Abstract
Objectives:
Beside having roles in energy homeostasis and endocrine modulation, adipose tissue (AT) is now considered a promising source of mesenchymal stromal cells (adipose-derived stromal cells or ASCs) for regenerative medicine. Despite numerous studies on cultured ASCs, native human ASCs are rarely investigated. Indeed, the phenotype of ASCs in their native state, their localization within AT and comparison with bone marrow-derived mesenchymal stromal cells (BM-MSCs) has been poorly investigated.
Design:
To address these issues, the stroma vascular fraction (SVF) of human AT was extracted and native cell subtypes were isolated by immunoselection to study their clonogenic potential in culture. Immunohistology on samples of human AT in combination with reconstruction of confocal sections were performed in order to localize ASCs.
Results:
Compared with BM-MNCs, all native ASCs were found in the CD34+ cell fraction of the AT-SVF. Native ASCs expressed classical mesenchymal markers described for BM-MSCs. Interestingly, CD34 expression decreased during ASC cell culture and was negatively correlated with cell proliferation rate. Immunohistological analysis revealed that native ASCs exhibited specific morphological features with protrusions. They were found scattered in AT stroma and did not express in vivo pericytic markers such as NG2, CD140b or alpha-smooth muscle actin, which appeared during the culture process. Finally, ASCs spontaneous commitment to adipocytic lineage was enhanced in AT from obese humans.
Conclusions:
The use of complementary methodological approaches to study native human ASCs revealed their immunophenotype, their specific morphology, their location within AT and their stemness. Furthermore, our data strongly suggest that human ASCs participate in adipogenesis during AT development.
Keywords: mesenchymal stromal cells, human, CD34, pericytes endothelial cells, confocal imaging
Introduction
Besides being involved in energy homeostasis, adipose tissue (AT) is an endocrine organ capable of secreting a large variety of regulatory proteins, including cytokines, chemokines and hormone-like factors such as leptin, adiponectin and resistin, referred to as adipokines.1, 2 Such proteins participate in many other functions such as inflammation, immunity and reproduction.3 Excessive AT development is thought to be the result of both adipocyte hypertrophy and apparent hyperplasia.4, 5, 6, 7, 8 As adipocytes are terminally differentiated cells, and, as such, are considered incapable of division,9, 10 the apparent increase in adipocyte number is thought to originate from adipogenesis (the proliferation/differentiation of adipocyte progenitor cells named preadipocytes). Thus, the expansion of adipose mass requires the presence of adipocyte precursor cells located in the stroma vascular fraction (SVF) of AT and the presence of which could also contribute to AT normal cell turnover during adulthood as recently suggested.11
The search for the origin of adipocyte progenitors revealed that AT hosts a population of multipotent progenitors called adipose-derived stromal cells (ASCs).12, 13 These progenitors can give rise to osteoblasts, chondrocytes and adipocytes.12 They also participate in and/or support angiogenesis or vascular repair in ischemic limbs14, 15, 16 and have immunomodulatory properties both in vitro and in vivo.17, 18 A number of studies of the characterization of ASCs have revealed disparate and inconsistent parameters. Recurrent questions concern the nature of native ASCs, their tissue location, and their comparison with bone marrow-derived mesenchymal stromal cells (BM-MSCs). Reports, including our own, strongly suggest that native ASCs belong to an AT-SVF-derived CD34+ cell population and are negative for haematopoietic (CD14, CD45) and endothelial markers (CD31).14, 16, 19, 20 However, other groups propose the existence of two progenitor cell populations within AT: ASCs derived from CD34+ cells and AT-MSCs derived from CD34− cells.18 Nevertheless, all reports agree with the classical BM-MSC surface immunophenotype of cultured ASCs: CD73+, CD90+, CD105+, CD45− and CD14−.21, 22, 23, 24, 25 Another debated question concerns the origin of native ASCs. Indeed, although Crisan et al.26 recently proposed that MSCs originate from a pericyte population lacking CD34 expression, Traktuev et al.27 suggested a CD34+ pericyte origin for ASCs. In this context, this study aimed to clarify and better define the surface immunophenotype of both native and cultured human ASCs as compared with BM-MSCs, to evaluate in situ their morphology and to define their tissue localization within intact human AT samples. We show herein that native human ASCs (i) are CD34 positive (the expression of which is dramatically decreased in vitro when cells proliferate) (ii) display both stromal and perivascular positions but (iii) do not express in situ pericyte markers such as NG2 and CD140b, (iv) and exhibit in situ a peculiar cell morphology with long protrusions. Furthermore we propose that ASCs support AT growth, as their number is diminished and the expression of adipogenic markers increase as obesity and adipocyte hyperplasia progress.
Materials and methods
Adipose tissue sampling and SVF isolation
Human subcutaneous AT samples were obtained from Caucasian women undergoing plastic surgery for lean (body mass index (BMI)<25 kg m−2) and obese class I (BMI 30–34.9 kg m−2) donors and undergoing bariatric surgery for classes II–III (BMI⩾35 kg m−2) obese subjects. The donors had a stable weight for at least 3 months before fat collection and their mean age was 43.7±1.8 years, 44.4±1.7 years and 43.4±1.6 years for lean, class I and class II–III patients, respectively (some clinical characteristics of some class II–III obese donors are presented in Supplementary data 1). All research subjects provided their informed written consent before their surgery allowing the use of their tissue specimens for research purposes. The protocol followed the Declaration of Helsinki guidelines and was approved by the local ethics committee. The SVF and mature adipocytes were isolated from subcutaneous AT by collagenase digestion as previously described.17, 28 As AT samples were derived from various surgical interventions (liposuction or dermolipectomy), ASC yield was compared depending on the surgical procedure. No statistically significant differences were found (1.3 × 10e5±7.1 × 10e4 vs 1.2 × 10e5±7.3 × 10e4 ASCs per g of AT for liposuction and dermolipectomy, respectively).
Adipocyte diameter determination
Once isolated, mature adipocytes were suspended in ECBM (Endothelial Cell Basal Medium, containing no growth factors)/0.5% BSA (Bovine Serum Albumin) (1V/10 V) and 5 μl of cell suspension were placed onto plastic slides. Three distinct calibrated fields were taken to measure manually adipocyte diameters with NIS software (Nikon, Champigny-sur-Marne, France). Fat cell-like shapes were labelled with the nucleus dye DAPI (4′,6-diamidino-2-phenylindole). Among them 94±1% were nucleated and identified as adipocytes (85±7% and 99±1%, for small (<60 μm) and large (>100 μm) adipocytes, respectively).
Adipose tissue CD34+ cell sorting and culture
Human AT-derived CD34+ and CD34+/CD31− cells were isolated from SVF using CD34 nanoparticles with MACS Cell Separation Technology (Myltenyi Biotec SAS, Paris, France) (CD34+ cell fraction purity 81.5±2.5% and <0.5% for positive and negative fractions, respectively) or StemCell Technologies SARL (Grenoble, France) (CD34+ cell fraction purity 92.9±0.8%) according to the manufacturer's instructions and as previously described.16 Crude SVF cells or magnetically sorted CD34+ cells were initially plated at a density of 4000 cells cm−2 in α-MEM medium supplemented with 2% or 10% fetal calf serum (FCS). After 24 h at 37 °C and 5% CO2, non-adherent cells were removed by phosphate-buffered saline (PBS) washing. The adherent fraction was then cultured in vitro in the same culture medium, with medium changed three times a week. After 8 days, ASC at passage 0 were harvested with trypsin EDTA (Invitrogen SARL, Cergy Pontoise, France). The number of viable cells was determined using trypan blue exclusion. To study the effect of cell passaging on immunophenotype, cells were then plated at a density of 2000 cells cm−2 and cultured for 6 additional days (passage 1). For kinetic cell growth and CD34 expression analysis, cells were plated at a density of 4000 cells cm−2 in α-MEM medium supplemented with 2 or 10% FCS, and harvested daily over an 8-day period. Cells were then counted and immunophenotyped by flow cytometry.
