Abstract
Septins are highly conserved cytoskeletal GTP-binding proteins implicated in numerous cellular processes from apoptosis to vesicle trafficking. Septins have been associated with leukemia and solid tumor malignancies, including breast, ovarian, and prostate. We previously reported that high SEPT9_i1 expression in human mammary epithelial cell lines (HMECs) led to malignant cellular phenotypes such as increased cell proliferation, invasiveness, motility, and genomic instability. Our goal here was to better understand how SEPT9_i1 expression might contribute to genomic instability and malignant progression. First, we confirmed that even transient expression of SEPT9_i1 was sufficient to increase aneuploidy in HMECs. We then analyzed SEPT9_i1 by immunoprecipitation and immunofluorescence studies and found that SEPT9_i1 interacts with both α and γ tubulin. SEPT9_i1 expressing cells demonstrated dramatic chromosome segregation defects, centrosome amplification and cytokinesis defects, suggesting two possible molecular mechanisms contributing to the development of genomic instability. This suggests that SEPT9_i1 may promote genomic instability through both cytokinesis and mitotic spindle defects which lead to chromosome missegregation.
Introduction
Since septins were discovered in budding yeast, they have been associated with cytoskeletal dynamics. Mammalian septins have been shown to play a role in mitosis and cytokinesis, through an interaction with microtubules (Sisson 2000). The human septin family is complex, with 14 known members that encode several alternative transcripts (Peterson and Petty, 2010). Of these, SEPT9 demonstrated cell-cycle dependent distribution and is implicated in microtubule-dependent functions through its interaction with α-tubulin (Surka et al., 2002; Nagata et al., 2003). In interphase cells, human septin SEPT9 (MSF) co-localized with actin, microtubules and other septins (Surka et al., 2002). One SEPT9 variant, SEPT9_i1 (previously called MSF-A and SEPT9_v1) specifically localized with microtubules. When SEPT9 or SEPT9_i1 was depleted by siRNA or microinjection of specific anti-MSF antibody, cytokinesis failure was observed resulting in the accumulation of binucleated cells (Surka et al., 2002; Gonzalez et al., 2007). Estey et al. also showed that knock down of SEPT9 caused cytokinesis defects, and introduction of SEPT9_i1 and SEPT9_i3 were able to rescue this phenotype (Estey et al., 2010). Nagata et al. showed localization of SEPT9_i1 to the mitotic spindle and midzone in human mammary epithelial cells (HMECs) and co-purified SEPT9_i1 interacted with α-tubulin and identified the GTP-binding domain of SEPT9 as critical for this interaction. In addition, Amir and Mabjeesh found that cancer cells with high SEPT9_i1 expression were resistant to microtubule-dependent HIF-1 inhibitors (2007). Together, these observations suggest that SEPT9_i1 may be important in mitosis via its interaction with microtubules and may have an important role in driving genomic instability.
Genomic instability is a characteristic feature of carcinogenesis, especially in human tumors of epithelial origin such as breast tumors. Genomic instability can be broadly classified into two categories: microsatellite instability (MIN) associated with mutations in DNA repair genes, and chromosome instability (CIN), which is recognized by numerical or structural chromosomal abnormalities (Charames and Bapat 2003). During normal mitosis, the parent somatic cell divides to generate two daughter cells with a diploid number of chromosomes. However, in many instances of carcinogenesis, abnormal chromosome segregation occurs during mitosis, which can lead to loss or gain of one or more chromosomes, a condition known as aneuploidy (Dey, 2004; Gisselsson et al., 2011; Thompson and Compton, 2010). These events have been associated with tumorigenesis by increasing the rate of chromosomal mutations, including deletions and amplifications of critical genes in cell proliferation and/or survival (Dey, 2004; Thompson and Compton, 2010; Gisselsson et al., 2011,).
Many mechanisms can result in aneuploidy, including mitotic spindle defects, defective attachment of chromatids and cytokinesis failure. Mitotic spindle defects, including unipolar or multipolar spindles, changes in microtubule stability and lagging chromosomes movement, can result in chromosome missegregation (Dey, 2004; Gisselsson et al., 2011; Thompson and Compton, 2010). Proper chromatid attachment is essential for faithful separation of the sister chromatids. Failures in chromatid attachment can result in nondisjunction, resulting in both sister chromatids going to one daughter cell. Cytokinesis failure results in giant bi- or multinucleated cells. The resulting tetraploid status has implications in future mitoses producing further aneuploidy.
