Abstract
Beside their haptic function, vibrissae of harbour seals (Phocidae) and California sea lions (Otariidae) both represent highly sensitive hydrodynamic receptor systems, although their vibrissal hair shafts differ considerably in structure. To quantify the sensory performance of both hair types, isolated single whiskers were used to measure vortex shedding frequencies produced in the wake of a cylinder immersed in a rotational flow tank. These measurements revealed that both whisker types were able to detect the vortex shedding frequency but differed considerably with respect to the signal-to-noise ratio (SNR). While the signal detected by sea lion whiskers was substantially corrupted by noise, harbour seal whiskers showed a higher SNR with largely reduced noise. However, further analysis revealed that in sea lion whiskers, each noise signal contained a dominant frequency suggested to function as a characteristic carrier signal. While in harbour seal whiskers the unique surface structure explains its high sensitivity, this more or less steady fundamental frequency might represent the mechanism underlying hydrodynamic reception in the fast swimming sea lion by being modulated in response to hydrodynamic stimuli impinging on the hair.
Keywords: hydrodynamic reception, whiskers, harbour seal, sea lion, underwater orientation, vortex-induced vibrations
1. Introduction
(a). Pinniped whiskers
Many pinniped species can be considered as opportunistic feeders, hunting on pelagic fishes as well as on benthic prey, for example flatfish or crustaceans. Similar to other predatory aquatic mammals such as toothed whales, seals and sea lions face the problem that despite their sophisticated visual system [1–3] they often cannot rely on vision for prey detection when foraging in dark and/or turbid waters [4]. While dolphins can cope with such conditions by the use of their active sonar system [5,6], this sensory ability has not been demonstrated in pinnipeds yet [7,8]. However, the extraordinarily well-developed vibrissae of pinnipeds have been considered as an alternative sensory system that may complement or even substitute vision during foraging trips [9].
Although the basic morphology of a vibrissal unit in pinnipeds resembles that of terrestrial mammals like the rat and the cat [10], they differ from those primarily with respect to the structure of the hair shaft, the more complex differentiation of the sinus system and the extraordinarily high degree of innervation (for detailed information, see [11]). Especially, the functional significance of the characteristic structure of vibrissal hair shafts in different pinniped species has largely been disregarded so far and thus will be addressed in the empirical part of this paper. While the vibrissal hair shaft in terrestrial mammals is round in cross section, those of all eared seals (sea lions and fur seals) are slightly flattened and appear oval in cross section (figure 1a, relation of the two ellipse axes 1 : 1.25). This flattening of the hair shaft is even more distinct in most, but not all phocid seals (also called ‘true seals’ or ‘earless seals’) like the harbour seal, whose vibrissae are almost disc-shaped in cross section (figure 1a). However, in contrast to the smooth outline of the vibrissal shaft in terrestrial mammals, all eared seals and some phocid seals (e.g. the bearded seal), the ratio of the major and minor axes changes periodically along the vibrissal hair shaft of harbour seals from about 1 : 2 to 1 : 4, thus giving the hair an undulated surface structure [9,11–14]. Vibrissae of walruses (Odobenidae) resemble those of eared seals, but are extraordinarily stout and usually short owing to active touch abrasion.
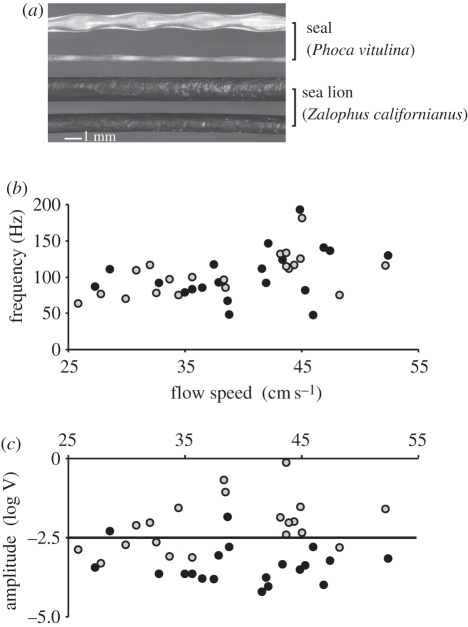
Figure 1.
(a) Photographic presentation of the structure of vibrissal hair shafts of the harbour seal (P. vitulina) and the California sea lion (Z. californianus). The vibrissae are presented in two views perpendicular to each other: top two, seal; bottom two, sea lion. The seal vibrissa is flattened and possesses an undulated shape. The sea lion vibrissa is oval and smooth in shape without undulation. (b) The basic frequency (in Hz) of whisker hairs in flow as a function of flow speed (in cm s–1). (c) The amplitudes of the dominant basic frequencies (in V, logarithmic scale) of the whiskers in flow as a function of flow speed (in cm s–1). Most signals of the seal whiskers possess amplitudes below 3 mV. For (b) and (c), black dots represent the data of the seal whiskers and grey dots the data of the sea lion whiskers. Each point reflects the mean value of 20 single measurements.
(b). Active touch in pinnipeds
In contrast to many terrestrial mammals [15,16], the pinniped species described so far possess only facial vibrissae, including some almost immobile supraorbital vibrissae above each eye and, in most phocid seals, two rhinal vibrissae vertically situated on the back of the muzzle. However, only the highly mobile mystacial vibrissae arranged in two pads on both sides of the snout have been studied so far with respect to active tactile sensing. Based on single-unit recordings from the infraorbital branch of the trigeminal nerve of harbour seals (Phoca vitulina) and grey seals (Halichoerus grypus), Dykes [17] suggested that the vibrissal system of these marine mammals should allow the haptic discrimination of for example surface structure as well as macrospatial tactile information derived from the shape and size of an object [17]. This hypothesis has been verified in the meantime for species of all three families of Pinnipedia. Fast and reliable haptic recognition of macrospatial information has been demonstrated for size and shape discrimination in the walrus [18], the California sea lion [19–21] and the harbour seal [22], whereas texture discrimination acuity has only been shown for harbour seals so far [23]. A characteristic of the haptic process in harbour seals and California sea lions is that they do not move their vibrissae during the exploration of an object, but protract the movable hairs as far forward as possible and keep them in this position [20–23]. Thus, these aquatic mammals do not show any whisking behaviour like that known from many rodent species [24–26]. However, as relative motion between the tactile organ and an object explored is essential for haptic perception [27], seals and sea lions achieve this by small-scale head movements while the vibrissae are in contact with the object. This might be different in the walrus that has been described as moving its whiskers in a forward and upward sweep when touching an object [18].