Isolation and culture of human bone marrow-mononuclear cells (BM-MNCs)
As, adhesion to plastic is the way to select MSCs from BM-MNCs, BM of healthy donors was obtained from filters used during the preparation of allogeneic haematopoietic grafts. They gave their written informed consent. The protocol followed the Declaration of Helsinki guidelines and was approved by the local ethics committee. BM-MNCs were obtained by density gradient (d=1.077 g cm−3) centrifugation of total BM cells. BM-MNCs were plated at 100 000–150 000 cells cm−2 in the same medium as AT CD34+ cells (α-MEM supplemented with 10% FCS). After 2 days, non-adherent cells were harvested, and adherent cells were detached by use of trypsin/EDTA in order to study their immunophenotype after culture and compare it with the one of AT-SVF.
Clonogenic assay
CD34+ and CD34− cells from AT were plated at low density (16 cells cm−2). After 2 weeks, cells were rinsed with PBS, fixed in methanol and stained with 6% Giemsa. Colonies containing more than 50 colony-forming unit fibroblasts (CFU-Fs) were counted. CFU-F frequency was calculated by dividing the colony number by the number of seeded cells. Two-day adherent and non-adherent BM-MNCs were seeded at 1000 and 40 000 cells cm−2, respectively, for 11 days. Cells were rinsed with PBS, fixed in methanol and stained with 6% Giemsa. Colonies containing more than 50 CFU-Fs were counted. CFU-F frequency was calculated by dividing the colony number by the number of seeded cells.
Adipogenic differentiation
CD34+ and CD34− cells from AT were induced to undergo adipogenic differentiation with α-MEM medium supplemented with 10% FCS, 1 μmol l−1 dexamethasone, 60 μmol l−1 indomethacine (adipogenic medium) and 450 μmol l−1 isobutylmethylxanthine for 3 days. After 3 days, medium was replaced by adipogenic medium. After 20 days, cultures were rinsed with PBS, fixed with 10% formaldehyde; lipid droplets were stained with Oil-red O solution.
Osteogenic differentiation
CD34+ and CD34− cells from AT were plated at 4000 cells cm−2 in osteogenic induction medium (α-MEM supplemented with 10% FCS, 0.1 μmol l−1 dexamethasone, 50 μmol l−1 ascorbic acid and 3 mmol l−1 sodium phosphate monobasic) for 2 weeks. Sodium phosphate monobasic was then replaced with β-glycerophosphate (10 mmol l−1), and cells were cultured for 1 week. Cultures were rinsed with PBS, fixed with 10% formaldehyde, and stained with alizarin red for visualizing matrix mineralization.
Flow cytometry
At least 1.105 cells (AT-CD34+, ASCs or BM-MNCs) in PBS/0.5% BSA and sodium azide (1/1000 w/v) were stained with various combinations of saturating amounts of monoclonal antibodies conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE) or PE-cyanin 5 (PC5) (Supplementary data 2) and their respective isotype controls (4 °C, 30 min, dark). The labelled cells were analyzed by multiparameter flow cytometry (EPICS XL flow cytometer, Beckman-Coulter, or FACScanto cytometer, BD Biosciences, Le Pont de Claix, France) and FlowJo (Ashland, OR, USA) (Tree Star) or FCS express software (DeNovo Software, Los Angeles, CA, USA).
Immunohistochemistry and immunocytochemistry and confocal microscopy
Intact human subcutaneous AT pieces (2–3 mm3) were fixed in neutral buffered 4% (w/v) paraformaldehyde (1 h, room temperature (RT)), blocked in PBS/3% BSA (30 min, RT), and incubated in PBS/0.1% BSA/0.2% triton/0.05% tween (IF buffer) with primary antibodies overnight at 4 °C (Supplementary data 3). After washing steps in IF buffer, AT samples were incubated for 1 h with secondary antibodies or with an antibody against lectin (Supplementary data 3). Nuclei were stained with Hoescht 33258 or TO-PRO-3 (Invitrogen SARL) (for confocal microscopy). Fluorescence analysis was performed utilizing a Nikon (Paris, France), inverted Eclipse TE300 microscope (20 × and 40 × objectives) or a LSM510 Confocal Laser Scanning microscope equipped with an Axiovert 200 M inverted microscope (Carl Zeiss, Jena, Germany). For confocal microscopy analysis, AT samples were visualized with an 63X objective lens (Plan-Apochromat, N.A. 1,4, Oil) and excited using three laser lines (488, 543 and 633 nm), and the emission was collected through appropriate narrow band-pass filters. Image stacks (25-μm thickness) were collected at 0.46-μm intervals along the optical axis. Each image was produced from an average of 60 Z-overlapping stacks using Zeiss LSM software (Carl Zeiss S.A.S., Le Pecq, France), and was processed to produce a surface-rendered three-dimensional model.
Human native CD34+ cells from AT were cytocentrifuged, placed onto glass slides, fixed in cold acetone (−20 °C, 20 min) and washed with Tris-buffered saline (TBS) for immunocytochemistry. Antigen demasking was realized in citrate buffer (30 min, 97 °C). Human CD34+ cells from AT were cultured in LabTek (BD Biosciences) chamber slides for 1 week and fixed in 4% paraformaldehyde (1 h, RT). After washing steps (TBS), permeabilization (TBS/0.1% Triton) and blocking, cells were incubated with anti-αSMA or anti-NG2 primary antibodies (Supplementary data 3) (1 h or overnight, respectively). Cells were incubated with corresponding secondary antibody (30 min, RT) after washing. Nuclei were stained with DAPI (4′,6-diamino-2-phenylindole dihydrochloride).
RNA isolation and real-time PCR
Total RNA was extracted from AT CD34+/CD31− cells by use of the Rneasy kit (QIAGEN S.A, Courtaboeuf, France), and RNA concentrations were determined by fluorimetric assay (Ribogreen, Invitrogen). RNA (0.5 μg) was reverse-transcribed (RT) by use of Superscript II (Invitrogen) (Random Hexamers and dNTPs were from Invitrogen). Room temperature was also carried out without the superscript enzyme on RNA samples. Primers for lipoprotein lipase (LPL), Glycerol 3-phosphate dehydrogenase (GPDH), Nanog, Oct-4 and hTERT were provided by Applied Biosystems (Assays on demand, Courtaboeuf, France). Runx2 and osteocalcin primers were designed by use of Primer Express v2.0 (Applied Biosystems) (Supplementary data 4). The amplification reaction was carried out with 15 ng complementary DNA samples in 96-well plates (Applied Biosystems) in a GeneAmp 7500 sequence detection system. The PCR mixture contained 5 μl of Taqman primers (5X prediluted in water) or 300 nmol l−1 for Runx2 or Osteocalcin and 10 μl of 2 × Taqman PCR master Mix or 10 μl of 2 × SyberGreen PCR Master Mix (Applied Biosystems). All reactions were performed at 50 °C (2 min), 95 °C (10 min), then 40 cycles of 95 °C (15 s) and 60 °C (1 min). The results were analyzed by use of GeneAmp 7500, and all values were normalized to that of 18S rRNA.
