Abstract
DKK1 modulates Wnt signaling, which is involved in the atherosclerosis. However, no data exist regarding the usefulness of measuring serum DKK1 concentration in predicting coronary atherosclerosis. A total of 270 consecutive patients (62.8 ± 11.2 yr; 70% male) were included. A contrast-enhanced 64-slice coronary MDCT was performed to identify the presence of atherosclerotic plaques. Agatston calcium scores (CS) were calculated to quantify the coronary artery calcification (CAC). DKK1 concentrations were measured by enzyme-linked immunosorbent assay. For each subsequent DKK1 quartile, there was a significant increase in CAC (P = 0.004) and the number of segments with coronary atherosclerosis (P < 0.001). In addition, DKK1 concentration was significantly higher in patients with atherosclerotic plaques, regardless of plaque composition (P = 0.01). Multivariate analysis identified DKK1 as an independent risk factor for the presence of coronary atherosclerotic plaque. The adjusted odds ratio for coronary atherosclerotic plaque was 4.88 (95% CI, 1.67 to 14.25) for highest versus lowest quartile of the DKK1 levels. Furthermore, patients with DKK1 concentrations ≥ 68.6 pg/mL demonstrated coronary atherosclerotic plaques even when they had low CS. Serum DKK1 concentrations correlate with the coronary atherosclerosis and play an independent role in predicting the presence of coronary atherosclerosis.
Keywords: Dickkopf-1, Atherosclerosis, Vascular calcification, Biomarker
INTRODUCTION
Coronary artery calcium is a marker of subclinical coronary atherosclerotic disease and predicts coronary events. As a result, there has been considerable interest in the potential use of the coronary artery calcium score (CACS) using computed tomography (CT) in models of risk prediction (1). Furthermore, with the advance of technology, contrast-enhanced CT angiography can identify both calcified and non-calcified coronary atherosclerotic plaques. The clinical usefulness of CACS and coronary CT angiography (CCTA) is well known and their use has recently increased (2, 3). However, the concern regarding radiation exposure from CCTA has also increased (4).
A clinical risk score such as the Framingham risk score is helpful in identifying the high-risk group. However, more than half of the cardiac events develop in low-risk patients (5). Accordingly, a more sophisticated method to stratify the cardiovascular risk needs to be developed. Finding new biomarkers that can identify and quantify the severity of coronary artery calcification (CAC) and atherosclerosis would be less expensive, avoid exposure to radiation, and be more accessible than imaging based methods.
The Wnt signaling pathways are involved in diverse developmental and physiological processes, including cell differentiation and tissue/organ morphogenesis (6). Recent evidence points to an important role of the Wnt signaling pathways in the regulation of inflammation (7). In addition, Wnt pathway activation enhances monocyte adhesion and regulates trans-endothelial migration of monocytes. As a result, the Wnt signaling pathway is involved in the process of atherosclerosis (8). Moreover, the Wnt signaling pathway plays an important role in the vascular calcification (9, 10).
The canonical Wnt pathway is regulated by multiple families of secreted antagonists such as the soluble frizzled related receptors and dickkopf-1 (DKK1). DKK1 regulates Wnt signaling by binding to the Wnt co-receptor, LRP 5/6. Recently, enhanced DKK1 expression was observed within advanced carotid plaques, suggesting that a DKK1-driven inflammatory loop could be operating within the atherosclerotic lesion (11). However, it has not been clearly demonstrated whether the serum concentration of DKK1 may be useful for predicting the extent of atherosclerosis or vascular calcification.
In this study, we aimed to evaluate the clinical significance of serum DKK1 concentration for predicting CAC and the presence of coronary atherosclerotic plaques.
MATERIALS AND METHODS
Study population
We studied 270 consecutive subjects who visited Seoul National University Bundang Hospital complaining of chest pain from July 2006 through July 2008. The exclusion criteria were acute myocardial infarction, uncontrolled arrhythmia (ventricular tachycardia/fibrillation, atrial flutter/fibrillation, or atrioventricular block greater than second degree), contrast allergy, and renal dysfunction (serum creatinine > 2.0 mg/dL). Clinical information was gathered by personal interview and a physical examination was performed by physicians. A biochemical evaluation and full medical examination were also performed.
The estimated pretest probability for coronary artery disease was estimated using the Duke clinical score, which includes type of chest discomfort, age, gender, and traditional risk factors. Subjects were categorized into a low (1% to 30%), intermediate (31% to 70%), or high (71% to 99%) risk group of having coronary artery disease (12).
