of ligand-binding sites on the cell surface was then determined by incubating cells at 4°C with the corresponding radiolabeled ligand for at least 1 hr. Biotinylation of cell surface proteins was performed as we described previously (Waterman et al. 1998).
Immunoprecipitation and immunoblotting analyses
Subconfluent CHO cells were grown in 10-cm culture dishes, washed briefly with PBS, and incubated for 10 min at 37°C with the indicated recombinant growth factors (each at 100 ng/ml). To stop activation, cells were washed with ice-cold PBS and kept on ice. Cell lysates were prepared at 4°C. Immunoprecipitation, gel electrophoresis, and immunoblotting were performed as we described previously (Levkowitz et al. 1996).
Immunofluorescence
Cells grown on cover-slips were rinsed with serum-free medium and then treated in the absence or presence of ligands for 5 min at 37°C. Thereafter, the medium was replaced and incubation was continued for the indicated time intervals. Cells were fixed for 15 min with 3% paraformaldehyde in PBS. For immunofluorescent labeling, cells were permeabilized for 10 min at 22°C with PBS containing 1% albumin and 0.2% Triton X-100. For double labeling, cover-slips were incubated for 1 hr at room temperature with mAb 111.1 or mAb 90 (anti-ErbB-1 or -3, respectively) in combination with anti-Cbl antibodies. After wash with PBS, the cover-slips were incubated with Cy3-conjugated goat-anti-rabbit F(ab′)2 and FITC-conjugated goat-anti-mouse F(ab′)2-specific antibodies (Jackson ImmunoResearch Laboratories) for an additional 1 hr. Finally, the coverslips were mounted in Elvanol (Hoechst, Frankfurt).
Quantitative immunofluorescence microscopy
The system used for quantitative fluorescence microscopy and image analysis will be described in detail elsewhere (E. Zamir, B.-Z. Katz, K. Yamada, B. Geiger, and Z. Kam, in prep.). Specimens were examined by use of a Zeiss Axioskope microscope (Oberkochen, Germany) with a 100/1.3 plan-Neofluar objective. Images were acquired by a scientific, 12-bit, charged-couple devise (CCD) camera (Model C220, Photometrics Co.). Particles were segmented by the Water algorithm following high-pass filter (subtracting from each pixel the average over 4 × 4 μm area around the particle). The parameters in Water were adjusted to the dimension of the particles, and were kept constant for all the analyses. To analyze the relationships between Cbl-labeled structures and ErbB-1-labeled vesicles, vesicles were segmented separately and fluorescence intensities measured for both Cbl- and ErbB1-segmented vesicles. The total number of Cbl- or ErbB1-containing vesicles was divided by the total cell area to yield the average number of vesicles per 103 μm2. Cbl-segmented vesicles that had FITC fluorescent intensity higher than a constant threshold were defined as Cbl-vesicles with ErbB1, and their percentage from the total number of Cbl-segmented vesicles was calculated. Similarly, the percentage of ErbB1-containing vesicles with Cbl from the total number of ErbB1-segmented vesicles was obtained.
Acknowledgments
In cherished memory of Yarden Weinberg (1994–1998) who died of rhabdomyosarcoma. We thank Drs. S. Yokota and A. Amsterdam for anti-ubiquitin antibodies, Amar Sahay for constructing the pcDNA3/F5 mutant, and Sara Lavi for technical help. This work was supported by a grant from the National Institutes of Health (CA72981).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked ‘advertisement’ in accordance with 18 USC section 1734 solely to indicate this fact.
Note added in proof
It has been reported very recently that Cbl enhances ubiquitination and degradation of the PDGF receptor [S. Miyake et al. (1998) Proc. Natl. Acad. Sci. 95: 7927–7932].
Footnotes
E-MAIL liyarden@weizmann.weizmann.ac.il; FAX 972-8-9344116.
