Summary
The protein kinase Bβ (Akt2) pathway is known to mediate insulin-stimulated glucose transport through increasing glucose transporter GLUT4 translocation from intracellular stores to the plasma membrane (PM). Combining quantitative phosphoproteomics with RNAi-based functional analyses, we show that a previously uncharacterized 138-kDa C2 domain-containing phosphoprotein (CDP138) is a substrate for Akt2, and is required for optimal insulin-stimulated glucose transport, GLUT4 translocation, and fusion of GLUT4 vesicles with the PM in live adipocytes. The purified C2 domain is capable of binding Ca2+ and lipid membranes. CDP138 mutants lacking the Ca2+-binding sites in the C2 domain or Akt2 phosphorylation site Ser197 inhibit insulin-stimulated GLUT4 insertion into the PM, a rate-limiting step of GLUT4 translocation. Interestingly, CDP138 is dynamically associated with the PM and GLUT4-containing vesicles in response to insulin stimulation. Together, these results suggest that CDP138 is a key molecule linking the Akt2 pathway to the regulation of GLUT4 vesicle - PM fusion.
Introduction
Insulin regulates glucose transport into skeletal muscle and adipose tissue by increasing the cell surface localization of the glucose transporter GLUT4 (Bryant et al., 2002; Huang and Czech, 2007). In the basal state, GLUT4 is retained within specific intracellular compartments and insulin rapidly increases the movement of GLUT4 from its intracellular compartment to the plasma membrane (PM), where it captures the extracellular glucose for internalization. This effect is essential to maintain glucose homeostasis in humans, and impaired insulin action contributes to the development of type II diabetes (Saltiel and Kahn, 2001).
Insulin binding to its tyrosine kinase receptor results in tyrosine phosphorylation of insulin receptor substrate (IRS) proteins. Phosphorylated IRS proteins bind to and activate phosphoinositide 3-kinase (PI3K), which phosphorylates polyphosphoinositides to form PI(3,4)P2, and PI(3,4,5)P3 (Cantley, 2002). The latter recruits the protein kinase B (Akt) and the phosphoinositide-dependent kinase (PDK-1) to the PM, where PDK-1 (Alessi et al., 1997) and the mTORC2 complex phosphorylate and activate Akt (Sarbassov et al., 2005). Several lines of evidence strongly suggest that activation of the PI3K - Akt2 pathway is necessary for insulin to induce GLUT4 translocation, and for glucose transport (Martin et al., 1996). First, PI(3,4,5)P3 formation is required for GLUT4 insertion into the PM (Tengholm and Meyer, 2002). Second, expression of constitutively active Akt in adipocytes increases glucose uptake (Kohn et al., 1996), and conversely, a dominant negative form of Akt inhibits insulin-induced GLUT4 translocation (Hill et al., 1999; Wang et al., 1999). Third, siRNA-mediated knockdown of Akt, particularly Akt2, significantly reduces insulin-stimulated glucose transport and GLUT4 translocation in cultured cells (Jiang et al., 2003). Fourth, a diabetes-like phenotype is observed in Akt2 knockout mice (Cho et al., 2001) and fifth, an inactivating mutation in Akt2 in humans leads to the development of severe insulin resistance and diabetes mellitus (George et al., 2004).
The GLUT4 exocytic pathway includes glucose storage vesicle (GSV) sorting, trafficking, docking, tethering, and finally fusion with the PM (Thurmond and Pessin, 2001). Accumulated evidence suggests that activation of Akt2 is involved in regulating both GSV mobilization to the periphery and membrane fusion between GSV and the PM, and this may occur through phosphorylation of different substrates. Akt has been shown to regulate GSV trafficking to and docking at the PM (Gonzalez and McGraw, 2006). This is consistent with the observation that Akt2 is recruited to and phosphorylates GSV components (Calera et al., 1998; Kupriyanova and Kandror, 1999). There is compelling evidence suggesting that Akt2 is also required for GSV – PM fusion (Chen et al., 2003; Koumanov et al., 2005; Ng et al., 2008; van Dam et al., 2005). Previous studies showed that Akt phosphorylation of AS160 stimulates GLUT4 trafficking (Eguez et al., 2005; Sano et al., 2003), but not the GSV–PM fusion step in cultured adipocytes (Bai et al., 2007; Jiang et al., 2008), suggesting that other Akt2 substrate(s) might be required for the last step of GLUT4 translocation.
In this study, we used quantitative phosphoproteomics and RNAi-based functional analyses to identify a previously uncharacterized 138 kDa C2 domain-containing phosphoprotein (CDP138) that is required for optimal GSV–PM membrane fusion during GLUT4 translocation, and for subsequent maximal insulin-stimulated glucose transport. Our data show that CDP138 functions downstream of Akt2 and dynamically associates with both the PM and GLUT4 vesicles. In addition, the C2 domain binds Ca2+ and membrane lipids. Both the intact C2 domain and the Akt2 phosphorylation site of CDP138 are necessary for full insulin-stimulated GLUT4 translocation. These observations establish CDP138 as a novel molecular link between insulin-stimulated Akt2 signaling and GLUT4 insertion into the PM.
Results
Identification of CDP138 as a novel Akt2 substrate using SILAC-based quantitative proteomics
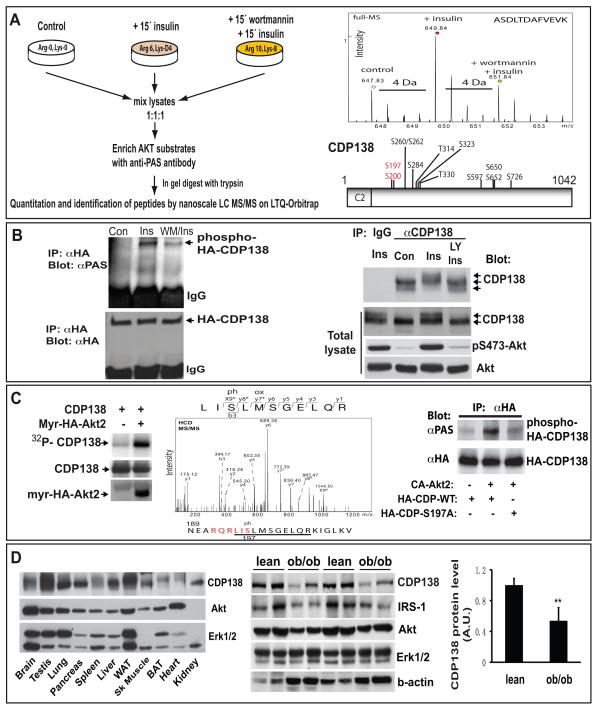
A mass spectrometry (MS)-based approach termed stable isotope labeling with amino acids in cell culture (SILAC) has provided a highly sensitive tool to identify and quantify phosphorylated proteins in cultured cells (Kruger et al., 2008; Olsen et al., 2006). To identify potential new Akt substrates in insulin-stimulated adipocytes, three parallel cultures of 3T3-L1 preadipocytes were metabolically labeled with different SILAC amino acids to make their proteomes distinguishable, before differentiated adipocytes were treated with or without the PI3K inhibitor wortmannin followed by insulin as outlined in Figure 1A. Equal amounts of total cell lysate from the three different samples were pooled and subjected to immunoprecipitation to enrich for phosphorylated Akt substrates (PAS) using antibodies (Ab) against the PAS motif RXRXXS/T (Alessi et al., 1996; Obata et al., 2000). The tryptic peptides were subsequently analyzed by tandem mass spectrometry (MS). Using this approach, we identified 128 proteins including 21 known Akt substrates enriched more than 1.5 fold from insulin-treated cells (Supplemetary Table S1). Among them, a previously uncharacterized 138-kDa C2 domain-containing phosphoprotein (CDP138), encoded by 5730419I09Rik (kiaa0528), was enriched from insulin-stimulated cells but was significantly inhibited in the cells pretreated with wortmannin (Figure 1A and Table S1). CDP138 contains a predicted C2 domain similar to those present in the Ca2+ receptor synaptotagmins, known to be required for vesicle exocytosis (Bai and Chapman, 2004).