Coronary multidetector computed tomography (MDCT) and coronary artery calcium scoring
All subjects were examined using the same CT unit and scanning protocols. All CT scans were performed using a 64-slice CCTA scanner (Brilliance 64, Philips Medical Systems, Best, The Netherlands) with 64 × 0.625-mm section collimation, 420-ms rotation time, 120-kV tube voltage, and 800-mA tube current under electrocardiographic-gated dose modulation. Before CCTA, all patients with a baseline heart rate > 70 beats/min received an intravenous esmolol of 10 to 30 mg (Jeil Pharm, Seoul, Korea). Nitroglycerin 0.6 mg was administered sublingually immediately before contrast injection (13). During CCTA acquisition, a bolus of 80 mL iomeprol (Iomeron 400, Bracco, Milan, Italy) was injected intravenously (4 mL/s) followed by a 50-mL saline chaser.
Coronary MDCT scans were analyzed independently by two experienced radiologists who were unaware of the clinical information and used a three-dimensional workstation (Brilliance, Philips Medical Systems). Agatston calcium scores (CS) were calculated to quantify the extent of CAC (14). The presence of coronary atherosclerotic plaque was evaluated according to the modified American Heart Association classification (15). The contrast-enhanced portion of the coronary lumen was semiautomatically traced at the maximal stenotic site and compared with the mean value of the proximal and distal reference sites. Structures that were > 1 mm2 within and/or adjacent to the vessel lumen were defined as plaques. Three groups of plaques were classified: lesions in which > 50% of the plaque area was occupied by calcified tissue (density > 130 Hounsfield unit in native scans) were classified as calcified, lesions with < 50% calcium as mixed, and lesions without any calcium were classified as noncalcified (16).
Measurement of serum DKK1 concentration
DKK1 concentrations were measured by enzyme-linked immunosorbent assay according to the manufacturer's instructions (DuoSet ELISA development kit, R&D Systems Inc., Minneapolis, MN, USA).
Definitions
Hypertension was defined as blood pressure ≥ 140/90 mmHg or taking any antihypertensive medications. Diabetes mellitus was defined by a fasting blood glucose ≥ 126 mg/dL or a history of or treatment for hyperglycemia. Hypercholesterolemia was defined by a total cholesterol ≥ 200 mg/dL or treatment for hypercholesterolemia. Ischemic heart disease was defined by a history of angina, myocardial infarction, or previous treatment with coronary medications or intervention for heart disease. Smoking status was classified into current smokers (smoked within the last month), ex-smokers (given up for more than one month), and non-smokers.
Statistical analysis
Statistical analyses were performed using SPSS (version 15.0, SPSS Inc., Chicago, IL, USA) or MedCalc (version 11.0, MedCalc software, Mariakerke, Belgium). Continuous variables are expressed as mean ± SD. Because serum DKK1 levels and CACS were not normally distributed, the values are also reported as median and interquartile range (IQR). Continuous variables were compared by either the unpaired Student's t-test or analysis of variance (ANOVA) followed by post-hoc comparison with the Scheffe test. Discrete variables are expressed as counts and percentages, and the chi-squared or Fisher's exact test was used to compare proportions. Correlation analyses were performed using the Pearson and Spearman coefficients of correlation for parametric and nonparametric variables, respectively. Multiple logistic regressions were employed to assess the independent association of DKK1 concentration and CACS with the presence of coronary atherosclerotic plaque. Differences in the predicted value were estimated by comparing the area under the receiver-operating characteristic curve (AUC), taking the correlation between the areas into account. We also calculated the c-statistic for models with conventional risk factors with and without CACS or DKK1. All statistical analyses were two-tailed, and P values < 0.05 were considered statistically significant.
Ethics statement
All subjects provided informed consent and the study was approved by the institutional review board at Seoul National University Bundang Hospital (IRB number: B-0807/059-004).