References
- Alroy I, Yarden Y. The ErbB signaling network in embryogenesis and oncogenesis: Signal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 1997;410:83–86. doi: 10.1016/s0014-5793(97)00412-2. [DOI] [PubMed] [Google Scholar]
- Basu SK, Goldstein JL, Anderson RGW, Brown MS. Monensin interrupts the recycling of low density lipoprotein receptors in human fibroblasts. Cell. 1981;24:493–502. doi: 10.1016/0092-8674(81)90340-8. [DOI] [PubMed] [Google Scholar]
- Boussiotis VA, Freeman GJ, Berezovskaya A, Barber DL, Nadler LM. Maintenance of human T cell anergy: Blocking of IL-2 gene transcription by activated Rap1. Science. 1997;278:124–128. doi: 10.1126/science.278.5335.124. [DOI] [PubMed] [Google Scholar]
- Bowtell DD, Langdon WY. The protein product of the c-cbl oncogene rapidly complexes with the EGF receptor and is tyrosine phosphorylated following EGF stimulation. Oncogene. 1995;11:1561–1567. [PubMed] [Google Scholar]
- Chang C-P, Lazar CS, Walsh BJ, Komuro M, Collawn JF, Kuhn LA, Tainer JA, Trowbridge IS, Farquhar MG, Rosenfeld MG, Wiley HS, Gill GN. Ligand-induced internalization of the epidermal growth factor receptor is mediated by multiple endocytic codes analogous to the tyrosine motif found in constitutively internalizing receptors. J Biol Chem. 1993;268:19312–19320. [PubMed] [Google Scholar]
- Chen WS, Lazar CS, Lund KA, Welsh JB, Chang C-P, Walton GM, der CJ, Wiley HS, Gill GN, Rosenfeld MG. Functional independence of the epidermal growth factor receptor from a domain required for ligand-induced internalization and calcium regulation. Cell. 1989;59:33–43. doi: 10.1016/0092-8674(89)90867-2. [DOI] [PubMed] [Google Scholar]
- Felder S, Miller K, Moehren G, Ullrich A, Schlessinger J, Hopkins CR. Kinase activity controls the sorting of the epidermal growth factor receptor within the multivesicular body. Cell. 1990;61:623–634. doi: 10.1016/0092-8674(90)90474-s. [DOI] [PubMed] [Google Scholar]
- French AR, Sudlow GP, Wiley HS, Lauffenburger DA. Postendocytic trafficking of epidermal growth factor-receptor complexes is mediated through saturable and specific endosomal interactions. J Biol Chem. 1994;269:15749–15755. [PubMed] [Google Scholar]
- Futter CE, Felder S, Schlessinger J, Ullrich A, Hopkins CR. Anexin I is phosphorylated in the multivesicular body during the processing of the epidermal growth factor receptor. J Cell Biol. 1993;120:77–83. doi: 10.1083/jcb.120.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galcheva-Gargova Z, Theroux SJ, Davis RJ. The epidermal growth factor receptor is covalently linked to ubiquitin. Oncogene. 1995;11:2649–2655. [PubMed] [Google Scholar]
- Galisteo ML, Dikic I, Batzer AG, Langdon WY, Schlessinger J. Tyrosine phosphorylation of the c-cbl proto-oncogene protein product and association with epidermal growth factor (EGF) receptor upon EGF stimulation. J Biol Chem. 1995;270:20242–20245. doi: 10.1074/jbc.270.35.20242. [DOI] [PubMed] [Google Scholar]
- Govers R, van Kerkhof P, Schwartz AL, Strous GJ. Linkage of the ubiquitin-conjugating system and the endocytic pathway in ligand-induced internalization of the growth hormone receptor. EMBO J. 1997;16:4851–4858. doi: 10.1093/emboj/16.16.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicke L, Riezman H. Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell. 1996;84:277–287. doi: 10.1016/s0092-8674(00)80982-4. [DOI] [PubMed] [Google Scholar]
- Honegger AM, Dull TJ, Felder S, van Obberghen E, Bellot F, Szapary D, Schmidt A, Ullrich A, Schlessinger J. Point mutation at the ATP binding site of EGF receptor abolishes protein-tyrosine kinase activity and alters cellular routing. Cell. 1987;51:199–209. doi: 10.1016/0092-8674(87)90147-4. [DOI] [PubMed] [Google Scholar]
- Hopkins CR, Gibson A, Shipman M, Miller K. Movement of internalized ligand-receptor complexes along a continuous endosomal reticulum. Nature. 1990;346:335–339. doi: 10.1038/346335a0. [DOI] [PubMed] [Google Scholar]
