Abstract
Primary tumors have been shown to prepare distal organs for later colonization of metastatic cells by stimulating organ-specific infiltration of bone marrow derived cells. Here we demonstrate that neutrophils accumulate in the lung prior to the arrival of metastatic cells in mouse models of breast cancer. Tumor-entrained neutrophils (TENs) inhibit metastatic seeding in the lungs by generating H2O2 and tumor secreted CCL2 is a critical mediator of optimal anti-metastatic entrainment of G-CSF-stimulated neutrophils. TENs are present in the peripheral blood of breast cancer patients prior to surgical resection but not in healthy individuals. Thus, while tumor-secreted factors contribute to tumor progression at the primary site, they concomitantly induce a neutrophil-mediated inhibitory process at the metastatic site.
Introduction
The leading cause of cancer related mortality is metastatic spread of tumor cells to distant sites. Metastasis is a multi step process consisting of intravasation, survival in the circulation, extravasation, survival in and colonization of distant tissues. The complexity of each step of the metastatic process makes it highly inefficient overall and although large numbers of cells disseminate from the primary tumor, only a small fraction of these cells end up forming distant tumors. However rare, disseminated tumor cells ultimately successfully form metastatic colonies and therefore present a critical therapeutic challenge.
Many tumor types are capable of metastasizing to distant sites however the location of these sites varies considerably among different primary tumors. Several studies have shown that the site of metastasis may be determined by a specific gene expression pattern, or signature, in primary tumor cells that mediates metastasis to specific distant organs (Gupta and Massague, 2006). In addition, tumor induced changes in the microenvironment of distal organs prior to colonization might make certain tissues more receptive to colonization of migrating tumor cells (Joyce and Pollard, 2009). Recent studies have suggested that factors secreted from the primary tumor may modulate the future site of metastasis in a directed fashion (Erler et al., 2009). Bone marrow derived cells are mobilized by the primary tumor and directed to the future site of metastasis (Kaplan et al., 2005) where they take part in the formation of a functional pre-metastatic niche. All of these processes are likely working in concert to determine the future site of metastasis.
Here we describe the accumulation and activation of neutrophils by the primary tumor during the pre-metastatic stage in mouse cancer models. Induced by the primary tumor, these tumor-entrained neutrophils (TENs) accumulate in the circulation and the pre-metastatic lung. Neutrophils are short-lived leukocytes that are part of the innate immune system. Owing to their highly motile nature, neutrophils are quick to respond and provide the first line of defense against infections through phagocytosis, extracellular degranulation and spreading of extracellular traps (Hickey and Kubes, 2009). Driven by cytokines and chemotactic factors, neutrophils are also recruited to sites of inflammation where they take part in the inflammatory process. The notion that tumors and sites of inflammation, such as wounds, share many similarities is now commonly accepted. Tumors might also be viewed as wounds that will not heal (Dvorak, 1986) and as such incite an inflammatory response by recruiting leukocytes through the production of various cytokines and chemokines (Coussens and Werb, 2002).
The tumor recruited leukocyte population is heterogeneous and consists, in part, of tumor-associated neutrophils (TANs). TANs were previously shown to have a pro-tumorigenic effect at the primary site by secreting pro-tumorigenic factors, promoting angiogenesis and suppressing immune responses (Pekarek et al., 1995; Shojaei et al., 2008; Youn et al., 2008). Interestingly, the pro-tumorigenic effects of neutrophils were shown to be TGF-β dependent and upon TGF-β blockade, neutrophils were shown to switch from the “N2” pro-tumorigenic phenotype to the “N1” anti-tumorigenic phenotype (Fridlender et al., 2009). Recent studies have also suggested a pro-metastatic role for neutrophils in the pre-metastatic niche. High numbers Gr-1+CD11b+ cells were shown to accumulate in the pre-metastatic lungs of mice bearing highly metastatic tumors. The Gr-1 epitope is present on both Ly-6G+ cells which are exclusively neutrophils (Daley et al., 2008) and Ly-6C+ cells in which the epitope is expressed at higher levels on monocytes than neutrophils (Zhu et al., 2007). Although not formally demonstrated by depletion of Gr-1+CD11b+ cells, this study suggests that tumor-mobilized lung associated Gr-1+CD11b+ cells contribute to the formation of a pro-metastatic niche by generating an immunosuppressive environment with abnormal vasculature (Yan et al., 2010). In another recent study it was shown that G-CSF stimulated neutrophils and monocytes increase the metastatic seeding of circulating tumor cells in the lungs and that both G-CSF and BV8 contribute to the formation of lung metastases. Furthermore, in one 4T1-cell derivative orthotopic model (66Cl4), Ly-6G-specific depletion of neutrophils was found to reduce the metastatic burden in the lungs (Kowanetz et al., 2010).
Through careful depletion and adoptive transfer experiments here we examine the role of TENs in a variety of breast cancer models and relate our findings to their presence in the peripheral blood of breast cancer patients.
Results
Neutrophils accumulate in the pre-metastatic lung
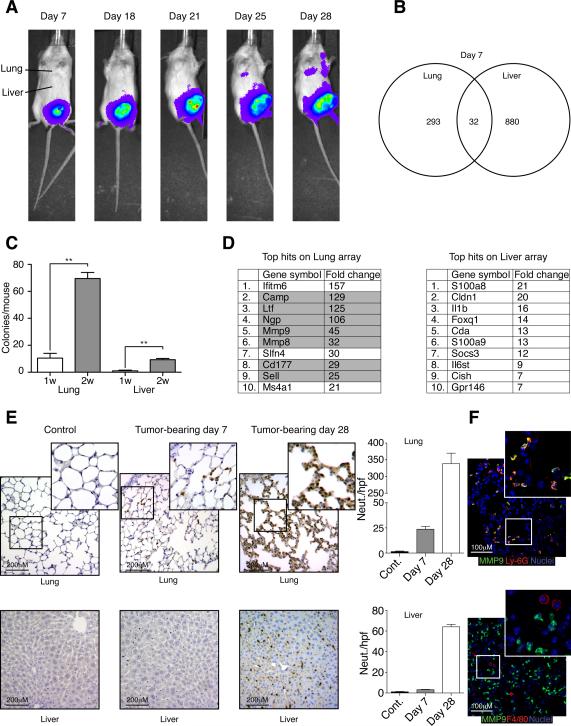
Orthotopically implanted 4T1 tumors spontaneously metastasize primarily to the lung whereas metastases to the liver, brain, and bones are less frequent (Fig. 1A) and arise much later (not shown). To determine if transcriptional changes take place in the lung prior to the arrival of metastatic cells which might modulate the behavior of these cells, we performed mRNA microarray analysis of lung and liver samples from sham-operated (orthotopically injected with PBS) versus tumor-bearing mice. We found no significant differences in gene expression 3 days after tumor engraftment. In contrast, the expression of 325 and 912 genes was significantly changed in the lung and liver, respectively, of tumor-bearing mice 7 days after tumor engraftment relative to sham-operated controls (Fig. 1B). Of the 325 genes significantly changed in the lung, 293 were lung specific and were not changed in the liver. Only a small number of tumor cells (<10) were found in the lungs or the liver at this point suggesting that tumor cell contribution to the expression array is negligible (Fig. 1C).
Figure 1. Early transcriptional changes in the pre-metastatic lung and liver.
A. Spontaneous metastatic progression from orthotopically engrafted luciferase-labeled 4T1 tumor cells to the lungs in syngeneic Balb/c mice. B. Venn diagram summarizing the transcriptional changes in the lung and the liver from sham operated vs. 4T1 tumor bearing mice (n=3 mice per group). C. Colony formation assay performed on lung and liver tissue from days 7 and 14 post tumor engraftment (n=4). D. Top 10 up-regulated genes in lung and liver expression array. Highlighted genes are expressed in myeloid cell lineages. E. Ly-6G immunohistochemistry performed on lung and liver tissues from tumor-bearing or sham-operated mice on day 7 post tumor engraftment. Graphs depicting the number of neutrophils/hpf in the lung and liver. F. Co-staining of lung tissue from tumor-bearing mice on day 7 shows that MMP9 (green) co-localizes with Ly-6G (red on upper panel) but not with F4/80 (red on lower panel). Error bars represent ± SEM. (** p<0.01). See also Figure S1.