RESULTS
Baseline characteristics of study subjects
A total of 270 consecutive patients with chest pain were included. The mean age was 62.8 ± 11.2 yr (range: 31-92 yr), and males comprised 70% of subjects. Of the 270 patients, 41 (15%) patients showed no evidence of coronary artery calcium. The mean value of CACS was 338.1 ± 518.7 (median 112.9, IQR 16.9-450.6). The mean serum concentration of DKK1 was 134.5 ± 127.2 pg/mL (median 99.8, IQR 61.6-158.5). Both CACS and DKK1 concentration showed skewed distributions. Clinical and laboratory characteristics of the patients are presented in Table 1 according to the quartile of DKK1 concentration. A significant increase in platelet count that correlated with increasing quartiles of DKK1 concentration was identified. All other variables were not different among the DKK1 quartiles.
Table 1.
Comparison of clinical and laboratory characteristics according to the DKK1 quartile group
Data are presented as mean (SD) or number (%). CACS are presented as median [IQR]. DKK1, dickkopf-1; HT, hypertension; DM, diabetes mellitus; FHx of IHD, family history of ischemic heart disease; BMI, body mass index; WBC, white blood cell; TG, triglyceride; HDL, high density lipoprotein; BUN, blood urea nitrogen; HbA1C, hemoglobin A1C; hsCRP, high sensitivity C-reactive protein; CACS, coronary artery calcium score. Cutoff values for DKK1 quartiles were 61.6 pg/mL, 99.8 pg/mL, and 158.5 pg/mL.
Association between DKK1 concentration and coronary atherosclerosis
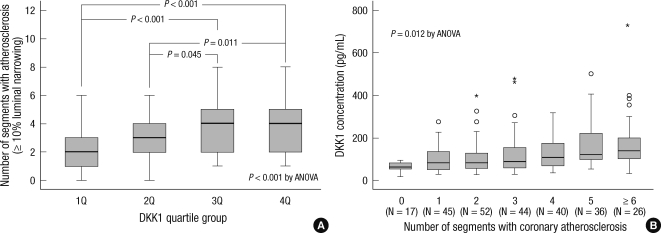
The serum concentration of DKK1 was positively but weakly correlated with CACS (Spearman's rho = 0.191, P = 0.002). CAC was significantly associated with the level of DKK1. The median (IQR) values of the CACS were 42.9 (0.0-224.8), 127.1 (22.2-612.3), 145.4 (38.5-639.3), and 154.1 (44.8-444.5) in the lowest, second, third, and highest quartiles of DKK1 level (P = 0.004). Also, the distribution of DKK1 and CACS quartiles were closely associated (P = 0.021). Overall, any coronary atherosclerotic plaque (≥ 10% luminal narrowing) was detected in 253 (94%) subjects, and the mean number of segments with coronary atherosclerotic plaques was 3.4 ± 1.8 per subjects. The number of segments with coronary atherosclerosis was significantly higher in groups with higher DKK1 concentrations (P < 0.001) (Fig. 1A). In addition, DKK1 concentration was significantly elevated according to the global coronary atherosclerotic burden (Fig. 1B).
Fig. 1.
Association between DKK1 concentration and coronary atherosclerotic plaque. Number of coronary artery segments with any atherosclerotic plaque (≥ 10% luminal narrowing) was evaluated in all the subjects, and 253 (94%) subjects showed more than one coronary atherosclerotic plaque. The number of segments with coronary atherosclerosis was significantly increased with increasing DKK1 quartiles (A). In addition, DKK1 concentration was significantly elevated according to the number of coronary artery segments with any atherosclerotic plaque (B). An outliers (open circles) are defined as a score that is between 1.5 and 3 box lengths away from the upper edge of the box. An extreme scores (asterisks) are defined as a score that is greater than 3 box lengths away from the upper edge of the box.
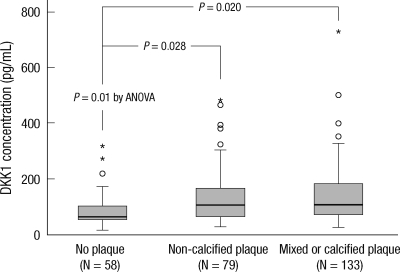
Significant coronary atherosclerotic stenosis (≥ 50% luminal narrowing) was identified in 212 (79%) subjects. Among these patients, 79 subjects had exclusively non-calcified plaques, 25 subjects had exclusively calcified plaques, and 108 subjects had both and, thus, were classified as having mixed plaques. DKK1 was significantly elevated in patients with coronary atherosclerotic stenosis (median [IQR] with DKK1 concentrations of 63.2 [52.7-102.8] pg/mL, 105.2 [64.4-169.1] pg/mL, and 108.5 [72.0-183.2] pg/mL in patients without plaque, with non-calcified plaque, and with mixed or calcified plaque, respectively) (P = 0.01) (Fig. 2).