Figure 1. An uncharacterized C2 domain-containing protein encoded by 5730419I09Rik is a novel phosphoprotein identified in insulin-stimulated adipocytes using a SILAC phosphoproteomic approach.
(A): Schematic procedure for SILAC quantitative proteomics used for identification and quantification of peptide from CDP138 (5730419I09Rik). Top right panel: quantification of CDP138 peptide from different groups of adipocytes. Lower right panel: Schematic diagram of CDP138 and the identified phosphorylation sites. (B): Confirmation of CDP138 phosphorylation induced by insulin. CHO-T cells expressing HA-CDP138, or differentiated 3T3-L1 adipocytes were treated with or without insulin (100 nM) for 15 min. A third sample was pretreated with wortmannin (100nM, WM) for 20 min or LY294002 (50 μM, LY) for 1 hour before insulin stimulation. Left panel: HA-CDP138 was immunoprecipitated with anti-HA Ab from CHO-T cells and blotted first with the phospho-Akt substrate PAS pAb (#9611, Lot 2, Cell Signaling), and then reblotted with an anti-HA Ab. Right panel: An anti-CDP138 peptide Ab was used for immunoprecipitation of CDP138 from cell lysates of adipocytes followed by immunoblotting with the same Ab. CDP138, pS473-Akt and Akt protein were also detected with total cell lysates. The arrows indicate the insulin-stimulated CDP138 mobility shift. (C): Constitutively active Akt2 (myr-HA-Akt2) directly phosphorylates HA-CDP138 in vitro kinase assays (left panel) and identification of Ser197 residue in CDP138 as the phosphorylation target of myr-HA-Akt2 with MS (middle panel) as described in S.I. Right panel: purified constitutively active Akt2 (Millipore) induces HA-CDP138-WT, but not HA-CDP-S197A, phosphorylation detected with PAS antibodies. (D): CDP138 protein expression in mouse tissues. Tissue protein extracts (25μg) from different tissues of lean C57BL/6J mice (left panel) or epididymal fat pads from ob/ob and lean male mice (24 weeks old, The Jackson Lab; middle panel) were analyzed by immunoblotting. Right panel: quantification of CDP138 protein levels in fat tissues from lean and obese mice. Data are mean ± SEM, ** P <0.01 lean vs ob/ob mice (n=4). WAT or BAT: white or brown adipose tissue. A, B & C are representatives of 2 to 3 independent experiments. See also Figure S1 and Table S1.
We constructed a cDNA clone (IMAGE: 40125694, GI:109658767) encoding the full-length human kiaa0528 protein tagged with three N-terminal HA epitopes. As shown in Figure 1B (left panel), insulin stimulates phosphorylation of HA-tagged CDP138 in CHOT cells, as detected with PAS antibodies. Insulin-stimulated phosphorylation was significantly inhibited by wortmannin. An antibody to a peptide from CDP138 was used to analyze endogenous protein in 3T3-L1 adipocytes by immunoprecipitation and immunoblotting (Fig. 1B right panel). CDP138 from insulin-treated cells migrated slower in SDS-PAGE than from control cells and the apparent size shift was reversed by LY294002, a PI3K inhibitor. This pattern of migration is consistent with CDP138 being phosphorylated in insulin-stimulated cells. CDP138 phosphorylation as detected with PAS antibodies reaches a maximum at 10 min and is sustained after 30 min upon insulin stimulation (Supplemental Figure S1). We detected multiple phosphorylation sites in CDP138 by mass spectrometric measurements (Figure 1A). To determine if Akt2 can directly phosphorylate CDP138, HA-CDP138 was expressed in HEK293 cells and immunoprecipitated with anti-HA Ab before being subjected to an in vitro kinase assay in the presence of constitutively active myristoylated Akt2 (myr-HA-Akt2) and γ-32P-ATP. Figure 1C shows that active Akt2 does induce CDP138 phosphorylation, demonstrating that CDP138 is an Akt2 substrate. MS analysis of an HA-CDP138 sample from the in vitro kinase assay revealed that active Akt2 induces CDP138 phosphorylation at serine (Ser)197, which lies within a consensus Akt substrate motif RQRLIS197 (Figure 1C). Conversion of Ser197 to alanine blocked active Akt2-induced CDP138 phosphorylation detected with PAS antibodies, further confirming Ser197 is the Akt2 phosphorylation site.
CDP138 protein is expressed in all tissues tested including insulin-sensitive tissues such as liver, muscle, and fat (Figure 1D, left panel). Interestingly, as shown in Figure 1D (middle & right panels), the CDP138 protein level, similar to that of IRS1, is significantly reduced in fat tissue from insulin resistant ob/ob mice, suggesting that CDP138 is a highly regulated protein in insulin sensitive tissues.
CDP138 is required for maximal insulin-stimulated glucose transport and GLUT4 translocation but not endocytosis
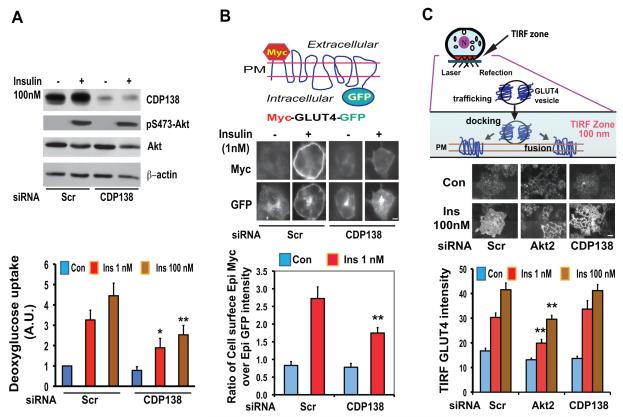
Since activation of the Akt2 pathway is important for insulin-stimulated glucose transport and C2 domain-containing proteins such as synaptotagmins are known to be involved in membrane trafficking, we next determined whether loss of CDP138 affects insulin-stimulated glucose transport in adipocytes. As shown in Figure 2A (upper panel), siRNA-induced silencing of CDP138 in 3T3-L1 adipocytes reduced protein levels by about 80% without significant effects on insulin-induced Akt phosphorylation or other protein expression, as compared with cells transfected with scrambled siRNA. The reduction in CDP138 protein levels was accompanied by a decrease in insulin-induced glucose transport by about 40-45% (Figure 2A lower panel), suggesting that CDP138 is required for glucose transport. To determine whether the reduced glucose transport was due to an effect on the GLUT4 exocytic pathway, we performed GLUT4 translocation assays in 3T3-L1 adipocytes transfected with CDP138 siRNA or the scrambled siRNA, together with the DNA construct encoding a myc-GLUT4-GFP fusion protein with a myc epitope inserted in the first exofacial loop and GFP at the C-terminus of GLUT4 (Figure 2B) (Jiang et al., 2002). GLUT4 translocation is quantified by measuring the ratio of cell surface myc signal detected by anti-myc immunofluorescent staining, to the total GFP intensity as the myc-GLUT4-GFP expression level in non-permeablized cells. At low concentrations (1 nM), insulin caused a 3-fold increase in GLUT4 translocation (Figure 2B), and CDP138 gene-specific silencing resulted in a 43% decrease in insulin-stimulated GLUT4 translocation. The effect of silencing of CDP138 on myc-GLUT4-GFP endocytosis was also tested. Although CDP138 knockdown reduces insulin-stimulated accumulation of myc-GLUT4-GFP on the cell surface before initiation of endocytosis, it does not significantly affect myc-GLUT4 endocytosis (Supplemental Figure S2), suggesting that CDP138 is specifically involved in the regulation of GLUT4 exocytosis.