Statistical analysis
Statistical analysis involved use of GraphPad (San Diego, CA, USA) and Statistica (Statsoft, Tulsa, OK, USA). Values are mean±standard error of the mean of (n) separate experiments. Comparisons among different groups were analyzed by ANOVA, then post-hoc Dunnet multiple comparison tests or Mann–Whitney test for two groups. Differences were considered statistically significant at P<0.05.
Results
Phenotypic changes of cells from human adipose tissue SVF and BM-MNCs during the adhesion phase of primary culture
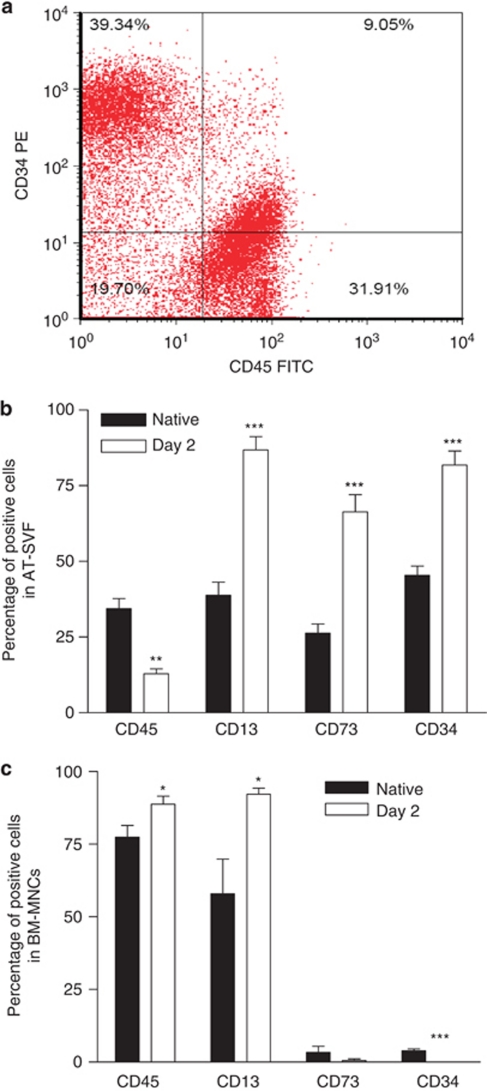
Flow cytometry analysis of freshly harvested human AT SVF stained with anti-CD45 and anti-CD34 antibodies confirmed that native human SVF is a heterogeneous fraction made up of several cell populations, as was expected and in accordance with previous work, including our own10, 13, 16 (Figure 1a). The cell populations in SVF included about 30% leukocytes, characterized as CD45+ cells, and a cell population defined as CD34+/CD45−, which represented about 40% of the cells from the SVF. Because adhesion to plastic is the most frequent way to select both ASCs and BM-MSCs, we used flow cytometry to investigate the evolution of CD34 and CD45 marker expression, together with that of standard MSC markers (CD73, CD13) in human SVF cells or BM-MNCs before and 2 days after adhesion to plastic. For the SVF, the adhesion phase was associated with an increased proportion of cells expressing CD13 and CD73 classic markers to identify BM-MSCs and a decreased proportion of cells positive for the leukocyte marker CD45 (Figure 1b), with a concomitant increase in proportion of cells expressing CD34 (80%). Freshly harvested BM-MNCs contained less than 5% of CD34+ cells, and in contrast to cells from the SVF, the adherent cell fraction bearing the main CFU-F activity (Supplementary data 5) showed no CD34+ cells 2 days after adhesion to plastic (Figure 1c).
Figure 1.
Phenotypic characterization by flow cytometry of native and short-term cultured AT-SVF and BM-MNCs. (a) Representative dot blot of flow-cytometry analysis of freshly harvested AT-SVF stained with CD45 Fluorescein IsoThioCyanate (FITC) and CD34 Phycoerythrin (PE) antibodies. (b and c) Immunophenotype of AT-SVF cells (b) and BM-MNCs (c) in their native state (black bars) or after 2 days of culture (white bars) using CD45 (FITC), CD13 (FITC), CD73 (PE) and CD34 Phycoerythrin-Cyanin 5.1 (PC5) cell surface markers. Results are percentage of positive cells and are expressed as means±s.e.m. for 27–30 separate experiments. *P<0.05, **P<0.01, ***P<0.001.
CD34 expression is downregulated during cell culture and is negatively correlated with cell expansion
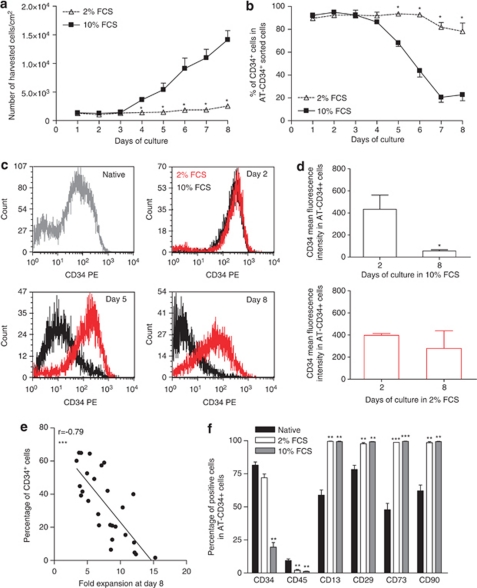
Because of conflicting results for CD34 expression in native ASCs, we hypothesized that changes in CD34 expression could be related to cell culture. Freshly harvested human AT-CD34+ cells were cultured in 2 or 10% FCS for 8 days, and CD34 expression was analyzed using flow cytometry. In parallel, the expression of standard MSCs and haematopoietic markers was studied before and after 8 days of culture. As expected, cell yield was greater than fivefold higher in 10 than 2% FCS medium (Figure 2a). Whatever the medium used (2 or 10% FCS) and in agreement with our previous observation, the proportion of CD34+ cells increased during the first 3 days of culture (Figure 2b). Then, as cell number began to increase, the proportion of CD34+ cells began to decrease in 10% FCS medium. In contrast, the proportion of CD34+ cells remained relatively stable when cell proliferation was low, that is, in 2% FCS medium (about 70% of the cells grown in 2% FCS medium expressed CD34 after 8 days of culture) (Figure 2b). Additionally, CD34 mean fluorescence intensity expressed by AT CD34+ cells was calculated during the culture of AT CD34+ cells in 2 or 10% FCS. As presented in Figures 2c and d, together with the decrease in the percentage of CD34+ cells there was a dramatic decrease in CD34 fluorescence intensity when AT-CD34+ cells were cultured in 10% FCS. Moreover, a negative correlation between cell expansion and expression of CD34 marker was found (Figure 2e). Furthermore, in freshly harvested SVF cells, the relative amount of CD34 mRNA normalized to that of ubiquitin was 103.2±8.3 (arbitrary units), whereas cell expansion led to a 80-fold decrease in CD34 mRNA level (1.3±1.0 arbitrary units) (data not shown). Collectively, these data suggest that both the quantity of CD34+ cells and the expression of CD34 protein are diminished when AT-CD34+ cells are expanded in culture. To note and as expected the proportion of AT CD34+ cells expressing classic BM-MSC markers such as CD13, CD29, CD73 and CD90 was increased after 8 days of culture whatever the medium used (Figure 2f).