Although aneuploidy occurs frequently in malignant tumors, its role in carcinogenesis is still controversial (Dey, 2004; Thompson and Compton, 2010; Gisselsson et al., 2011). Aneuploidy may be an important mechanism that drives tumorigenesis or merely a non-specific secondary state that occurs during the process of cancer formation. There are several compelling lines of evidence, however, that support the hypothesis that aneuploidy plays an active role in carcinogenesis (Hermsen, et al., 2002; Perez de Castro et al., 2007; Baretton et al., 1993; Rubin et al., 1994). Identifying genes and proteins that contribute to aneuploidy, and thus possibly carcinogenesis, will be an important focus in the future of cancer research.
We hypothesize that high SEPT9_i1 expression increases genomic instability through its association with cytoskeletal proteins relevant to chromosome segregation and cell division. Here, we investigate whether SEPT9_i1 expression can impact tumorigenesis in mammary epithelial cells by promoting mitotic defects through its regulation of chromosome segregation and/or cytokinesis.
Materials and Methods
Cell lines
MCF-10A and HCT116 cell lines were purchased from American Type Culture Collection (ATCC, Manassas, VA). HPV 4-12 was developed and provided by S.P Ethier (Karmanos Cancer Institute, Wayne State University, Detroit, MI). MCF-10A was maintained in F12/DMEM 1:1 media containing L-glutamine, 15mM HEPES buffer and supplemented with 5% horse serum, 20 ng/ml of epidermal growth factor (EGF), 8 μg/ml of insulin, 500 ng/ml of hydrocortisone and 100 ng/ml of Cholera toxin (CT). HPV4-12 cells were maintained in F12 Nutrient Mixture with 5% fetal bovine serum (FBS), 10ng/ml EGF, 8 ug/ml insulin, 1 μg/ml of hydrocortisone and 100 ng/ml of CT. HCT116 cells were maintained in McCoy’s 5A medium (modified) with L-glutamine and with 10% FBS.
Plasmid Constructs
SEPT9_v1 cDNA was subcloned into pCMV-3tag-1A (Stratagene, Santa Clara, CA) using Hind III and Sal I restriction sites, which encoded for SEPT9_i1 protein tagged with 3 Flag epitopes at the N terminus.
Transient transfection
MCF-10A and HCT116 cells were transiently transfected with pCMV-3tag-1A or pCMV-3tag-1A-SEPT9_v1 plasmids using Fugene® HD (Promega, Madison, WI) transfection reagent following manufacturer’s instruction. Briefly, 18 μl of Fugene® HD reagent was mixed with 6 μg of plasmid in Opti-MEM reduced serum media and incubated for 15 minutes at room temperature. The transfection complex was added dropwise to ~ 1.75 × 106 cells grown in 100 mm tissue culture plates. Cells were collected for immunofluorescence, metaphase spreads and western blot analysis at different time points after transfection dictated by the doubling time of the cells. Flag-SEPT9_i1 expression, aneuploidy analysis for immunofluorescence was determined for each time point in triplicate.
Aneuploidy analysis
HPV4-12 and MCF-10A cells retrovirally transduced with empty vectors or SEPT9_v1 were grown to 70% confluence. Cells were treated with colcemid at 0.02 μg/ml for 18 hours to enrich for cells in metaphase. Then, cells were trypsinized and collected by centrifugation at 1000 rpm for 5 min. Cells were then washed with 1X PBS and incubated with a pre-warmed hypotonic solution (0.4 % KCL + 0.4 % sodium citrate) for 7 min at 37 °C with subsequent centrifugation. The resuspended pellet was fixed twice by dropwise addition of a 3:1 mixture of methanol and glacial acetic acid for 30 min and centrifugation between each fixation. Fresh fixative was then added, and resuspended cell pellets were dropped onto clean microscope slides. Slides were air dried, stained in 0.54 mg/ml Giemsa solution, and destained in deionized water. After air drying, chromosomes were visualized and counted using an Olympus BX60 model microscope with a 100x oil objective. Twenty-five metaphases were counted for aneuploidy, in triplicate, for each sample.