Figure 2. Knockdown of CDP138 in 3T3-L1 adipocytes inhibits insulin-stimulated glucose transport (A) and myc-GLUT4-GFP translocation (B), but not endogenous GLUT4 movement to the periphery detected in TIRF zone (C).
(A): Differentiated adipocytes at day 5 were transfected with siRNAs against mouse 5730419I09Rik or the scrambled siRNA (Scr) as described earlier (Jiang et al., 2003) for 60 hrs, then serum starved overnight. Cells were then treated with or without insulin (1 nM and 100nM) for 30 min for the glucose uptake assay, or 15 min for immunoblotting of CDP138, pAkt, Akt and β-actin. Glucose transport data are presented as mean ± SD of 4 independent experiments. * P < 0.05 vs Scr Insulin (1nM) group; ** P < 0.01 vs Scr Insulin (100nM) group. (B): Day 5 adipocytes were transfected with siRNAs and myc-GLUT4-GFP for 60 hrs and then serum starved overnight. Cells were then treated with insulin (1 nM) for 20 min. Cell surface myc-GLUT4-GFP was detected with anti-myc monoclonal Ab (9E10) and Alexa Fluor 568-labeled goat anti-mouse IgG in non-permeablized cells. The myc signal and GFP signal were quantified as previously described (Jiang et al., 2002). Data presented are representative microscopic images and mean ± SD of about 160 GFP-positive cells in each group from three independent experiments. ** P < 0.01 vs Scr Insulin (1 nM) group. (C): Endogenous GLUT4 accumulation in the TIRF zone in fixed adipocytes treated with or without insulin for 20 min. GLUT4 was detected with a goat anti-GLUT4 Ab and Alexa Fluor 488-conjugated donkey anti-goat Ab in permeabilized cells. Top and middle panels show 100nm TIRF zone and representative GLUT4 TIRFM images, respectively. Data are mean ± SD of 3 independent experiments with 300 plus cells in each group. ** P < 0.01 vs Scr Insulin groups. Scare Bars: 5 μm. See also Figure S2.
CDP138 is not required for endogenous GLUT4 accumulation at the periphery of the adipocytes
We also quantified endogenous GLUT4 redistribution to the PM using total internal reflection fluorescence microscopy (TIRFM) with a setting of about 100nm distance from the coverslip (Figure 2C, top panel). For this experiment, endogenous GLUT4 was detected by immunofluorescent staining with a goat anti-GLUT4 Ab that recognizes the cytoplasmic C-terminus of GLUT4. The fluorescent signal therefore reflects the presence of GLUT4 in the TIRF zone, either inserted in the PM and/or GSV docked at or free near the PM. Figure 2C shows the TIRFM images and quantification of GLUT4 distribution in the TIRF zone. In 3T3-L1 adipocytes transfected with scrambled siRNA, 100nM insulin enhanced the GLUT4 signal in the TIRF zone by about 2.5-fold. Cells transfected with Akt2 siRNA showed a significant reduction in insulin-stimulated GLUT4 distribution to the periphery, consistent with the concept that Akt2 plays a role in GLUT4 trafficking. In contrast, knockdown of CDP138 did not inhibit insulin-stimulated GLUT4 accumulation at the periphery (Figure 2C). This finding appears inconsistent with the results obtained with the myc-GLUT4-GFP translocation assay that showed reduction of GLUT4 on the cell surface by silencing CDP138 (Figure 2B). However, both observations are consistent with the possibility that CDP138 is specifically required for the insertion of GLUT4 into the PM, but not GSV movement to the periphery, whereas Akt2 is known to be involved in both steps.
CDP138 is a key factor involved in the process of fusion between GSV and the PM
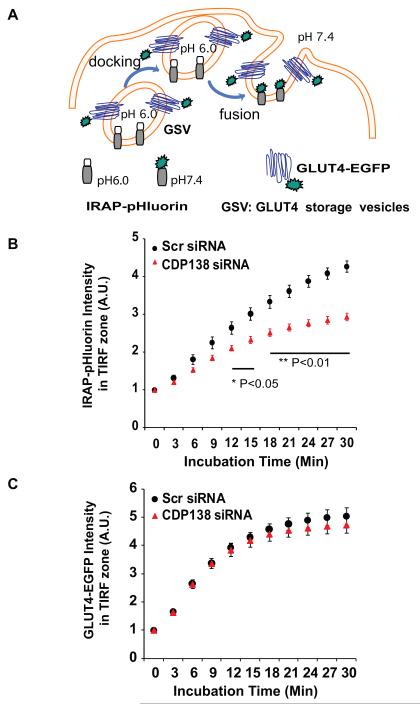
To determine whether CDP138 is required for insulin-stimulated fusion between GLUT4 vesicles and the PM, we used a TIRFM-based live cell fusion assay (Jiang et al., 2008). The assay is based on the expression of a fusion protein of insulin-responsive aminopeptidase (IRAP) tagged with the pH-sensitive green fluorescence protein pHluorin at its luminal terminus. The resultant molecular probe (IRAP-pHluorin) co-localizes with the insulin-responsive GSV in 3T3-L1 adipocytes. IRAP-pHluorin is essentially non-fluorescent at pH 6.0 within the GSV, but is very brightly fluorescent at the pH 7.4 environment when GSV have fused with the PM, as illustrated in Figure 3A (Jiang et al., 2008; Lopez et al., 2009). Therefore, this molecular probe provides a sensitive tool to monitor dynamic changes in GSV – PM fusion, with the pHluorin fluorescence intensity reflecting the fusion event. As reported by Lopez et al (2009), the Akt1/2 inhibitor Akti partially but significantly inhibited both insulin-induced membrane fusion in live adipocyes and myc-GLUT4-GFP translocation to cell surface in fixed cells (Supplemental Figure S3). In comparison to scrambled siRNA-treated cells, knockdown of CDP138 significantly diminished the pHluorin intensity within 30 min period of insulin treatment (Figure 3B and Supplemental Movie S1 & S2). Quantitatively, silencing of CDP138 inhibited the insulin-induced IRAP-pHluorin signal by about 35% (Figure 3B). This partial inhibitory effect could be due to incomplete knockdown of CDP138 and the presence of other factors or pathways that are also required for GLUT4 – PM fusion (Lopez et al, 2009). However, knockdown of CDP138 did not significantly affect insulin-stimulated GLUT4-EGFP accumulation in the TIRF zone in live adipocytes (Figure 3C). Taken together, our data suggests that CDP138 is specifically required for insulin-stimulated GSV fusion with the PM, but not for the movement of the vesicles from intracellular stores to the TIRF zone.
Figure 3. Knockdown of CDP138 in live 3T3-L1 adipocytes inhibits insulin-stimulated membrane fusion between GLUT4 storage vesicles (GSV) and the PM, but not GLUT4-EGFP trafficking to the TIRF zone.
(A): Schematic illustration of the molecular probes and TIRF microcopy-based live cell assays for GLUT4 trafficking and GSV - PM fusion. (B) and (C): The effect of CDP138 knockdown on insulin-stimulated IRAP-pHluorin insertion into the PM (B), and accumulation of GLUT4-EGFP in the TIRF zone (C). Adipocytes (day 4) were transfected by electroporation with plasmid DNA encoding IRAP-pHluorin or GLUT4-EGFP, together with either the scrambled siRNA or smartPool siRNA against mouse 5730419I09Rik. Cells were reseeded for 72 hrs, serum starved for 2 hr then stimulated with 100 nM insulin for 30 min. Analyses were performed in a cell warmer adapted for a Nikon TiE with fully motorized combined dual laser (488 and 561nm). Images were acquired every 3 min immediately after addition of insulin and analyzed as described in the S.I.. Perfect focus system and multiple points capture program were used to acquire images from multiple positively transfected cells at each time point. Data are mean ± SEM of three independent experiments (n=3) with total 159 cells (43,46,70/exp, Scr siRNA) or 154 cells (43, 37, 74/exp, CDP138 siRNA) in the GLUT4-EGFP trafficking assay; and 125 cells (41, 48, 36/exp, Scr siRNA) or 120 cells (43, 46, 31/exp, CDP138 siRNA) in the GSV - PM fusion assay. P value: CDP138 siRNAs vs scrambled siRNA. Live cell movies for IRAP-pHluorin membrane fusion assay are provided in S.I. See also Figure S3, Movie S1 and Movie S2.