(c). Functional significance of hydrodynamic information for pinnipeds
While the efficient haptic function of the vibrissal system of pinnipeds may be important during benthic feeding, it cannot explain how the animals find pelagic prey when vision is impeded. As water disturbances caused, for example, by moving organisms provide a prevalent and reliable source of sensory information and many aquatic species developed hydrodynamic receptor systems [28], Dehnhardt et al. [29] hypothesized that seal whiskers' function is analogous to the fish lateral line. In this study, as well as in follow-up experiments [11], a harbour seal and a California sea lion were presented with dipole water movements generated by a constant-volume oscillating sphere (hydrodynamic dipole stimuli) positioned 5–50 cm in front of the vibrissae of the visually and acoustically masked experimental animal. Absolute detection thresholds below 1 µm water particle displacement determined for sphere vibrations around 50 Hz confirmed that the vibrissae in both species can be considered as hydrodynamic receptor systems comparable to those of other aquatic species (for comparison, see [28]). However, although hydrodynamic dipole stimuli are well suited for the characterization of a sensory system, they are far from being natural hydrodynamic information, and the seals' sensitivity to them cannot explain pelagic prey detection and capture. While a dipole water movement attenuates rapidly right after termination of sphere oscillations, the wake of a swimming fish represents a complex flow field [30–34] persisting over a considerable amount of time after the fish has disappeared. Particle image velocimetry (PIV) revealed that the flow field even behind a small swimming fish of about 10 cm body length can show a distinct vortex structure for at least 30 s and particle velocities above background noise for more than 5 min [33,34]. Thus, water disturbances in the wake of a swimming fish constitute a hydrodynamic trail that could be traceable by a piscivorous predator equipped with a corresponding hydrodynamic receptor system as long as the fish is continuously swimming and other hydrodynamic events do not disturb the trail. Accordingly, Dehnhardt et al. [35] conducted behavioural experiments requiring the blindfolded harbour seal Henry to search for and track the hydrodynamic trail of a miniature submarine. Although trails of the submarine lack the three-dimensional vortex structure of fish trails, the seal almost spontaneously followed even complex trails containing changes in the main course, thus demonstrating for the first time that hydrodynamic information can be used for long-distance object location. Introducing delays between the start of the submarine and the start of the seal's search, the animal was presented with ageing trails, this way simulating trails as long as 40 m. Even with these long trails, the seal's trail following was almost congruent to the course of the trail generator up to its final position. As during haptic object exploration, the seal protracted its vibrissae to the most forward position while searching for and following a hydrodynamic trail, indicating that this application of the hairs is a general feature for active tactile sensing in these aquatic mammals. In experiments with biogenic hydrodynamic trails generated by a second seal, the tracking behaviour of the trail-following seal corresponded to that described for trials with the miniature submarine, but the accuracy and success of trail following was even better [36]. However, the high accuracy and success rate of hydrodynamic trail following can be negatively affected by the trail generator performing a ‘burst-and-glide’ swimming style [37]. This behaviour has been described for many fish species [38,39] and, in addition to energy saving, might also serve as an anti-predation strategy.
Generally fish trails consist of vortices arranged in a highly complex three-dimensional structure. However, the specific pattern is strongly correlated with the body shape and the swimming style of the species [34,40–44]. As the spatial arrangement of mystacial vibrissae allows a seal to perform simultaneous multiple-point velocity measurements in the wake of a swimming fish from which this three-dimensional vorticity can be derived, a seal might be able to read qualitative information about the trail generator from a hydrodynamic trail. This has been confirmed in recent behavioural experiments partly performed under PIV control, demonstrating that a harbour seal can accurately recognize the direction of the hydrodynamic trail generated by a fin-like paddle [45]. PIV analysis revealed that the seal might have obtained directional information from the structure and spatial arrangement of the vortices in the trail and, additionally, from the high water velocities between two counter-rotating vortices, which were directed towards the moving direction of the paddle. Furthermore, the seal was able to discriminate objects differing in size or shape by their respective hydrodynamic signature [46]. Here, PIV analysis suggests that the seal used the spatial extension of the trail and the size of single vortices within the wake to discriminate object size, while shape discrimination could have been based on the spatial arrangement of the vortices.
Swimming speeds of the harbour seals in our trail-following experiments were typically 0.5–2 m s−1. Similarly the swimming speed of grey seals, estimated under different experimental foraging conditions, was about 2 m s−1 [47]. Considering that at this speed, the pliable vibrissae are protracted to the most forward position largely perpendicular to the swimming direction, it remained undefined how these animals are able to accurately analyse hydrodynamic events in front of them. Because of the high flow resistance resulting from their own locomotion, the vibrissal hair shaft was expected to be bent backwards and, additionally, to vibrate because of vortex shedding from the hair (vortex-induced vibrations, VIV) However, a recent study by Hanke et al. [14] demonstrated that the undulated surface structure and flattening of the hair shaft described for the vibrissae of harbour seals represent a hydrodynamic specialization that reduces hair bending and vibrations. Compared with a circular cylinder or an oval cross-section whisker of a sea lion, vibrissae of harbour seals are subject to significantly lower hydrodynamic forces and thus can glide almost quiescent through the water. Thus, it can be concluded that harbour seals keep their vibrissae as still as possible while searching for relevant hydrodynamic information.
