Outline
Sleep consists of quiescent periods with reduced responsiveness to external stimuli. Despite being maladaptive in that when asleep, animals are less able to respond to dangerous stimuli, sleep behavior is conserved in all animal species studied to date. Thus, sleep must be performing at least one fundamental, conserved function that is necessary, and/ or whose benefits outweigh its maladaptive consequences. Currently, there is no consensus on what that function might be. Over the last 10 years, multiple groups have started to characterize the molecular mechanisms and brain structures necessary for normal sleep in Drosophila melanogaster. These researchers are exploiting genetic tools developed in Drosophila over the past century to identify and manipulate gene expression. Forward genetic screens can identify molecular components in complex biological systems and once identified, these genes can be manipulated within specific brain areas to determine which neuronal groups are important to initiate and maintain sleep. Screening for mutations and brain regions necessary for normal sleep has revealed that several genes that affect sleep are involved in synaptic plasticity and have preferential expression in the mushroom bodies (MB). Moreover, altering MB neuronal activity alters sleep. Previous genetic screens found that the same genes enriched in MB are necessary for learning and memory. Increasing evidence in mammals, including humans, points to a beneficial role for sleep in synaptic plasticity, learning and memory. Thus, results from both flies and mammals suggest a strong link between sleep need and wake plasticity.
Using Drosophila to understand sleep mechanisms and functions
Sleep is conserved across all the animal species that have been carefully studied so far (Cirelli and Tononi 2008), and is necessary to maintain cognitive function and performance (Killgore 2010). Yet, why and how sleep benefits the brain remains unclear. This surprising deficit arises from a simple fact, the brain is extremely complicated and the methods to directly assessing the effects of sleep on brain functioning with high (cellular) resolution are limited and still suffer from many technical limitations. For instance, repeated in vivo two-photon imaging have recently been used in zebrafish (Appelbaum et al. 2010) and mice (Maret et al. 2011) to study how axonal terminals and single dendritic spines are affected by sleep and wake, but the analysis remains so far confined to few superficial areas of the brain. Most sleep studies have been conducted in mammals, especially rats and mice, and, to a much less extent, in birds (Lesku et al. 2011). Sleep in mammals and birds shows electroencephalographic (EEG) patterns similar to those observed in human sleep: during non-rapid eye movement (NREM) sleep, which accounts for most of sleep, large slow waves predominate, while REM sleep is characterized by an “activated” high frequency low voltage pattern similar to that of wake. The rodent brain, however, is still very complex and genetic molecular techniques have only recently been developed to probe the cellular mechanisms underlying sleep functions. This is why the use of simpler model organisms, including the zebrafish Danio reiro and the nematode Caenorhabditis elegans, has been of great help (Cirelli and Tononi 2008, Zimmerman et al. 2008, Harbison et al. 2009, Crocker and Sehgal 2010).
Here we focus on Drosophila melanogaster as a simple system to investigate sleep. Work over the last 30 years has demonstrated that fruit flies are a practical system to explore complex behavior, including circadian rhythms. By using a combination of forward and reverse genetics to isolate fly mutants with abnormal circadian behavior, neurogenetists were able to characterize the complex transcriptional feedback system at the basis of the circadian molecular clock, and to identify mammalian orthologues (Peschel and Helfrich-Forster 2011). Current studies of fly sleep mainly rely on the same infrared-based technology originally designed to study circadian behavior, but with higher temporal resolution (secs/mins rather than hours/days). Sleep/wake are defined based on measures of locomotor activity: sleep is defined as any period of immobility > 5 min, because flies quiescent for > 5 min have a reduced arousal threshold, which is the essential feature that distinguishes sleep from quiet wake (Cirelli et al. 2005a, Bushey et al. 2007, Bushey et al. 2009).
The first papers on sleep/rest in Drosophila confirmed that the rest behavior observed in fruit flies shares most of the features of sleep in humans (Hendricks et al. 2000, Shaw et al. 2000). Quiescent periods in flies were entrained by the 24-hour circadian cycle, occurring primarily at night. Interfering with these quiescent periods resulted in a homeostatic response with increased rest the following day. Sleep deprivation also resulted in decreased performance. Hypnotics and some stimulants (i.e. caffeine and modafinil) produced similar affects on sleep in Drosophila as they did in mammals. Moreover, quiescent periods were more abundant in young flies than in older flies. Together these results suggested that sleep behavior is present in flies, and may involve at least some of the same biochemical pathways known to affect sleep in mammals (reviewed in (Cirelli 2003, Shaw 2003, Ho and Sehgal 2005)). Based on these encouraging results, several laboratories started genetic screens, using both reverse and forward approaches, searching for fly mutants with either reduced sleep time or with alterations in the homeostatic response to sleep deprivation.
Successful (and less successful) screenings of sleep phenotypes in flies
As we will discuss below, the sleep screens are primarily concentrating on total sleep time over the 24-hour period (Cirelli et al. 2005a, Koh et al. 2008, Wu et al. 2008). Our group has also tried to identify mutations that specifically affect the homeostatic regulation of sleep by studying the response to 24 hours of sleep loss. Flies were allowed to recover starting at light onset, i.e. in the morning when they are normally awake. This is because flies sleep a lot during the night even in baseline conditions, and thus a further increase in sleep after sleep deprivation may not occur due to a ceiling effect. Almost all mutant lines tested so far showed an increase in sleep duration and a decrease in sleep fragmentation after 24 hours of sleep deprivation. As in wild-type flies, the sleep rebound was most pronounced during the first 4–6 hours immediately after the end of the sleep deprivation period, and in most cases did not persist the second day after sleep loss. Similarly to wild-type flies, most mutant lines only recovered a small fraction (10–40%) of the sleep lost. We have over the past several years identified several putative “sleep deprivation” mutants, but in all cases this phenotype was not confirmed after repeated testing. Most likely, this is because even in wild-type flies sleep rebound is extremely variable. Genetic screens work best when variation falls within a very narrow range and thus outliers can be readily identified. When a phenotype is variable and outliers are not easily discernable, false positives cannot be tested quickly enough to efficiently identify variations due to mutations. So thus far, screening specifically for homeostatic mutants has not been successful (but mutants that are both short sleeping and show abnormal response to sleep loss have been identified, see below).