The C2 domain of CDP138 has two Ca2+-binding sites that are critical for membrane lipid binding
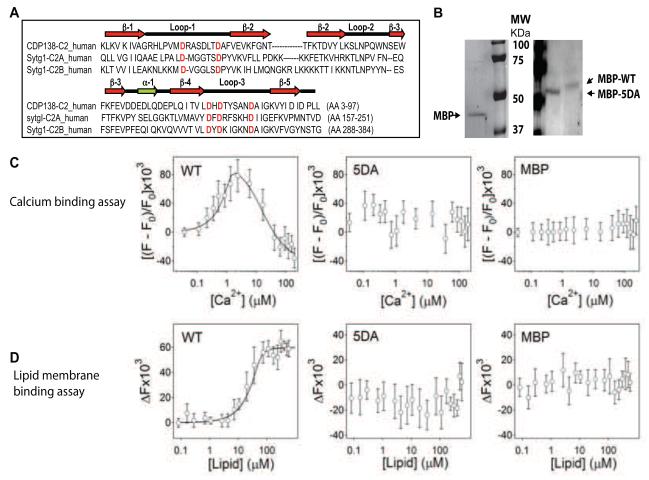
The primary amino acid sequence of the CDP138 C2 domain is similar to the C2A and C2B domains from synaptotagamin-1, with 5 conserved aspartate residues in loop 1 and loop 3 regions (Figure 4A) that may interact with Ca2+. To test this possibility, we performed biophysical and functional analyses on the isolated C2 domain fused to maltose-binding protein (MBP). MBP, MBP-C2-WT and MBP-C2-5DA, a mutant lacking five aspartate residues (Figure 4B), have been analyzed for their Ca2+ and lipid membrane binding properties. The mutant C2-5DA domain fusion protein migrates faster in SDS-PAGE than the wild-type fusion protein, because the substitution of 5 aspartate residues with alanine changes the molecular weight and charge of the protein.
Figure 4. The purified C2 domain from CDP138 is capable of binding Ca2+ ions and lipid membranes.
(A): Diagram and amino acid alignment of the C2 domains of CDP138 (CDP138-C2) and synaptotagmin-1 (Sytg1-C2A & Sytg-C2B). β: beta strands; α: alpha helix; loop: coil loop. The conserved potential Ca2+-binding aspartate residues are highlighted. (B): Gel images of purified MBP (42kDa), MBP-C2-WT domain, and MBP-C2-5DA mutant. (C): Calcium binds to the wild-type C2-domain but not to the 5DA or the MBP proteins. Change in tryptophan fluorescence intensity at 340 nm as a signal of calcium interaction with the MBP-C2-WT fusion protein, MBP-C2-5DA mutant fusion protein, and MBP measured at 37°C. The calcium-binding isotherm was constructed as described in S.I.. Calcium exerts a biphasic effect on tryptophan fluorescence of the wild-type protein, but has little effect on the 5DA or MBP proteins. The curve of calcium binding to MBP-C2-WT was constructed using two independent binding sites per wild-type C2 domain, with dissociation constants of KD,1 = 0.03±0.012 μM and KD,2 = 15.0±6 μM. (D): Fluorescence resonance energy transfer (RET) from protein tryptophan residues to Py-PE in membrane indicates membrane binding by the MBP-C2 WT fusion protein but not by the MBP-5DA-C2 mutant or MBP proteins. Change in tryptophan fluorescence intensity as a function of lipid concentration, corrected for the effect of membranes without energy acceptor Py-PE, measured at 37°C. The solid line describing membrane binding of MBP-C2-WT was simulated using a lipid-to-protein stoichiometry N = 20 and a dissociation constant KD = 0.06±0.015 μM. All data is presented as mean ± SD from three independent experiments. See also Figure S4.
Ca2+-binding property of the C2 domain
Calcium binding to the fusion proteins was directly measured by assessing Ca2+-induced changes in tryptophan fluorescence (Supplemental Figure S4A). The data in Figure 4C show that increasing Ca2+ concentrations exert a biphasic effect on the fluorescence of the wild-type protein; fluorescence intensity increases at Ca2+ concentrations up to 2-3 μM and then decreases at higher Ca2+ concentrations. Analysis of the biphasic effect indicated that the wild-type protein has a high affinity (KD = 0.03 μM) and a lower affinity (KD = 15.0 μM) Ca2+-binding sites. The effect of Ca2+ ions on the fluorescence of the 5DA mutant protein and on MBP was negligible (Figure 4C).
Binding of C2 domain to lipid membranes
Protein-lipid membrane interactions were studied by resonance energy transfer (RET), as described earlier (Qin et al., 2004). The fusion proteins were incubated with lipid vesicles containing 2% Py-phosphatidylethanolamine (Py-PE) and tryptophans were excited at 290 nm. If the protein binds to the membrane, the energy of the excited electrons of tryptophan’s indole ring can be transferred to the pyrene group of Py-PE. The data shown in Figure 4D demonstrate clearly that the RET effect is only observed with the wild-type C2 domain, but not the 5DA mutant or MBP proteins. The spectra sets collected when the proteins were titrated are presented in Supplemental Figure S4B. These data were used to determine lipid concentration-dependence of the relative change in tryptophan fluorescence intensity at 340 nm, ΔF340. For the wild-type C2 domain the data predict lipid-to-protein stoichiometry of N = 20 and a dissociation constant of KD = 0.06 μM. Data for the 5DA and MBP proteins did not indicate membrane binding.
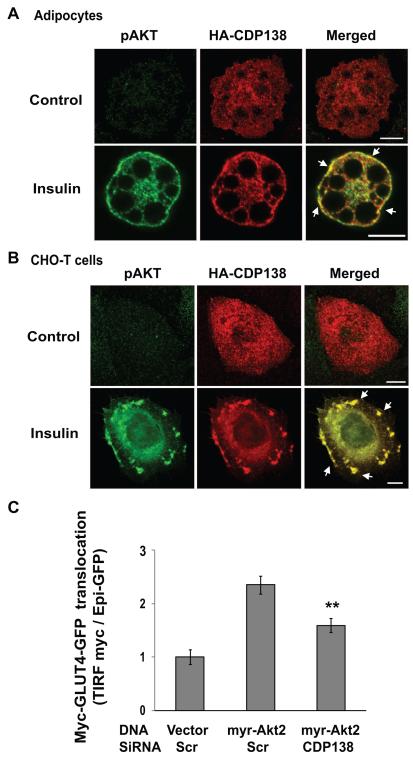
CDP138 is partially co-localized with active Akt and facilitates constitutively active Akt2-induced GLUT4 translocation
To examine if CDP138 colocalizes with Akt, adipocytes and CHOT cells were transfected with HA-CDP138 expression vector. We consistently observed insulin-stimulated HA-CDP138 co-localization with active Akt, detected with phospho-S473 Akt specific antibody, at the PM cortical area in adipocytes, and at the membrane ruffles in CHOT cells (Figure 5A & B).
Figure 5. CDP138 co-localizes with phospho-Akt (A & B), and is required for constitutively active myr-Akt2-induced GLUT4 translocation (C).