Several neutrophil-specific genes (such as cathelicidin and lactoferrin) are acutely upregulated in the pre-metastatic lung but not in the liver (Fig. 1D) and although we cannot rule out the possibility that some of the mRNAs in the array can come from the parenchyma or microenvironment we speculated that neutrophils are recruited to the pre-metastatic lung. This hypothesis was confirmed by immunohistological staining with the neutrophil specific antibody Ly-6G which showed an increase in Ly-6G+ neutrophils in the lung by day 7 post tumor engraftment (Fig. 1E). Further immunohistochemical analysis was performed on pre-metastatic lung tissue to test whether acutely upregulated genes co-localize with neutrophils. As can be seen in figure 1F, MMP9 (upregulated 45-fold) co-localizes with the neutrophil marker Ly-6G, as well as with other neutrophil markers such as cathelicidin (Fig. S1A), but not with the pan macrophage marker F4/80. Neutrophils continue to accumulate in the lungs over a period of 28 days and start accumulating in the liver on day 28 post tumor engraftment (Fig. 1E). The increase in lung neutrophils was also found in athymic mice bearing other pre-metastatic xenografts as well as in mice bearing MMTV-Wnt1 and MMTV-PyMT-driven spontaneous mammary tumors prior to the appearance of overt lung metastases. (Fig. S1B–C). Interestingly, we found that neutrophils do not accumulate in large numbers in the pre-metastatic lungs (or the circulation) of mice bearing the 4T1 derivative tumor 66Cl4 (Fig. S1D–F, see also Discussion).
Neutrophil depletion in tumor-bearing mice
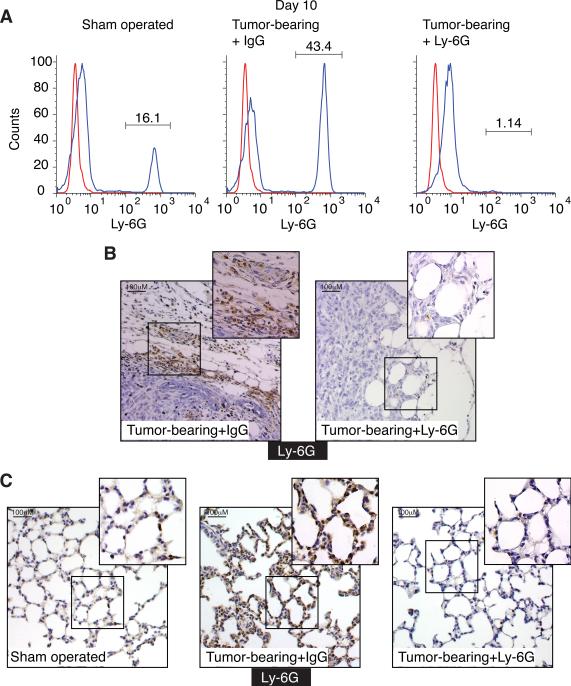
In order to unequivocally determine the role of neutrophils in the lung pre-metastatic site, we first established our ability to efficiently deplete neutrophils in tumor bearing animals. Balb/c mice were orthotopically injected with 4T1 cells and were then treated with either control (IgG) or the neutrophil-specific depleting antibody Ly-6G (Daley et al., 2008). Control mice were sham-operated and treated with the control antibody (IgG). Ly-6G antibody treated tumor-bearing mice show a dramatic reduction in circulating neutrophil numbers from 43% in tumor-bearing mice treated with control IgG down to 2% (Fig. 2A). Neutrophils are also dramatically depleted in the primary tumor rim and lungs (Fig. 2B–C). As expected, we observed similar results when using the Gr-1 antibody for neutrophil depletion (not shown). Analysis of lung associated CD11b+ cells shows a significant increase in CD11b+MMP9+ cells (which are almost exclusively neutrophils, note Ly-6G-MMP9 co-localization in Fig. 1F) in the lungs of tumor-bearing mice (Fig. S2A) compared with sham-operated mice. Upon neutrophil depletion there is a dramatic reduction in lung associated CD11b+MMP9+ cells (neutrophils, Fig. S2B) but no significant effect on the CD11b+MMP9− population (Fig. S2C). This observation suggests that lung associated CD11b+ cell populations, other than neutrophils, are not dramatically affected by neutrophil depletion. Interestingly, we found no significant differences in the number of lung-associated Ly-6C+ monocytes in either tumor-bearing mice or neutrophil-depleted tumor-bearing mice compared to tumor free mice (Fig. S2D).
Figure 2. Ly-6G antibody administration depletes neutrophils from the circulation, the primary tumor and the pre-metastatic lung.
A. Circulating Ly-6G+ neutrophils in 10 days post tumor engraftment in sham-operated and tumor-bearing mice treated with control IgG or Ly-6G antibody. B. Ly-6G immunohistochemistry performed on tumors from IgG or Ly-6G antibody treated mice. C. Ly-6G immunohistochemistry performed on lung tissue from sham operated mice and tumor-bearing mice treated with control IgG or Ly-6G antibody. See also Figure S2.
Tumor entrained neutrophils inhibit seeding in the pre-metastatic lung
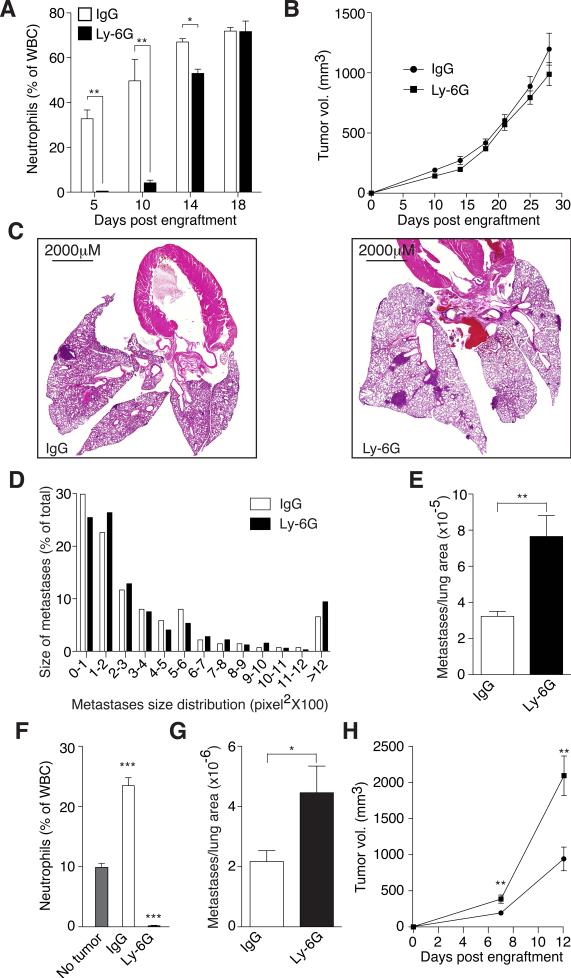
We next tested the long-term effects of neutrophil depletion on spontaneous metastasis from synchronous mammary 4T1 tumors in syngeneic Balb/C mice. Neutrophil depletion in the circulation was highly effective until day 14 post tumor engraftment, after which neutrophil levels were not significantly different in control or neutrophil depleted mice, perhaps due to enhanced neutrophil release from the bone marrow induced by the growing tumor (Fig. 3A). Importantly, metastatic seeding of the lung takes place during the period of effective neutrophil depletion. While neutrophil depletion had no significant effect on apoptosis (Fig. S3A–B) or the growth rate of the primary tumor (Fig. 3B), neutrophil-depleted tumor-bearing mice had increased metastatic burden compared with controls (Fig. 3C and E). A thorough histological examination shows that while there is no significant difference in metastases size distribution (Fig. 3D) there is about a 3-fold increase in the number of metastatic events (Fig. 3E) suggesting that tumor entrained neutrophils (TENs) inhibit seeding in the pre-metastatic lung. Interestingly, neutrophil depletion had no significant effect on the number of circulating tumor cells (data not shown) suggesting the effect of neutrophils on seeding is confined to the pre-metastatic site. Similar results were observed in 4T1 tumor bearing athymic mice suggesting that the anti metastatic effect of TENs is T-cell independent (Fig. S3C–H). In addition, enhancement of metastatic seeding in the lungs in neutrophil-depleted mice was similarly observed when using the Gr-1 antibody, potentially depleting not only Ly-6G+ neutrophils but also a subset of Ly-6C+ monocytes (Fig. S3C–H). This observation suggests that in 4T1 tumor bearing mice, the anti-metastatic effect of neutrophils dramatically outweighs any contribution by Ly-6C+ monocytes. The low rate of spontaneous metastasis to other organs (liver and brain) was unaffected by neutrophil depletion but neutrophil accumulation at these sites was at least 10-fold lower than the lungs (Fig. 1E and data not shown).
Figure 3. TENs attenuate the spontaneous formation of lung metastases.
A. Blood differentials showing neutrophils in control and anti-Ly-6G treated tumor-bearing mice. B. Primary tumor growth in control (IgG, n=9) and neutrophil depleted (Ly-6G, n=9) mice. C. Representative lung H&E images of control (IgG) and neutrophil depleted (Ly-6G) mice by day 25 post tumor engraftment. D. Metastatic foci size distribution in control (IgG) and neutrophil depleted (Ly-6G) mice. E. Metastatic events per lung area in control (IgG) and neutrophil depleted (Ly-6G) mice. F. Circulating Ly-6G+ neutrophils in tumor free (No tumor), control (IgG treated, n=5) and neutrophil depleted (anti-Ly-6G treated, n=5) MMTVPyMT/MMTV-cMyc tumor-bearing mice. G. Metastatic events per lung area in control (IgG) and neutrophil depleted (Ly-6G) mice. H. Primary tumor growth in control (IgG) and neutrophil depleted (Ly-6G) mice. Error bars represent ± SEM. See also Figure S3.