- Jeffers M, Taylor GA, Weidner KM, Omura S, Vande Woude GF. Degradation of the Met tyrosine kinase receptor by the ubiquitin-proteasome pathway. Mol Cell Biol. 1997;17:799–808. doi: 10.1128/mcb.17.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly M, Kazlauskas A, Corvera S. Phosphatidylinositol 3′-kinase activity is required at a postendocytic step in platelet-derived growth factor receptor trafficking. J Biol Chem. 1995;270:13225–13230. doi: 10.1074/jbc.270.22.13225. [DOI] [PubMed] [Google Scholar]
- Jongeward GD, Clandinin TR, Sternberg PW. sli-1, a negative regulator of let-23-mediated signaling in C. elegans. Genetics. 1995;139:1553–1566. doi: 10.1093/genetics/139.4.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurten RC, Cadena DL, Gill GN. Enhanced degradation of EGF receptors by a sorting nexin. Science. 1996;272:1008–1010. doi: 10.1126/science.272.5264.1008. [DOI] [PubMed] [Google Scholar]
- Langdon WY, Hartley JW, Klinken SP, Ruscetti SK, Morse HC., III v-cbl, an oncogene from a dual-recombinant murine retrovirus that induces early B-lineage lymphomas. Proc Natl Acad Sci. 1989;86:1168–1172. doi: 10.1073/pnas.86.4.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkowitz G, Klapper LN, Tzahar E, Freywald A, Sela M, Yarden Y. Coupling of the c-Cbl protooncogene product to ErbB-1/EGF-receptor but not to other ErbB proteins. Oncogene. 1996;12:1117–1125. [PubMed] [Google Scholar]
- Lund KA, Opresko LK, Starbuck C, Walsh BJ, Wiley HS. Quantitative analysis of the endocytic system involved in hormone-induced receptor internalization. J Biol Chem. 1990;265:15713–15723. [PubMed] [Google Scholar]
- Lupher ML, Jr, Songyang Z, Shoelson SE, Cantley LC, Band H. The Cbl phosphotyrosine-binding domain selects a D(N/D)XpY motif and binds to the Tyr292 negative regulatory phosphorylation site of ZAP-70. J Biol Chem. 1997;272:33140–33144. doi: 10.1074/jbc.272.52.33140. [DOI] [PubMed] [Google Scholar]
- Meisner H, Conway BR, Hartley D, Czech MP. Interactions of Cbl with Grb2 and phosphatidylinositol 3′- kinase in activated Jurkat cells. Mol Cell Biol. 1995;15:3571–3578. doi: 10.1128/mcb.15.7.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisner H, Daga A, Buxton J, Fernandez B, Chawla A, Banerjee U, Czech MP. Interactions of Drosophila Cbl with epidermal growth factor receptors and role of Cbl in R7 photoreceptor cell development. Mol Cell Biol. 1997;17:2217–2225. doi: 10.1128/mcb.17.4.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Tanaka K, Omura S, Saito Y. Degradation process of ligand-stimulated platelet-derived growth factor beta-receptor involves ubiquitin-proteasome proteolytic pathway. J Biol Chem. 1995;270:29447–29452. doi: 10.1074/jbc.270.49.29447. [DOI] [PubMed] [Google Scholar]
- Opresko LK, Chang CP, Will BH, Burke PM, Gill GN, Wiley HS. Endocytosis and lysosomal targeting of epidermal growth factor receptors are mediated by distinct sequences independent of the tyrosine kinase domain. J Biol Chem. 1995;270:4325–4333. doi: 10.1074/jbc.270.9.4325. [DOI] [PubMed] [Google Scholar]
- Ota Y, Samelson LE. The product of the proto-oncogene c-cbl: A negative regulator of the Syk tyrosine kinase. Science. 1997;276:418–420. doi: 10.1126/science.276.5311.418. [DOI] [PubMed] [Google Scholar]
- Perrimon N, Perkins LA. There must be 50 ways to rule the signal: The case of the Drosophila EGF receptor. Cell. 1997;89:13–16. doi: 10.1016/s0092-8674(00)80177-4. [DOI] [PubMed] [Google Scholar]
- Pinkas-Kramarski R, Soussan L, Waterman H, Levkowitz G, Alroy I, Klapper L, Lavi S, Seger R, Ratzkin B, Sela M, Yarden Y. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J. 1996;15:2452–2467. [PMC free article] [PubMed] [Google Scholar]