Another likely reason why screening for sleep deprivation mutants has failed is due to the fact that in flies it is easier to assess sleep quantity than sleep quality (intensity). Even in mammals, the strongest and most consistent effects of sleep loss are not on total sleep duration (which may or may not increase, depending on the duration of sleep deprivation and on the time of day when animals are allowed to recover sleep), but on sleep depth: after sleep deprivation, sleep is more consolidated (with less fragmentation due to brief awakenings), and more “intense”. In birds and mammals, this intensity is best measured using slow wave activity (SWA), which is defined as the EEG power spectrum in the 0.5 to 4 Hz range during NREM sleep: the longer and/or more “intense” is wake, the higher is SWA at sleep onset (Achermann and Borbely 2003). Moreover, SWA changes are larger in the brain areas more directly affected by the wake experience. For example, birds watching for the first time a movie with only one eye open show increased SWA in the visual brain controlateral to the open eye (Lesku et al. 2011). Also, in humans and rats, learning a motor task increases SWA specifically in the trained cortical area, and does so more than performing a previously learned task (Huber et al. 2004a, Hanlon et al. 2009). Interestingly, social enrichment increases sleep time also in Drosophila, demonstrating that experience in flies also contributes to sleep need (Ganguly-Fitzgerald et al. 2006). In flies, however, we can measure some aspects of sleep depth (e.g. brief awakenings)(Huber et al. 2004b), but sleep/wake electrophysiological recordings are invasive and difficult (Nitz et al. 2002, van Swinderen et al. 2004), and no attempt has been done so far to implement “high-density” recordings as it is done in mammals.
A likely source of variability when studying the sleep deprivation phenotype also stems from the complex interaction between circadian and homeostatic factors, the influence of which can vary to a variable extent across individual flies. When allowing flies to sleep rebound in the morning, the homeostatic process will promote sleep, while the circadian process will oppose it. The influence of the latter can be removed genetically by using mutations affecting key canonical circadian genes. Some of these mutant flies (tim01, per01, Clkjrk), which are arrhythmic (they sleep throughout the 24-hour cycle), show prominent (100%, as compared to 30–40% in wild-type canton-S flies) rebound after 12–24 hours of sleep deprivation in dark only conditions (Shaw et al. 2002). cyc01 mutants are unique, in that they show an exaggerated sleep rebound after as little as 3 hours of sleep deprivation, and die after longer periods of sleep loss (Shaw et al. 2002). cyc01 mutants have reduced heat shock protein expression and mutations affecting hsp83, a heat shock chaperone, also result in an exaggerated homeostatic response and death after sleep deprivation, demonstrating an important role for stress response genes in protecting against the lethal effects of sleep loss (Shaw et al. 2002).
Sleep can be extremely variable because it is a complex behavior dependent on both genetic factors and experience in both mammals and Drosophila. Genetic background can be controlled for by testing flies in a common genetic background. Over successive generations mutants can be backcrossed into a specific background and compared to wild-type flies from the same background, or comparisons can be made between siblings that inherit a common background. However, it is much harder to control for “quality of wake”, i.e. individual experiences. Experience in the small glass tubes where flies are normally housed for sleep recordings (one fly/tube) is theoretically uniform, but the visual/mechanical/acoustic stimuli used during sleep deprivation may introduce a new level of variation. On the other hand, the glass tubes represent a very impoverished environment, where flies can only move a few cm, and cannot fly. As we will see below, compelling evidence in mammals and flies now shows that sleep is necessary to process/mitigate the effects of experience on neuronal function (e.g. (Huber et al. 2004a, Ganguly-Fitzgerald et al. 2006, Hanlon et al. 2009). If so, then testing in a uniform, impoverished environment may reduce our ability to assess the effects of sleep on brain functions. In line with this, learning mutants do not show increases in sleep time after social experience (Ganguly-Fitzgerald et al. 2006). Unfortunately, it is difficult to design an automated fly tracking system that can also monitor multiple flies in complex environment, but an effort in this direction should definitively be made.
A Reverse Genetic Screen Demonstrates the Role of the cAMP/PKA/CREB Pathway in Sleep Regulation
In flies a role in sleep regulation was first demonstrated for the cAMP/PKA/CREB pathway using reverse genetics (Hendricks et al. 2001). In reverse genetics a candidate gene is mutated first, and then the effects of the mutation on sleep are assessed (from genotype to phenotype). Candidate genes are chosen based on their known function, which makes them likely to be relevant for sleep. The cAMP/PKA/CREB pathway was a good candidate because it was known that cAMP levels and CREB activity are important for learning and memory (discussed below), and that CREB expression in the fly brain shows a circadian pattern. Furthermore, CREB responsive transcription has a diurnal cycle. Hendricks and colleagues (Hendricks et al. 2001) tested previously identified mutations and found that increasing cAMP or CREB activity decreased both sleep time and sleep rebound after sleep deprivation, while inhibiting cAMP or CREB had the opposite effect (Table 1). Furthermore, they found that sleep deprivation increased CREB driven reporter expression. A later study in mice found that mutations knocking down 2 of the 3 CREB mouse isoforms increased sleep time (Graves et al. 2003). Finally, a more recent study in mice found that a few hours of sleep deprivation selectivity impair long-term synaptic potentiation (LTP) in the hippocampus, and do so specifically for the LTP forms that require cAMP and PKA signaling (Vecsey et al. 2009). The study also demonstrated that the LTP impairment due to sleep loss resulted from increased nucleotide phosphodiesterase (PDE) activity (which breaks down cAMP), because selective PDE inhibition was able to rescue the LTP deficits, as well as the deficits in a hippocampus-dependent memory task.
Table 1.