Fig. 2.
Comparison of DKK1 concentration according to the type of coronary atherosclerotic plaque. DKK1 was significantly elevated in patients with both calcium-containing and non-calcified coronary atherosclerotic plaques compared to the patients without plaque. The central line represents distribution median, the boxes span from 25th to 75th percentiles, and the error bars extend from 10th to 90th percentiles. An outliers (open circles) are defined as a score that is between 1.5 and 3 box lengths away from the upper edge of the box. An extreme scores (asterisks) are defined as a score that is greater than 3 box lengths away from the upper edge of the box.
The association between DKK1 concentration and coronary atherosclerotic stenosis was not different according to the pretest risk profile evaluated using the Duke clinical score. The frequency of coronary atherosclerotic stenosis was significantly increased according to the level of DKK1, both in the low to intermediate-risk group (n = 72) and in the high-risk group (n = 198).
Comparison of CACS and DKK1 in predicting the presence of coronary atherosclerotic stenosis
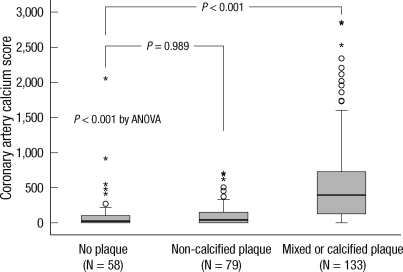
The levels of CACS were significantly higher in patients with calcified or mixed plaque. However, the values were not different in patients with non-calcified plaque compared to patients without plaques (Fig. 3).
Fig. 3.
CACS according to the coronary atherosclerotic plaque classification. CACS was only increased in patients with calcium-containing plaques compared to the patients without plaque. This difference showed the clinical advantage of DKK1 over CACS in predicting the presence of coronary atherosclerotic plaques without calcium deposits. Shown are box plots representing median and interquartile range (equivalently the 25th and 75th percentiles). An outliers (open circles) are defined as a score that is between 1.5 and 3 box lengths away from the upper edge of the box. An extreme scores (asterisks) are defined as a score that is greater than 3 box lengths away from the upper edge of the box.
The AUC for the DKK1 concentration was 0.678 (95% CI: 0.619-0.734), which was comparable to that of CACS (AUC 0.729, 95% CI: 0.672-0.782) (P = 0.260). The sensitivity and specificity of DKK1 levels ≥ 68.6 pg/mL for the presence of coronary atherosclerotic plaques were 77% (71%-82%) and 55% (42%-68%), respectively.
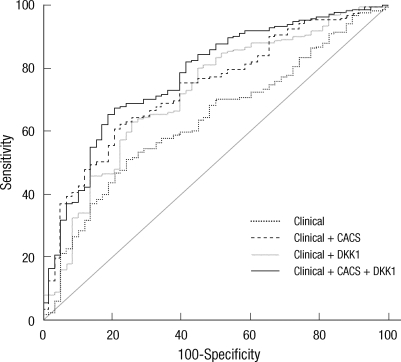
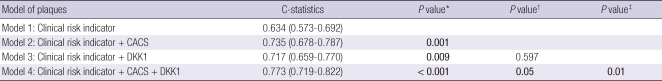
For the prediction of coronary atherosclerotic plaques, improvement in discrimination compared with the model with conventional risk factors was observed with inclusion of CACS (c-statistic = 0.735) in the model and with DKK1 (c-statistic = 0.717). Addition of both CACS and DKK1 resulted in a statistically significant increase of the c-statistic compared with the model based on clinical risk factor or clinical risk factor plus either CACS or DKK1 (Fig. 4, Table 2).
Fig. 4.
Role of DKK1 in predicting the presence of coronary atherosclerotic plaque. The receiver operating characteristic curves illustrate clinical risk indicator including age, body mass index, hypertension, diabetes, and dyslipidemia alone or plus CACS and/or DKK1 concentration. Areas under the curves are 0.634 for clinical risk indicator alone, 0.735 for clinical risk indicator plus CACS, 0.717 for clinical risk indicator plus DKK1, and 0.773 for clinical risk indicator plus CACS and DKK1. The area under the curve for the receiver operating characteristic curve for clinical risk indicator plus CACS or DKK1, and CACS & DKK1 are significantly greater than that of the clinical risk indicator alone.