A & B: HA-CDP138-WT was transfected into adipocytes (A) and CHO-T cells (B) for 48 hrs before serum starvation overnight. Cells were then treated with or without insulin (100nM) for 10 min. Cells were fixed and permeabilized before immunostaining with mouse anti-HA and rabbit anti-phospho-Akt (S473) antibodies followed by goat anti-mouse (Alexa Fluor568) and goat anti-rabbit (Alexa Fluor488) secondary antibodies, respectively. The white arrow indicates co-localization of phospho-Akt and HA-CDP138. Scale bar: 10μm. C: Differentiated adipocytes were transfected by electroporation with the scrambled siRNA or CDP138 siRNAs together with plasmid DNAs encoding myc-GLUT4-GFP and myr-HA-Akt2 or HA-empty vector. Cells were reseeded for 60 hours before serum starvation overnight. Myc-GLUT4 translocation assays were carried out with TIRF microscopy as described in the Experimental Procedures. Data are mean ± SEM of four independent experiments. ** P< 0.01 myr-Akt2 / CDP138 siRNA vs myr-Akt2 / Scr siRNA.
It is established that constitutively active Akt induces GLUT4 translocation independent of insulin stimulation. To determine if CDP138 functions downstream of Akt2, differentiated adipocytes were transfected with active myr-HA-Akt2 and myc-GLUT4-GFP together with either the scrambled siRNA, or siRNA against mouse CDP138. TIRFM was then used to quantify the effect of active myr-HA-Akt2 on the translocation of myc-GLUT4-GFP to the cell surface. As shown in Figure 5C, overexpression of active Akt2 stimulated GLUT4 translocation by about 2.5-fold in adipocytes transfected with the scrambled siRNA. However, siRNA-induced knockdown of CDP138 significantly inhibited the effect of constitutively active Akt2 on GLUT4 translocation. As noted above, knockdown of CDP138 did not alter insulin-stimulated Akt phosphorylation (Figure 2A). Together, these data confirm that CDP138 functions downstream of the Akt2 pathway and is required for maximal Akt2-induced GLUT4 translocation.
The C2 domain and Akt phosphorylation site are both required for the normal function of CDP138 in GLUT4 translocation
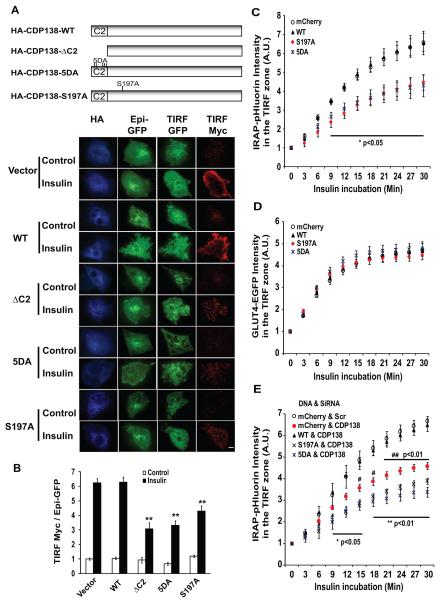
We next determined if the C2 domain and Akt2 phosphorylation site in CDP138 are necessary for GLUT4 translocation. Myc-GLUT4-GFP translocation was tested in the presence of the HA-CDP138-WT or mutant proteins that lack (a) the C2 domain (ΔC2 AA1-108), (b) the Ca2+-binding aspartate residues (5DA), or (c) the Akt2 phosphorylation site Ser197 (S197A). Insulin-stimulated myc-GLUT4-GFP translocation was quantified as the ratio of cell surface myc signal (detected with TIRFM) to the total GFP signal in the widefield image (Epi-GFP). As shown in Figure 6A and 6B, overexpression of HA-CDP138-WT did not significantly alter GLUT4 translocation. However, overexpression of all three constructs (HA-CDP138-ΔC2, HA-CDP138-5DA, and HA-CDP138-S197A) inhibited the insulin-stimulated translocation of myc-GLUT4-GFP to the cell surface, with the ΔC2 construct being the most inhibitory. Our phosphopeptide analysis showed that CDP138 is also phosphorylated at the Ser200 residue. Thus, we compared the effects of overexpressed mutants lacking either Ser197 or Ser200 on insulin-stimulated myc-GLUT4-GFP translocation. Interestingly, only S197A, but not S200A, blocked GLUT4 translocation (Supplemental Figure S5), further suggesting that Akt2-dependent phosphorylation of Ser197 is necessary for CDP138 function but phosphorylation of Ser200 is not.
Figure 6. The effects of CDP138-ΔC2, CDP138-5DA and CDP138-S197A mutants on myc-GLUT4-GFP translocation and GSV-PM fusion in 3T3-L1 adipocytes.
Plasmid DNAs for pCMV5-HA, HA-CDP138-WT, HA-CDP138-ΔC2, HA-CDP138-5DA, or HA-CDP138-S197A were transfected by electroporation into adipocytes together with the myc-GLUT4-GFP expression vector. Cells were reseeded for 48 hrs and serum-starved overnight. Cells were then treated with or without insulin (100nM) for 30 min, before immunostaining with rabbit anti-myc and mouse anti-HA antibodies. (A): Schematic diagram for CDP138 (wild-type and mutants) and representative images of the myc-GLUT4-GFP translocation assay using TIRF microscopy. Scare Bars: 5 μm. (B): GLUT4 translocation to the cell surface of fixed adipocytes is shown as the ratio of surface TIRF myc signal to total Epi GFP. Data are presented as mean ± SEM of three independent experiments. ** P< 0.01 (n=3); ΔC2, 5DA, or S197A vs HA vector. (C) & (D): The effects of CDP138-mCherry constructs on insulin-induced GSV - PM fusion and GLUT4-EGFP trafficking in live adipocytes, respectively. Data are expressed in arbitrary units as the ratio of pHluorin or EGFP intensity to the basal intensity at time zero. Data are mean ± SEM of three independent experiments (total 65 to 137 cells each group). P value (n=3): S197A or 5DA vs mCherry vector. (E): Overexpression of human WT but not mutant CDP138-mCherry rescures siRNA-induced inhibition of GSV - PM fusion in live adipocytes. Adipocytes (day 4) were transfected with the scrambled siRNA or smartPool siRNA against mouse 5730419I09Rik together with plasmid DNAs encoding IRAP-pHluorin and mCherry alone, CDP138-WT-mCherry, CDP138-S197A-mCherry or CDP138-5DA-mCherry for 72 hrs before GSV – PM fusion assay. Data are mean ± SEM of three independent experiments (total 137 to 153 cells each group). P value (n=3): * and ** CDP138 siRNA plus S197A or 5DA vs Scr siRNA plus mCherry vector. # P < 0.05 or ## P < 0.01: CDP138 siRNA plus mCherry vector vs Scr siRNA plus mCherry vector. See also Figure S5.
We have constructed similar mutants of CDP138 as described above but with mCherry fused at their C-terminus, and compared their effect on insulin-stimulated GLUT4 trafficking and GSV - PM fusion, as detected with TIRFM in live adipocytes using GLUT4-EGFP and IRAP-pHluorin as the molecular probes, respectively. Our data show that the CDP138-5DA-mCherry and CDP138-S197A-mCherry mutants inhibited membrane fusion, but the CDP138-WT-mCherry or mCherry control vector had no effect (Figure 6C). Despite their effect on membrane fusion, none of the constructs significantly affected the insulin-stimulated accumulation of GLUT4-EGFP in the TIRF zone (Figure 6D). These data suggest that Akt2-induced phosphorylation and Ca2+-binding by CDP138 are both important for GSV - PM fusion, but not GSV trafficking in adipocytes.
To evaluate the rescue effects of CDP138-mCherry on CDP138 siRNA-induced inhibition of GSV – PM fusion, mouse CDP138 siRNAs were co-transfected into 3T3-L1 adipocytes together with IRAP-pHluorin and siRNA-resistant human CDP138-mCherry (WT or mutants). Figure 6E shows that overexpressed human WT CDP138-mCherry reverses the CDP138 siRNA-induced inhibitory effect on the membrane fusion while overexpressed S197A or 5DA mutant further enhances the inhibition, suggesting the effect of CDP138 on GSV – PM fusion is gene specific.