- Sepp Lorenzino L, Ma Z, Lebwohl DE, Vinitsky A, Rosen N. Herbimycin A induces the 20 S proteasome- and ubiquitin–dependent degradation of receptor tyrosine kinases. J Biol Chem. 1995;270:16580–16587. doi: 10.1074/jbc.270.28.16580. [DOI] [PubMed] [Google Scholar]
- Soler C, Beguinot L, Sorkin A, Carpenter G. Tyrosine phosphorylation of ras GTPase-activating protein does not require association with the epidermal growth factor receptor. J Biol Chem. 1993;268:22010–22019. [PubMed] [Google Scholar]
- Sorkin A, Waters CM. Endocytosis of growth factor receptors. BioEssays. 1993;15:375–382. doi: 10.1002/bies.950150603. [DOI] [PubMed] [Google Scholar]
- Sorkin A, Helin K, Waters CM, Carpenter G, Beguinot L. Multiple autophosphorylation sites of the epidermal growth factor are essential for receptor kinase activity and internalization. Contrasting significance of tyrosine 992 in the native and truncated receptors. J Biol Chem. 1992;267:8672–8678. [PubMed] [Google Scholar]
- Sorkin A, Mazzotti M, Sorkina T, Scotto L, Beguinot L. Epidermal growth factor receptor interaction with clathrin adaptors is mediated by the Tyr974-containing internalization motif. J Biol Chem. 1996;271:13377–13384. doi: 10.1074/jbc.271.23.13377. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Neff L, Baron R, Levy JB. Tyrosine phosphorylation and translocation of the c-Cbl protein after activation of tyrosine kinase signaling pathways. J Biol Chem. 1995;270:14347–14351. doi: 10.1074/jbc.270.24.14347. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Amling M, Neff L, Peyman A, Uhlmann E, Levy JB, Baron R. c-Cbl is downstream of c-Src in a signaling pathway necessary for bone resorption. Nature. 1996;383:528–531. doi: 10.1038/383528a0. [DOI] [PubMed] [Google Scholar]
- Thien CB, Langdon WY. EGF receptor binding and transformation by v-cbl is ablated by the introduction of a loss-of-function mutation from the Caenorhabditis elegans sli-1 gene. Oncogene. 1997;14:2239–2249. doi: 10.1038/sj.onc.1201193. [DOI] [PubMed] [Google Scholar]
- Trowbridge IS, Collawn JF, Hopkins CR. Signal-dependent protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- Tzahar E, Waterman H, Chen X, Levkowitz G, Karunagaran D, Lavi S, Ratzkin BJ, Yarden Y. A hierarchical network of inter-receptor interactions determines signal transduction by NDF/neuregulin and EGF. Mol Cell Biol. 1996;16:5276–5287. doi: 10.1128/mcb.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada I, Lai WH, Posner BI, Bergeron JM. Association of the tyrosine phosphorylated epidermal growth factor receptor with a 55-kD tyrosine phosphorylated protein at the cell surface and in endosomes. J Cell Biol. 1992;116:321–330. doi: 10.1083/jcb.116.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yeung YG, Langdon WY, Stanley ER. c-Cbl is transiently tyrosine-phosphorylated, ubiquitinated, and membrane-targeted following CSF-1 stimulation of macrophages. J Biol Chem. 1996;271:17–20. doi: 10.1074/jbc.271.1.17. [DOI] [PubMed] [Google Scholar]
- Wang Z, Moran MF. Requirement for the adapter protein GRB2 in EGF receptor endocytosis. Science. 1996;272:1935–1938. doi: 10.1126/science.272.5270.1935. [DOI] [PubMed] [Google Scholar]
- Waterman H, Sabanai I, Geiger B, Yarden Y. Alternative intracellular routing of ErbB receptors may determine signaling potency. J Biol Chem. 1998;273:13819–13827. doi: 10.1074/jbc.273.22.13819. [DOI] [PubMed] [Google Scholar]
- Wiley HS, Herbst JJ, Walsh BJ, Lauffenburger DA, Rosenfeld MG, Gill GN. The role of tyrosine kinase activity in endocytosis, compartmentation, and down-regulation of the epidermal growth factor receptor. J Biol Chem. 1991;266:11083–11094. [PubMed] [Google Scholar]
- Yoon CH, Lee J, Jongeward GD, Sternberg PW. Similarity of sli-1, a regulator of vulval development in C. elegans, to the mammalian proto-oncogene c-Cbl. Science. 1995;269:1102–1105. doi: 10.1126/science.7652556. [DOI] [PubMed] [Google Scholar]