This specialized hair type found in 15 out of 18 phocid seal species (but not in the phocid bearded seal and the Monk seals, as well as all otariids and walrusses) seems to be crucial for the perception of hydrodynamic information while swimming at high speed. However, the question remains whether those pinniped species whose vibrissal hair shafts are much less flattened and smooth in outline are capable of hydrodynamic trail following when the high flow resistance experienced under such conditions affect the whiskers. A corresponding experiment with a blindfolded California sea lion revealed that the animal is also capable of hydrodynamic trail following and that its accuracy is thoroughly comparable to that of a harbour seal [48]. However, in contrast to harbour seals, the performance of the sea lion was more affected by the ageing of the trail, indicating that its whiskers are less sensitive to decaying hydrodynamic information inherent in a trail.
Although two hydrodynamic sensory systems developed in the suborder Pinnipedia, comparative studies addressing the respective function of different hair types for hydrodynamic stimulus transduction are still missing. In the study at hand, we therefore compared the hydrodynamic properties of isolated whiskers of harbour seals and California sea lions in a rotational flow channel and describe their capability to decode a hydrodynamic event in terms of their signal-to-noise ratios (SNRs). The data obtained contribute to a better understanding of the respective sensory function of the two different whisker types.
2. Material and methods
(a). Whisker material
Measurements were performed on six single hairs, three harbour seal vibrissae (P. vitulina; labelled as PvV1, PvV2, PvV3) and three vibrissae of a California sea lion (Zalophus californianus; labelled as ZcV1, ZcV2, ZcV3). Vibrissae used in this study stem from animals that had died from natural causes about a year before the study was implemented. The whiskers of a juvenile harbour seal were provided by the Research Centre Büsum (University of Kiel, Germany) and the sea lion whiskers were obtained from the Natural History Museum Münster, Germany. For each species, vibrissae of different sizes were used, taken from comparable positions on the vibrissal pads (row D, columns 1, 2 and 3 in the harbour seal and columns 2, 4 and 6 in the sea lion). The sea lion whiskers measured 93.6 mm (ZcV1), 71.9 mm (ZcV2) and 45.1 mm (ZcV3) in length with a bending angle of 9–15°. The lengths of the seal's vibrissae were 85 mm (PvV1), 66.5 mm (PvV2) and 50.8 mm (PvV3), with bending angles ranging from 20° to 35°. All whiskers were kept under dry conditions and therefore immersed in water for 2 h before starting the measurement.
(b). Experimental set-up
The experimental set-up consisted of two basic components: a round fluid tank (diameter 124 cm) and a steel mounting rack to which the measuring devices were attached and which served to fixate the whiskers. To avoid heterodyning of the measuring signals with external vibrations (e.g. from the drive), both components were decoupled mechanically from each other using heavy weights and vibration dampers. The fluid tank was filled with water to a level of 20 cm and was centred with respect to the rack. A 300 W DC motor served to rotate the tank around its vertical axis, leading to a rotation of the fluid at a constant angular speed after an initial break-in phase. Flow velocity in the flume depended on the radial distance. Angular speed was surveyed throughout the experiments by an optical sensor at the tank's fringe. An adjustable holder was attached to the rack and placed above the flume to mount the vibrissae and to allow the immersion of the whisker into the water at variable distances from the axis of rotation. This way the whisker could be exposed to varying flow speeds without any further break-in intervals, as it would occur in a linear tank. Radial variation of flow velocity within the diameter of the whiskers as well as within the diameter of the disturbing cylinders was below 1 per cent.
The whisker was fixed in a hollow piezoceramic cylinder, which transformed mechanical vibrations into corresponding electric charge fluctuations. The transducer was coupled at the base of the hair shaft in order to incorporate all structural and material parameters of the whisker during the measurement. The transducer output was connected to a charge amplifier that generated a voltage proportional to the momenta present at the root of the hair shaft. In order to assess comparative measurements, the voltage signal was not necessarily calibrated to applied absolute forces. The vibration signal of each whisker was recorded by a data acquisition card and analysed with the data analysis software LabVIEW (both: National Instruments, Austin, TX, USA). All whiskers were submerged in the tank for about two-thirds of their total length. Disturbing effects caused by the air–water interface along the hairshaft would have appeared as additional noise contribution to the measured signals in both hair types and thus both whisker signals were equally biased.
During hydrodynamic as well as haptic reception, harbour seals and sea lions protract their whiskers and then usually keep the hairs in the most forward position. This way, most whiskers in the harbour seal face a flow field right in front of the animal with their narrow side, while in the sea lion, the wide side of the whiskers is directed towards the flow field. In our experimental set-up, the orientation of the single whiskers was in accordance with this in situ situation.
(c). Flow measurements
After passing a critical Reynolds number (Re), vortices detach, alternating from the right and the left side of a cylindrical object in the flowing fluid. This phenomenon is called a Kármán vortex street. The generation of artificial and reproducible hydrodynamic disturbances was achieved by immersing aluminium cylinders in the flow tank at a depth of 10 cm in front of the vibrissae at a distance of 5 cm. The cylinders were 100 cm in length and were either hollow cylinders with a cross section of 16 mm or solid cylinders with diameters of 8 and 4 mm. Within a Reynolds number range of 300–10 000, the Strouhal number is constant (St = 0.2). With the knowledge of the characteristic width (cylinder diameter) D and flow speed U∞, the frequency of vortex shedding from the cylinder fVS was calculated using the equation St = fVS×D/U∞. The Kármán vortex street behind a cylinder is suitable to serve as a model for alternating patterns of water movements as generated by fish, although it differs from the hydrodynamic trail of a swimming fish in that it is not three dimensional, and rotation direction is inverted. For additional measurements with sea lion whiskers, a flat, rectangular profile (40 mm wide, 2 mm thick and 50 mm long) was used to generate a hydrodynamic event causing the collapse of the vibrissal self-oscillation.
Each of the six vibrissae was used to detect the vortex-shedding frequency fVS of the Kármán vortex streets generated by the different cylinders. Every measurement was repeated at three different flow speeds within the maximum range of the flume from 15 to 55 cm s–1. Data were collected over a time interval of approximately 120 s. The first 60 s served to record the wanted signal including noise with the cylinder fixed upstream of the vibrissae. Then the cylinder was removed. Continuous monitoring of the vibrissal signal allowed us to wait until the residual wake of the cylinder was attenuated. After a delay of usually 10–20 s this way, only the noise was recorded for another 40–50 s.