Consistent with the observations made with the 4T1 tumor model, neutrophil depletion in MMTV-PyMT/MMTV-cMyc tumor bearing mice results in enhanced metastatic seeding in the lungs (Fig. 3F–G). However, in this model neutrophil depletion also resulted in a modest enhancement in primary tumor growth (Fig. 3H) therefore the increase in metastasis could be due in part to the larger tumor volume at the primary site in this model.
In another widely used experimental model of metastasis we injected tumor cells into the tail vein of tumor-bearing mice and sham controls to examine the number of seeding tumor cells in the lungs, with and without neutrophil depletion (Fig. S3I–L). While the presence of a primary tumor increases the seeding efficiency of tumor cells in the pre-metastatic lung (perhaps due to previously described processes (Hiratsuka et al., 2006)) there is a further increase in tumor cell seeding in neutrophil depleted tumor bearing mice (Fig. S3K). Together, our observations in both spontaneous and experimental models of metastasis support the notion that although a subset of Gr-1+Cd11b+ cells (which contain monocytes and neutrophils) might play a pro-metastatic role (Yan et al.), the net effect of TENs is inhibition of tumor cell seeding in the premetastatic lung.
TENs are sufficient for anti-metastatic protection
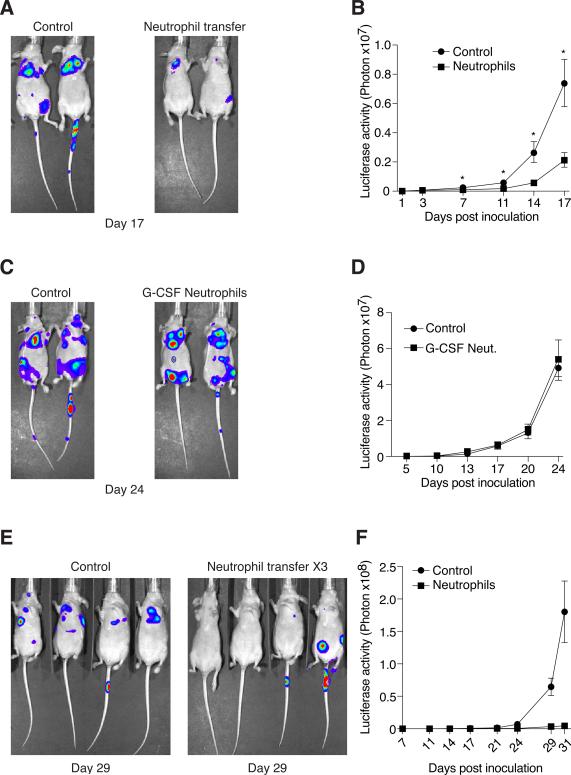
We sought to determine whether TENs are sufficient to provide anti-metastatic protection independent of T-cell activity. To this end, athymic mice were injected with 2×105 4T1 cells via the tail vein to induce experimental metastases and then, 4 hours later, these mice were treated with 5×106 TENs purified from 4T1 tumor-bearing mice. The transfer of TENs results in a significant delay in the formation of lung foci (Fig. 4A–B). In contrast, transfer of neutrophils purified from G-CSF treated animals had no significant effect on the formation of lung foci (Fig. 4C–D). When TENs were transfused on 3 consecutive days, at 4, 24 and 48 hours after introduction of tumor cells, lung foci were nearly entirely ablated (Fig. 4E–F). These observations suggest that while tumor-naïve, G-CSF induced neutrophils do not affect the formation of lung foci, TENs can provide anti-metastatic protection in vivo and dramatically reduce the seeding of disseminating tumor cells in the lungs.
Figure 4. TENs but not G-CSF stimulated neutrophils attenuate the formation of experimental metastases.
A. Representative images showing that transfer of TENs attenuates the formation of lung foci after tail-vein injection of tumor cells (n=5 per group). B. Quantification of lung specific luciferase activity shows that TENs significantly delay the formation of lung foci. C. Representative images showing that transfer of G-CSF stimulated neutrophils has no effect on the formation of lung foci (n=5 per group). D. Quantification of lung specific luciferase activity shows that G-CSF induced neutrophils do not delay the formation of lung foci. E. Multiple TENs transfers after introduction of tumor cells result in near complete ablation of lung foci formation (n=4 per group). F. Quantification of lung specific luciferase activity shows that lung foci formation in mice treated with multiple TENs transfers is ablated. Error bars represent ± SEM. (* p<0.05, ** p<0.01).
TENs acquire a cytotoxic phenotype and kill tumor cells
Since neutrophils are armed with an arsenal of toxic peptides and molecules we postulated that TENs might be able to kill tumor cells directly and thereby provide anti-metastatic protection. We therefore tested the tumor-cell killing capacity of TENs in vitro. Neutrophils were purified from sham-operated and tumor-bearing mice and co-cultured with luciferase-labeled 4T1 cells (to allow selective quantification) at a 20:1 neutrophil to tumor cell ratio. This co-culture ratio is probably an under estimation of the actual in-vivo ratio since tumor bearing mice (by day 7 post tumor engraftment) have approximately 106 neutrophils/ ml of blood and they have already accumulated in the lungs in large numbers (Fig. 1E) while at that time there are fewer than 10 tumor cells in the lungs (Fig. 1C).
While control neutrophils had no significant cytotoxic effect, TENs were highly cytotoxic (Fig. 5A). Such cytotoxicity was not observed when tumor cells were co-cultured with a mixture of lymphocytes and monocytes purified from either tumor bearing or control mice. No killing was observed when the neutrophils and the tumor cells were separated by a membrane demonstrating that physical contact is necessary for neutrophil mediated tumor-cell killing (Fig. 5B). A similar killing pattern was observed with MCF7 cells co-cultured with TENs from 4T1-tumor bearing mice (Fig. 5B) suggesting that this is a broader phenomenon. Furthermore, neutrophils purified from C57/B6 mice bearing B16 melanoma tumors, but not from control mice, gained the ability to kill both B16 and 4T1 cells (Fig. S4A–B).
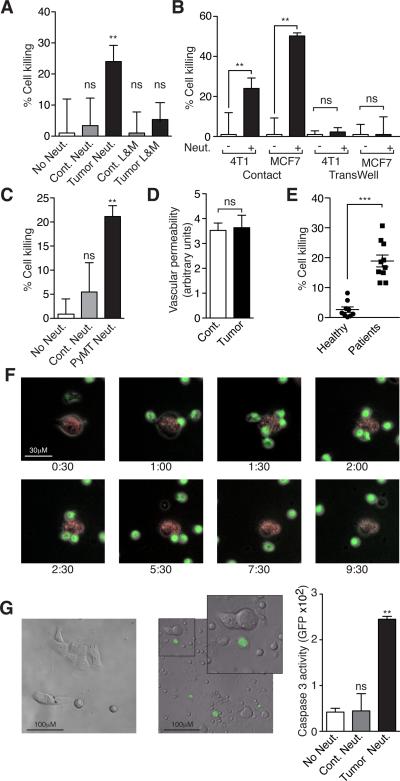
Figure 5. TENs gain a cytotoxic phenotype and kill tumor cells by initiating physical contact.
A. Co-culture of 4T1 cells with neutrophils (Neut.) or a mixture of Lymphocytes and Monocytes (L&M) purified from sham-operated (Cont.) or 4T1 tumor-bearing (Tumor) mice. B. TENs in co-culture with 4T1 or MCF7 cells allowing physical contact or in a TransWell plate. C. Neutrophils purified from wild type (Cont. Neut.) or mammary tumor-bearing MMTV-PyMT mice in co-culture with 4T1 cells. D. Vascular permeability in lungs of sham operated (Cont.) and tumor-bearing (Tumor) mice. E. Neutrophils purified from healthy human volunteers (Healthy) or breast cancer patients (Patients) in co-culture with MDA-MB-231 cells. F. Time-lapse microscopy showing a co-culture of GFP labeled TENs (green) and 4T1 cells (red). G. Representative images showing (Cleaved Caspase 3 activity (NucView 488) in 4T1 cells cultured alone (top) or in co-culture with TENs (bottom). GFP intensity as an estimate for apoptosis in 4T1 cells cultured alone (No Neut.), with control neutrophils (Cont. Neut.) or TENs (Tumor Neut.). These experiments were repeated at least 3 times with similar results. Error bars represent ± SEM. (* p<0.05, ** p<0.01, ***p<0.001). See also Figure S4, Movies S1 and S2.