Mutations affecting Drosophila sleep, and their effects on learning and memory.
| Total sleep time |
Arousal Threshold |
Response to sleep deprivation (homeostatic response) |
References (effects on sleep) |
Learning/memory | References (effects on learning/memory) |
Brain areas with high expression |
Reference | Demonstrated ability to rescue memory deficits |
References | |
|---|---|---|---|---|---|---|---|---|---|---|
| Potassium Channels and genes affecting Shaker currents | ||||||||||
| Shaker | Reduced sleep (LOF) | Normal | Normal | (Cirelli et al. 2005a) | Impaired courtship conditioning; Impaired aversive olfactory learning; Impaired short-term memory | (Bushey et al. 2007) (Cowan and Siegel 1984) (Cowan and Siegel 1986) |
MB | (Rogero et al. 1997) (Schwarz et al. 1990) |
||
| quiver/sleepless | Reduced sleep (LOF) | No response to sleep deprivation | (Koh et al. 2008) | MB | (Wu et al. 2010) | |||||
| Hyperkinetic | Reduced sleep (LOF) | Normal | Normal | (Bushey et al. 2007) | Impaired short-term memory | (Bushey et al. 2007) | ||||
| Neurotransmitters | ||||||||||
| Dopamine transporter (DAT) | Reduced sleep (fumin) | Reduced at low stimulation | Normal | (Kume et al. 2005) (Wu et al. 2008) |
Poor memory retention | (Zhang et al. 2008) (Seugnet et al. 2008) (Waddell 2010) (Schwaerzel et al. 2003) |
D1 receptor expression | (Seugnet et al. 2008) | ||
| Octopamine biosynthesis: Tyrosine decarboxylase 2 (Tdc2) / Tyramine β hydroxylase (Tbh) | LOF mutations increase sleep; feeding flies octopamine decreases sleep | Increased | (Crocker and Sehgal 2008) | Impaired appetitive olfactory memory | (Schwaerzel et al. 2003) | Octopamine receptor enriched in MB | (Han et al. 1998) | |||
| d5-HT1A serotonin receptor | Reduced sleep time Increasing serotonin levels increases sleep | (Yuan et al. 2006) | ||||||||
| cAMP\PKA\CREB pathway | ||||||||||
| Dunce (3',5'-cyclic-AMP phosphodiesterase activity) | Reduced sleep (LOF) | (Hendricks et al. 2001) | Impaired aversive olfactory memory | (Dudai et al. 1976) (Qiu and Davis 1993) |
MB | (Qiu and Davis 1993) | ||||
| Rutabaga (adenylate cyclase activity) | Increased sleep (LOF); LOF mutations prevent experience dependent increases in sleep | (Hendricks et al. 2001, Donlea et al. 2009) | Impaired memory | (Schwaerzel et al. 2003) (Blum et al. 2009) |
MB | (Han et al. 1992) | MB, PDF | (Schwaerzel et al. 2003) (Blum et al. 2009, Donlea et al. 2009) blist |
||
| Cyclic-AMP response element binding protein 2 (Creb2) | Increased/decreased sleep | Increased sleep rebound after sleep loss | (Hendricks et al. 2001) | Impaired (OE enhances long-term memory?) | (Yin et al. 1995) (Perazzona et al. 2004) (Sakai et al. 2004) |
Enriched in cell bodies, not in neuropil | (Yin et al. 1995) | |||
| cAMP-dependent protein kinase 1 (PKA-C1) | Expression of a constitutively active form reduces sleep | (Joiner et al. 2006) | Impaired learning; Heterozygotes have normal memory and are resistant to age-related memory impairment | (Skoulakis et al. 1993) (Yamazaki et al. 2007) |
MB | (Skoulakis et al. 1993) | ||||
| Epidermal growth factor | ||||||||||
| Epidermal growth factor receptor (EGFR) | LOF mutations prevent experience dependent increases in sleep | (Donlea et al. 2009) | ||||||||
| rhomboid (Rho) | OE increases sleep (in combination with star); LOF mutations reduce sleep | Dominant negative mutants recover less sleep after sleep deprivation | (Foltenyi et al. 2007) | Pars Intercerebralis | (Foltenyi et al. 2007) | |||||
| Star | OE increases sleep (in combination with rho) | (Foltenyi et al. 2007) | ||||||||
| s-Spitz | OE mutations increase sleep | (Foltenyi et al. 2007) | ||||||||
| Steroids | ||||||||||
| ecdysone receptor (EcR) | LOF mutations reduce sleep | LOF mutations reduce change in sleep bout duration after sleep loss | (Ishimoto and Kitamoto 2010) | Impaired courtship | (Ishimoto et al. 2009) | OE in MB increases sleep; LOF in MB impairs long-term memory | (Ishimoto et al. 2009) | |||
| molting defective (mld, DTS-3) | LOF mutations reduce sleep | LOF mutations reduce change in sleep bout duration after sleep loss | (Ishimoto and Kitamoto 2010) | |||||||
| mRNA transport and control | ||||||||||
| dFmr1 | LOF mutations increase sleep; OE decreases sleep | LOF or OE increase arousal threshold | LOF or OE reduce sleep rebound | (Bushey et al. 2009) | Olfactory learning and memory impaired, Impaired courtship | (Bolduc et al. 2008) (McBride et al. 2005) |
MB | (Schenck et al. 2002) | Greatest effect on sleep is in MB | (Bushey et al. 2009) |
| Heat shock proteins/chaperones | ||||||||||
| Binding immunoglobulin protein (BiP) | No effect | OE increases sleep rebound, LOF mutations reduce sleep rebound | (Naidoo et al. 2007) | |||||||
| hsp83 | No effect | LOF mutations increase sleep rebound | (Shaw et al. 2002) | |||||||
| Circadian genes/genes expressed in circadian neurons | ||||||||||
| cycle | LOF mutations increases sleep during starvation | LOF mutations show high mortality rate after sleep deprivation and increased sleep rebound | (Shaw et al. 2002) (Keene et al. 2010) |
|||||||
| period | Necessary for experience dependent increases in sleep | Normal | (Donlea et al. 2009) (Shaw et al. 2002) (Thimgan et al. 2010) |
Impaired long-term memory and courtship (OE enhances it) | (Sakai et al. 2004) | |||||
| Clock | LOF mutations increases sleep during starvation | (Keene et al. 2010) | ||||||||
| blister (bs, serum response factor) | LOF mutations show no increase in sleep after social enrichment | (Donlea et al. 2009) | Expressed throughout the brain but rescue in sLNvs | (Donlea et al. 2009) | Expressed throughout brain but rescue in sLNvs | (Donlea et al. 2009) | ||||
| Pigment-dispersing factor (Pdf) | LOF mutations increase sleep | (Parisky et al. 2008) | ||||||||