Table 2.
C-Statistics: Incremental prognostic value of CACS and DKK1 concentration regarding the presence of coronary atherosclerotic plaques
Clinical risk indicator includes age, body mass index, hypertension, diabetes, and hypercholesterolemia. *P value comparing with model 1; †P value comparing with model 2; ‡P value comparing with model 3. DKK1, dickkopf-1; CACS, coronary artery calcium score.
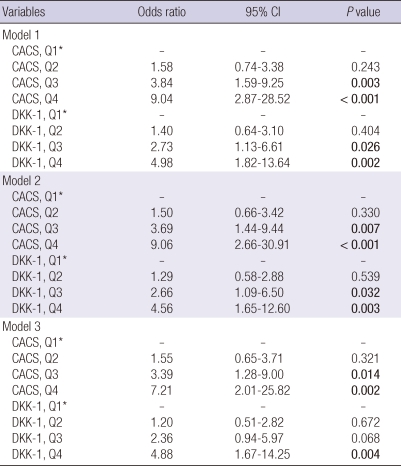
In multivariate analysis, CACS and DKK1 concentrations retained a strong association with the presence of coronary atherosclerotic plaque after adjustment for age, gender, and other variables (Table 3). Interestingly, the patients who had low CACS (Agatston CS < 154.1, cut-off value after the receiver-operating characteristic curve analysis) but DKK1 ≥ 68.6 pg/mL showed a similar probability of having coronary atherosclerotic plaque as patients with high CACS (Agatston CS ≥ 154.1) (Fig. 5).
Table 3.
Unadjusted and adjusted OR and 95% confidence intervals derived from logistic regression analysis for the association between CACS and DKK1 concentration with the presence of coronary atherosclerotic plaques
The dependent variable was the presence of coronary atherosclerotic plaque causing more than 50% stenosis. *The first quartile (Q1) was used as the reference value. Cut-off values were 16.9, 112.9, and 450.6 for CACS quartiles and 61.6 pg/mL, 99.8 pg/mL, and 158.5 pg/mL for DKK1 quartiles. Model 1, unadjusted; Model 2, age and gender adjusted; Model 3, age, gender, hypertension, diabetes mellitus, hypercholesterolemia, smoking, calcium, creatinine, fibrinogen, hsCRP, and platelet count adjusted.
Fig. 5.
Complementary role of DKK1 and CACS in identifying coronary atherosclerotic plaque. Patients who had low CACS (Agatston CS < 154.1) but high DKK1 concentration (DKK1 ≥ 68.6 pg/mL) show a similar probability of having coronary atherosclerotic plaque as patients with high CACS.
DISCUSSION
In this study, we demonstrated that DKK1 concentration significantly correlated with CAC and the presence of coronary atherosclerotic plaque. Furthermore, in patients with a low CACS, DKK1 concentration could be useful to identify the presence of coronary plaque.
CACS alone can rank the risk of coronary heart disease independently of the clinical risk profile and shows good correlation with calcified atherosclerotic plaque. However, the efficacy of CACS for predicting non-calcified coronary plaques has shown limited potential (17). Moreover, a zero CACS result does not exclude significant coronary artery stenosis or the need for coronary revascularization (18).
Contrary to CACS, DKK1 can identify non-calcified as well as calcium-containing plaques without radiation exposure. In addition, DKK1 provides incremental information regarding the prediction of coronary atherosclerotic plaque beyond traditional risk factors. All of these results merit further investigation of DKK1 measurement in various clinical situations for identifying coronary atherosclerosis.
The hallmark of the DKK family is its ability to modulate Wnt signaling. The founding member of the family, DKK1, was discovered by its ability to block Wnt signaling (19, 20). The Wnt signaling pathway is involved in inflammation and atherosclerosis. In addition, the role of Wnt signaling during vascular calcification has been established. Aicher et al. have shown that DKK1 induces the osteoclast differentiation factor RANKL, which has an important role in vascular calcification (21, 22). Moreover, Ueland et al. (11) have demonstrated that DKK1 expression is enhanced in advanced carotid plaques and that DKK1 is a novel mediator in platelet-mediated endothelial cell activation, which could occur within the atherosclerotic lesions. All of these findings suggest that DKK1 may modulate vascular calcification and atherosclerosis. However, until now there was no concrete evidence showing the clinical implication of measuring serum DKK1 concentrations in the patients.