To conclusively exclude the possibility that neutrophil cytotoxicity in mice bearing implantable tumors is a result of a non specific anti graft immune response we evaluated neutrophil cytotoxicity in mice bearing spontaneous MMTV-PyMT driven mammary tumors. As shown in figure 5C, neutrophils purified from PyMT-induced tumor bearing mice are highly cytotoxic. Furthermore, neutrophil entrainment was not a result of a general response to wounding since neutrophils purified from wounded mice were found to be non-cytotoxic (Fig. S4C–E). Importantly, although large numbers of TENs accumulate in the pre-metastatic lung, we found no gross histological evidence for tissue damage (Fig. S4F). Furthermore, using Evans Blue staining we found no indication for increased micro vascular permeability in lungs from tumor-bearing mice compared to controls (Fig. 5D) suggesting that TENs can discriminate between tumor cells and normal cells (i.e. the lung endothelium) in vivo.
Finally, we wanted to ascertain whether the generation of TENs is relevant to human disease. While neutrophils isolated from healthy human volunteers were non-cytotoxic, neutrophils isolated from newly diagnosed breast cancer patients (Fig. S5A) prior to tumor removal or chemotherapy were highly cytotoxic (Fig. 5E) suggesting that neutrophil entrainment is not unique to murine tumor models but also occurs during the natural course of the human disease. Furthermore, although pre-metastatic lung tissue samples from breast cancer patients are unavailable, high myeloperoxidase staining in the metastatic lung parenchyma (in 5/5 cases examined) suggests that neutrophils accumulate in the lungs of breast cancer patients (Fig. S5B). Taken together, these observations support the notion that processes similar to those seen in murine cancer models, i.e. neutrophil accumulation, lung sequestration and activation also occur during the natural course of human disease.
Real time visualization of TENs cytotoxicity
We then monitored the interaction between TENs and tumor cells using time-lapse microscopy. As early as 60 minutes after their addition, GFP labeled TENs can be seen converging on the adherent 4T1 cells (red, Fig. 5F). The neutrophils initiate physical contact with the tumor cell at which time the tumor cell undergoes dramatic morphological changes (Fig. 5F). To ascertain whether the interaction between TENs and tumor cells results in tumor cell apoptosis, tumor cells were labeled with a Caspase 3 fluorescent substrate prior to the addition of TENs. As can be seen in figure 5G there were no Caspase 3 positive cells (GFP positive) in the control culture while GFP positive cells can be seen in the 4T1+TEN co-culture. Quantification of GFP intensity as an indicator of Caspase 3 activation shows a dramatic increase in the presence of TENs but not in the presence of control neutrophils (Fig. 5G). Using time-lapse microscopy we are able to show the TENs induced activation of Caspase 3 in tumor cells in real time (Movie S1). Control neutrophils do not form prolonged interaction with tumor cells and fail to activate Caspase 3 and induce apoptosis (Movie S2). These data suggest that TENs inhibit tumor cell seeding in the lungs by inducing apoptosis however the high rate of spontaneous apoptosis of incoming tumor cells in the lung capillaries precludes direct assessment of tumor cell killing by TENs in situ.
TENs cytotoxicity is mediated by hydrogen peroxide
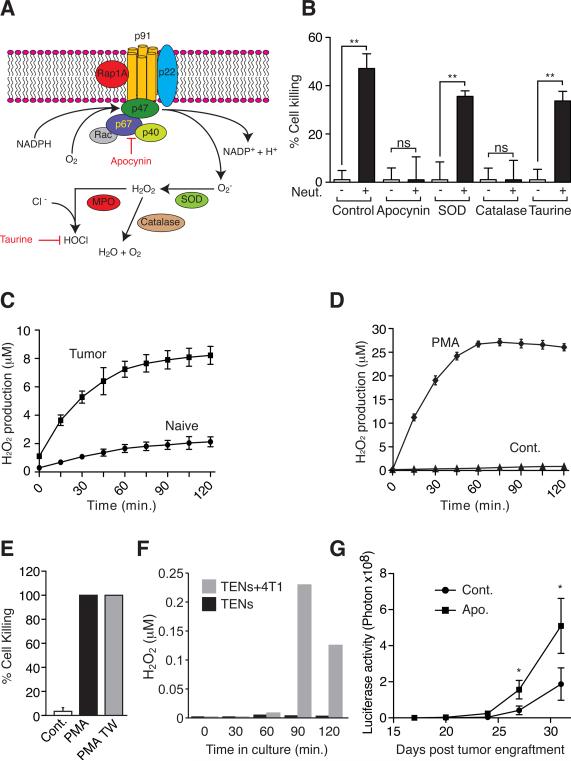
Next we sought to characterize the mechanism by which neutrophils kill tumor cells. Neutrophils can generate reactive oxygen species (ROS) through the activity of the NADPH Oxidase complex and induce cell death by an oxidative burst (Fig. 6A). Indeed, co-culture of TENs with tumor cells in the presence of Apocynin, a specific inhibitor of the NADPH Oxidase complex, completely inhibits the neutrophil mediated tumor-cell killing (Fig. 6B). Addition of exogenous superoxide dismutase did not inhibit cell death suggesting that superoxides per se are not directly involved in cell killing. In contrast, catalase (which converts H2O2 to H2O and O2) completely inhibits cell killing (Fig. 6B). The presence of the hypochlorous acid scavenger, taurine, did not reduce tumor-cell killing by stimulated neutrophils suggesting the killing is by H2O2 (Fig. 6B). This notion is further supported by the observation that TENs generate more H2O2 than control neutrophils (Fig. 6C). To gain further insight into the killing mechanism we treated naïve neutrophils with PMA, a potent activator of H2O2 production. As expected, PMA induced a dramatic increase in H2O2 production in naïve neutrophils (Fig. 6D). Consistent with previous reports (Dallegri et al., 1983) we found that when in co-culture with 4T1 cells, PMA-treated naïve neutrophils become highly cytotoxic killing close to 100% of tumor cells (Fig. 6E). However, we found that unlike TENs, PMA-stimulated neutrophils have the capacity to kill tumor cells even when separated by a membrane in a transwell system (Fig. 6E). This result suggests that PMA stimulated neutrophils spontaneously secrete H2O2 in levels high enough to kill tumor cells via a mechanism that does not require physical contact. Since TENs require physical contact for tumor cell killing (Fig. 5B) we hypothesized that rather than spontaneous H2O2 release, physical contact triggers the secretion of H2O2 in TENs. To test this hypothesis we measured H2O2 levels in the supernatant of TENs cultured alone or in co-culture with 4T1 cells. We found that although TENs generate high levels of H2O2 (see Fig. 6C) they do not secrete detectable levels of H2O2 when cultured alone (Fig. 6F). Consistent with our hypothesis, we were able to detect H2O2 in nM concentration in the supernatant when TENs were co-cultured with 4T1 cells, suggesting H2O2 secretion by TENs is triggered in the presence of tumor cells (Fig. 6F).
Figure 6. TENs cytotoxicity is mediated through the NADPH Oxidase – H2O2 pathway and triggered by physical contact.
A. The NADPH Oxidase complex - superoxides generated by oxidizing NADPH converted into H2O2 by cellular superoxide dismutase (SOD). Myeloperoxidase (MPO) converts H2O2 to hypochlorous acid (HOCl). Apocynin inhibits the formation of the NADPH Oxidase complex, catalase catalyzes the formation of H2O and O2 from H2O2 and taurine eliminates HOCl. B. Co-culture of 4T1 tumor cells with TENs in the presence of the Apocynin, Superoxide Dismutase (SOD), or Taurine. C. H2O2 production in purified control neutrophils (naïve) and TENs (Tumor). D. H2O2 production in naïve neutrophils in the presence of PMA or vehicle control (Cont.). E. Naïve neutrophils co-cultured with 4T1 cells treated with vehicle (Cont.), or PMA allowing physical contact (PMA) or in a TransWell plate (PMA-TW). F. H2O2 concentration in the supernatant of TENs cultured alone (TENs) or in co-culture with 4T1 cells (TENs+4T1). G. Quantification of lung specific luciferase activity to follow metastatic progression in control (Cont. n=5) and Apocynin (Apo. n=5) treated tumor-bearing mice (Cont. n=5). These experiments were repeated at least 2 times with similar results. Error bars represent ± SEM. (* p<0.05, ** p<0.01).
Finally, to test whether the process through which neutrophils inhibit metastasis is also relevant in vivo we tested the effect of Apocynin on spontaneous metastasis. We show that Apocynin treatment enhances metastasis and that Apocynin treated mice developed spontaneous lung metastases earlier than control mice (Fig. 6G). These results suggest that neutrophils induce tumor cell death through generation of ROS by the NAPDH Oxidase complex. However, it is the generation and contact triggered release of H2O2 rather than superoxides or the H2O2 chlorine metabolite, hypochlorous acid that mediates killing.