A quantitative comparison of sensors, in our case the two different whisker types, can be achieved by calculating their SNRs. The SNR describes the quality of the transmission of a wanted signal that is overlaid by background noise. It can be defined as the ratio of the mean power of wanted and noise signals. The mean power of wanted and noise signals was determined by the root mean square (RMS) of the voltage output of the charge amplifier.
(d). Data analysis
For signal conditioning, a notch filter blocked the 50 Hz noise of the mains electricity in the whisker signal. Then the whisker signal passed through a high-pass filter (fifth-order Butterworth), which blocked additionally all frequencies below 70 Hz. Finally, the maximum frequency and its amplitude were extracted out of the filtered whisker signal by means of a fast Fourier transformation (FFT) algorithm (sampling rate: 10 kHz, sample length: 1024, frequency resolution less than 1 Hz, window: Hanning). This frequency is caused by VIVs, which were identified and characterized that way.
In parallel, but before high-pass filtering, the signal was fed into a low-pass filter (fifth-order Butterworth), which blocked all frequencies above an adjustable threshold (usually 20 Hz). Hence, this extracted signal reflects the comparatively low frequency of vortex shedding from the cylinder (fVS). Every frequency and amplitude values for further processing were obtained by automatic averaging over 20 samples of single values.
To determine the SNR, the signal was tapped before and after the low-pass filter. The effective amplitudes (RMS) of the signal parts in the time domain were used to calculate the SNR. The SNR can be calculated in two different ways. If the signal is large and the noise is comparatively small (S ≫ N), the wanted signal usually contains the noise, which can be neglected (S + N ≈ S, when S ≫ N). In this case, the noise value has to be measured with no signal present, which means without disturbing the cylinder in the flow tank. This procedure was used with seal whiskers. However, if the noise level is higher than the signal (S ≪ N), the signal has to be filtered out by a band-pass filter. Then, for the determination of the SNR, the noise can be provided by using the unfiltered signal, which also contains the wanted signal (N ≈ N + S, when N ≫ S). This procedure was used with sea lion whiskers.
3. Results and discussion
(a). Vortex-induced vibrations
Excluding any artificial flow perturbation by immersed cylinders, only forces induced by the laminar flow on the whisker occur, such as VIV. The measured amplitude of these vibrissal oscillations is proportional to the force acting on the hair and thus reflects the intensity of fluid–structure interaction. Spectral analysis via FFT showed the existence of a dominant frequency depending on whisker length and flow speed. The frequency varied between 47.6 and 192.6 Hz at a flow speed range from 17 to 52 cm s–1 as demonstrated in figure 1b. The amplitudes ranged from 0.06 to 13.98 mV for seal whiskers and from 0.02 to 723.20 mV for sea lion whiskers. There was no significant difference between the hairs of harbour seals and sea lions in frequency dependence as a function of flow speed. In both species, a positive trend for higher frequencies at higher flow speeds was observed. Owing to differences in whisker size, length and curvature, the pooled data show a large variation. For a detailed analysis of a relation between oscillation frequency and the length of a whisker or between frequency and flow velocity, more data have to be obtained. The dominant frequencies documented for sea lion vibrissae are more intense than those of the harbour seal. Whereas the majority of frequencies measured for harbour seal whiskers possess amplitudes below 3 mV, frequencies of sea lion whiskers reached values approximately 10 times higher (figure 1c). Thus, the forces acting at the base of a seal whisker were on average more than 10 times lower than those at the hair base of a sea lion whisker. Hence, flow-induced vibrissal oscillations occurred with both vibrissal types, but the intensity of the oscillation was about a magnitude smaller in seals compared with sea lions.
(b). Detection of hydrodynamic disturbances
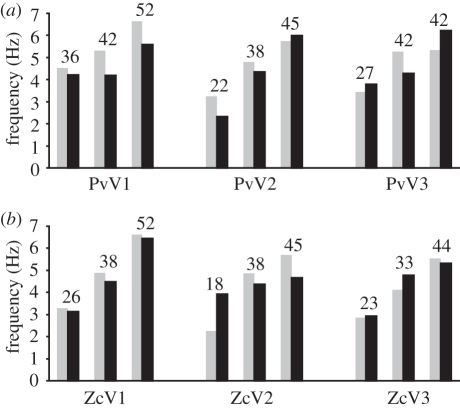
One aim of this study was to show the ability to detect hydrodynamic disturbances with isolated pinniped vibrissae and to characterize their sensory performance. By analysing the vibrissal signal, the measured frequency of vortex shedding (fVS) produced by the cylinders was extracted and could be compared with the calculated value. Corresponding pairs of calculated and measured frequencies for each whisker at different flow speeds using the perturbing cylinder with a diameter of 16 mm are illustrated in figure 2a,b. Over a flow speed range from 18 to 45 cm s–1, the deviations of calculated and measured frequencies were less than 30 per cent. The result of the sea lion's whisker ZcV2 at a flow speed of 18 cm s–1 was deviating owing to unknown reasons. Generally, fVS increases with higher flow speed. Tests with cylinders of 4 and 8 mm diameter revealed equivalent results. The mean amplitudes of fVS were 5.3 mV for seals and 7.7 mV for sea lions.
Figure 2.
Vortex shedding frequencies (fVS in Hz) of the Kármán vortex streets generated by a cylinder theoretically predicted for as well as measured by (a) seal whiskers (PvV1, PvV2, PvV3) and (b) sea lion whiskers (ZcV1, ZcV2, ZcV3). Black bars represent the frequencies determined from the vibrissal signal. Grey bars show the expected values calculated by means of flow velocity and cylinder diameter (16 mm). Above each value pair, the flow velocity (in cm s−1) at which the data were obtained and which was used for the calculation is indicated.
Using the hydrodynamic disturbances investigated in this study as a model for the vortex structure present in a fish trail, one can conclude that the whiskers of both species are capable of perceiving basic characteristics of a wake, such as frequency and amplitude. Because the generated perturbing signals were the same for both whisker types, the intensities of corresponding deflections of the whiskers were expected to be similar. However, the measured amplitudes for the sea lion vibrissae were slightly higher, which could be due to a higher stiffness of these vibrissae that would result in a stronger coupling of the forces exerted by water disturbances to the force sensor at the base of the vibrissae.