| Resistant to dieldrin (Rdl) | Reduced sleep (LOF); increased sleep (GOF) | (Parisky et al. 2008) (Agosto et al. 2008) |
||||||||
| Chromatin Structure/Microtu bule dynamics | ||||||||||
| Elongator Protein 3 | LOF mutations reduce sleep | (Singh et al. 2010) | (no studies on memory) Increases bouton number at NMJ | (Singh et al. 2010) | ||||||
| Triglyceride Storage | ||||||||||
| Lipid Storage Droplet 2 (Lsd-2) | LOF mutations reduce sleep | LOF mutations show no sleep rebound | (Thimgan et al. 2010) | Fat Body | (Gronke et al. 2003) | |||||
| brummer | No effect | LOF mutations show increased sleep rebound | (Thimgan et al. 2010) | Fat Body | (Gronke et al. 2005) |
LOF, loss of function; GOF, gain of function; OE, overexpression
A Forward Genetic Screen Identifies Shaker
A major barrier to studying sleep is the ignorance concerning the specific molecular events occurring during this behavioral state as compared to wake. Forward genetics is an unbiased approach in which prior knowledge of the “important” genes is not required, and novel genes can thus be discovered (Cirelli 2009). The starting point is the phenotype, and a significant part of the work involves going back to the genotype to identify the responsible gene (from phenotype to genotype). Forward genetic methods include quantitative trait loci (QTLs) analysis and mutagenesis screening. In the latter random small mutations are induced over the entire genome, and hundreds/thousands of mutated individuals are screened for the phenotype of interest. Insertional mutagenesis uses transposable elements (in flies) to induce mutations, while chemical mutagenesis uses ethylmethane sulfonate (EMS, in flies) or N-ethyl N-nitrosourea (ENU, in mice).
In our screen mutant flies are continuously recorded for one week, including 2–3 baseline days, 24 hours of sleep deprivation, and 1–3 days of recovery after sleep deprivation. Ten to sixteen flies (4–7 day old at the beginning of the experiment) are tested for each line. This relatively high number of flies is needed because sleep pattern and sleep amount, although consistent across different days in each individual adult fly, may vary among different flies. The analysis of thousand of lines (> 15,000) has confirmed a significant difference between male and female flies: females sleep almost exclusively during the night, while males also show a long period of sleep in the middle of the day. The daily amount of sleep in the mutant lines tested so far shows a normal distribution, with female flies for most lines sleeping between 400 and 800 min/day, with a mean of ~ 600 min, similar to that of wild-type flies.
Our first EMS screen looked for short sleeping male flies. Males were the first choice because hemizygous (containing only the mutagenized X-chromosome) could be generated and tested. In genetic screens, the likelihood of identifying a mutation depends on the number of individual mutagenized chromosomes that can be tested. This is why Drosophila is so amply used in genetic screens, since hundreds of individuals containing a given mutagenized chromosome can be quickly generated and tested. The first X-chromosome EMS screening identified a short sleeping line carrying minisleep, a mutation in Shaker (Sh), a gene coding the alpha subunit of a voltage-dependent potassium channel (Cirelli et al. 2005a)(Table 1). The screen used restrictive criteria to select short sleeping mutants, with sleep being required to decrease by at least two standard deviations from the mean of all tested lines. Thus, the screen did not identify other genes on the X chromosome, such as dunce and Hyperkinetic (Hk), whose loss also results in a short sleeping phenotype (Hendricks et al. 2001, Bushey et al. 2007), but not as pronounced as that seen in Shaker mutants. Of note, Hk encodes the beta (regulatory) subunit of the Shaker channel, and null Hk mutations decrease but do not abolish the Shaker current (since the alpha subunit is still functioning). Both Sh and Hk loss of function (null/hypomorphic) mutants that have a short sleeping phenotype also have short-term memory deficits in the heatbox paradigm, as well as decreased lifespan (Bushey et al. 2010). Further studies also found that short sleeping Sh mutants were also more resistant to volatile anesthetics (Tinklenberg et al. 1991, Weber et al. 2009).
Channels homologous to Sh in vertebrates have similar properties and, in both mammals and flies, the Sh current plays a major role in the control of membrane repolarization and transmitter release. Consistent with the results in flies, knocking out the Sh orthologue in mice, Kcna2, results in reduced NREM sleep (Douglas et al. 2007). However, the Kcna2 short sleeping phenotype is far from being as dramatic as in Shaker flies, perhaps because of redundancy - there is one Shaker gene in Drosophila, but at least 16 genes code for alpha subunits of voltage-dependent potassium channels in mammals (Misonou and Trimmer 2004, Yuan and Chen 2006). Finally in mice, the injection of an antibody against the kv1.2 potassium channel into the central medial thalamus induces arousal from anesthesia (Alkire et al. 2009).
After the identification of minisleep an independent genetic screen using insertional mutagenesis identified Sleepless, a mutation in the quiver (qvr) locus, which also shows very significantly reduced sleep time (to only ~ 2 hours a day, 85% less than controls) (Koh et al. 2008). qvr codes a ly-6/neurotoxin family member and its loss reduces Shaker localization, kinetics, and current density (Wu et al. 2010). Thus, two independent genetic screens identified a major role for the Shaker current in sleep regulation. There are nevertheless some interesting differences between Shaker and Sleepless flies, most notably that only the latter show a reduced homeostatic response, as indicated by no changes in sleep duration after sleep deprivation.