Tumor-secreted CCL2 is a critical mediator of neutrophil entrainment
What is the mechanism through which neutrophils are activated by the primary tumor? 4T1 tumor bearing mice have been shown to have high levels of serum G-CSF that may account for neutrophil accumulation (DuPre and Hunter, 2007). Indeed, in vivo rhG-CSF administration induces a significant increase in circulating and lung-associated neutrophils in tumor-naïve animals (Fig. S5C–F). However, it does not induce a cytotoxic neutrophil response in vivo (Fig 4C–D) or in vitro (Fig. 7A). These observations suggest that G-CSF secretion is sufficient for both accumulation in the circulation and lung sequestration of neutrophils however these neutrophils require further activation for the entrainment of their anti-metastatic behavior.
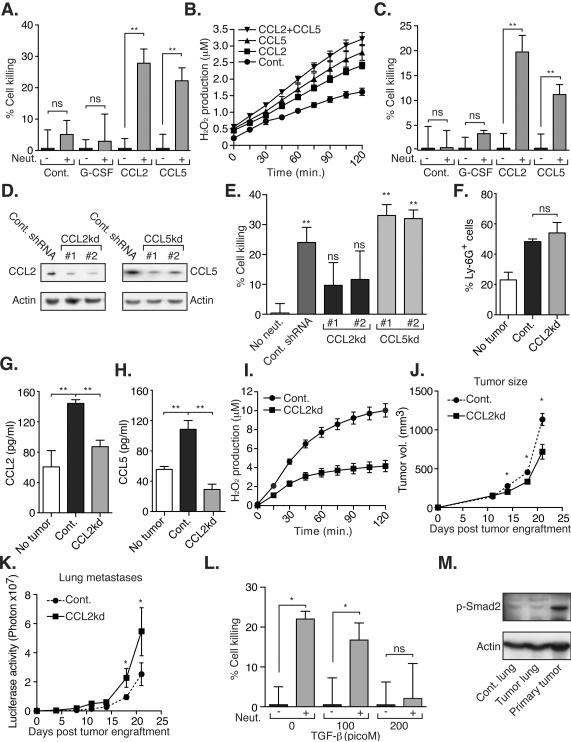
Figure 7. Tumor secreted CCL2 is both required and sufficient for neutrophil entrainment.
A. Neutrophils purified from naïve mice were co-cultured with 4T1 cells in the presence or absence of mCCL2, mCCL5 and rhG-CSF. B. H2O2 production in neutrophils from naïve mice in the presence or absence of mCCL2 and mCCL5. C. Neutrophils purified from healthy human volunteers were co-cultured with MDA-MB-231cells in the presence or absence of mCCL2, mCCL5 and rhG-CSF. D. CCL2 and CCL5 expression in 4T1 cells transduced with a non-targeting shRNA (cont.), CCL2 specific shRNA (#1 and #2), and CCL5 specific shRNA (#1 and #2). E. Co-culture of 4T1 cells with TENs purified from mice bearing control (Cont. shRNA), CCL2kd (#1 and #2) or CCL5kd (#1 and #2) tumors. F. Circulating Ly-6G+ neutrophils in sham operated (no tumor) and mice bearing control (Cont.) or CCL2kd tumors. G–H. Circulating levels of CCL2 and CCL5 in tumor free mice (no tumor), and in mice bearing control shRNA (Cont.) and CCL2kd (CCL2kd) tumors. I. H2O2 production in TENs purified from mice bearing control or CCL2kd tumors. J. Primary tumor growth in control (n=5) and CCL2kd (n=5) tumor-bearing mice. K. Lung specific luciferase activity measuring metastatic progression to the lungs in mice bearing control or CCL2kd tumors. L. Co-culture of TENs and 4T1 cells in the presence or absence of TGF-α. M. Phosphorylation of Smad2 in normal lung (Cont. lung), pre-metastatic lung (Tumor lung) and the primary tumor. These experiments were repeated at least 2 times with similar results. Error bars represent ± SEM. (* p<0.05, ** p<0.01). See also Figure S5.
The expression array performed on pre-metastatic lungs showed a 7.9 and 4.6 fold increase in CCR1 and CCR2 respectively, both of which are expressed in murine neutrophils (Reichel et al., 2006) (and Fig. S5G). Since 4T1 tumors were shown to secrete CCL2 and CCL5 (DuPre et al., 2007) that are potential ligands for CCR2 and CCR1 respectively, we tested whether these chemokines can induce neutrophil-mediated tumor-cell killing. Addition of either mCCL2 or mCCL5 can stimulate naïve neutrophils and induce tumor cell killing in vitro suggesting that both are sufficient for neutrophil entrainment (Fig. 7A). This observation is further substantiated by the fact that CCL2 and CCL5 induce an increase in H2O2 production in naïve neutrophils (Fig. 7B). Similarly, both hCCL2 and hCCL5 can stimulate human neutrophils purified from healthy volunteers and induce the killing of human MDA-MB-231 in vitro (Fig. 7C). Since CCL2 and CCL5 expression has been documented in several human tumors (see for example (Soria and Ben-Baruch, 2008)) this observation suggests that neutrophil entrainment by tumor-secreted factors might also be relevant in human malignancies. Moreover, we find that human chemokines such as CCL3, CXCL1, CXCL12 and CXCL16 also have the capacity of dramatically inducing neutrophil cytotoxicity (Fig. S5H). This observation further expands the scope of human malignancies where neutrophil entrainment might be relevant.
We compared the expression profiles of TENs, GCSF treated, CCL2, and CCL5 treated neutrophils all relative to naïve neutrophils and the results are presented in figure S5I–J. The top 10 most highly regulated genes in TENs compared to naïve neutrophils were similarly altered in the GCSF treated neutrophils despite the GCSF treated cells having little activity in the in vitro assay. CCL2 and CCL5 treated neutrophils, while potently activated, have no transcriptional changes above 2 fold including the 10 genes altered in TENS and GCSF treated neutrophils. From this analysis, we can conclude that GCSF has the most potent effect on neutrophil transcriptional profiles but the activating changes induced by CCL2 and CCL5 are likely not transcriptional. Combined effects of GCSF + CCL2 or 5 also has little effect above the GCSF profiles (Fig. S5I–J) consistent with this hypothesis.
CCL2 knockdown in tumors, with 2 independent shRNAs, blocks neutrophil entrainment (Fig. 7D and 7E) without affecting accumulation (Fig. 7F). Knockdown of CCL2 leads to a reduction in both CCL2 and CCL5 levels in the circulation (Fig. 7G and 7H). CCL5 knockdown on the other hand is insufficient to block entrainment perhaps due to redundancy with CCL2. Indeed, CCL2 knockdown is shown to reduce CCL5 levels in 4T1 tumor bearing mice consistent with this notion (Fig. 7H). Neutrophils accumulating in CCL2kd tumor-bearing mice were not activated as evidenced by their reduced H2O2 production when compared to neutrophils isolated from mice bearing control tumors (Fig. 7I).
To further understand the role of tumor secreted CCL2 on tumor growth and metastasis in vivo, we compared the growth and spontaneous metastasis of orthotopically implanted CCL2 knockdown (CCL2kd) and control 4T1 tumors. Consistent with the pro-tumorigenic properties attributed to CCL2 (Loberg et al., 2007; Lu and Kang, 2009) we found that primary CCL2kd tumors show growth retardation when compared to control tumors (Fig. 7J). However, we found that spontaneous metastasis from the CCL2kd tumors occurs earlier (Fig. 7K) suggesting that these tumors have increased metastatic potential. Taken together, these observations suggest that while CCL2 secretion by the primary tumor has a beneficial effect and enhances growth at the primary site, it is concomitantly capable of inducing an anti-metastatic neutrophil response and inhibiting tumor cell seeding at a distant site. Furthermore, these observations support the notion that a specific tumor secreted factor, namely CCL2, rather than a non-specific host versus tumor immune response, is an important mediator of neutrophil entrainment. As demonstrated above, neutrophils express CCR2 however, since CC ligands such as CCL2 are known to be promiscuous in binding CC receptors, the possibility of neutrophil entrainment through CC receptors other than CCR2 cannot be excluded.
As mentioned above, neutrophil depletion does not affect the growth of the 4T1 primary tumors (Fig. 3, S3E and S3L), suggesting that although TENs are induced by the primary tumor, neutrophils are not cytotoxic at the primary site. To gain further insight into the different function of CCL2 at the primary site and the pre-metastatic site we tested whether other tumor secreted factors have the capacity to modulate TENs cytotoxic activity. We found that TGF-β, which is known to be secreted by 4T1 tumors (Zhang et al., 2008), dramatically inhibits TENs cytotoxicity towards tumor cells (Fig. 7L). This result is consistent with previous observations showing that TGF-β blockade induces neutrophil-specific cytotoxicity at the primary tumor site (Fridlender et al., 2009). Since 4T1 tumors secrete TGF-β its activity in the vicinity of the primary tumor is high (Fig. 7M) likely resulting in inhibition of TENs cytotoxicity. However, at distant sites such as the pre-metastatic lung, TGF-β activity is low (Fig. 7M) and should permit TENs cytotoxicity providing a plausible explanation for the different effects of CCL2 at the primary and the pre-metastatic sites.