(c). Signal-to-noise ratio
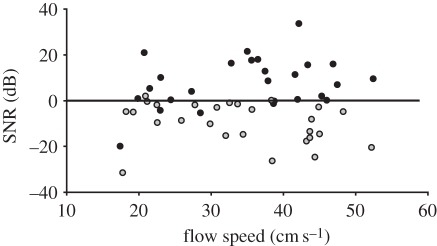
The sensory performance of a whisker as a hydrodynamic signal detector can be described by its SNR as displayed in figure 3 for the investigated whiskers. The performance of the harbour seal whiskers contrasts strongly with the performance of the sea lion whiskers. The SNR during the detection of a hydrodynamic disturbance for isolated seal vibrissae is much higher than for isolated sea lion vibrissae. In seals, the SNR values of the whiskers were mostly positive, adopting a mean value of +7 dB in comparison to the whiskers of the sea lions that possess an averaged SNR of −9 dB. These results hold regardless of flow speed or cylinder diameter.
Figure 3.
Signal-to-noise ratios (in dB) of the whiskers while detecting the vortex-shedding frequency fVS of the generated Kármán vortex street as a function of the flow speed (in cm s–1). Conventions as in figure 1.
Both hair types successfully detected the generated vortex-shedding frequency, but the vibrissae of seals showed a much better performance in terms of SNR. This can be explained by the 10 times lower VIVs in seal whiskers. These vibrations were part of the noise level. Since the generated hydrodynamic disturbances induced comparably intense measuring signals, higher noise lead to smaller SNR values in sea lion whiskers. Thus, at this point, it has to be preliminarily concluded that seal whiskers are the better system at least for the detection of Kármán vortex streets. In harbour seals, it was previously shown that the undulated surface structure of the whiskers efficiently suppresses the generation of VIVs at the whisker [14]. The present results indicate that the effective reduction of dynamic forces acting on the hair shaft leads to substantial noise reduction (cf. [49]). As a consequence, a seal whisker should be largely undisturbed even when the animal is swimming at high speed during prey pursuit. Accordingly, we could show that the signal at a seal whisker directly reflects an external hydrodynamic event without the need of extracting the noise produced by VIV.
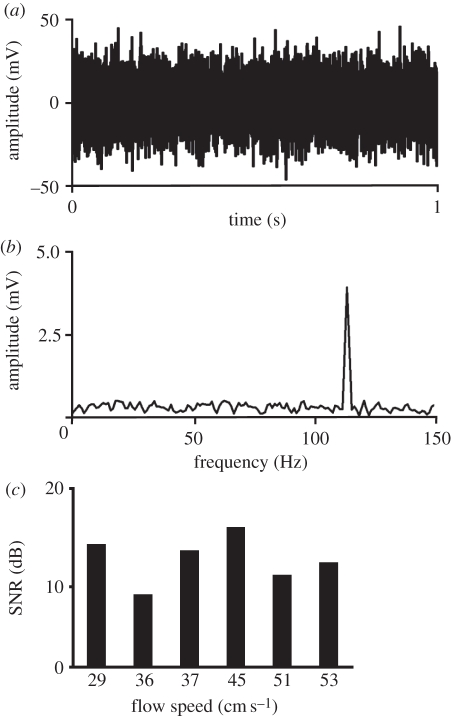
However, high noise signals of sea lion whiskers can be interpreted in a different way and thus may reveal the mechanism underlying hydrodynamic reception in the fast-swimming sea lions. Each noise signal contained a dominant frequency, which depended on flow velocity (figure 4a,b). We suggest that this frequency acts as a carrier signal and thus should not be interpreted as noise. Instead, this more or less steady fundamental frequency should be modulated by a hydrodynamic event impinging on the hair, for example a vortex, and this modulation would be the crucial information for the sea lion. To test this hypothesis, additional measurements were made with the sea lion whisker ZcV2. In order to suppress the fundamental frequency, artificial hydrodynamic events were not produced by cylinders, but by a flat perturbing body located at a distance of about 5 cm upstream of the vibrissa. This strong turbulence caused the carrier frequency to collapse. Without the characteristic carrier signal, the remaining measuring signal was defined as noise. After removing the perturbing body, fluid movement returned to laminar flow and the incipient carrier signal was exploited as the desired signal level. Hence, new SNRs resulted from these measurements, which were similar to those of seal vibrissae (figure 4c). At a mean value of +13 dB, they appear even better than in seals; however, the experimental conditions were not the same, and a complete breakdown of the carrier signal may rarely occur in nature.
Figure 4.
Additional measurements of a California sea lion whisker assuming a modulated carrier signal. All results were obtained from the sea lion whisker ZcV2. (a) Amplitude (in mV) of the signal including the carrier for one exemplary measurement at a flow speed of 53 cm s–1. (b) Frequency distribution obtained by spectral analysis of the measurement illustrated in (a) with a peak frequency at 114 Hz. (c) Signal-to-noise ratios (in dB) with the carrier frequency defined as the wanted signal.
Besides the perception of external fluid fluctuations, the existence of a flow-depending carrier frequency holds additional information. A calibration of this frequency as a function of flow speed should enable the animal to determine the velocity of its self-motion relative to ambient water. This might be useful for navigational tasks such as path integration. Corresponding measurements are currently being conducted in our laboratory.