Immunofluorescence
For immunofluorescence analysis, cells were grown on two-chambered slides and fixed with 4% paraformaldehyde for 40 min at room temperature. Alternatively, cells were fixed 10 min with cold methanol at −20 °C followed by 1 min with acetone at −20 °C. Slides were then washed thrice in 1X PBS for 10 min, blocked for 1 h in blocking solution (5% dry milk, 1% BSA, 0.025% Triton X-100 in 1X PBS) at room temperature and incubated overnight at 4°C with polyclonal rabbit anti-SEPT9_i1 (1:50) (BioCarta, San Diego, CA), monoclonal mouse anti-α-tubulin (1:100), polyclonal rabbit-γ-tubulin (1:5,000), monoclonal anti-Flag (1:500) (Sigma-Aldrich Corp. St. Louis, MO) and/or polyclonal rabbit anti -Ki67 (1:50) (Abcam Inc. Cambridge, MA) primary antibodies diluted in blocking solution. Phalloidin conjugated to Alexa Fluor 568 was used to identify filamentous actin (F-actin). Alexa Fluor 488, Alexa Fluor 596 or Alexa Fluor 633 were used as secondary antibodies (Molecular Probes, Invitrogen, Carlsbad, CA) at a 1:500 dilution in blocking solution for 1 h at room temperature. Slides were preserved in ProLong Gold (Invitrogen) mounting media with 4,6-diamidino-2-phenylindole (DAPI) to stain the DNA. Cells were visualized using an Olympus BX60 model microscope (100 x objectives).
Western blot
Western blot analysis was done using 50 μg of whole-cell lysates. The following antibodies were used: monoclonal mouse anti-Flag at 1:1000 dilution, monoclonal mouse anti-actin, peroxidase conjugated (Sigma-Aldrich, Inc.) at 1:10,000 dilution and/or mouse monoclonal anti-GAPDH (Abcam) at 1:10,000, SEPT9_i1 specific antibody (Gonzalez et al., 2007 and 2009) at 1:5,000, anti-α-tubulin at 1:1,000, anti-γ-tubulin at 1:1,000 and anti-MAD2 at 1:1,000. Goat anti-rabbit:HRP secondary antibody (1:000 dilution) and a goat anti-mouse:HRP (1:1000 dilution) antibody were both purchased from Cell Signaling Technology (Beverly, MA). All antibodies were diluted in 5% milk, 0.05% Tween 20 in 1X TBS. SuperSignal West Pico Chemiluminescent kit (Pierce Biotechnology, Rockford, IL) was used for detection before exposure to Kodak XAR-5 film.
Immunoprecipitation
Cells were grown in 100mm dishes to 70% confluence before medium was removed, then the cells were washed twice with ice cold 1x PBS and incubated for five minutes at 4°C in M-PER lysis buffer (Pierce Biotechnology). The cells were collected and lysates were cleared by centrifugation at 13,000 rpm for 30 minutes at 4°C. Approximately 400 μg of solubilized lysate was used for each immunoprecipitation using the Protein G Immunoprecipitation kit (Sigma Aldrich, St. Louis, MO), and immunoprecipitations were performed according to manufacturer’s instructions. The immunoprecipitated proteins were analyzed by SDS-PAGE and probed with the antibody described in the assay.
Cell synchronization
Cells were synchronized with a double thymidine block as described previously (Fan, et al. 2000). Briefly, cells were incubated in medium containing 2 mM thymidine for 12 hours, released into normal medium 10 hours and then incubated for 12 hours in medium with 2 mM thymidine. After the last incubation in thymidine cells were released again to enrich for mitoses.