Identified sleep mutants suggest a link between sleep and brain plasticity, especially in the mushroom bodies
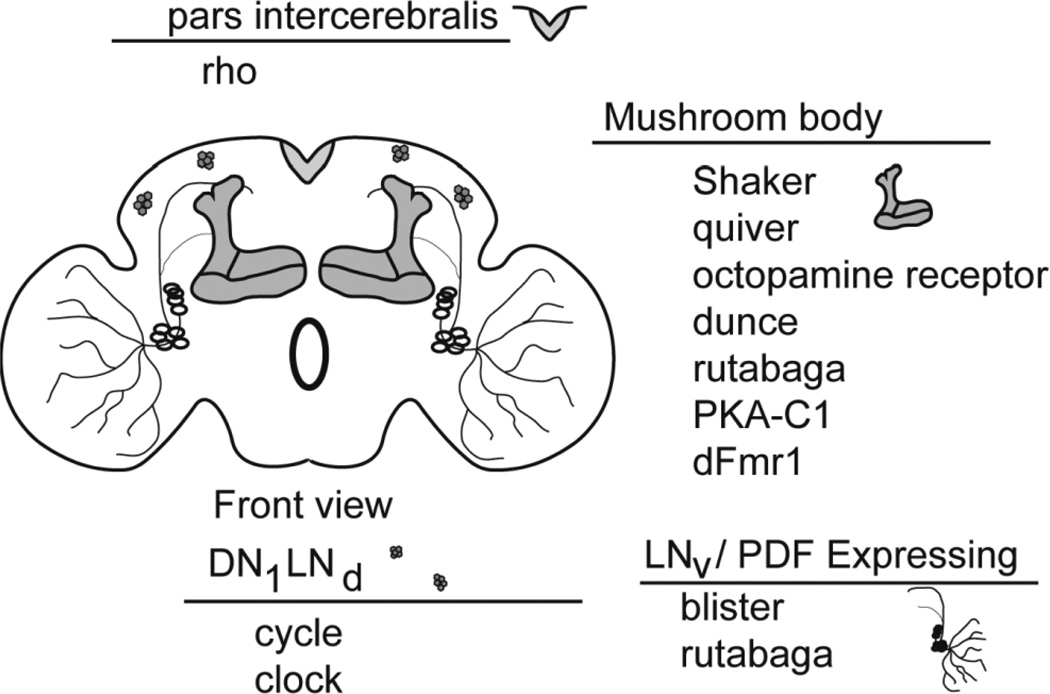
Table 1 lists most of the sleep mutants identified in either forward or reverse genetic screens, and the distribution of most of the corresponding genes is shown in Figure 1. The isolated genes include those coding for the voltage-dependent potassium channel Shaker, several neurotransmitters, molecules that are part of the cAMP pathway, steroids, heat shock proteins/chaperones, circadian proteins, and proteins involved in mRNA transport (Fmr1) and chromatin structure. In most cases, previous research had found that these “sleep” genes are also important for learning and/or memory, and many are enriched in the mushroom bodies (MB), a brain area crucial for olfactory learning and memory. A genetic screen that restricted UAS-shiTS1 expression within specific neuronal groups, to block synaptic transmission in specific brain regions, found that silencing activity in the MB produced the greatest reduction in sleep time (Pitman et al. 2006). Another group independently demonstrated the importance of the MB in sleep regulation after screening GAL4 lines in combination with the UAS-mc* transgene, which expresses a constitutively active PKAc isoform (Joiner et al. 2006). Finally, blocking neurotransmission within the MB protects against the impairment in learning caused by sleep deprivation (Li et al. 2009).
Figure 1.
“Sleep” genes and their distribution in the fly brain.
For most of these mutations, transgenic rescue experiments confirm that the gene products are indeed necessary within the MB to restore normal learning and memory (Table 1). The dual role in sleep and learning of two neurotransmitters, octopamine and dopamine, and their effects on the MB through activation of the PKA pathway, has been especially well characterized. Octopamine and dopamine are necessary for normal learning in appetitive and aversive learning, respectively (Schwaerzel et al. 2003), and PKA may interact with Shaker and quiver to modulate excitability in the MB (Yao and Wu 2001). The fragile x-mental retardation (Fmr1) gene product is also enriched in the MB (Schenck et al. 2002). Fmr1 protein product, FMRP, is present in dendritic spines (Feng et al. 1997) and a hallmark of loss of function Fmr1 mutations, in both flies and mammals, is the failure to remove immature synapses (Hinton et al. 1991, Comery et al. 1997, Irwin et al. 2002, Pan et al. 2004, Restivo et al. 2005). Fmr1 overexpression in flies results in the opposite phenotype, with dendritic and axonal underbranching and loss of synapse differentiation (Pan et al. 2004, Pan and Broadie 2007). In a previous study we found that sleep duration increases when Fmr1 function is lost and decreases when Fmr1 is overexpressed, even when overexpression is confined to the MB of adult flies (Bushey et al. 2009).
MB are also closely involved in the temporal and spatial reorganization of cellular memory “traces”, as identified using calcium imaging (Berry et al. 2008). Aversive olfactory conditioning results in immediate memory traces (3–6 min) in the antennal lobe, a region necessary for short-term memory (Yu et al. 2004), while intermediate memory traces occur in the DPM neuron that innervates the MB 30 min to 1 hour after training (Yu et al. 2005). After aversive olfactory conditioning designed to trigger long-term memory, calcium influx occurs in the alpha/beta lobes of the MB within 3 and 9 hours after training, and persists for 24 hours (Yu et al. 2006). Of note, neurotransmission from the alpha/beta lobes is also necessary for memory retrieval (Krashes et al. 2007). The fact that these memory traces can persist over a 24-hour period suggests that sleep may have a functional role, perhaps to favor the reorganization of memories across different brain circuits, similar to the “reactivation and redistribution of hippocampus-dependent memories to neocortical sites” proposed to occur during NREM sleep, at least in mammals (Diekelmann and Born 2010, Rattenborg et al. 2010). Unfortunately, calcium imaging experiments are invasive, and not conducive to analysis of physiological behavioral states. Thus, it remains unknown whether sleep is important for the occurrence/transfer of these memory traces.