4. Conclusions
This study indicates that the different types of vibrissae of harbour seals and California sea lions use different mechanisms for the detection of external hydrodynamic information. Seals maximally reduce the whisker's basic noise by means of an undulating surface structure of the hair that optimizes its SNR and thus enhances sensory performance [14,49]. In contrast, the sea lion's whiskers were shown to possess a basic carrier signal. By a modulation of this signal through external stimuli, they could gain information about the hydrodynamic event. Both mechanisms enable the respective animals to orient themselves in the marine environment, for example, when tracking fish trails while foraging [35,37,48]. Although, finally, the SNR values of seal and sea lion whiskers were similar, differences in temporal resolution might occur between species. In sea lions, each external event causes the destabilization of the carrier signal. The carrier frequency at the whisker has to recover before a new modulation can occur, that is, before a new hydrodynamic event can be perceived. This effect of the transient recovery time probably reduces the temporal resolution of the whisker while scanning for hydrodynamic information. A deficit in temporal resolution will also cause an impaired spatial perception of vortex patterns in the moving sea lion and thus might explain why the hydrodynamic trail following in this species is more affected by the ageing of trails [48]. However, as the sea lion in the study by Gläser et al. [48] still performed well during hydrodynamic trail following, further studies are needed on the information a sea lion can read from a trail, for example trail direction as well as the geometry of the trail generator, as has recently been shown for harbour seals [45,50].
(a). Biomimetics and future directions
The mechanism to suppress VIV by modifying the shape of a cylindrical structure may have enormous biomimetic potential (cf. [14] and references therein). Nature-inspired examples of flow-controlling or flow-influencing shapes were already deduced from humpback whale flippers [49,51,52]. Owing to their undulated leading edge, stall is delayed. Applications of biomimetic flow control in engineered propellers have been considered [49].
Besides the design of technical constructions with improved behaviour while exposed to fluid flow, sensory applications are a focus of further investigations of pinniped vibrissae. In particular, flow sensors in marine robotics could support already existing tools for orientation and navigation. The correlation of carrier frequency and flow speed offers a technical usage as a velocity sensor. However, in order to combine hydrodynamic and velocity perception, the modulation process in sea lion whiskers has to be analysed in more detail. Morphological and biomechanical parameters have to be studied in order to build artificial whiskers which technically satisfy the characteristic requirements of aquatic environments (cf. [53]). We suggest that in contrast to a single hair, a complete whisker pad providing a sensor array will be suitable for instantaneous volume measurement and gradient analysis.
Acknowledgements
The authors would like to express their gratitude towards Prof. Nogge (Cologne) and Prof. Rechenberg (Berlin) who made some of the experimental facilities available. A. Stein (Institute of Human Genetics, Bonn) photographed figure 1. This study was funded by grants from the VolkswagenStiftung to G.D., and the German Research Foundation (DFG, SPP1207) to G.D., A.L. and W.H.
References
- 1.Hanke F. D., Hanke W., Scholtyssek C., Dehnhardt G. 2009. Basic mechanisms in pinniped vision. Exp. Brain Res. 199, 299–311 10.1007/s00221-009-1793-6 (doi:10.1007/s00221-009-1793-6) [DOI] [PubMed] [Google Scholar]
- 2.Hanke F. D., Dehnhardt G. 2009. Aerial visual acuity in harbor seals (Phoca vitulina) as a function of luminance. J. Comp. Physiol. A 195, 643–650 10.1007/s00359-009-0439-2 (doi:10.1007/s00359-009-0439-2) [DOI] [PubMed] [Google Scholar]
- 3.Hanke F. D., Scholtyssek C., Hanke W., Dehnhardt G. 2011. Contrast sensitivity in a harbor seal (Phoca vitulina). J. Comp. Physiol. A 197, 203–210 10.1007/s00359-010-0600-y (doi:10.1007/s00359-010-0600-y) [DOI] [PubMed] [Google Scholar]
- 4.Weiffen M., Möller B., Mauck B., Dehnhardt G. 2006. Effect of water turbidity on the visual acuity of harbor seals (Phoca vitulina). Vis. Res. 46, 1777–1783 10.1016/j.visres.2005.08.015 (doi:10.1016/j.visres.2005.08.015) [DOI] [PubMed] [Google Scholar]
- 5.Au W. W. L., Ford J. K. B., Horne J. K., Allman K. A. N. 2004. Echolocation signals of free-ranging killer whales (Orcinus orca) and modeling of foraging for chinook salmon (Oncorhynchus tshawytscha). J. Acoust. Soc. Am. 115, 901–909 10.1121/1.1642628 (doi:10.1121/1.1642628) [DOI] [PubMed] [Google Scholar]
- 6.Nachtigall P. E., Supin A. Y. 2008. A false killer whale adjusts its hearing when it echolocates. J. Exp. Biol. 211, 1714–1718 10.1242/jeb.013862 (doi:10.1242/jeb.013862) [DOI] [PubMed] [Google Scholar]
- 7.Dehnhardt G. 2002. Sensory systems. In Marine mammal biology (ed. Hoelzel A. R.), pp. 116–141 Oxford, UK: Blackwell Publishing [Google Scholar]