CDP138 is dynamically associated with the PM and GLUT4 vesicles
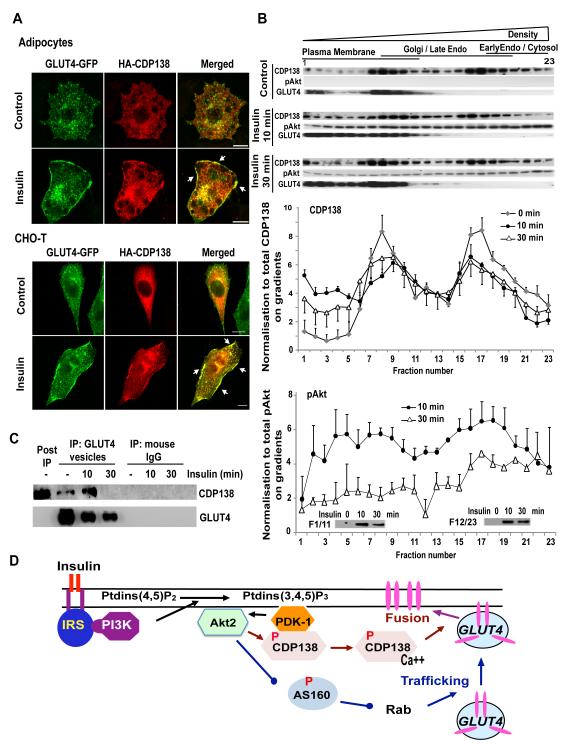
To examine the intracellular distribution of CDP138, we co-expressed myc-GLUT4-GFP and HA-CDP138 in adipocytes. As shown in Figure 7A, in the basal state intracellular staining of HA-CDP138-WT was punctuate and only partially overlapped with GLUT4 vesicles in intracellular stores. Within 10 minutes of insulin stimulation, GLUT4 and CDP138 can be seen co-localized at the PM. A similar pattern was observed in stable CHO-T cell lines expressing myc-GLUT4-GFP (Figure 7A). Next, we used self-generated Opti-prep iodixanol gradient fractionation (Chen et al, 2007) to examine the subcellular distribution of CDP138 and GLUT4 in adipocytes (Supplemental Figure S6A for method characterization). CDP138 was partially redistributed from high density fractions to the lower density portion of the PM fractions within 10 min of insulin stimulation (Figure 7B & Supplemental Figure S6B). After 30 minutes, CDP138 had partially redistributed within PM fractions. We analyzed CDP138 distribution in GLUT4 vesicles enriched with conjugated monoclonal anti-GLUT4 Ab (1F8). Interestingly, we also detected a small increase of CDP138 with enriched GLUT4 vesicles within 10 min (Figure 7C). However, this association was undetectable 30 min after stimulation when total GLUT4 content in the enriched vesicles was also reduced by about 35% (Figure 7C). These data suggest that CDP138 dynamically interacts with the PM and GLUT4 vesicles.
Figure 7. CDP138 is colocalized with GLUT4 at the PM (A) and dynamically associated with the PM fractions (B) and GLUT4 vesicles (C).
HA-CDP138-WT plasmid DNA was transfected alone into a CHO-T cell line stably expressing myc-GLUT4-GFP, or into adipocytes together with the myc-GLUT4-GFP vector. Forty-eight hr later, serum starved cells were treated with or without insulin for 10 min before immunofluorescent staining with anti-HA Ab, as described for Figure 5. Scare Bars: 10 μm. (B): Post nuclear supernatants from 3T3-L1 adipocytes treated with or without insulin 100nM were fractionated with iodixanol gradients (10-20-30% Optiprep) as described in S.I. Equal volumes of each fraction were resolved in 4-20% SDS-PAGE followed by immunoblotting with antibodies against CDP138, p-Akt and GLUT4. For quantification, the results were normalized to the total level of the indicated protein. Data are mean ± SEM of 3 independent experiments. (C): GLUT4 vesicles were enriched by immunoabsorption with anti-GLUT4 Ab (1F8) from adipocytes after removal of nuclear fraction as described in S.I. Samples were then immunoblotted with anti-CDP138 and anti-GLUT4 antibodies. Images are representative of three independent experiments (A, B & C). (D): Akt substrate CDP138 is a potential link between Akt2 activation and GSV - PM fusion. See also Figure S6.
Discussion
It is known that activation of Akt2 is required for insulin-stimulated GLUT4 translocation, and that Akt2 acts by regulating mobilization of GSV and fusion between GSV and the PM (Zaid et al., 2008). It has been reported previously that Akt2 controls GLUT4 retention and trafficking by phosphorylating the RabGAP AS160. However, the molecular mechanism by which Akt2 regulates GLUT4 insertion into the PM, a rate-limiting step of GLUT4 translocation, remains unclear. In the present study, we used a SILAC quantitative phosphoproteomic approach to identify CDP138, a previously unknown C2 domain-containing phosphoprotein, and confirmed that it is an Akt2 substrate. RNAi-based functional assays revealed that CDP138 is required for maximal insulin-stimulated glucose transport and GLUT4 translocation to the PM, but not for GLUT4 movement to the periphery in adipocytes. We used both pH-sensitive IRAP-pHluorin and GLUT4-EGFP as molecular probes to demonstrate in live adipocytes that CDP138 is critical for optimal membrane fusion between GSV and the PM, but not for GSV trafficking to the TIRF zone. Collectively, these complementary functional analyses demonstrate that the novel phosphoprotein CDP138 is involved in regulating GLUT4 translocation, most likely at the GSV – PM fusion step. Thus, CDP138 represents a novel link between Akt2 activation and GLUT4 insertion into the PM. It is possible that Akt2 regulates the GLUT4 trafficking and membrane fusion steps in adipocytes through the RabGAP AS160 and CDP138, respectively (Figure 7D).
Our results are consistent with the hypothesis that CDP138 is a downstream target of Akt2 and is involved in the regulation of GLUT4 translocation. First, insulin stimulated phosphorylation of CDP138 in cultured cells, and the phosphorylation was partially blocked by the PI3K inhibitors. We also detected several phosphorylation sites in CDP138 by mass spectrometry, which suggests that insulin might induce CDP138 phosphorylation at different sites through both PI3K-dependent and -independent pathways. Second, we showed that constitutively active Akt2 induced CDP138 phosphorylation at a Ser197 residue within a consensus Akt substrate motif. Over-expression of a mutant CDP138 lacking the Ser197 phosphorylation site, but not Ser200, significantly inhibited insulin-stimulated myc-GLUT4-GFP translocation to the cell surface and GSV - PM fusion in adipocytes, suggesting that Ser197 phosphorylation is important to the glucose transporter system. Third, overexpressed human WT but not S197A or 5DA mutant CDP138 rescues the mouse CDP138 siRNA-induced inhibitory effect on the membrane fusion in 3T3-L1 adipocytes. Fourth, our results showed that CDP138 co-localizes with phospho-Akt in insulin-stimulated cells. We also observed that CDP138 interacts with Akt2 upstream kinase PDK1 in a proteomics study and this was confirmed in a co-immunoprecipitation study (Data not shown). These observations suggest the interesting possibility that the CDP138-PDK1 interaction might bring CDP138 and phospho-Akt2 in close proximity at the PM. This might be mediated through PI3K-derived PI(3,4,5)P3, which interacts with the PH domains of both PDK1 and Akt2 in insulin-stimulated cells. If this occurs, CDP138 would become accessible to phosphorylation by active Akt2. Furthermore, RNAi-induced gene specific knockdown of CDP138 did not affect insulin-stimulated Akt phosphorylation but significantly inhibited GLUT4 translocation induced by constitutively active Akt2, suggesting this novel phosphoprotein functions downstream of the Akt2 pathway.