Results
High SEPT9_i1 expression increases aneuploidy in mammary epithelial cells
We assayed the cells with high ectopic SEPT9_i1 expression for aneuploidy, a characteristic of genomic instability in many cancers. Ectopic expression of SEPT9_i1 increased the amount of aneuploid cells to ~45% as compared to 5 and 12%% in parental and empty vector controls respectively (Figure 1a) in both immortalized mammary epithelial cells (IHMEC) lines studied, MCF-10A and HPV 4-12. Similar results were observed for polyclonal populations of MCF-10A cells with high SEPT9_i1 expression (Figure 1b). Interestingly, high SEPT9_i1 expression gave rise to a striking bimodal distribution of cells with extra chromosomes (data not shown). One population showed generalized aneuploidy with few extra chromosomes, while the second population showed a gross number of extra chromosomes closer to a tetraploid status. This difference suggested that SEPT9_i1 might impact genomic instability by at least two different mechanisms including mitotic spindle defects and/or incomplete cell division.
Figure 1. High SEPT9_i1 increases aneuploidy in IHMECs.
(a) Ectopic expression of SEPT9_i1 increases genomic instability in MCF-10A and HPV 4-12 cells in that ~45% of the cells in the population are aneuploid. Columns, percentage of aneuploid cells from 25 metaphases in each of three independent experiments; bars, SE *p =.0213, ** p= .0012, ***p= .0001, **** p=<.0001 Student t-test. (b) Multiple polyclonal populations of MCF-10A cells over-expressing SEPT9_i1 showed similar results with ~40% of cells being aneuploid. Columns, percentage of aneuploid cells from 25 metaphases in each of three independent experiments; bars, SE., **** p=<.0001 (c.) Ectopic expression of SEPT9_i1 increases mitotic spindle defects in ~20% of MCF-10A cells as depicted in the graph. Columns, percentage of cells with mitotic spindle defects in three independent experiments; bars, SDev. (d.) Immunofluorescence studies were used to stain cells for DNA (DAPI; blue), α-tubulin (red) and γ-tubulin (green). SEPT9_i1 over-expression promotes mitotic spindle defects as compared to vector control: disorganization of the mitotic spindle (i vs vi), chromatin outside of the metaphase plate (ii vs vii), multipolar spindles (iii vs viii), lagging chromosomes at the midbody (iv vs ix) and centrosome amplification (v vs x). (e.). Graph showing that ~4.5% of SEPT9_v1-cells become multinucleated as compared to the empty vector control. Columns, mean of three independent experiments; bars, SDev. *p=.0383 (f.) Over-expression of SEPT9_i1 increases multinucleated giant cells. Immunofluorescence was used to stain cells for DNA (DAPI; blue) and α-tubulin (red). Empty vector not shown.
High SEPT9_i1 expression promotes mitotic spindle defects
SEPT9_i1 transductants and empty vector control MCF-10A cells were enriched in mitosis after a 10 hour release from a double thymidine block. Cells highly expressing SEPT9_il showed an increase in mitotic spindle defects of ~20% (Figure 1c). Cells were stained for anti- α-tubulin (red), anti- γ-tubulin (green) and DAPI for DNA (Figure 1d). SEPT9_i1 promoted chromosome mis-segregation showed by misaligned chromosomes (vi, vii), multipolar spindles (viii), chromosome lagging (ix) and centrosome amplification (x) in 25% of cells as compared to empty vector control.
High SEPT9_i1 expression promotes cytokinesis failure
To assess whether over-expression of SEPT9_i1 can affect cell division, MCF-10A-SEPT9_i1, and empty vector controls were stained with anti-α-tubulin (red) and DAPI (blue) for immunofluorescence and the number of bi-nucleated cells counted. SEPT9_i1 over-expression had an effect on cell division: approximately 4.5% of SEPT9_i1 transductants were bi-nucleated as compared to empty vector control (Figure 1e and f). These results suggested that over-expression of the SEPT9_i1 transcript prevents proper cell division at the end of mitosis by disrupting cytoskeleton dynamics and/or protein involved in the completion of cytokinesis.
Transient expression of Flag-SEPT9_v1 construct was sufficient to increase aneuploidy
To determine if transient transfection was sufficient to drive aneuploidy, MCF-10A and HCT116 cells were transiently transfected with a construct expressing Flag-SEPT9_i1 and an empty vector control. SEPT9_i1 expression was assayed by Western blot at different time points post-transfection (Figure 2a). The timepoints were chosen by the doubling time of each cell line. At every time point, metaphases spreads were collected for aneuploidy studies.