A recently characterized general mechanism that acts systemically to control memory formation and sleep need is that of the steroid hormone ecdysone (Table 1). Ecdysone coordinates post-embryonic development and is necessary for neuronal rewiring (Hewes 2008). Administering ecdysone to adult flies increases sleep time in a dose dependent manner, while blocking ecdysone synthesis (DTS-3) or the ecdysone receptor (EcR) reduces sleep time (Ishimoto and Kitamoto 2010). Adding ecdysone during training also enhances courtship long-term memories, while adding ecdysone after training impairs memory (Ishimoto and Kitamoto 2010). Both experience in the courtship conditioning assay and sleep deprivation increase ecdysone levels, while overexpressing the ecdysone receptor within the MB increases sleep time, and targeted knockdown using RNAi impairs long-term memory. Finally, treatment with ecdysone enhances CREB-dependent expression (Ishimoto and Kitamoto 2010). Overall, these results show that the ecdysone system may represent a systemic mechanism capable not only of controlling brain plasticity in response to environmental stimulation, especially in the MB, but also of directly affecting sleep.
Distinct circadian neurons promote either wake or sleep
Like humans, flies are more active during the day, and show the longest and most consolidated sleep bouts at night, even in constant darkness. Treating flies with a GABA antagonist results in increased sleep latency and decreased total sleep time, while mutations in the Drosophila GABAA receptor (Rdl) that increase the time the channel remains open result in reduced sleep latency and longer sleep (Agosto et al. 2008)(Table 1). The Rdl GABAA receptors are expressed in the ventral lateral clock neurons (LNvs) (Parisky et al. 2008), which express the peptide pigment dispersing factor (PDF). PDF immunoreactivity peaks at the beginning of the light period (Park et al. 2000), consistent with a role for PDF in initiating and/or maintaining arousal. In line with this, reducing PDF expression and enhancing neuronal excitability decrease sleep time (Parisky et al. 2008). It has been suggested that PDF may play in Drosophila a role similar to that played in mammals by the arousal-promoting peptide orexin/hypocretin (Sakurai 2007).
Drosophila clock neurons have been shown to regulate sleep time in a complex way that is more than a simple reflection of the time of day. For instance, social enrichment in older flies results in longer sleep, but only if the expression of Rutabaga and period in PDF expressing neurons is normal (Donlea et al. 2009). Also, starvation increases wakefulness in Drosophila as it does in mammals, (Keene et al. 2010, Thimgan et al. 2010), but the clock neurons that do not express PDF (DN1s or LNds neurons) promote sleep during starvation and their loss enhances sleep loss during starvation (Keene et al. 2010). Of note, sleep loss due to starvation is not followed by a sleep rebound when flies are placed back on their normal diet (Thimgan et al. 2010). Also, in contrast to sleep deprivation due to mechanical or other kinds of stimulation, starvation does not impair performance in an aversive phototaxis memory test (Thimgan et al. 2010). Moreover, lipid storage droplet 2 (Lsd-2) mutants that show reduced triglycerides storage sleep less on normal media and are not significantly impaired in the aversive phototaxis memory test after sleep deprivation (performance in Lsd2 mutant flies is slightly lower than wild-type controls, but still in the range of wild-type flies) (Thimgan et al. 2010). However, starvation in these experiments never lasted more than 12 hours, leaving open the possibility that longer fasting may cause sleep rebound and/or memory impairment. Altogether, these results show that different groups of circadian neurons in Drosophila can either promote wake or sleep, in addition to affect neuronal plasticity in response to social enrichment.
i.
Fly sleep and hypotheses on sleep functions
Sleep is perhaps the only major behavior still in search of a function. While the entire body certainly benefits from sleep (Knutson et al. 2007), most researchers agree that sleep may be especially important for the brain and supply something not provided by quiet wake. There is great uncertainty, however, when it comes to which chemical or molecular pathway in the brain may be depleted during wake and restored during sleep or, alternatively, about which toxic substance might accumulate during wake and dissipate during sleep. For instance, it was suggested that sleep favors the replenishment of glycogen in glial stores (Benington and Heller 1995), but recent evidence show that this may be the case only in a few brain regions, and not in all strains of mice (Franken et al. 2003, Franken et al. 2006). The demonstration that flies sleep, and the subsequent identification of several fly sleep mutants (Table 1), have already contributed to the ongoing debate concerning sleep functions, as will be discussed below.
As mentioned above, growing evidence in mammals including humans, birds, and flies points to a link between sleep need and neuronal plasticity, but why and how sleep may benefit the brain by modifying synapses remains unclear. There are three main hypotheses on how sleep could do so, which suggest a role for sleep in synaptic homeostasis (Tononi and Cirelli 2006), macromolecule synthesis (Mackiewicz et al. 2009), and memory consolidation (Diekelmann and Born 2010). Importantly, these hypotheses are not mutually exclusive. For instance, there is compelling evidence that sleep benefits several forms of memory (Diekelmann and Born 2010), but the underlying mechanisms remain unclear, and could involve the specific strengthening of a few synapses already potentiated during wake, and/or, as hypothesized by the synaptic homeostasis hypothesis, a more generalized downregulation of synaptic strength during sleep, with subsequent increase in the signal to noise ratio (details in (Tononi and Cirelli 2006)).
Some early clues about the functions of sleep were obtained by considering the extensive changes in brain gene expression that occur between sleep and wake or after sleep deprivation (Cirelli and Tononi 2000b, Terao et al. 2003a, Terao et al. 2003b, Cirelli et al. 2004, Cirelli et al. 2005c, Cirelli et al. 2006, Terao et al. 2006, Zimmerman et al. 2006, Mackiewicz et al.,Maret et al. 2007, Jones et al. 2008, Mackiewicz et al. 2009, Nikonova et al. 2010). In all species studied so far (flies, mice, rats, hamsters, and sparrows) wake leads to the upregulation of transcripts involved in the response to cellular stress and in activity-dependent processes of synaptic potentiation. By contrast, transcripts expressed at higher levels during sleep are involved in synaptic depression, in the synthesis/maintenance of membranes and in lipid metabolism, including the synthesis and transport of cholesterol (Cirelli et al. 2004, Mackiewicz et al. 2007, Mackiewicz et al. 2009). Lesions of the locus coeruleus that deplete the cerebral cortex of noradrenaline, which promotes arousal, attention and the response to novelty (Sara 2009), blunt the homeostatic response to sleep deprivation and abolish the upregulation of a few wake-related genes, mostly plasticity-related genes (Cirelli et al. 1996, Cirelli and Tononi 2004, Cirelli et al. 2005b). Of note, adrenalectomy has almost opposite effects: sleep homeostasis is not affected, but ~2/3 of all wake-related transcripts are no longer upregulated by sleep deprivation, with the notable exception of some stress response genes including BiP and plasticity-related genes (Mongrain et al. 2010). While these results may at first appear disparate, they may also reflect a coherent set of functional changes at the cellular level. One way to make sense of these findings is in terms of plastic processes. Specifically, in the synaptic homeostasis hypothesis we have suggested that during wake there is a net increase in synaptic strength in many brain areas; these plastic changes are a major determinant of sleep need and sleep would be needed to renormalize such changes (Tononi and Cirelli 2003, Tononi and Cirelli 2006). Why would wake result in a net increase in synaptic strength? Because the awake brain is always “learning”, whether it is performing a learning task or simply adapting to an ever-changing environment, and novelty exposure, enrichment and learning mostly occur through synaptic potentiation, not synaptic depression (Nithianantharajah and Hannan 2006, Feldman 2009). Moreover, wake is associated with high levels of acetylcholine and noradrenaline (Jones 2005), which together favor synaptic potentiation (Cirelli et al. 1996, Cirelli and Tononi 2000a, Seol et al. 2007).