To understand the molecular mechanism by which CDP138 regulates GLUT4 translocation, we also analyzed the biochemical and functional interactions of the C2 domain-containing protein. Deletion of the C2 domain from CDP138 significantly inhibited insulin-stimulated GLUT4 translocation, suggesting the C2 domain is crucial for this process. The C2 domain of CDP138 is similar to those of known membrane fusion proteins such as synaptotagmin. Biophysical analyses revealed that the purified C2 domain of CDP138 is able to bind Ca2+ and liposomes with a lipid composition that mimics the cytoplasmic face of plasma membranes. It is interesting to note that the C2 domain contains two Ca2+-binding sites of differing affinity, presumably one each in loop 1 and 3. The mutant C2 domain lacking five aspartate residues in loop1 and 3 regions is unable to bind Ca2+ or membrane lipids, suggesting that interaction of the C2 domain with lipids is Ca2+-dependent. It is possible that Ca2+-binding to the C2 domain results in exposure of nonpolar residues that mediate membrane binding. Alternatively, Ca2+ ions may serve as ionic bridges between acidic residues of the protein and negatively charged membranes. Interestingly, in the studies using GLUT4 vesicles and density gradient fractionation of membrane compartments, we also observed that CDP138 associated with GLUT4 vesicles and the lower density PM-containing fractions within 10 min of treatment of adipocytes with insulin. Surprisingly, CDP138 dissociated from the vesicles and redistributed in the PM-containing fractions in adipocytes after 30 min. These dynamic interactions further support the notion that CDP138 may be involved in the regulation of GLUT4 vesicle fusion with the PM either directly or indirectly. Since CDP138 is a highly regulated phosphoprotein, it is unique among proteins known to be involved in membrane fusion processes. Further studies are needed to understand the molecular basis by which CDP138 regulates membrane fusion.
Experimental Procedures
DNA constructs, siRNAs, and antibodies
Constitutively active HA-myr-Akt2 in the pcDNA3 expression vector was from Addgene (Cambridge, MA). IRAP-pHluorin and GLUT4-EGFP were kindly provided by Dr. Tao Xu (Institute of Biophysics, Beijing, China). The pCMV5 plasmid DNA encoding myc-GLUT4-GFP was constructed as described (Jiang et al., 2002). The cDNA clone of human KIAA0528 was used to make a full-length CDP138 tagged with a HA epitope at the N-terminus and mCherry at the C-terminus as described in Supplemental Information (S.I.). The mutants of HA-CDP138 and CDP138-mCherry, including ΔC2, 5DA, Ser197Ala and Ser200Ala were made by PCR-based mutation. SiRNA duplexes against mouse 5730419I09RIK and Akt2 are described in S.I. Antibodies used for immunoprecipitation and immunoblotting are described earlier (Zhou et al., 2010) and in S.I.
Cell culture, siRNA and gene transfection, and SILAC medium
3T3-L1 adipocytes were transfected with siRNA duplexes or DNA constructs by electroporation as described (Jiang et al., 2003). Oligofectamin-2000™ reagents (Invitrogen) for transfection of plasmid DNA into CHO-T and HEK293 cells. DMEM containing Arg-0/Lys-H4, Arg-6/Lys-D4, and Arg-10/Lys-8 were used for SILAC experiments.
Mass spectrometric analysis
After SDS/PAGE, in-gel trypsin digestion, and peptide extraction, samples were processed for LC-MS as described in S.I.
CDP138 C2 domain purification, calcium and lipid membrane binding assays
MBP- WT1-118 and MBP-5DA1-118 in pMal-C2 vector (Amersham) were expressed in BL21 bacteria and purified as described in S.I. Binding of C2 domain fusion proteins to lipid membranes and Ca2+ were determined by fluorescence resonance energy transfer as described earlier (Qin et al., 2004) and by tryptophan fluorescence spectra as described in S.I., respectively.
Deoxyglucose uptake assay
To detect the effect of gene silencing on insulin-stimulated glucose transport, [3H]-deoxyglucose uptake assays were carried out in 3T3-L1 adipocytes as described earlier (Jiang et al., 2003) and in S.I.
In vitro protein kinase assay
For the in vitro Akt2 kinase assays, either purified constitutively active His6-Akt2 (ΔPH, S474D and PDK1 activated) or overexpressed HA-tagged myr-Akt2 was used for inducing HA-CDP138 phosphorylation as described in S. I.
Isolation of GLUT4-containing vesicles and subcellular fractionation
Serum-starved 3T3-L1 adipocytes were stimulated with or without 100 nM insulin for 10 min or 30 min. GLUT4 vesicles were enriched by immunoadsorption with anti-GLUT4 Ab (1F8) from adipocytes after removal of nuclear and the PM fractions as previously described (Kandror and Pilch, 2006). Post-nuclear subcellular fractionation was performed by ultracentrifugation with continuous iodixanol gradients as described (Chen et al., 2007) and in S.I.
GLUT4 translocation detection with widefield fluorescence and TIRF microscopy
Both wide field and TIRF microscopy were used to detect myc-GLUT4-GFP translocation to the cell surface as previously described (Jiang et al., 2002) and in S.I.
Analysis of GLUT4 trafficking and GSV – PM fusion in live cells with TIRF microscopy
Differentiated adipocytes were transfected by electroporation with the plasmid DNA encoding IRAP-pHluorin or GLUT4-EGFP and siRNAs, or pcDNA3-mCherry constructs encoding CDP138 fusion protein and its mutants. Live cell imaging was performed to measure membrane fusion and GLUT4 trafficking with IRAP-pHluorin and GLUT4-EGFP as molecular probes, respectively, as described in S.I.
Statistics
For all the quantified data, population averages are given as mean and standard deviation (SD) or standard error of the mean (SEM). Statistical significance was tested using unpaired two-tailed Student’s t-test.