Figure 2.
Phenotype of native and cultured AT-derived CD34+ cells. AT-derived CD34+ cells were plated in α-MEM supplemented with 2% or 10% FCS and harvested every day for 8 days. (a) The number of harvested cells was counted everyday. Data are mean±s.e.m. of harvested cells per cm2 from four to six independent experiments. *P<0.05, 2 versus 10% FCS. Native immunoselected CD34+ cells following 8 days of culture with either 2 or 10% SVF were analysed by flow cytometry for the expression of CD34. The percentage of adherent cells expressing CD34 antigen is presented in panel b. Data are expressed as mean±s.e.m. of four to six independant experiments. *P<0.05, 2 versus 10% FCS. (c) Representative histograms showing the mean fluorescence intensity for CD34 marker by native immunoselected CD34+ cells after 2, 5 or 8 days of culture with 2% (red line) or 10% FCS (black line). (d) Quantitative analysis of CD34 mean fluorescence intensity after 2, 5 or 8 days of culture with 2% (white histograms) or 10% FCS (black histograms). Data are presented as mean mean±s.e.m. of four independant experiments. *P<0.05, day 0 versus day 8. (e) The proportion of CD34+ cells was evaluated by flow cytometry, and number of harvested cells per cm2 was counted. Results are expressed as percentage CD34+ cells after 1 week of culture versus the fold expansion at day 8 (number of harvested cells divided by the number of seeded cells at day 0). (f) Isolated AT-CD34+ cells were analysed by flow cytometry with CD34 (PC5), CD45 (FITC), CD13 (PE), CD29 (FITC), CD73 (PE) and CD90 (FITC) antibodies, before (black bars) and after culture (8 days) in α-MEM supplemented with 2% (white bars) or 10% FCS (grey bars). Data are mean±s.e.m. percentage of positive-stained cells from 3–16 separate experiments. **P<0.01 and ***P<0.001, native versus 8-day-cultured cells.
Native human adipose tissue-derived CD34+ cells contain CFU-F activity, adipogenic and osteogenic potentials
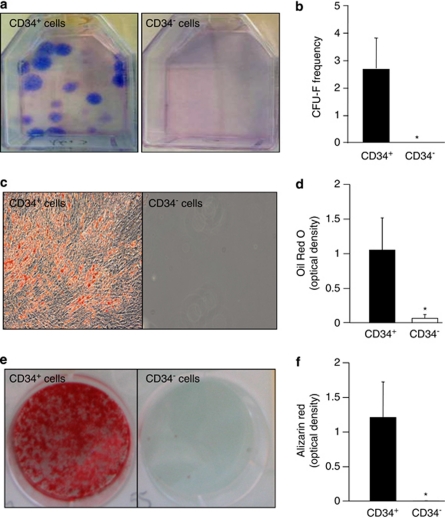
CFU-F activity was assessed in freshly harvested CD34+ and CD34− cell populations from AT. CFU-F activity was restricted to the CD34+ cell population (Figures 3a and b). After inducing adipogenic differentiation in confluent cells, we detected lipid accumulation by Oil-red O staining and triglyceride content (data not shown) in the CD34+ cell fraction (Figures 3c and d). At 3 weeks after the induction of osteogenic differentiation, matrix mineralization was observed in the CD34+ cell fraction (Figures 3e and f). Under the same conditions, the CD34− cell fraction from AT revealed neither osteogenesis nor adipogenesis (Figures 3c–f).
Figure 3.
CFU-F activity and adipogenic and osteogenic differentiation of AT-derived CD34+ and CD34− cells. CFU-F) assay of AT-CD34+ and -CD34− cells. (a) Images of a representative experiment and (b) percentage of CFU-Fs in the CD34+ and CD34− fractions of the SVF (n=6, *P<0.001). AT-derived CD34+ and CD34− cells were cultured under adipogenic conditions for 3 weeks; Oil-red O staining for lipid accumulation (c) and quantification (d) (n=6, *P<0.001). AT-derived CD34+ and CD34− cells were cultured under osteogenic conditions for 3 weeks; alizarin-red staining for matrix mineralization (e) and quantification (f) (n=6, *P<0.001).
Phenotypic characterization of native and cultured ASCs
According to the results shown above, we assumed that native ASCs were in the CD34+CD45− cell fraction of the AT derived-SVF. We studied the expression of various membrane markers in the freshly harvested cell population before culture and after 8 days of culture. Cell culture led to a clear phenotype homogenization (Table 1 and Supplementary data 6 and 7), with no marked phenotype evolution between the first and second cell passage (data not shown). More than 95% of cultured ASCs expressed a large variety of MSCs markers (CD9, CD10, CD13, CD49e, CD73, CD90 and CD166) but no haematopoietic markers (CD3, CD11c, CD14, CD15, CD16, CD18 and CD41) (Table 1).
Table 1. Phenotypic characterization of native and cultured ASCs.
| Native ASCs | Cultured ASCs | |
|---|---|---|
| Mesenchymal markers | ||
| CD9 | 51±0.2 | 97.4±1.5 |
| CD10 | 63.6±3.4 | 98.9±0.3 |
| CD13 | 81.9±3.4 | 99.7±0.04 |
| CD49e | 80.3±3.7 | 100±0.05 |
| CD55 | 42.1±6.9 | 99.7±0.06 |
| CD73 | 70±4.01 | 98.4±0.4 |
| CD90 | 73.7±4.9 | 99.6±0.2 |
| CD166 | 0.2±0.05 | 97.3±0.7 |
| Haematopoietic markers | ||
| CD3 | 0.2±0.2 | 0.3±0.1 |
| CD11c | 0±0 | 0.4±0.06 |
| CD14 | 0.2±0.2 | 0.6±0.1 |
| CD15 | 0.6±0.2 | 1.5±0.6 |
| CD16 | 0.4±0.2 | 0.1±0.1 |
| CD18 | 0.3±0.2 | 0.5±0.2 |
| CD41 | 0.9±0.3 | 1.7±0.8 |
Abbreviation: ASCs, adipose-derived stromal cells.
Data are expressed as mean percentage±s.e.m.