Discussion
In recent years, significant efforts have been directed at understanding events that take place during the metastatic process. It has become clear that key events in the metastatic cascade, such as migration, intravasation, extravasation and reinitiation at the future site of metastasis, are determined at least in part in a cell autonomous manner (Gupta and Massague, 2006). On the other hand, tumor cells are surrounded by non-malignant tumor stroma, consisting of extracellular matrix components, bone marrow derived cells, fibroblasts and other cell types (McAllister and Weinberg, 2010) and the function of these cells is modified by the malignant cells to generate a favorable environment for tumor initiation and progression (Polyak et al., 2009). The protumorigenic tumor-stroma interaction has consequences not only on primary tumor growth but also on invasion and metastasis as these processes have been found to be actively promoted by stromal cells (Joyce and Pollard, 2009). Finally, tumor mobilized bone marrow derived cells were shown to have protumorigenic effects at the metastatic site where they promote seeding of circulating tumor cells and support the formation of macrometastases (Kaplan et al., 2005).
The role of neutrophils in tumorigenesis and metastasis
Our analysis of the earliest transcriptional events that take place in the pre-metastatic lung indicated that neutrophils induced by the primary tumor arrive early to this site. The contribution of neutrophils to primary tumor growth and metastasis is somewhat controversial. Neutrophils have been shown to promote primary tumor growth (Pekarek et al., 1995) and have a pro-tumorigenic effect through enhanced angiogenesis (Nozawa et al., 2006; Shojaei et al., 2008), increased degradation of the ECM (De Larco et al., 2004), and immune suppression (Youn et al., 2008). Furthermore, CD11b+ bone marrow derived cells, a heterogeneous myeloid cell population, have been associated with priming of the pre-metastatic lung and enhanced seeding of circulating tumor cells (Erler et al., 2009; Yan et al.) and cells expressing MMP9 (upregulated 45 fold in the pre-metastatic lung expression array- Fig. 1D) were shown to potentiate the formation of lung metastases (reviewed in (van Kempen and Coussens, 2002)). In contrast, an anti-tumorigenic role has been observed following immunologic (Hicks et al., 2006) or cytokine activation (Colombo et al., 1992) as well as through blockade of TGF-β (Fridlender et al., 2009).
To unequivocally assess their contribution, neutrophils were depleted using the Ly-6G antibody shown in previous studies to effectively and specifically deplete neutrophils (Daley et al., 2008). Our data shows that in the absence of neutrophils, the size distribution of lung metastases is not significantly changed while the number of metastatic events is significantly increased suggesting that neutrophils limit the number of circulating tumor cells that can seed the lungs productively. We also found that while the presence of a primary tumor increases the seeding efficiency of circulating tumor cells when compared to tumor-free mice, depletion of neutrophils results in a further increase in seeding efficiency. In the context of tissue injury, neutrophil depletion was found to indirectly lead to a reduction in the number of macrophages present at the sight of injury (Daley et al., 2008). However, we found no evidence for increased numbers of monocytes/macrophages in the pre-metastatic lung of tumor bearing animals relative to controls and no changes in monocyte/macrophage numbers were detected following neutrophil depletion. These results suggest that the observed effects on metastatic seeding are primarily attributable to neutrophils rather than to other cell populations. The dramatic accumulation of anti-metastatic TENs in the lungs seemingly contradicts the fact that the lungs are the major site of metastasis from the mammary fat-pad. However, although TENs contribute to the inefficiency of metastatic seeding in the lungs, this protective mechanism eventually fails and metastatic growth ensues. Interestingly, neutrophil depletion did not increase the metastatic burden in other tissues such as the liver in the 4T1 model. However, in our hands we very rarely saw spontaneous metastasis to tissues other than the lungs in either IgG treated or neutrophil depleted mice. Thus, while neutrophils have no obvious protective effect in this setting, this idea still needs to be tested in a model where there is a more reliable spontaneous rate of metastasis to other organs in order to draw firm conclusions.
TENs have cytotoxic properties and unlike TANs (Tumor Associated Neutrophils), they are not necessarily associated with a specific organ but are circulating in the periphery. TENs are CD11b+Ly-6G+ cells, generate increased amounts of H2O2 and are identified in cancer patients and tumor-bearing mice. These features are common the both TENs and granulocytic Myeloid Derived Suppressor Cells (G-MDSCs) (Kusmartsev and Gabrilovich, 2006; Youn et al., 2008) and raise the interesting possibility that TENs might also have immune suppressive qualities or alternatively represent a distinct subpopulation of cells. Through depletion experiments however, we show that the net contribution of Ly-6G+ neutrophils is anti-metastatic outweighing any pro-metastatic effect that might result from immune suppression by these cells.
Kowanetz and colleagues have shown recently that simultaneous depletion of both Ly-6G+ neutrophils and Ly-6C+ monocytes reduce spontaneous metastasis in a variety of tumor models including 4T1. Such a result could be consistent with the results presented here assuming a strongly pro-metastatic role of Ly-6C+ monocytes, which would counteract the effects of neutrophil depletion. As discussed above, specific depletion of neutrophils with the Ly-6G antibody is required to make a definitive assessment and this experiment was performed by Kowenetz et, al. only in 66Cl4 tumors where metastatic burden was reported to be reduced by approximately 35%. 66Cl4 tumor-bearing mice however are observed to have very few circulating and lung associated neutrophils compared with controls (Fig. S1D–F), in agreement with Kowenetz et, al. (see Figure 1C and Supplemental Figure S2F) and with previous reports (Pande et al., 2009). These observations suggest that the anti metastatic effect of neutrophils is not evident in the 66Cl4 model simply because of insufficient neutrophil accumulation.
Neutrophil accumulation and entrainment
Under normal circumstances, neutrophils play a crucial role as a component of the innate immune system fighting infections and taking part in the inflammatory process. As demonstrated above, neutrophil function can be modulated by the primary tumor and an increase in lung associated neutrophil numbers can be seen in several tumor models during the pre-metastatic phase. Administration of G-CSF was previously shown to be sufficient for neutrophil accumulation (Molineux et al., 1990) and is in fact currently used successfully as a therapy for increasing the number of circulating neutrophils in patients with neutropenia (Metcalf, 2010). The accumulation of large neutrophil numbers in tumor-bearing mice is therefore probably caused by colony stimulation factor secreted by the primary tumor (DuPre and Hunter, 2007). Our data shows that G-CSF administration is not only sufficient for neutrophil accumulation but is also sufficient for lung sequestration of these neutrophils even in the absence of a tumor. This observation suggests that neutrophils are not actively directed to the pre-metastatic lung by the primary tumor but instead, an increase in circulating neutrophils is passively responsible for the increase in lung-arrested neutrophils. Still, our data shows that G-CSF stimulated neutrophils require further activation in order to acquire a cytotoxic phenotype.
In the 4T1 model, we have shown that secretion of CCL2 by the primary tumor is involved in neutrophil entrainment. Our data and that of others suggest that CCL2 may have a pro-tumorigenic effect at the primary site and that CCL2 blockade reduces tumorigenesis (Loberg et al., 2007; Lu and Kang, 2009). Furthermore, systemic CCL2 blockade was recently shown to reduce both tumor growth and metastasis a NSCLC tumor model in a T-cell dependent manner (Fridlender et al., 2011). CCL2 was further found to play a pro-metastatic role in a process mediated by inflammatory monocytes (Qian et al., 2011). Our data shows that reduced CCL2 expression by the primary tumor results in enhanced metastatic seeding in a breast cancer model in a T-cell independent manner. These data highlight the complexity of the host response to chemokines likely due to the many different cell populations that respond to these factors.
The role of chemokines in human disease is similarly complex. Several studies have shown that CCL2 has a pro-tumorigenic effect and that high CCL2 serum levels in cancer patients are associated with advanced disease and a poor prognosis (Soria and Ben-Baruch, 2008). Conversely, other clinical studies show that high CCL2 levels are associated with a better prognosis for breast cancer patients (Dehqanzada et al., 2006) and a prolonged overall survival in pancreatic, gastric and colon cancer patients (Monti et al., 2003; Tonouchi et al., 2002; Watanabe et al., 2008). These latter studies are consistent with our data and provide evidence for the relevance of CCL2 expression to human disease. However, we also show that chemokines other than CCL2 can stimulate a cytotoxic response in naïve human neutrophils (Fig. S5H) an observation that suggests that other chemokines might be involved in neutrophil entrainment in human disease. We show that hCXCL12 (SDF-1) is twice as potent as hCCL2 in activating human neutrophils. 4T1 tumors do not express high levels of CXCL12, however induced CXCL12 expression in 4T1 cells results in reduced tumor growth and reduced metastasis (Williams et al., 2010). Furthermore, high CXCL12 expression in breast cancer patients was found to be strongly associated with a better prognosis (Mirisola et al., 2009). As many of the chemokines tested are involved in inflammatory processes it seems that cytotoxic neutrophils with anti-metastatic properties may also be generated during states of systemic inflammation. Whether the anti-tumorigenic/anti-metastatic effect of CXCL12 or any other inflammatory chemokines associated with better prognosis is mediated through the activation of neutrophils remains to be explored.