Why would then sleep be needed to revert the net increase of synaptic strength at the end of wake? Because such increase would result in higher energy consumption (Attwell and Laughlin 2001, Rothman et al. 2003), in larger synapses that take up precious space (Chklovskii et al. 2002), and in the saturation of the capacity to learn. Also, a net strengthening of synapses likely represents a major source of cellular stress, due to the need to synthesize and deliver cellular constituents ranging from mitochondria to synaptic vesicles to various proteins and lipids (Mackiewicz et al. 2009). In this view, then, sleep would be necessary to downregulate synapses to a baseline level that is sustainable and ensures cellular homeostasis. Importantly, downregulation would have to occur off-line, i.e. during sleep, because it should affect most synapses, whether or not they are engaged in behavior. How would sleep bring about a net decrease in synaptic strength? One obvious cellular mechanism is the reduced level of neuronal excitability that characterizes most neurons during most of sleep. During NREM sleep, which accounts for 70–80% of all sleep in mammals, neurons are relatively hyperpolarized and fire less as compared to wake (Steriade 2003), including neurons that release noradrenaline, serotonin, hypocretin and histamine (Jones 2005, Saper et al. 2010). Synaptic downregulation may be favored by periods of neuronal silence (Kemp and Bashir 2001, Werk and Chapman 2003, Birtoli and Ulrich 2004, Rosanova and Ulrich 2005, Werk et al. 2006, Czarnecki et al. 2007, Lubenov and Siapas 2008), as well as by the low levels of noradrenaline, serotonin, hypocretin and histamine (Harley 1991, Seol et al. 2007). These electrophysiological and biochemical changes across the sleep/wake cycle are well characterized in mammals, but recent evidence shows that they also occur in flies (Crocker and Sehgal 2010). For instance, Drosophila neurons in the medial brain, as well as LNvs neurons, which release the wake-promoting neuropeptide PDF (pigment dispersing factor), are more active in wake than in sleep (Nitz et al. 2002, Sheeba et al. 2008). Moreover, noradrenaline is wake-promoting in mammals as octopamine, its equivalent in insects, is wake-promoting in flies, an effect mediated in both species by the cAMP/PKA/CREB signaling pathway (Hendricks et al. 2001, Graves et al. 2003, Crocker and Sehgal 2008, Crocker et al. 2010). Specific molecular mechanisms that may mediate synaptic renormalization are also conserved between mammals and flies, including the gene Fmr1 (Fragile X mental retardation 1), for which extensive evidence is available (Hinton et al. 1991, Comery et al. 1997, Irwin et al. 2002, Pan et al. 2004, Restivo et al. 2005).
The evidence supporting the synaptic homeostasis hypothesis comes from mammals as well as flies. In rats, a recent study examined molecular and electrophysiological markers of synaptic function during sleep and wake (Vyazovskiy et al. 2008). It was found that the levels of AMPA receptors in cortical synaptoneurosomes decrease by ~40% after several hours of sleep (Vyazovskiy et al. 2008). Phosphorylation changes of AMPA receptors, and of the enzymes CamKII and GSK3β, were also in line with a net decrease in synaptic strength during sleep. Electrophysiologically, it was shown using cortical electrical stimulation and local field potential recordings that both slope and amplitude of cortical evoked responses (classical in vivo measures of synaptic strength) also decrease after sleep (Vyazovskiy et al. 2008). Similar observations have been made in humans using transcranial magnetic stimulation and high-density electroencephalogram (EEG) analysis (Bellina et al. 2008). Direct evidence for a net decrease in synaptic strength after sleep comes from a recent study in which miniature excitatory postsynaptic currents (mEPSCs) were recorded from frontal cortex slices of mice and rats. Changes in mEPSCs frequency are thought to result from modification of the presynaptic component of synaptic transmission, while amplitude changes indicate alterations in the postsynaptic component (e.g. (Ungless et al. 2001)). It was found that, in both rats and mice, the frequency and amplitude of mEPSCs increase after wake and decrease after sleep (Liu et al. 2010). Recovery sleep after sleep deprivation also decreases mEPSCs, suggesting that sleep brings about a net decrease in synaptic strength (Liu et al. 2010). Finally, mean firing rates in the rat cerebral cortex increase after periods of wake and decrease after periods of sleep, consistent with a net change in synaptic strength (Vyazovskiy et al. 2009). In line with this observation, the levels of glutamate in the rat cortical extrasynaptic space also increase progressively during wake and decrease during NREM sleep (Dash et al. 2009). In line with these mammalian studies, we recently found in flies that overall protein levels of both pre- and postsynaptic components of central synapses are high after wake and low after sleep (Gilestro et al. 2009). These changes are related to behavioral state rather than time of day and occur in all major areas of the Drosophila brain. Moreover, the decrease of synaptic markers during sleep is progressive (Gilestro et al. 2009), consistent with the synaptic homeostasis hypothesis. Another study also found morphological evidence, in a specific neural circuit (large LNvs), for synaptic growth in flies that were sleep deprived for 48h after chronic social enrichment, compared to flies that were left undisturbed (Donlea et al. 2009). Furthermore, new data from our laboratory show that that number/size of synapses in 3 different Drosophila neural circuits is higher after wake and lower after sleep, and that synaptic renormalization can only occur if flies are allowed to sleep, but not if they are sleep deprived (Bushey et al. 2011). Finally, a recent study in zebrafish larvae (Appelbaum et al. 2010) found that presynaptic terminals of hypocretin neurons projecting to the pineal gland undergo both circadian and sleep-wake dependent structural changes, the latter consistent with sleep-dependent downregulation. Finally, the findings from fly mutagenesis screens that genes necessary for brain plasticity are also necessary for sleep are in line with the synaptic homeostasis hypothesis.