In situ localization of native ASCs
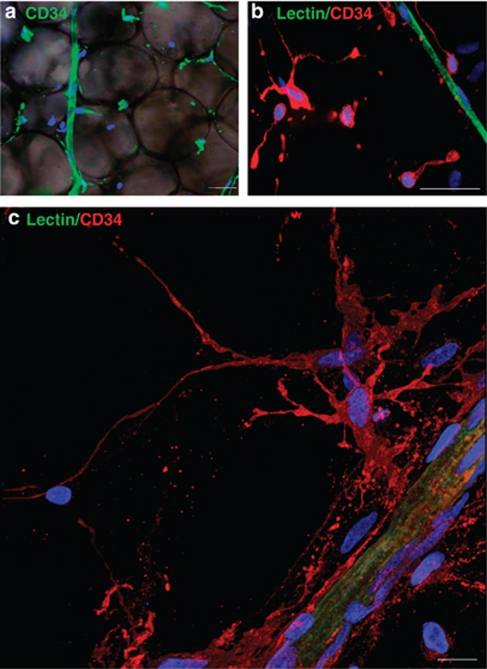
To examine the spatial organization of ASCs within human AT, we investigated the vascular network in intact human AT by anti-lectin or -CD31 antibody staining, which produced identical patterns (data not shown). Native ASCs were localized with anti-CD34 antibodies and were distinguished from leukocytes and endothelial cells with the use of anti-CD45 and anti-lectin antibodies, respectively. Adipocytes were detected by phase contrast imaging. We found human AT capillaries, characterized by aligned cells positive for lectin or CD34, distributed among adipocytes (Figure 4a). Interestingly, CD34 staining revealed CD34+ cells mainly located in AT stroma and sometimes near capillaries (Figure 4a). To discriminate ASCs from AT capillary endothelial cells, double staining with anti-lectin and -CD34 antibodies was performed (Figure 4b). A large proportion of ASCs were visualized as scattered throughout AT stroma, some were found close to capillaries and seldom adhering to AT capillaries (Figures 4a and b). To verify that ACS stromal location was indeed devoid of capillaries and because of AT intrinsic structure, that is the presence of large mature adipocytes sometimes reaching diameters of 100–150 μm, reconstruction of confocal sections on 25-μm thickness from human AT sample was performed. Human AT was labelled with anti lectin and CD34. The optical sectioning capability of confocal imaging confirmed that human native ASCs located in the stroma of AT were not close to capillaries. Moreover, in their native microenvironment human ASCs exhibited a branched morphology with several lateral protrusions, which could reach a length of 80 μm (Figure 4c and Supplementary data movie). Furthermore, some cell branches, already revealed in Figures 4a and b, could form a network completely surrounding mature adipocytes (Figure 4c and Supplementary data movie).
Figure 4.
In situ ASC localization. Human subcutaneous AT samples were stained with anti-CD34 (green), and adipocytes were visualized by phase contrast imaging (a) (20 × magnification). (b) Capillaries were stained with anti-lectin (green), and ASCs were localized with use of anti-CD34 antibody (red). Nuclei were stained with Hoescht 33258 (blue) (40 × magnification). Representative microscopy results from at least 10 independent experiments (bar, 50 μm) (c) Representative 3D-stack projection of human AT. Capillaries were stained with anti-lectin (green), and ASCs were localized with use of anti-CD34 antibody (red). Nuclei were stained with TO-PRO 3 Iodide (blue) (63 × magnification). Representative microscopy results from at least five independent experiments (bar, 10 μm).
In situ and in vitro analysis of pericyte marker expression in native ASCs
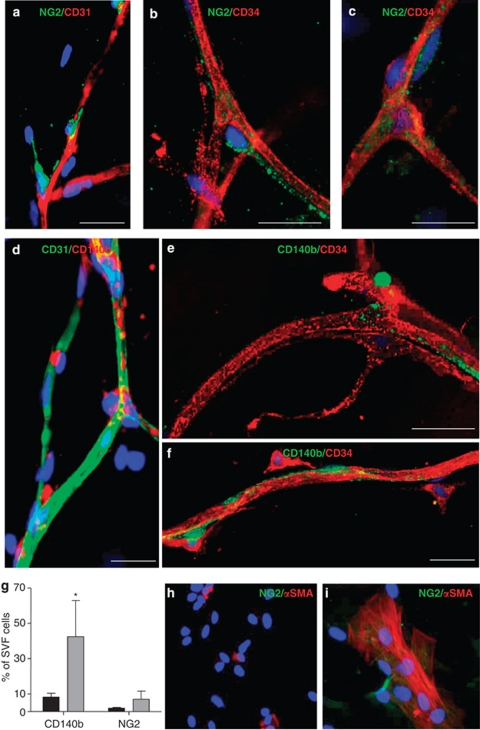
To better characterize native ASCs, we analyzed the expression of the pericyte markers NG2 and CD140b in intact human AT. Double staining of AT samples with anti-CD31 and -NG2 or -CD140b antibodies revealed positive staining for perivascular NG2 (Figure 5a) and CD140b (Figure 5d) on AT capillaries (CD31+ network). Further CD34 staining of NG2+ and CD140b+ cells revealed these cells in the perivascular location in intact human AT, but cells were not CD34+ (Figures 5b, c, e and f for NG2 and CD140b, respectively). Further flow cytometry analysis of NG2 and CD140b expression in cells freshly isolated from the SVF with anti-CD34, -CD31, -CD140b and -NG2 antibodies revealed few cells expressing pericyte markers: 1.8±0.4% and 12.7±3.5% of the total cells were positive for NG2 and CD140b, respectively, and < 2% of these were CD34+ (data not shown). Additionally, flow cytometry results of in vitro experiments to study the effect of culture on the expression of pericyte markers NG2, CD140b and α-SMA by CD34+ cells revealed the expression of NG2 and CD140b increased by the culture process (3.5- and 5-fold increase, respectively, Figure 5g). The increase in NG2 and α-SMA expression by ASCs during cell culture was confirmed using immunocytochemistry (Figures 5h and i).
Figure 5.
Pericyte marker expression in AT. Human subcutaneous AT samples were stained with anti-NG2 (green) and anti-CD31 (red) (a) or anti-CD34 (red) antibodies (b, c); with anti-CD31 (green) and -CD140b (red) antibodies (d); or anti-CD34 (red) and -CD140b (green) antibodies (e, f). Representative microscopy results from five independent experiments (bar, 25 μm). (g) Flow cytometry analysis of pericyte markers CD140b and NG2 expression in native (black bars) and cultured (grey bars) AT-SVF. Data are mean±s.e.m. percentage of positive cells from three separate experiments. *P<0.05 native vs cultured SVF cells. (h) CD34+ cells were centrifuged on cytospin slides before culture or after culture on Labtek chamber slides for 1 week (i). Slides were double-labelled with anti-NG2 (green) and α smooth muscle actin (red) antibodies. Nuclei were stained with DAPI (blue).