TGF-β blocks neutrophil cytotoxicity
Neutrophil depletion in 4T1 tumor-bearing mice has no significant effect on primary tumor growth while stimulating metastasis suggesting that the primary tumor is protected from TENs cytotoxicity. Neutrophils were previously shown to have a cytotoxic effect at the primary site, however this N1 phenotype was manifested only when TGF-β signaling was blocked suggesting that the so-called “N2” pro-tumorigenic neutrophil phenotype is maintained at the primary site by exposure to TGF-β (Fridlender et al., 2009). As demonstrated here, the situation at the metastatic site is considerably different. Neutrophils arrive early at the premetastatic site prior to the arrival of detectable metastatic tumor cells and are activated in response to chemokines CCL2/5 (and perhaps others) secreted by the primary tumor. These neutrophils are not exposed to the inhibitory effect of tumor secreted TGF-β, acquire an anti-tumorigenic “N1” phenotype and are capable of killing metastatic cells. This process is independent of activated T cells since it is observed in both immunocompetent and athymic mice. Thus CCL2 secreted by the primary tumor may act as a double-edged sword, promoting growth of the primary tumor where TGF-β activity is high and neutrophil killing is inhibited, while at the same time inducing a neutrophil mediated anti-metastatic response in the lung where TGF-β activity is lower. We predict therefore that advanced metastatic disease would be refractory to neutrophil killing due to high TGF-β levels in larger metastatic masses but that TGF-β blockade prior to adoptive transfer of TENs might enhance the killing activity. Such predictions can now be readily tested experimentally.
Our data suggests that during the natural course of cancer progression, a unique population of cytotoxic neutrophils is generated. These neutrophils are entrained by the primary tumor and exert their cytotoxic activity in distant tissues thereby providing anti-metastatic protection. Neutrophil entrainment consists of two crucial events: neutrophil accumulation and chemokine induced activation. Both are driven by tumor-secreted factors that are necessary for optimal tumor growth. Unfortunately, the anti-metastatic protection provided by TENs is not complete as eventually tumor cells outcompete TENs and form metastases. Our results however suggest a means of entraining neutrophils to suppress metastatic tumor cell growth that may be exploited therapeutically in adoptive transfer procedures like those described here for the management of micrometastatic disease.
Experimental Procedures
Animals
Balb/c, C57/B6, FVB and Balb/c–Ubc-GFP mice were purchased from Taconic, Jackson lab and Harlan. MMTV-Wnt1 mice were a kind gift from Dr. Harold Varmus. MMTV-PyMT mice were a kind gift from Dr. Johanna Joyce. MMTV-PyMT MMTV-cMyc mice were a kind gift from Dr. Jacqueline Bromberg. All experiments involving animals were approved by MSKCC's Institutional Animal Care and Use Committee (IACUC).
Cell lines
4T1, B16-F10, Lewis Lung, MCF7 and MDA-MB-231 cell lines were purchased from the ATCC. The human 4175 (LM2) cell line was a kind gift from Dr. Joan Massagué and the mouse 66Cl4 cell line was a kind gift from Dr. Yibin Kang. CCL2 and CCL5 knockdown 4T1 cells were generated by lentiviral transduction with CCL2 and CCL5 specific shRNAs from the SKI shRNA lentiviral collection. 4T1 and 4175 cells were orthotopically injected into the mammary fat pad. B16-F10 and LLC cells were injected intradermally. Sham operated mice were orthotopically injected with PBS.
Neutrophil depletion
Neutrophil depletion was achieved using daily IP injections of 12.5μg Rat anti-Gr-1 antibody (BD) or Rat anti-Ly-6G antibody (BD) starting on day 3 post tumor engraftment. Starting on day 14, neutrophil depleting antibodies were administered twice daily. Control mice were injected with 12.5μg Rat isotype control (BD). Neutrophil depletion was monitored by manual blood differentials and FACS analysis.
Colony formation assay
Female nude mice (n=4) were orthotopically injected with 5×106 puromycin resistant 4T1 cells. On days 7 and 14 post tumor engraftment the mice were sacrificed and perfused with PBS. The lungs and livers were removed, washed with PBS and dissociated with Collagenase A (Roche). The single cell suspension was then plated onto puromycin containing media and selected for 14 day. Colonies were stained with Methylene-Blue and counted manually. This experiment was repeated 2 times with similar results.
Mouse neutrophil purification
Whole blood was collected by cardiac puncture using heparinized (Sigma) syringe. The blood was diluted with PBS-BSA (0.5%) and subjected to a discontinuous Histopaque (Sigma) gradient (1.077 and 1.119). Neutrophils were collected from the 1.077–1.119 interface, lymphocytes and monocytes collected from the plasma-1.077 interface. RBCs were eliminated by hypotonic lysis. The cells were washed twice with PBS-BSA and re-suspended in OptiMEM (Invitrogen) 0.5% FBS in a final concentration of 2×106 cells/ml. Neutrophil purity and viability were determined visually and were consistently >98%.
Human neutrophil purification
Approval was obtained from the institutional review board of Memorial Sloan-Kettering Cancer Center. Blood samples were collected from healthy volunteers or from patients who were already having blood drawn for pre-surgical testing. Patients were consented accordingly as part of the approved protocol. Heparinzed blood (20U/ml final) was mixed with an equal volume of Dextran 500 (3% in saline). The leukocyte-rich supernatant was layered on top of histopaque 1077 (Sigma) and centrifuged. Neutrophils, collected in the pellet fraction, were resuspended in 0.2% hypotonic buffer to remove contaminating erythrocytes. Neutrophils were than washed three times in HBSS and resuspended in RPMI+FCS 2%.
In vitro killing assay
Luciferase labeled cells (5,000/well) were plated on a 96-well in OptiMEM 0.5% FBS (mouse neutrophils) or RPMI 2% FBS (human neutrophils). 4 hours later, purified neutrophils (100,000/well for mouse neutrophils and 5000 for human neutrophils) were added to the plated tumor cells and co-cultured overnight. Chemokines or inhibitors (Apocynin (100μM), Catalase (1,000u/ml), Superoxide Dismutase (1,000u/ml), Taurine (50mM) and PMA (50ng/ml) all from Sigma) were added to the culture immediately after neutrophils were added. Following overnight incubation, luciferase activity was measured using the Clarity (Bio-Tek) microplate luminescence reader. In vitro killing experiments were repeated at least 3 times.
Neutrophil transfer
Female nude mice were injected with 5×104 luciferase labeled 4T1 cells to the tail vein. 4 hours later, the mice were injected with 5×106 TENs or G-CSF stimulated neutrophils into the tail vein. Control mice were injected with vehicle. Formation of lung metastases was monitored using the IVIS-200 optical in vivo imaging system. Neutrophil transfer experiments had at least 5 mice per group and were repeated 3 times with similar results.
Chemokine administration
rhG-CSF (Neupogen, Amgen) was administered daily (250μg/kg/day) s.c. for 4 consecutive days. In vitro neutrophil stimulation; naïve mouse were co-cultured with tumor cells in the presence of mCCL2, mCCL5 (R&D), rhG-CSF (20ng/ml) or with TGF-β (200 pMolar, R&D). Human neutrophils purified from healthy volunteers were stimulated in vitro with hCCL2, hCCL3, hCCL4, hCCL5, CXCL1, CXCL12 and CXCL16 (R&D at 100ng/ml) or with rhG-CSF (20ng/ml).
Statistical analysis
For statistical analysis, the data is presented as mean ± SEM and were analyses using Student's t tests. Differences were considered significant when p < 0.05.
Supplementary Material
Highlights
Tumor entrained neutrophils (TENs) accumulate in the pre-metastatic lung
TENs provide anti-metastatic protection by eliminating disseminated tumor cells
Tumor-secreted factors are both required and sufficient for neutrophil entrainment
Tumor-secreted TGF-β protects the primary tumor from neutrophil cytotoxicity
Significance.
Metastasis represents the final stage in cancer progression and is the primary cause for cancer related mortality. Disseminated cells that are capable of colonizing distant organs and forming metastases therefore present an important therapeutic challenge. Our data shows that during the pre-metastatic phase in multiple murine breast cancer models, neutrophils stimulated by the primary tumor accumulate in the lung. These neutrophils acquire a cytotoxic phenotype and provide anti-metastatic protection by eliminating disseminated tumor cells. Although the neutrophils are eventually outcompeted by continued influx of metastatic cells, infusion of exogenous neutrophils effectively blocks metastasis and therefore represents a potential therapeutic strategy for management of micro-metastatic disease.