Supplementary Material
Acknowledgements
We wish to thank Dr. Tao Xu for IRAP-pHluorin and GLUT4-EGFP constructs, Dr. Jennifer Lippincott-Schwartz for the fast switching TIRFM platform, Harry Dolan (Cell Signaling Technology) for Lot-2 stock of PAS (# 9611) antibody, and Supriyo Ray for preparing lipid vesicles. We appreciate Drs. Tim Osborne, Dan Kelly and Tod Gulick for critical reading of the manuscript and suggestions, and Shonna Hyde for administrative support. These studies were supported by a SBMRI fund to ZYJ. Initial SILAC proteomic studies were supported by a Junior Faculty Award from American Diabetes Association to ZYJ & by a NIH program project grant DK060564 to MPC. XX is supported by James & Esther King Postdoctoral Research Fellowship. ZYJ, MK did SILAC proteomics with MM, FG and MPC. ZG, MK, LMB, KM, ZYJ did protein phosphorylation analysis. XX did TIRFM and confocal imaging analysis. QZ did glucose transport assay. ZYJ did wide field GLUT4 translocation assay and analyzed endogenous GLUT4 distribution with TIRFM. VMA did membrane fractionation. VMA, SAT & SD did Ca2+- and lipid-binding assays. ZYJ, MK, XX, ZG, VMA, QZ, & SAT designed experiments and analyzed data. ZYJ wrote the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alessi DR, Caudwell FB, Andjelkovic M, Hemmings BA, Cohen P. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 1996;399:333–338. doi: 10.1016/s0014-5793(96)01370-1. [DOI] [PubMed] [Google Scholar]
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- Bai J, Chapman ER. The C2 domains of synaptotagmin--partners in exocytosis. Trends Biochem Sci. 2004;29:143–151. doi: 10.1016/j.tibs.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Bai L, Wang Y, Fan J, Chen Y, Ji W, Qu A, Xu P, James DE, Xu T. Dissecting multiple steps of GLUT4 trafficking and identifying the sites of insulin action. Cell Metab. 2007;5:47–57. doi: 10.1016/j.cmet.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol. 2002;3:267–277. doi: 10.1038/nrm782. [DOI] [PubMed] [Google Scholar]
- Calera MR, Martinez C, Liu H, Jack AK, Birnbaum MJ, Pilch PF. Insulin increases the association of Akt-2 with Glut4-containing vesicles. J Biol Chem. 1998;273:7201–7204. doi: 10.1074/jbc.273.13.7201. [DOI] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Chen X, Al-Hasani H, Olausson T, Wenthzel AM, Smith U, Cushman SW. Activity, phosphorylation state and subcellular distribution of GLUT4-targeted Akt2 in rat adipose cells. J Cell Sci. 2003;116:3511–3518. doi: 10.1242/jcs.00675. [DOI] [PubMed] [Google Scholar]
- Chen XW, Leto D, Chiang SH, Wang Q, Saltiel AR. Activation of RalA is required for insulin-stimulated Glut4 trafficking to the plasma membrane via the exocyst and the motor protein Myo1c. Dev Cell. 2007;13:391–404. doi: 10.1016/j.devcel.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- Eguez L, Lee A, Chavez JA, Miinea CP, Kane S, Lienhard GE, McGraw TE. Full intracellular retention of GLUT4 requires AS160 Rab GTPase activating protein. Cell Metab. 2005;2:263–272. doi: 10.1016/j.cmet.2005.09.005. [DOI] [PubMed] [Google Scholar]
- George S, Rochford JJ, Wolfrum C, Gray SL, Schinner S, Wilson JC, Soos MA, Murgatroyd PR, Williams RM, Acerini CL, et al. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science. 2004;304:1325–1328. doi: 10.1126/science.1096706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez E, McGraw TE. Insulin signaling diverges into Akt-dependent and - independent signals to regulate the recruitment/docking and the fusion of GLUT4 vesicles to the plasma membrane. Mol Biol Cell. 2006;17:4484–4493. doi: 10.1091/mbc.E06-07-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MM, Clark SF, Tucker DF, Birnbaum MJ, James DE, Macaulay SL. A role for protein kinase Bbeta/Akt2 in insulin-stimulated GLUT4 translocation in adipocytes. Mol Cell Biol. 1999;19:7771–7781. doi: 10.1128/mcb.19.11.7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab. 2007;5:237–252. doi: 10.1016/j.cmet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Jiang L, Fan J, Bai L, Wang Y, Chen Y, Yang L, Chen L, Xu T. Direct quantification of fusion rate reveals a distal role for AS160 in insulin-stimulated fusion of GLUT4 storage vesicles. J Biol Chem. 2008;283:8508–8516. doi: 10.1074/jbc.M708688200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZY, Chawla A, Bose A, Way M, Czech MP. A phosphatidylinositol 3-kinase-independent insulin signaling pathway to N-WASP/Arp2/3/F-actin required for GLUT4 glucose transporter recycling. J Biol Chem. 2002;277:509–515. doi: 10.1074/jbc.M108280200. [DOI] [PubMed] [Google Scholar]
- Jiang ZY, Zhou QL, Coleman KA, Chouinard M, Boese Q, Czech MP. Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc Natl Acad Sci U S A. 2003;100:7569–7574. doi: 10.1073/pnas.1332633100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandror KV, Pilch PF. Isolation of GLUT4 STorage Vesicles (John Wiley & Sons, Inc.) Curr Protoc Cell Biol. 2006:3.20.1–3.20.13. doi: 10.1002/0471143030.cb0320s30. [DOI] [PubMed] [Google Scholar]
- Kohn AD, Summers SA, Birnbaum MJ, Roth RA. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- Koumanov F, Jin B, Yang J, Holman GD. Insulin signaling meets vesicle traffic of GLUT4 at a plasma-membrane-activated fusion step. Cell Metab. 2005;2:179–189. doi: 10.1016/j.cmet.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Kruger M, Kratchmarova I, Blagoev B, Tseng YH, Kahn CR, Mann M. Dissection of the insulin signaling pathway via quantitative phosphoproteomics. Proc Natl Acad Sci U S A. 2008;105:2451–2456. doi: 10.1073/pnas.0711713105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupriyanova TA, Kandror KV. Akt-2 binds to Glut4-containing vesicles and phosphorylates their component proteins in response to insulin. J Biol Chem. 1999;274:1458–1464. doi: 10.1074/jbc.274.3.1458. [DOI] [PubMed] [Google Scholar]
- Lopez JA, Burchfield JG, Blair DH, Mele K, Ng Y, Vallotton P, James DE, Hughes WE. Identification of a distal GLUT4 trafficking event controlled by actin polymerization. Mol Biol Cell. 2009;20:3918–3929. doi: 10.1091/mbc.E09-03-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SS, Haruta T, Morris AJ, Klippel A, Williams LT, Olefsky JM. Activated phosphatidylinositol 3-kinase is sufficient to mediate actin rearrangement and GLUT4 translocation in 3T3-L1 adipocytes. J Biol Chem. 1996;271:17605–17608. doi: 10.1074/jbc.271.30.17605. [DOI] [PubMed] [Google Scholar]
- Ng Y, Ramm G, Lopez JA, James DE. Rapid activation of Akt2 is sufficient to stimulate GLUT4 translocation in 3T3-L1 adipocytes. Cell Metab. 2008;7:348–356. doi: 10.1016/j.cmet.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Obata T, Yaffe MB, Leparc GG, Piro ET, Maegawa H, Kashiwagi A, Kikkawa R, Cantley LC. Peptide and protein library screening defines optimal substrate motifs for AKT/PKB. J Biol Chem. 2000;275:36108–36115. doi: 10.1074/jbc.M005497200. [DOI] [PubMed] [Google Scholar]
- Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–648. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Qin S, Pande AH, Nemec KN, Tatulian SA. The N-terminal alpha-helix of pancreatic phospholipase A2 determines productive-mode orientation of the enzyme at the membrane surface. J Mol Biol. 2004;344:71–89. doi: 10.1016/j.jmb.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem. 2003;278:14599–14602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Sun XJ, Rothenberg P, Kahn CR, Backer JM, Araki E, Wilden PA, Cahill DA, Goldstein BJ, White MF. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991;352:73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- Tengholm A, Meyer T. A PI3-kinase signaling code for insulin-triggered insertion of glucose transporters into the plasma membrane. Curr Biol. 2002;12:1871–1876. doi: 10.1016/s0960-9822(02)01223-x. [DOI] [PubMed] [Google Scholar]
- Thurmond DC, Pessin JE. Molecular machinery involved in the insulin-regulated fusion of GLUT4-containing vesicles with the plasma membrane (review) Mol Membr Biol. 2001;18:237–245. doi: 10.1080/09687680110082400. [DOI] [PubMed] [Google Scholar]
- van Dam EM, Govers R, James DE. Akt activation is required at a late stage of insulin-induced GLUT4 translocation to the plasma membrane. Mol Endocrinol. 2005;19:1067–1077. doi: 10.1210/me.2004-0413. [DOI] [PubMed] [Google Scholar]
- Wang Q, Somwar R, Bilan PJ, Liu Z, Jin J, Woodgett JR, Klip A. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol Cell Biol. 1999;19:4008–4018. doi: 10.1128/mcb.19.6.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaid H, Antonescu CN, Randhawa VK, Klip A. Insulin action on glucose transporters through molecular switches, tracks and tethers. Biochem. J. 2008;413:201–215. doi: 10.1042/BJ20080723. [DOI] [PubMed] [Google Scholar]
- Zhou QL, Jiang ZY, Mabardy AS, Del Campo CM, Lambright DG, Holik J, Fogarty KE, Straubhaar J, Nicoloro S, Chawla A, et al. A novel pleckstrin homology domain-containing protein enhances insulin-stimulated Akt phosphorylation and GLUT4 translocation in adipocytes. J Biol Chem. 2010;285:27581–27589. doi: 10.1074/jbc.M110.146886. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.