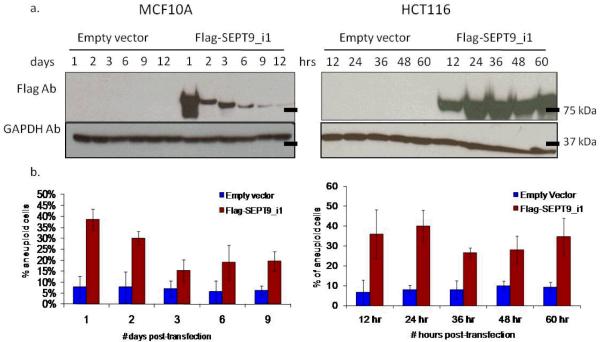
Figure 2. Transient transfection of SEPT9_i1 increases aneuploidy.
(a) Western blot analysis showing transient expression of an empty vector control and a Flag-SEPT9_v1 construct in MCF-10A (left) and HCT116 (right). GAPDH was used as a loading control. (b) Metaphase spreads were counted at each time point after transfection for both cell lines, MCF-10A (left) and HCT116 (right). MCF-10A and HCT116 transfected with Flag-SEPT9_i1 showed an increase in aneuploidy up to ~37% of cell lines one time point after transfection. Columns, percentage of aneuploid cells from 25 metaphases in each of three independent experiments; bars, SDev.
Western blot analysis showed that for MCF-10A the expression of Flag-SEPT9_i1 was reduced over time with no detectable signal on the empty vector control. Surprisingly, SEPT9_i1 transient expression increased aneuploidy to ~37% one day post-transfection (approximately one doubling time) (Figure 2b; left panel). Aneuploidy started decreasing three days after transfection, similar to the decrease of Flag-SEPT9_i1 expression over time as shown by the Western blot. HCT116 studies showed the same pattern; approximately 35% of HCT116-SEPT9_i1 cells became aneuploid 12 hours post-transfection (one doubling time) (Figure 2b; right panel).
High SEPT9_i1 expression correlates with Aurora A foci amplification
To confirm that SEPT9_i1 was associated with centrosome amplification, we investigated the relationship with Aurora A (major component of centrosomes) and SEPT9_i1 expression by immunofluorescence. MCF-10A-SEPT9_i1 and vector control cells were synchronized in mitosis by a 10 hour release after a double thymidine block. Cells were stained with anti-SEPT9_i1 (green), Aurora A (red) and DAPI (blue) (Figure 3a). High SEPT9_i1 expression was associated with amplification of Aurora A kinase foci in ~20% of SEPT9_i1-mitotic cells as compared to ~2% of the vector control (Figure 3b).
Figure 3. Increased SEPT9_i1 expression showed Aurora A kinase amplification.
(a.) MCF-10A-SEPT9_i1 and vector control cells were synchronized in mitosis by a 10 hour release after a double thymidine block. Immunofluorescence was used to stain for DNA (DAPI; blue), SEPT9_v1 (green) and Aurora A kinase (red). SEPT9_v1 over-expression increased Aurora A kinase foci in mitotic cells (bottom panel) as compared to vector control (top panel). (b.) SEPT9_i1 transductants show that ~20% of the cells have Aurora A kinase amplification. Columns, percentage of cells with >2 foci of three independent experiments; bars, SDev. *p=.0422, Student t-test
High SEPT9_i1 expression promotes increased incidence of micronuclei and nuclear fragmentation
While analyzing cells by immunofluorescence for mitotic spindle defects or evidence of cytokinesis failure, we observed novel phenotypes specific to SEPT9_i1 transductants when compared to empty vector controls. MCF-10A-SEPT9_i1 transductants demonstrated micronuclei (~3.5%) (Figure 4 a,b) and nuclear fragmentation (~1.5%) (Figure 4 c,d). Even though these phenotypes were found at low frequency, the empty vector controls did not demonstrate either of these phenotypes. The relevance of these phenotypes in terms of genomic instability and cancer needs to be characterized further; however, previous studies have associated the presence of micronuclei with loss of DNA material in one or both daughter cells during mitosis (Shackney et al., 1989).
Figure 4. SEPT9_i1 transductants showed nuclear atypia.