In this study, we observed a positive correlation between the serum concentration of DKK1 and CAC. Furthermore, the DKK1 concentration was significantly increased in patients with CAC and atherosclerotic plaques. Interestingly, DKK1 concentration was significantly different between patients without plaque and with non-calcified plaque; however, CACS showed no difference between these patients.
With the advance of technological improvement, CCTA could characterize coronary atherosclerotic plaques. New technologies enable us to identify non-calcified coronary plaques, but there are concerns about the associated radiation exposure. Accordingly, a new method to identify both non-calcified and calcium-containing plaques needs to be developed. From this point of view, the measurement of DKK1 concentration in addition to CACS would be beneficial for the prediction of coronary artery disease in patients with chest pain. However, it is not obvious whether this result could also be applied to other populations, such as asymptomatic individuals or other ethnic backgrounds. Furthermore, it is not clear whether DKK1 is also useful to predict systemic atherosclerosis other than coronary atherosclerosis. Accordingly, the association between DKK1 concentration and systemic atherosclerosis in carotid, aorta, and peripheral vessels need to be evaluated.
We observed a significant increase in platelet count that correlated with increasing quartiles of DKK1 concentration. Previous report demonstrated that DKK1 is a mediator in platelet-dependent endothelial activation (11). However, the association between DKK1 and coronary atherosclerotic plaques remained significant after adjustment for platelet count in multivariate analysis.
In conclusion, serum DKK1 concentrations correlate with the presence of CAC and play a role in predicting the presence of coronary atherosclerotic plaques. The measurement of DKK1 merits further investigation as a simple test for identifying coronary atherosclerosis without the risk of radiation exposure.
Footnotes
This work was supported by the Korea Research Foundation Grant, funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-2008-331-E00106) and by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (2009-0063258).
AUTHOR SUMMARY
A Novel Biomarker of Coronary Atherosclerosis: Serum DKK1 Concentration Correlates with Coronary Artery Calcification and Atherosclerotic Plaques
Kwang-Il Kim, Kyoung Un Park, Eun Ju Chun, Sang Il Choi, Young-Seok Cho, Tae-Jin Youn, Goo-Yeong Cho, In-Ho Chae, Junghan Song, Dong-Ju Choi and Cheol-Ho Kim
Here, we show that DKK1 concentration is significantly associated with coronary artery calcification and coronary atherosclerotic plaques. Furthermore, in patients with low calcium scores, DKK1 concentration is valuable in differentiating the presence of coronary plaques. Accordingly, measuring the serum DKK1 concentration is clinically useful for identifying coronary atherosclerosis.
References
- 1.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 2.Rubinshtein R, Halon DA, Gaspar T, Jaffe R, Karkabi B, Flugelman MY, Kogan A, Shapira R, Peled N, Lewis BS. Usefulness of 64-slice cardiac computed tomographic angiography for diagnosing acute coronary syndromes and predicting clinical outcome in emergency department patients with chest pain of uncertain origin. Circulation. 2007;115:1762–1768. doi: 10.1161/CIRCULATIONAHA.106.618389. [DOI] [PubMed] [Google Scholar]
- 3.Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 4.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007;298:317–323. doi: 10.1001/jama.298.3.317. [DOI] [PubMed] [Google Scholar]
- 5.Bamberg F, Dannemann N, Shapiro MD, Seneviratne SK, Ferencik M, Butler J, Koenig W, Nasir K, Cury RC, Tawakol A, Achenbach S, Brady TJ, Hoffmann U. Association between cardiovascular risk profiles and the presence and extent of different types of coronary atherosclerotic plaque as detected by multidetector computed tomography. Arterioscler Thromb Vasc Biol. 2008;28:568–574. doi: 10.1161/ATVBAHA.107.155010. [DOI] [PubMed] [Google Scholar]