Acknowledgements
RB is supported by grants from the NIH and the Breast Cancer Research Foundation. We thank Dr. Edi Brogi, Dr. Juan-Manuel Schvartzman, Sho Fujisawa, Oren Litvin and Yvette Chin for technical assistance, Dr. Eric Pamer and Dr. Lindy Barrett (MSKCC, New York) for critical reading of this manuscript and Isaac Kaplan for his assistance with image analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession Numbers The accession number in the Gene Expression Omnibus public database for expression array experiments is GSE30888.
References
- Colombo MP, Lombardi L, Stoppacciaro A, Melani C, Parenza M, Bottazzi B, Parmiani G. Granulocyte colony-stimulating factor (G-CSF) gene transduction in murine adenocarcinoma drives neutrophil-mediated tumor inhibition in vivo. Neutrophils discriminate between G-CSF-producing and G-CSF-nonproducing tumor cells. J Immunol. 1992;149:113–119. [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- Dallegri F, Frumento G, Patrone F. Mechanisms of tumour cell destruction by PMA-activated human neutrophils. Immunology. 1983;48:273–279. [PMC free article] [PubMed] [Google Scholar]
- De Larco JE, Wuertz BR, Furcht LT. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res. 2004;10:4895–4900. doi: 10.1158/1078-0432.CCR-03-0760. [DOI] [PubMed] [Google Scholar]
- Dehqanzada ZA, Storrer CE, Hueman MT, Foley RJ, Harris KA, Jama YH, Kao TC, Shriver CD, Ponniah S, Peoples GE. Correlations between serum monocyte chemotactic protein-1 levels, clinical prognostic factors, and HER-2/neu vaccine-related immunity in breast cancer patients. Clin Cancer Res. 2006;12:478–486. doi: 10.1158/1078-0432.CCR-05-1425. [DOI] [PubMed] [Google Scholar]
- DuPre SA, Hunter KW., Jr. Murine mammary carcinoma 4T1 induces a leukemoid reaction with splenomegaly: association with tumor-derived growth factors. Exp Mol Pathol. 2007;82:12–24. doi: 10.1016/j.yexmp.2006.06.007. [DOI] [PubMed] [Google Scholar]
- DuPre SA, Redelman D, Hunter KW., Jr. The mouse mammary carcinoma 4T1: characterization of the cellular landscape of primary tumours and metastatic tumour foci. Int J Exp Pathol. 2007;88:351–360. doi: 10.1111/j.1365-2613.2007.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlender ZG, Kapoor V, Buchlis G, Cheng G, Sun J, Wang LC, Singhal S, Snyder LA, Albelda SM. Monocyte chemoattractant protein-1 blockade inhibits lung cancer tumor growth by altering macrophage phenotype and activating CD8+ cells. Am J Respir Cell Mol Biol. 2011;44:230–237. doi: 10.1165/rcmb.2010-0080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Hickey MJ, Kubes P. Intravascular immunity: the host-pathogen encounter in blood vessels. Nat Rev Immunol. 2009;9:364–375. doi: 10.1038/nri2532. [DOI] [PubMed] [Google Scholar]
- Hicks AM, Riedlinger G, Willingham MC, Alexander-Miller MA, Von Kap-Herr C, Pettenati MJ, Sanders AM, Weir HM, Du W, Kim J, et al. Transferable anticancer innate immunity in spontaneous regression/complete resistance mice. Proc Natl Acad Sci U S A. 2006;103:7753–7758. doi: 10.1073/pnas.0602382103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–1375. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowanetz M, Wu X, Lee J, Tan M, Hagenbeek T, Qu X, Yu L, Ross J, Korsisaari N, Cao T, et al. Inaugural Article: Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1015855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusmartsev S, Gabrilovich DI. Role of immature myeloid cells in mechanisms of immune evasion in cancer. Cancer Immunol Immunother. 2006;55:237–245. doi: 10.1007/s00262-005-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loberg RD, Ying C, Craig M, Day LL, Sargent E, Neeley C, Wojno K, Snyder LA, Yan L, Pienta KJ. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res. 2007;67:9417–9424. doi: 10.1158/0008-5472.CAN-07-1286. [DOI] [PubMed] [Google Scholar]
- Lu X, Kang Y. Chemokine (C-C motif) ligand 2 engages CCR2+ stromal cells of monocytic origin to promote breast cancer metastasis to lung and bone. J Biol Chem. 2009;284:29087–29096. doi: 10.1074/jbc.M109.035899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister SS, Weinberg RA. Tumor-host interactions: a far-reaching relationship. J Clin Oncol. 2010;28:4022–4028. doi: 10.1200/JCO.2010.28.4257. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The colony-stimulating factors and cancer. Nat Rev Cancer. 2010;10:425–434. doi: 10.1038/nrc2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirisola V, Zuccarino A, Bachmeier BE, Sormani MP, Falter J, Nerlich A, Pfeffer U. CXCL12/SDF1 expression by breast cancers is an independent prognostic marker of disease-free and overall survival. Eur J Cancer. 2009;45:2579–2587. doi: 10.1016/j.ejca.2009.06.026. [DOI] [PubMed] [Google Scholar]
- Molineux G, Pojda Z, Dexter TM. A comparison of hematopoiesis in normal and splenectomized mice treated with granulocyte colony-stimulating factor. Blood. 1990;75:563–569. [PubMed] [Google Scholar]
- Monti P, Leone BE, Marchesi F, Balzano G, Zerbi A, Scaltrini F, Pasquali C, Calori G, Pessi F, Sperti C, et al. The CC chemokine MCP-1/CCL2 in pancreatic cancer progression: regulation of expression and potential mechanisms of antimalignant activity. Cancer Res. 2003;63:7451–7461. [PubMed] [Google Scholar]
- Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande K, Ueda R, Machemer T, Sathe M, Tsai V, Brin E, Delano MJ, Van Rooijen N, McClanahan TK, Talmadge JE, et al. Cancer-induced expansion and activation of CD11b+ Gr-1+ cells predispose mice to adenoviral-triggered anaphylactoid-type reactions. Mol Ther. 2009;17:508–515. doi: 10.1038/mt.2008.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekarek LA, Starr BA, Toledano AY, Schreiber H. Inhibition of tumor growth by elimination of granulocytes. J Exp Med. 1995;181:435–440. doi: 10.1084/jem.181.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their microenvironment. Trends Genet. 2009;25:30–38. doi: 10.1016/j.tig.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011 doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CA, Khandoga A, Anders HJ, Schlondorff D, Luckow B, Krombach F. Chemokine receptors Ccr1, Ccr2, and Ccr5 mediate neutrophil migration to postischemic tissue. J Leukoc Biol. 2006;79:114–122. doi: 10.1189/jlb.0605337. [DOI] [PubMed] [Google Scholar]
- Shojaei F, Singh M, Thompson JD, Ferrara N. Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proc Natl Acad Sci U S A. 2008;105:2640–2645. doi: 10.1073/pnas.0712185105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267:271–285. doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Tonouchi H, Miki C, Tanaka K, Kusunoki M. Profile of monocyte chemoattractant protein-1 circulating levels in gastric cancer patients. Scand J Gastroenterol. 2002;37:830–833. [PubMed] [Google Scholar]
- van Kempen LC, Coussens LM. MMP9 potentiates pulmonary metastasis formation. Cancer Cell. 2002;2:251–252. doi: 10.1016/s1535-6108(02)00157-5. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Miki C, Okugawa Y, Toiyama Y, Inoue Y, Kusunoki M. Decreased expression of monocyte chemoattractant protein-1 predicts poor prognosis following curative resection of colorectal cancer. Dis Colon Rectum. 2008;51:1800–1805. doi: 10.1007/s10350-008-9380-7. [DOI] [PubMed] [Google Scholar]
- Williams SA, Harata-Lee Y, Comerford I, Anderson RL, Smyth MJ, McColl SR. Multiple functions of CXCL12 in a syngeneic model of breast cancer. Mol Cancer. 2010;9:250. doi: 10.1186/1476-4598-9-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HH, Pickup M, Pang Y, Gorska AE, Li Z, Chytil A, Geng Y, Gray JW, Moses HL, Yang L. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 2010;70:6139–6149. doi: 10.1158/0008-5472.CAN-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Berndt BE, Chen JJ, Kao JY. Expression of a soluble TGF-beta receptor by tumor cells enhances dendritic cell/tumor fusion vaccine efficacy. J Immunol. 2008;181:3690–3697. doi: 10.4049/jimmunol.181.5.3690. [DOI] [PubMed] [Google Scholar]
- Zhu B, Bando Y, Xiao S, Yang K, Anderson AC, Kuchroo VK, Khoury SJ. CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J Immunol. 2007;179:5228–5237. doi: 10.4049/jimmunol.179.8.5228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.