(a) SEPT9_i1 increases the presence of micronuclei in ~3.5% of the cells as compared to the vector control. (b) Immunofluorescence was used to stain cells for DNA (Dapi; blue) and α-tubulin (red) shows the existence of micronuclei. (c) SEPT9_i1 transductants showed nuclear fragmentation in ~1.5% of the cells as compared to the vector control. (d) Immunofluorescence was used to stain cells for DNA (Dapi; blue) and α-tubulin (red) which shows the presence of nuclear fragmentation. Three hundred cells were counted per triplicate. Columns, mean of three independent experiments; bars, SDev. *p=0.01.
SEPT9_i1 interacts with proteins important in mitosis and cell division
To begin examining the mechanism by which SEPT9_i1 promotes genomic instability in breast cancer cells, we searched for potential SEPT9_i1 interactions with components of the mitotic spindle, such as tubulins, and/or proteins involve in the spindle checkpoint, such as MAD2. Tubulins are key players of the mitotic machinery and cytoskeleton dynamics in mammalian cells, and MAD2 is a key player in the organization of the spindles and proper chromosome segregation. Therefore, we hypothesized that SEPT9_i1 over-expression affects the expression and/or localization of mitotic spindle proteins, which might impact proper cell division or chromosome segregation.
Previous studies showed an interaction between septins and tubulins; therefore, to explore further the interaction of SEPT9_i1 and α-tubulin, co-immunoprecipitation was performed. Whole cell lysates from MCF-10A-SEPT9_i1 transductants and empty vector control cells were subjected to immunoprecipitation analyses with SEPT9_i1 antibody. Co-immunoprecipitation of α-tubulin and SEPT9_i1 was confirmed in IHMECs (Figure 5a). Additionally, we found that SEPT9_i1 co-immunoprecipitated with γ-tubulin (Figure 5b). We did not observe co-immunoprecipitation of SEPT9_i1 with either MAD2, BUBR1, or β-actin (Figure 5b and data not shown).
Figure 5. SEPT9_i1 interacts with components of the mitotic spindle.
(a.) SEPT9_i1 co-immunoprecipitated with α- tubulin. Whole cell lysates (left panel) from vector control (pLNCX2) and SEPT9_i1 transductants were used for immunoprecipitation. SEPT9_i1 (middle panel) and α-tubulin (right panel) antibodies were used for immunoprecipitation and the samples were subsequently immunoblotted for SEPT9_i1 or α-tubulin. (b.) SEPT9_i1 co-immunoprecipitated with γ-tubulin (second panel), but not with β-actin (third panel) or MAD2 (fourth panel). Whole cell lysates from vector control (pLNCX2) and SEPT9_i1 transductants were used for immunoprecipitation. The SEPT9_i1 antibody was used for immunoprecipitation and the samples were immunoblotted for SEPT9_i1, γ-tubulin, β-actin and MAD2.
Discussion
We explored in further detail the roles of SEPT9_i1 in tumorigenesis by describing two independent mechanisms impacting genomic instability. When metaphase spreads of cells with high SEPT9_i1 were categorized by the number of extra chromosomes, a bimodal distribution was observed, with a population of cells with ploidy status close to tetraploidy and another population with more generalized aneuploidy. A closer look at mitotic cells showed that deregulation of SEPT9_i1 expression promotes mitotic spindle defects associated with chromosome mis-segregation including disorganization of the chromosomes in respect to the mitotic spindles, multipolar spindles, and chromosome lagging during telophase. In addition, a small but significant population of high SEPT9_i1 expressing cells became bi-nucleated demonstrating a failure of faithful division (cytokinesis) into two daughter cells. This suggested the possibility of two types of mitotic errors promoted by high SEPT9_i1 expression: mitotic spindle defects and failure to complete cell division at cytokinesis. Estey et al. (2010) showed SEPT9 plays an important role in midbody abscission. While, knockdown of SEPT9 and subsequent rescue with SEPT9_i1 or SEPT9_i3 is sufficient to rescue the defect. One can speculate that the level of expression of each isoform is an important factor for successful cell division and that each isoform has a distinct function. More research needs to be done to look at the regulation of septins and the relationships, either temporal or spatial, between the different family members.