Native ASC microenvironment changes modify both the content of ASCs in subcutaneous AT and the expression of adipocyte lineage genes by native ASCs
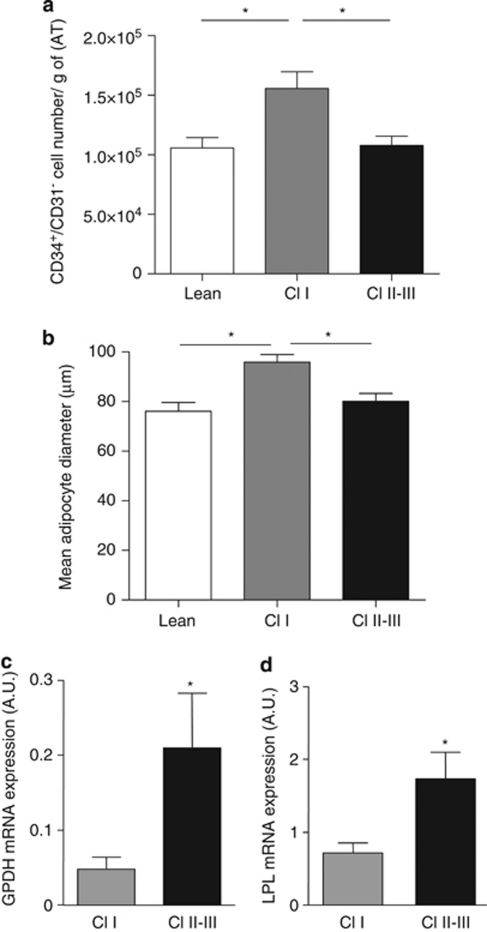
First, the gene expression of transcription factors characteristic of embryonic/pluripotent stem cells genes such as Nanog and Oct-4 were analyzed in human native ASCs and compared with human embryonic stem cells (ESCs). Native ASCs showed expression of Oct-4 and Nanog (30.5±19.2 and 2.2±1, respectively; 2.5- to 3-fold less expressed than in ESCs, P<0.05, n=3, data not shown) but not hTERT. Second, the impact of tissue microenvironment changes on the differentiation potentials of ASCs was studied. The number of ASCs by flow-cytometry analyses was examined and the expression of several genes involved in adipogenic and osteogenic programs in freshly harvested ASCs from donors exhibiting class I to class III obesity by real-time PCR analyses was investigated. Subcutaneous AT from class I obese individuals exhibited more ASCs than lean individuals (1.1 × 10e5±8.7 × 10e3 vs 1.6 × 10e5±1.4 × 10e4 ASCs per g of AT in lean vs class I obese donors, respectively, P<0.05, n=50 and 38, respectively) and class II–III obese individuals (1.5-fold decrease, n=59, P<0.05, Figure 6a). The lower number of ASCs in class II–III obese subjects was accompanied by a reduced mean diameter of subcutaneous mature adipocytes (Figure 6b), but with higher transcript levels of mRNA encoding for the adipocyte markers LPL and GPDH as compared with subjects with class I obesity (Figures 6c and d). To note, no expression of osteogenic genes such as Runx2 and osteocalcin were detected in native ASCs and no change in their expression was found whatever the degree of obesity (data not shown).
Figure 6.
Influence of obesity on ASC cell number, adipogenic and osteogenic gene expression. (a) Freshly harvested AT-SVF underwent two-colour flow cytometry with fluorescent-labelled antibodies against CD34 (PerCP) and CD31 (FITC). CD34+/CD31− cell number (normalized per gram of AT) was determined in lean (BMI<25 kg m−2, n=50) class I obese (BMI 30–34.9 kg m−2, n=38) or class II–III obese (BMI⩾35 kg m−2, n=59) donors. (b) Mature adipocytes were isolated for mean diameter determination in lean (n=10), class I obese (n=8) and class II–III obese (n=18) donors. (c) The mRNA expression of adipocyte markers GPDH and LPL (d) were determined in freshly harvested ACSs cells by real-time RT-PCR. The results were normalized to 18S rRNA levels and expressed as arbitrary units (AU). Data are mean±s.e.m. from 4–8 independent experiments, *P<0.05, class I versus classes II–III.
Discussion
In this study, we show that native ASCs are contained in the CD34+ cell population of AT and that the expression of CD34 marker is negatively correlated with cell expansion in vitro. In intact human AT, ASCs were found scattered in AT stroma, occasionally close to capillaries but interestingly without expressing pericytic markers in situ, as previously reported. Excessive AT development, as seen in humans with severe obesity (class II–III), was associated with a lower ASC number and smaller mean adipocyte diameter together with a marked increase in the expression of adipogenic molecular markers (LPL and GPDH) in native ASCs from subcutaneous AT suggesting activation of an adipogenic differentiation program presumably to support the adipocyte hyperplasia seen in severe forms of obesity.
CD34 belongs to the family of single-pass transmembrane sialomucin proteins that contains three members: CD34, podocalyxin and endoglycan.29 For 25 years, CD34 has been widely used to identify and isolate haematopoietic stem and progenitor cells.30 More recently, CD34 has been used to help identify other tissue-specific stem or progenitor cells such as satellite muscle cells and epidermal precursors.29 It is also widely used as a marker of vascular endothelial cells,31, 32, 33 endothelial progenitor cells,34 fibrocytes,35 human decidual stromal cells,36 satellite cells37 and keratocytes.38 Culture-expanded BM-MSCs are generally considered negative for CD34 marker, but the expression of CD34 in freshly harvested MSCs is still debated. Indeed, almost all studies have found MSCs in the BM-derived CD34− fraction, which led the International Society for Cell Therapy to formulate a position statement indicating that MSCs are CD34−.39 A limited number of studies have reported human CD34+ BM-derived cells that give rise to fibroblast-like stromal elements bearing osteogenic and chondrogenic differentiation potentials.40, 41, 42 In this paper we show that the adherent fraction of BM-MNCs did not contain CD34+ cells (although was highly enriched in CFU-F activity).
The expression of CD34 in human ASCs is still debated. In this study, we show that only CD34+ cells from human AT (and not CD34− cells) were able to show fibroblast-forming activity, as well as lipid-droplet formation or matrix mineralization under adipogenic or osteogenic culture conditions. Our data are in agreement with our previous results14, 16, 19 and those of other groups that studied these cells before culture.20, 24, 43, 44 Moreover, we showed that CD34 expression negatively correlated with ASCs expansion. In haematopoietic stem or progenitor cells, CD34 membrane expression is known to be downregulated as haematopoiesis progresses.30 Such downregulation seems to be a consistent feature of other stromal cells, such as stromal-derived keratocytes, initially isolated as CD34+ cells, which lose their CD34 expression during in vitro expansion.38 In this work we demonstrate that in their native state and in contrast to human BM-MSCs, human ASCs are contained in the CD34+ cell fraction. Whether the downregulation of CD34 expression is an in vitro consequence or the result of a more committed phenotype as compared with what occurs with the differentiation of haematopoietic stem cells remains undefined.