- 8.Schusterman R. J., Kastak D., Levenson D. H., Kastak C. R., Southall B. L. 2004. Pinniped sensory systems and the echolocation issue. In Echolocation in bats and dolphins (eds Thomas J. A., Vater C. M. M.), pp. 531–535 Chicago, IL: University of Chicago Press [Google Scholar]
- 9.Hyvärinen H. 1989. Diving in darkness: whiskers are sense organs of the ringed seal (Phoca hispida saimensis). J. Zool. 218, 663–678 10.1111/j.1469-7998.1989.tb05008.x (doi:10.1111/j.1469-7998.1989.tb05008.x) [DOI] [Google Scholar]
- 10.Ebara S., Kumamoto K., Matsuura T., Mazurkiewicz J. E., Rice F. L. 2002. Similarities and differences in the innervation of mystacial vibrissal follicle–sinus complexes in the rat and cat: a confocal microscopic study. J. Comp. Neurol. 449, 103–119 10.1002/cne.10277 (doi:10.1002/cne.10277) [DOI] [PubMed] [Google Scholar]
- 11.Dehnhardt G., Mauck B. 2008. Mechanoreception in secondarily aquatic vertebrates. In Sensory evolution on the threshold: adaptations in secondarily aquatic vertebrates (eds Thewissen J. G. M., Nummela S.), pp. 295–314 Berkeley, CA: University of California Press [Google Scholar]
- 12.Watkins W. A., Wartzok D. 1985. Sensory biophysics of marine mammals. Mar. Mamm. Sci. 1, 219–260 10.1111/j.1748-7692.1985.tb00011.x (doi:10.1111/j.1748-7692.1985.tb00011.x) [DOI] [Google Scholar]
- 13.Ginter C. C., Fish F. E., Marshall C. D. Morphological analysis of the bumpy profile of phocid vibrissae. Mar. Mamm. Sci. 26, 733–743 10.1111/j.1748-7692.2009.00365.x (doi:10.1111/j.1748-7692.2009.00365.x) [DOI] [Google Scholar]
- 14.Hanke W., Witte M., Miersch L., Brede M., Oeffner J., Michael M., Hanke F., Leder A., Dehnhardt G. 2010. Harbor seal vibrissa morphology suppresses vortex-induced vibrations. J. Exp. Biol. 213, 2665–2672 10.1242/jeb.043216 (doi:10.1242/jeb.043216) [DOI] [PubMed] [Google Scholar]
- 15.Hyvärinen H., Kangasperko H., Peura R. 1977. Functional structure of the carpal and ventral vibrissae of the squirrel (Sciurus vulgaris). J. Zool. 182, 457–466 [Google Scholar]
- 16.Fundin B. T., Arvidsson J., Rice F. L. 1995. Innervation of nonmystacial vibrissae in the adult rat. J. Comp. Neurol. 357, 501–512 10.1002/cne.903570402 (doi:10.1002/cne.903570402) [DOI] [PubMed] [Google Scholar]
- 17.Dykes R. W. 1975. Afferent fibers from mystacial vibrissae of cats and seals. J. Neurophysiol. 38, 650–662 [DOI] [PubMed] [Google Scholar]
- 18.Kastelein R. A., van Gaalen M. A. 1988. The sensitivity of the vibrissae of a Pacific walrus (Odobenus rosmarus divergens). Aquat. Mamm. 14, 123–133 [Google Scholar]
- 19.Dehnhardt G. 1990. Preliminary results from psychophysical studies on the tactile sensitivity in marine mammals. In Sensory abilities of cetaceans (eds Thomas J. A., Kastelein R. A.), pp. 435–446 New York, NY: Plenum Press [Google Scholar]
- 20.Dehnhardt G. 1994. Tactile size discrimination by a California sea lion (Zalophus californianus) using its mystacial vibrissae. J. Comp. Physiol. A 175, 791–800 [DOI] [PubMed] [Google Scholar]
- 21.Dehnhardt G., Dücker G. 1996. Tactual discrimination of size and shape by a California sea lion (Zalophus californianus). Anim. Learn. Behav. 24, 366–374 10.3758/BF03199008 (doi:10.3758/BF03199008) [DOI] [Google Scholar]
- 22.Dehnhardt G., Kaminski A. 1995. Sensitivity of the mystacial vibrissae of harbour seals (Phoca vitulina) for size differences of actively touched objects. J. Exp. Biol. 198, 2317–2323 [DOI] [PubMed] [Google Scholar]
- 23.Dehnhardt G., Mauck B., Hyvärinen H. 1998. Ambient temperature does not affect the tactile sensitivity of mystacial vibrissae of harbour seals. J. Exp. Biol. 201, 3023–3029 [DOI] [PubMed] [Google Scholar]
- 24.Welker C. I. 1964. Analysis of sniffing in the albino rat. Behaviour 22, 223–244 10.1163/156853964X00030 (doi:10.1163/156853964X00030) [DOI] [Google Scholar]
- 25.Wineski L. E. 1985. Facial morphology and vibrissal movement in the golden hamster. J. Morphol. 183, 199–217 10.1002/jmor.1051830208 (doi:10.1002/jmor.1051830208) [DOI] [PubMed] [Google Scholar]
- 26.Carvell G. E., Simons D. J. 1990. Biometric analyses of vibrissal tactile discrimination in the rat. J. Neurosci. 10, 2638–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lederman S. J., Klatzky R. L. 1987. Hand movements—a window into haptic object recognition. Cogn. Psychol. 19, 342–368 10.1016/0010-0285(87)90008-9 (doi:10.1016/0010-0285(87)90008-9) [DOI] [PubMed] [Google Scholar]
- 28.Bleckmann H. 1994. Reception of hydrodynamic stimuli in aquatic and semiaquatic animals, 41st edn, pp. 1–115 Stuttgart, Germany: Gustav Fischer Verlag [Google Scholar]
- 29.Dehnhardt G., Mauck B., Bleckmann H. 1998. Seal whiskers detect water movements. Nature 394, 235–236 10.1038/28303 (doi:10.1038/28303) [DOI] [Google Scholar]
- 30.Blickhan R., Krick C. M., Zehren D., Nachtigall W. 1992. Generation of a vortex chain in the wake of a subundulatory swimmer. Naturwissenschaften 79, 220–221 10.1007/BF01227131 (doi:10.1007/BF01227131) [DOI] [Google Scholar]
- 31.Müller U. K., van den Heuvel B. L. E., Stamhuis E. J., Videler J. J. 1997. Fish foot prints: morphology and energetics of the wake of a continuously swimming mullet. J. Exp. Biol. 200, 2893–2906 [DOI] [PubMed] [Google Scholar]