- 6.Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis: a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 7.Sen M, Ghosh G. Transcriptional outcome of Wnt-Frizzled signal transduction in inflammation: evolving concepts. J Immunol. 2008;181:4441–4445. doi: 10.4049/jimmunol.181.7.4441. [DOI] [PubMed] [Google Scholar]
- 8.Christman MA, 2nd, Goetz DJ, Dickerson E, McCall KD, Lewis CJ, Benencia F, Silver MJ, Kohn LD, Malgor R. Wnt5a is expressed in murine and human atherosclerotic lesions. Am J Physiol Heart Circ Physiol. 2008;294:H2864–H2870. doi: 10.1152/ajpheart.00982.2007. [DOI] [PubMed] [Google Scholar]
- 9.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest. 2005;115:1210–1220. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirton JP, Crofts NJ, George SJ, Brennan K, Canfield AE. Wnt/beta-catenin signaling stimulates chondrogenic and inhibits adipogenic differentiation of pericytes: potential relevance to vascular disease? Circ Res. 2007;101:581–589. doi: 10.1161/CIRCRESAHA.107.156372. [DOI] [PubMed] [Google Scholar]
- 11.Ueland T, Otterdal K, Lekva T, Halvorsen B, Gabrielsen A, Sandberg WJ, Paulsson-Berne G, Pedersen TM, Folkersen L, Gullestad L, Oie E, Hansson GK, Aukrust P. Dickkopf-1 enhances inflammatory interaction between platelets and endothelial cells and shows increased expression in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1228–1234. doi: 10.1161/ATVBAHA.109.189761. [DOI] [PubMed] [Google Scholar]
- 12.Pryor DB, Shaw L, McCants CB, Lee KL, Mark DB, Harrell FE, Jr, Muhlbaier LH, Califf RM. Value of the history and physical in identifying patients at increased risk for coronary artery disease. Ann Intern Med. 1993;118:81–90. doi: 10.7326/0003-4819-118-2-199301150-00001. [DOI] [PubMed] [Google Scholar]
- 13.Chun EJ, Lee W, Choi YH, Koo BK, Choi SI, Jae HJ, Kim HC, So YH, Chung JW, Park JH. Effects of nitroglycerin on the diagnostic accuracy of electrocardiogram-gated coronary computed tomography angiography. J Comput Assist Tomogr. 2008;32:86–92. doi: 10.1097/rct.0b013e318059befa. [DOI] [PubMed] [Google Scholar]
- 14.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 15.Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, McGoon DC, Murphy ML, Roe BB. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975;51(4 Suppl):5–40. doi: 10.1161/01.cir.51.4.5. [DOI] [PubMed] [Google Scholar]
- 16.Choi EK, Chun EJ, Choi SI, Chang SA, Choi SH, Lim S, Rivera JJ, Nasir K, Blumenthal RS, Jang HC, Chang HJ. Assessment of subclinical coronary atherosclerosis in asymptomatic patients with type 2 diabetes mellitus with single photon emission computed tomography and coronary computed tomography angiography. Am J Cardiol. 2009;104:890–896. doi: 10.1016/j.amjcard.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 17.Scholte AJ, Schuijf JD, Kharagjitsingh AV, Jukema JW, Pundziute G, van der Wall EE, Bax JJ. Prevalence of coronary artery disease and plaque morphology assessed by multi-slice computed tomography coronary angiography and calcium scoring in asymptomatic patients with type 2 diabetes. Heart. 2008;94:290–295. doi: 10.1136/hrt.2007.121921. [DOI] [PubMed] [Google Scholar]
- 18.Gottlieb I, Miller JM, Arbab-Zadeh A, Dewey M, Clouse ME, Sara L, Niinuma H, Bush DE, Paul N, Vavere AL, Texter J, Brinker J, Lima JA, Rochitte CE. The absence of coronary calcification does not exclude obstructive coronary artery disease or the need for revascularization in patients referred for conventional coronary angiography. J Am Coll Cardiol. 2010;55:627–634. doi: 10.1016/j.jacc.2009.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- 20.Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 21.Panizo S, Cardus A, Encinas M, Parisi E, Valcheva P, Lépez-Ongil S, Coll B, Fernandez E, Valdivielso JM. RANKL increases vascular smooth muscle cell calcification through a RANK-BMP4-dependent pathway. Circ Res. 2009;104:1041–1048. doi: 10.1161/CIRCRESAHA.108.189001. [DOI] [PubMed] [Google Scholar]
- 22.Aicher A, Kollet O, Heeschen C, Liebner S, Urbich C, Ihling C, Orlandi A, Lapidot T, Zeiher AM, Dimmeler S. The Wnt antagonist Dickkopf-1 mobilizes vasculogenic progenitor cells via activation of the bone marrow endosteal stem cell niche. Circ Res. 2008;103:796–803. doi: 10.1161/CIRCRESAHA.107.172718. [DOI] [PubMed] [Google Scholar]