Conventional techniques used to visualize tissue structure generally involve excision and sectioning of the tissue; however, such techniques do not facilitate direct visualization of the complex cellular interactions taking place in 3-D. Hence, to better characterize native ASCs, we used immunohistochemistry of intact human AT analyzed by fluorescent microscopy and also confocal microscopy. This method preserves the 3-D structure and allowed us to finely investigate ASC distribution within the complex 3-D architecture of human AT (thickness of 25 μm). We found human ASCs to exhibit specific morphological features with very long protrusions allowing them to form networks surrounding mature adipocytes. Native human ASCs were found scattered within AT. Indeed, human ASCs were largely located in the stroma of human subcutaneous AT, although some were found near the capillary network. However, they rarely exhibited a pericytic position. Interestingly, similar results were found in mouse subcutaneous AT, with mouse ASCs found in the deep layers of AT as well as near microvessels.45 The findings are not consistent with earlier studies showing that MSCs from multiple organs, including AT, have a perivascular origin and exhibit pericyte features.26, 27 Traktuev et al.27 proposed that ASCs are pericytes because culture-expanded ASCs were positive for CD140b and NG2 pericyte markers, and CD140b+ and NG2+ cells were localized close to capillaries in human AT. However, the authors did not provide immunohistochemical evidence showing that perivascular CD34+ cells are CD140b+. In this study, we show that ASCs in their native microenvironment are negative for CD140b and NG2 pericyte markers and that cell culture induces the expression of both markers. Therefore, although some CD140b+ or NG2+ cells were found in the pericytic position in AT and some native ASCs could be found close to capillaries, in situ native ASCs were invariably negative for CD140b or NG2 markers. Our results agree with those from a recent study involving immunohistology of the AT endothelial network and showing that CD34 staining did not co-localize with pericytic markers in human AT.46, 47 To better define the stemness of ASCs, we investigated the mRNA expression of Oct4 and Nanog genes. Indeed, the molecular mechanisms governing ESC pluripotency have provided insights into the role of transcription factors such as Oct4 and Nanog in maintaining ESCs in the undifferentiated state. Remarkably, these pluripotent factors have been shown to participate in the reprogramming of differentiated cells back to the pluripotent state.48, 49 Oct4 and Nanog genes have been detected in various somatic tissue-derived cells, the most often-cited cell type being BM-MSCs50, 51, 52, 53 and culture-expanded ASCs. Although more poorly expressed in ASCs than in ESCs, Oct4 and Nanog genes were found in human native ASCs (a hallmark of pluripotency). However, we cannot rule out that Oct4 retropseudogenes, unable to compensate for loss of the original Oct4 gene, are actively transcribed in both normal and transformed cells and could contribute to false-positive signals.54, 55 Similar to Oct4 and Nanog, the catalytic subunit of human telomerase (hTERT) is described more as a stem cell marker for ESCs than for adult stem/progenitor cells. Telomerase is expressed in cultured ASCs.56, 57, 58, 59 We report for the first time that hTERT is not detected in human native ASCs.
Finally, we studied the effects of AT microenvironment changes on ASC adipogenic or osteogenic commitment. Excessive AT development, as found in humans with obesity, was associated with increased expression of adipogenic differentiation genes and no change in expression of osteogenic differentiation genes. The excessive development of AT results from both hypertrophy of pre-existing adipocytes and adipocyte hyperplasia due to the formation of new adipocytes.5, 10 Because adipocytes are terminally differentiated cells and therefore considered incapable of division.9, 10 The apparent increase in adipocyte number is thought to originate from adipogenesis and/or the proliferation or differentiation of adipocyte progenitor cells (pre-adipocytes). We have recently demonstrated a correlation between excessive AT development in humans and increased number of mature adipocytes, together with increased progenitor-cell proliferation.60 Our current results indicate that the number of native ASCs in subcutaneous AT was lower in subjects with class II–III obesity who also exhibited lower mean adipocyte diameter than class I obese donors. Our unpublished observations indicate no association between the number of ASCs and the duration of obesity. Furthermore, the mRNA levels of adipogenic markers LPL and GPDH were markedly higher in native ASCs from donors with class II or III obesity than in those with class I obesity. These findings suggest that ASCs may support the adipocyte hyperplasia that occurs in severe obesity as shown by the lower mean adipocyte diameter compared with class I obese patients.
In conclusion, our use of complementary methodological approaches to study native human ASCs from subcutaneous AT revealed data regarding their immunophenotype, their location within human AT, their 3-D morphology and their stemness. Furthermore, our data strongly suggest that human ASCs participate in adipogenesis, which occurs during excessive AT development. Further studies are needed to better understand how changes in AT microenvironment could participate in ASC commitment and therefore help elucidate the optimal conditions for developing viable and safe tools for tissue engineering.
Acknowledgments
We are grateful to Pauline Decaunes and David Estève (INSERM U858), Mireille André and Emmanuelle Arnaud (CNRS UMR 5241) for their invaluable technical assistance. We are grateful to Dr Dylan Thompson (University of Bath, UK) for fruitful discussions and critical reading of the manuscript. This work was supported by the Fondation pour la Recherche Médicale (DCV20070409252, programme Vieillissement Cardiovasculaire Normal et Pathologique), the Région Midi-Pyrénées (programme n°07006299), Communauté de Travail des Pyrénées, programme R9390173; Appel d'offre Coopération, Recherche – Développement (2007), the Prix de Recherche de l'Association Française d'étude et de recherches sur l'obésité (Aféro) and Roche (2007).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on International Journal of Obesity website (http://www.nature.com/ijo)
Supplementary Material
References
- Wang P, Mariman E, Renes J, Keijer J. The secretory function of adipocytes in the physiology of white adipose tissue. J Cell Physiol. 2008;216:3–13. doi: 10.1002/jcp.21386. [DOI] [PubMed] [Google Scholar]
- Halberg N, Wernstedt-Asterholm I, Scherer PE.The adipocyte as an endocrine cell Endocrinol Metab Clin North Am 200837753–768.x-xi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafontan M. Fat cells: afferent and efferent messages define new approaches to treat obesity. Annu Rev Pharmacol Toxicol. 2005;45:119–146. doi: 10.1146/annurev.pharmtox.45.120403.095843. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P, Gustafson A, Persson B. Adipose tissue fat cell size and number in relation to metabolism in endogenous hypertriglyceridemia. Acta Med Scand. 1971;190:363–367. doi: 10.1111/j.0954-6820.1971.tb07444.x. [DOI] [PubMed] [Google Scholar]
- Hirsch J, Batchelor B. Adipose tissue cellularity in human obesity. Clin Endocrinol Metab. 1976;5:299–311. doi: 10.1016/s0300-595x(76)80023-0. [DOI] [PubMed] [Google Scholar]
- van Harmelen V, Skurk T, Rohrig K, Lee YM, Halbleib M, Aprath-Husmann I, et al. Effect of BMI and age on adipose tissue cellularity and differentiation capacity in women. Int J Obes Relat Metab Disord. 2003;27:889–895. doi: 10.1038/sj.ijo.0802314. [DOI] [PubMed] [Google Scholar]