- 32.Nauen J. C., Lauder G. V. 2002. Quantification of the wake of a rainbow trout using three-dimensional stereoscopic particle image velocimetry. J. Exp. Biol. 205, 3271–3279 [DOI] [PubMed] [Google Scholar]
- 33.Hanke W., Brücker C., Bleckmann H. 2000. The ageing of the low-frequency water disturbances caused by swimming goldfish and its possible relevance to prey detection. J. Exp. Biol. 203, 1193–1200 [DOI] [PubMed] [Google Scholar]
- 34.Hanke W., Bleckmann H. 2004. The hydrodynamic trails of Lepomis gibbosus (Centrarchidae), Colomesus psittacus (Tetraodontidae) and Thysochromis ansorgii (Cichlidae) measured with scanning particle image velocimetry. J. Exp. Biol. 207, 1585–1596 10.1242/jeb.00922 (doi:10.1242/jeb.00922) [DOI] [PubMed] [Google Scholar]
- 35.Dehnhardt G., Mauck B., Hanke W., Bleckmann H. 2001. Hydrodynamic trail following in harbor seals (Phoca vitulina). Science 293, 102–104 10.1126/science.1060514 (doi:10.1126/science.1060514) [DOI] [PubMed] [Google Scholar]
- 36.Schulte-Pelkum N., Wieskotten S., Hanke W., Dehnhardt G., Mauck B. 2007. Tracking of biogenic hydrodynamic trails in a harbor seal (Phoca vitulina). J. Exp. Biol. 210, 781–787 10.1242/jeb.02708 (doi:10.1242/jeb.02708) [DOI] [PubMed] [Google Scholar]
- 37.Wieskotten S., Dehnhardt G., Mauck B., Miersch L., Hanke W. 2010. The impact of glide phases on the trackability of hydrodynamic trails in harbour seals (Phoca vitulina). J. Exp. Biol. 213, 3734–3740 10.1242/jeb.047134 (doi:10.1242/jeb.047134) [DOI] [PubMed] [Google Scholar]
- 38.Blake R. W. 1983. Functional design and burst-and-coast swimming in fishes. Can. J. Zool. 61, 2491–2494 10.1139/z83-330 (doi:10.1139/z83-330) [DOI] [Google Scholar]
- 39.Videler J. J., Weihs D. 1982. Energetic advantages of burst-and-coast swimming of fish at high speeds. J. Exp. Biol. 97, 169–178 [DOI] [PubMed] [Google Scholar]
- 40.Drucker E. G., Lauder G. V. 2002. Experimental hydrodynamics of fish locomotion: functional insights from wake visualization. Integr. Comp. Biol. 42, 243–257 10.1093/icb/42.2.243 (doi:10.1093/icb/42.2.243) [DOI] [PubMed] [Google Scholar]
- 41.Nauen J. C., Lauder G. V. 2002. Hydrodynamics of caudal fin locomotion by chub mackerel, Scomber japonicus (Scombridae). J. Exp. Biol. 205, 1709–1724 [DOI] [PubMed] [Google Scholar]
- 42.Fish F. E., Lauder G. V. 2006. Passive and active flow control by swimming fishes and mammals. Annu. Rev. Fluid Mech. 38, 193–224 10.1146/annurev.fluid.38.050304.092201 (doi:10.1146/annurev.fluid.38.050304.092201) [DOI] [Google Scholar]
- 43.Standen E. M., Lauder G. V. 2007. Hydrodynamic function of dorsal and anal fins in brook trout (Salvelinus fontinalis). J. Exp. Biol. 210, 325–339 10.1242/jeb.02661 (doi:10.1242/jeb.02661) [DOI] [PubMed] [Google Scholar]
- 44.Tytell E. D., Standen E. M., Lauder G. V. 2008. Escaping Flatland: three-dimensional kinematics and hydrodynamics of median fins in fishes. J. Exp. Biol. 211, 187–195 10.1242/jeb.008128 (doi:10.1242/jeb.008128) [DOI] [PubMed] [Google Scholar]
- 45.Wieskotten S., Dehnhardt G., Mauck B., Miersch L., Hanke W. 2010. Hydrodynamic determination of the moving direction of an artificial fin by a harbour seal (Phoca vitulina). J. Exp. Biol. 213, 2194–2200 10.1242/jeb.041699 (doi:10.1242/jeb.041699) [DOI] [PubMed] [Google Scholar]
- 46.Wieskotten S., Mauck B., Miersch L., Dehnhardt G., Hanke W. 2011. Hydrodynamic discrimination of wakes caused by objects of different size or shape in a harbour seal (phoca vitulina). J. Exp. Biol. 214, 1922–1930 10.1242/jeb.053926 (doi:10.1242/jeb.053926) [DOI] [PubMed] [Google Scholar]
- 47.Gallon S. L., Sparling C. E., Georges J. Y., Fedak M. A., Biuw M., Thompson D. 2007. How fast does a seal swim? Variations in swimming behaviour under differing foraging conditions. J. Exp. Biol. 210, 3285–3294 10.1242/jeb.007542 (doi:10.1242/jeb.007542) [DOI] [PubMed] [Google Scholar]
- 48.Gläser N., Wieskotten S., Otter C., Dehnhardt G., Hanke W. 2011. Hydrodynamic trail following in a California sea lion (Zalophus californianus). J. Comp. Physiol. A 197, 141–151 10.1007/s00359-010-0594-5 (doi:10.1007/s00359-010-0594-5) [DOI] [PubMed] [Google Scholar]
- 49.Fish F. E., Howle L. E., Murray M. M. 2008. Hydrodynamic flow control in marine mammals. Integr. Comp. Biol. 48, 788–800 10.1093/icb/icn029 (doi:10.1093/icb/icn029) [DOI] [PubMed] [Google Scholar]
- 50.Wieskotten S., Mauck B., Miersch L., Dehnhardt G., Hanke W. 2011. Hydrodynamic discrimination of wakes caused by objects of different size or shape in a harbour seal (Phoca vitulina). J. Exp. Biol. 214, 1922–1930 10.1242/jeb.053926 (doi:10.1242/jeb.053926) [DOI] [PubMed] [Google Scholar]
- 51.Miklosovic D. S., Murray M. M., Howle L. E., Fish F. E. 2004. Leading-edge tubercles delay stall on humpback whale (Megaptera novaeangliae) flippers. Phys. Fluids 16, L39–L42 10.1063/1.1688341 (doi:10.1063/1.1688341) [DOI] [Google Scholar]
- 52.Fish F. E., Miklosovic D. S., Murray M. M., Howle L. E. 2003. Delayed stall due to leading edge tubercles of the humpback whale flipper. Integr. Comp. Biol. 43, 903 [Google Scholar]
- 53.Solomon J. H., Hartmann M. J. 2006. Robotic whiskers used to sense features. Nature 443, 525. 10.1038/443525a (doi:10.1038/443525a) [DOI] [PubMed] [Google Scholar]