To determine whether SEPT9_i1 affected general mitotic spindle assembly and function or interfered with proper regulation of the mitotic spindle checkpoint, several candidates for protein interaction were assayed. Interactions with α-tubulin and γ-tubulin were observed by immunoprecipitation. Tubulins are major components of spindle microtubules and centrosomes, which are microtubule organizer centers (MTOCs). This is supported by previous studies that showed that SEPT9_i1 localized with microtubules at the midplane in metaphase during mitosis and in the midbody and cleavage furrow during cytokinesis (Nagata et al., 2003; Surka et al., 2002). No interaction with essential spindle checkpoint protein MAD2 was found, suggesting that SEPT9_i1 might affect mitotic spindle dynamics and/or organization but does not directly interfere with the mitotic spindle checkpoint. Additionally, we assayed several other mitotic spindle checkpoint proteins including, BUBR1, Aurora A and B, and CENPE and no interaction was found (data not shown). High expression of SEPT9_i1 did, however, result in an increase in the number of Aurora A foci during mitosis. Overexpression of Aurora A has been shown to override the checkpoint, allowing cells to proceed through the cell cycle, which results in cytokinesis failure and large, multinucleated cells (Anand et al., 2003). This suggests that SEPT9_i1 may regulate Aurora A expression either indirectly through a signaling cascade separate from the mitotic spindle checkpoint or through a feedback mechanism dependent on temporal or spatial regulation. More research needs to be done to analyze the relationship between Aurora A and SEPT9_i1.
We also showed that transient transfection of SEPT9_i1 was sufficient to drive aneuploidy and that the degree of aneuploidy correlated with the amount of SEPT9_i1 expression. Aneuploidy in both MCF-10A and HCT116 arose within one doubling time after expression. HCT116 cells, a colon cancer cell line, was utilized because of its stable karyotype, to confirm that any effect of chromosome number was due to SEPT9_i1 high expression and not due to specific artifacts in MCF-10A cells.
Here we demonstrate that SEPT9_i1 increases genomic instability in mammary epithelial cells through two mechanisms: defective chromosome segregation and cytokinesis failure. In addition, SEPT9_i1 interacts with α- and γ-tubulin, important members of the mitotic spindle. SEPT9_i1 may also have an indirect effect on the mitotic spindle checkpoint through an increase in Aurora A expression. We also showed that cellular proliferation and aneuploidy occur through two distinct mechanisms. Further analysis to determine the mechanism through which SEPT9_i1 is driving aneuploidy is important and could lead to the development of targeted chemotherapeutic drugs.
Acknowledgements
This work was supported by the NIH National Research Service Award #5-T32-GM07544 from the National Institute of General Medicine Sciences and the NRSA Predoctoral Individual Fellowship to Promote Diversity in Health-Related Research # 1F31CA123639-01-A1 to E.A. Peterson, the Department of Defense Breast Cancer Research Program Post-doctoral Grant #W81XWH-10-1-0545 to L. Stanbery and by the NIH National Cancer Institute grant RO1CA072877 to E.M. Petty. We want to thank Sam Straight from the Center for Live-Cell Imaging for helpful technical support with microscopy.