- Arner E, Westermark PO, Spalding KL, Britton T, Ryden M, Frisen J, et al. Adipocyte Turnover: Relevance to Human Adipose Tissue Morphology. Diabetes. 2010;59:105–109. doi: 10.2337/db09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo J, Gavrilova O, Pack S, Jou W, Mullen S, Sumner AE, et al. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput Biol. 2009;5:e1000324. doi: 10.1371/journal.pcbi.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterwith SC. Regulators of adipocyte precursor cells. Poult Sci. 1997;76:118–123. doi: 10.1093/ps/76.1.118. [DOI] [PubMed] [Google Scholar]
- Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obes Rev. 2001;2:239–254. doi: 10.1046/j.1467-789x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- Miranville A, Heeschen C, Sengenes C, Curat CA, Busse R, Bouloumie A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118–129. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- Yanez R, Lamana ML, Garcia-Castro J, Colmenero I, Ramirez M, Bueren JA. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24:2582–2591. doi: 10.1634/stemcells.2006-0228. [DOI] [PubMed] [Google Scholar]
- Sengenes C, Lolmede K, Zakaroff-Girard A, Busse R, Bouloumie A. Preadipocytes in the human subcutaneous adipose tissue display distinct features from the adult mesenchymal and hematopoietic stem cells. J Cell Physiol. 2005;205:114–122. doi: 10.1002/jcp.20381. [DOI] [PubMed] [Google Scholar]
- Boquest AC, Shahdadfar A, Fronsdal K, Sigurjonsson O, Tunheim SH, Collas P, et al. Isolation and transcription profiling of purified uncultured human stromal stem cells: alteration of gene expression after in vitro cell culture. Mol Biol Cell. 2005;16:1131–1141. doi: 10.1091/mbc.E04-10-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, et al. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Shigeura T, Matsumoto D, Sato T, Takaki Y, Aiba-Kojima E, et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;208:64–76. doi: 10.1002/jcp.20636. [DOI] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, et al. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- Sengenes C, Miranville A, Maumus M, de Barros S, Busse R, Bouloumie A. Chemotaxis and differentiation of human adipose tissue CD34+/CD31- progenitor cells: role of stromal derived factor-1 released by adipose tissue capillary endothelial cells. Stem Cells. 2007;25:2269–2276. doi: 10.1634/stemcells.2007-0180. [DOI] [PubMed] [Google Scholar]
- Nielsen JS, McNagny KM. Novel functions of the CD34 family. J Cell Sci. 2008;121 (Part 22:3683–3692. doi: 10.1242/jcs.037507. [DOI] [PubMed] [Google Scholar]
- Weissman IL, Shizuru JA. The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood. 2008;112:3543–3553. doi: 10.1182/blood-2008-08-078220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fina L, Molgaard HV, Robertson D, Bradley NJ, Monaghan P, Delia D, et al. Expression of the CD34 gene in vascular endothelial cells. Blood. 1990;75:2417–2426. [PubMed] [Google Scholar]
- Baumhueter S, Dybdal N, Kyle C, Lasky LA. Global vascular expression of murine CD34, a sialomucin-like endothelial ligand for L-selectin. Blood. 1994;84:2554–2565. [PubMed] [Google Scholar]
- Young PE, Baumhueter S, Lasky LA. The sialomucin CD34 is expressed on hematopoietic cells and blood vessels during murine development. Blood. 1995;85:96–105. [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Barth PJ, Westhoff CC. CD34+ fibrocytes: morphology, histogenesis and function. Curr Stem Cell Res Ther. 2007;2:221–227. doi: 10.2174/157488807781696249. [DOI] [PubMed] [Google Scholar]
- Garcia-Pacheco JM, Oliver C, Kimatrai M, Blanco FJ, Olivares EG. Human decidual stromal cells express CD34 and STRO-1 and are related to bone marrow stromal precursors. Mol Hum Reprod. 2001;7:1151–1157. doi: 10.1093/molehr/7.12.1151. [DOI] [PubMed] [Google Scholar]
- Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, et al. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- Espana EM, Kawakita T, Liu CY, Tseng SC. CD-34 expression by cultured human keratocytes is downregulated during myofibroblast differentiation induced by TGF-beta1. Invest Ophthalmol Vis Sci. 2004;45:2985–2991. doi: 10.1167/iovs.04-0201. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Simmons PJ, Torok-Storb B. CD34 expression by stromal precursors in normal human adult bone marrow. Blood. 1991;78:2848–2853. [PubMed] [Google Scholar]
- Chen JL, Hunt P, McElvain M, Black T, Kaufman S, Choi ES. Osteoblast precursor cells are found in CD34+ cells from human bone marrow. Stem Cells. 1997;15:368–377. doi: 10.1002/stem.150368. [DOI] [PubMed] [Google Scholar]
- Kaiser S, Hackanson B, Follo M, Mehlhorn A, Geiger K, Ihorst G, et al. BM cells giving rise to MSC in culture have a heterogeneous CD34 and CD45 phenotype. Cytotherapy. 2007;9:439–450. doi: 10.1080/14653240701358445. [DOI] [PubMed] [Google Scholar]
- D'Andrea F, De Francesco F, Ferraro GA, Desiderio V, Tirino V, De Rosa A, et al. Large-scale production of human adipose tissue from stem cells: a new tool for regenerative medicine and tissue banking. Tissue Eng Part C Methods. 2008;14:233–242. doi: 10.1089/ten.tec.2008.0108. [DOI] [PubMed] [Google Scholar]
- Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Akamatsu H, Hasegawa S, Yamada T, Nakata S, Ohkuma M, et al. Isolation of multipotent stem cells from mouse adipose tissue. J Dermatol Sci. 2007;48:43–52. doi: 10.1016/j.jdermsci.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Lin YF, Jing W, Wu L, Li XY, Wu Y, Liu L, et al. Identification of osteo-adipo progenitor cells in fat tissue. Cell Prolif. 2008;41:803–812. doi: 10.1111/j.1365-2184.2008.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CS, Xin ZC, Deng CH, Ning H, Lin G, Lue TF. Defining adipose tissue-derived stem cells in tissue and in culture. Histol Histopathol. 2010;25:807–815. doi: 10.14670/HH-25.807. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- D'Ippolito G, Diabira S, Howard GA, Menei P, Roos BA, Schiller PC. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004;117 (Part 14:2971–2981. doi: 10.1242/jcs.01103. [DOI] [PubMed] [Google Scholar]
- Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J, et al. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20:857–869. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- Lin G, Garcia M, Ning H, Banie L, Guo YL, Lue TF, et al. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008;17:1053–1063. doi: 10.1089/scd.2008.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke S, Enczmann J, Waclawczyk S, Wernet P, Kogler G. Oct4 and its pseudogenes confuse stem cell research. Cell Stem Cell. 2007;1:364–366. doi: 10.1016/j.stem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Lengner CJ, Welstead GG, Jaenisch R. The pluripotency regulator Oct4: a role in somatic stem cells. Cell Cycle. 2008;7:725–728. doi: 10.4161/cc.7.6.5573. [DOI] [PubMed] [Google Scholar]
- Kang SK, Putnam LA, Ylostalo J, Popescu IR, Dufour J, Belousov A, et al. Neurogenesis of Rhesus adipose stromal cells. J Cell Sci. 2004;117 (Part 18:4289–4299. doi: 10.1242/jcs.01264. [DOI] [PubMed] [Google Scholar]
- Jun ES, Lee TH, Cho HH, Suh SY, Jung JS. Expression of telomerase extends longevity and enhances differentiation in human adipose tissue-derived stromal cells. Cell Physiol Biochem. 2004;14:261–268. doi: 10.1159/000080335. [DOI] [PubMed] [Google Scholar]
- Rodriguez AM, Pisani D, Dechesne CA, Turc-Carel C, Kurzenne JY, Wdziekonski B, et al. Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J Exp Med. 2005;201:1397–1405. doi: 10.1084/jem.20042224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madonna R, Willerson JT, Geng YJ. Myocardin a enhances telomerase activities in tissue mesenchymal cells and embryonic stem cells undergoing cardiovascular myogenic differentiation. Stem Cells. 2008;26:202–211. doi: 10.1634/stemcells.2007-0490. [DOI] [PubMed] [Google Scholar]
- Maumus M, Sengenes C, Decaunes P, Zakaroff-Girard A, Bourlier V, Lafontan M, et al. Evidence of in situ proliferation of adult adipose tissue-derived progenitor cells: influence of fat mass microenvironment and growth. J Clin Endocrinol Metab. 2008;93:4098–4106. doi: 10.1210/jc.2008-0044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.