References
- Amir S, Wang R, Matzkin H, Simons JW, Mabjeesh NJ. MSF-A interacts with hypoxia-inducible factor-1alpha and augments hypoxia-inducible factor transcriptional activation to affect tumorigenicity and angiogenesis. Cancer Res. 2006;66(2):856–66. doi: 10.1158/0008-5472.CAN-05-2738. [DOI] [PubMed] [Google Scholar]
- Amir S, Mabjeesh NJ. SEPT9_v1 protein expression is associated with human cancer cell resistance to microtubule-disrupting agents. Cancer Biol Ther. 2007;6:1926–31. doi: 10.4161/cbt.6.12.4971. [DOI] [PubMed] [Google Scholar]
- Baretton G, Vogt T, Valina C, Schneiderbanger K, Lohrs U. [Prostate cancers and potential precancerous conditions: DNA cytometric investigations and interphase cytogenetics] Verh Dtsch Ges Pathol. 1993;77:86–92. [PubMed] [Google Scholar]
- Charames GS, Bapat B. Genomic instability and cancer. Curr Mol Med. 2003;3(7):589–96. doi: 10.2174/1566524033479456. [DOI] [PubMed] [Google Scholar]
- Dey P. Aneuploidy and malignancy: an unsolved equation. J Clin Pathol. 2004;57(12):1245–9. doi: 10.1136/jcp.2004.018952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan M, Du L, Stone AA, Gilbert KM, Chambers TC. Modulation of mitogen-activated protein kinases and phosphorylation of Bcl-2 by vinblastine represent persistent forms of normal fluctuations at G2-M1. Cancer Res. 2000;60(22):6403–7. [PubMed] [Google Scholar]
- Gisselsson D. Aneuploidy in Cancer. Cell Cycle. 2011;10(3):1–3. doi: 10.4161/cc.10.3.14518. [DOI] [PubMed] [Google Scholar]
- Gonzalez ME, Peterson EA, Privette LM, Loffreda-Wren JL, Kalikin LM, Petty EM. High SEPT9_v1 expression in human breast cancer cells is associated with oncogenic phenotypes. Cancer Res. 2007;67(18):8554–64. doi: 10.1158/0008-5472.CAN-07-1474. [DOI] [PubMed] [Google Scholar]
- Hermsen M, Postma C, Baak J, Weiss M, Rapallo A, Sciutto A, Roemen G, Arends JW, Williams R, Giaretti W, others Colorectal adenoma to carcinoma progression follows multiple pathways of chromosomal instability. Gastroenterology. 2002;123(4):1109–19. doi: 10.1053/gast.2002.36051. [DOI] [PubMed] [Google Scholar]
- Nagata K, Kawajiri A, Matsui S, Takagishi M, Shiromizu T, Saitoh N, Izawa I, Kiyono T, Itoh TJ, Hotani H, others Filament formation of MSF-A, a mammalian septin, in human mammary epithelial cells depends on interactions with microtubules. J Biol Chem. 2003;278(20):18538–43. doi: 10.1074/jbc.M205246200. [DOI] [PubMed] [Google Scholar]
- Nelsen CJ, Rickheim DG, Timchenko NA, Stanley MW, Albrecht JH. Transient expression of cyclin D1 is sufficient to promote hepatocyte replication and liver growth in vivo. Cancer Res. 2001;61(23):8564–8. [PubMed] [Google Scholar]
- Perez de Castro I, de Carcer G, Malumbres M. A census of mitotic cancer genes: new insights into tumor cell biology and cancer therapy. Carcinogenesis. 2007;28(5):899–912. doi: 10.1093/carcin/bgm019. [DOI] [PubMed] [Google Scholar]
- Peterson EA, Petty EP. Conquering the complex world of human septins: Implications for health and disease. Clinical Genetics. 2010;77(6):511–524. doi: 10.1111/j.1399-0004.2010.01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin EM, DeRose PB, Cohen C. Comparative image cytometric DNA ploidy of liver cell dysplasia and hepatocellular carcinoma. Mod Pathol. 1994;7(6):677–80. [PubMed] [Google Scholar]
- Shackney SE, Smith CA, Miller BW, Burholt DR, Murtha K, Giles HR, Ketterer DM, Pollice AA. Model for the genetic evolution of human solid tumors. Cancer Res. 1989;49(12):3344–54. [PubMed] [Google Scholar]
- Sisson JC, Field C, Ventura R, Royou A, Sullivan W. Lava lamp, a novel peripheral golgi protein, is required for Drosophila melanogaster cellularization. J Cell Biol. 2000;151(4):905–18. doi: 10.1083/jcb.151.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surka MC, Tsang CW, Trimble WS. The mammalian septin MSF localizes with microtubules and is required for completion of cytokinesis. Mol Biol Cell. 2002;13(10):3532–45. doi: 10.1091/mbc.E02-01-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SL, Compton DA. Chromosomes and cancer cells. Chrom Res Epub ahead of print. 2010 doi: 10.1007/s10577-010-9179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Wang F, Yan F, Yao PY, Du J, Gao X, Wang X, Wu Q, Ward T, Li J, others Septin 7 Interacts with Centromere-associated Protein E and Is Required for Its Kinetochore Localization. J Biol Chem. 2008;283(27):18916–25. doi: 10.1074/jbc.M710591200. [DOI] [PMC free article] [PubMed] [Google Scholar]