The results from fly mutagenesis screens, on the other hand, so far do not provide direct evidence that sleep is necessary for the synthesis of macromolecules such as brain lipids (Mackiewicz et al. 2009). This, however, may be due to a bias for these screens to focus on short sleeping mutants, while loss of function mutations decreasing lipid synthesis should produce long sleepers. Finally, the finding that in both mammals and flies prolonged wake is associated with increased expression of heat shock proteins and chaperones such as BiP may suggest that sleep loss causes the accumulation of unfolded proteins, which then need to be processed and eliminated during sleep. The fact that Drosophila mutations that affect the expression of BiP and hsp83 result in an exaggerated sleep rebound (Table 1) is consistent with this idea. However, there are no data showing that the overexpression of heat shock proteins reduces sleep time in normal physiological conditions (Shaw et al. 2002, Naidoo et al. 2007), suggesting perhaps that abnormal accumulation of unfolded proteins occurs only when wake is prolonged beyond its physiological duration. Yet, in both flies and mammals BiP expression also increases during spontaneous wake, and in sleep deprived mice BiP is still induced after adrenalectomy (Mongrain et al. 2010), suggesting that BiP induction may not be simply a sign of cellular stress. Long-term sensitization training in Aplysia induces BiP (Kuhl et al. 1992), and BiP may be involved in the trafficking of glutamatergic AMPA receptors (Rubio and Wenthold 1999). Moreover, the induction of BiP and of the unfolded protein response promotes the surface expression of GluR1-containing AMPA receptors (Vandenberghe et al. 2005). Thus, BiP induction in the context of the unfolded protein response during wake/sleep deprivation may be the result of a physiological increase in glutamatergic signaling and long-term potentiation.
Conclusion
A decade of screening and studying sleep in Drosophila has provided insight into the molecular pathways involved in both sleep and wake. Consistent with data in mammals including humans, studies in flies show that sleep need is a function of brain plasticity, since genes necessary for neuronal plasticity and brain regions where this plasticity occurs are directly involved in sleep homeostasis. Thus, any hypothesis about sleep functions must explain the strong link between sleep need and plastic changes.
Acknowledgements
Supported by NIGMS (R01 GM075315 to CC), Army Research Office (DURIP Award W911NF-08-1-0169 to CC), and Canadian Institutes of Health Research (to DB).
References
- Achermann P, Borbely AA. Mathematical models of sleep regulation. Front Biosci. 2003;8:s683–s693. doi: 10.2741/1064. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12700054. [DOI] [PubMed] [Google Scholar]
- Agosto J, Choi JC, Parisky KM, Stilwell G, Rosbash M, Griffith LC. Modulation of gabaa receptor desensitization uncouples sleep onset and maintenance in drosophila. Nat Neurosci. 2008;11(3):354–359. doi: 10.1038/nn2046. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18223647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkire MT, Asher CD, Franciscus AM, Hahn EL. Thalamic microinfusion of antibody to a voltage-gated potassium channel restores consciousness during anesthesia. Anesthesiology. 2009;110(4):766–773. doi: 10.1097/aln.0b013e31819c461c. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19322942. [DOI] [PubMed] [Google Scholar]
- Appelbaum L, Wang G, Yokogawa T, Skariah GM, Smith SJ, Mourrain P, Mignot E. Circadian and homeostatic regulation of structural synaptic plasticity in hypocretin neurons. Neuron. 2010;68(1):87–98. doi: 10.1016/j.neuron.2010.09.006. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20920793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21(10):1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Bellina V, Huber R, Rosanova M, Mariotti M, Tononi G, Massimini M. Cortical excitability and sleep homeostasis in humans: A tms/hd-eeg study. J Sleep Res. 2008;17 Suppl.1:39. [Google Scholar]
- Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Progress in Neurobiology. 1995;45:347–360. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- Berry J, Krause WC, Davis RL. Olfactory memory traces in drosophila. Prog Brain Res. 2008;169:293–304. doi: 10.1016/S0079-6123(07)00018-0. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18394482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birtoli B, Ulrich D. Firing mode-dependent synaptic plasticity in rat neocortical pyramidal neurons. J Neurosci. 2004;24(21):4935–4940. doi: 10.1523/JNEUROSCI.0795-04.2004. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15163685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum AL, Li W, Cressy M, Dubnau J. Short- and long-term memory in drosophila require camp signaling in distinct neuron types. Curr Biol. 2009;19(16):1341–1350. doi: 10.1016/j.cub.2009.07.016. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19646879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolduc FV, Bell K, Cox H, Broadie KS, Tully T. Excess protein synthesis in drosophila fragile x mutants impairs long-term memory. Nat Neurosci. 2008;11(10):1143–1145. doi: 10.1038/nn.2175. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18776892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushey D, Huber R, Tononi G, Cirelli C. Drosophila hyperkinetic mutants have reduced sleep and impaired memory. J Neurosci. 2007;27(20):5384–5393. doi: 10.1523/JNEUROSCI.0108-07.2007. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17507560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushey D, Hughes KA, Tononi G, Cirelli C. Sleep duration affects lifespan in drosophila. BMC Neurosci. 2010;11:56. doi: 10.1186/1471-2202-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushey D, Tononi G, Cirelli C. The drosophila fragile x mental retardation gene regulates sleep need. J Neurosci. 2009;29(7):1948–1961. doi: 10.1523/JNEUROSCI.4830-08.2009. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19228950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushey D, Tononi G, Cirelli C. Increased pre-synaptic size and post-synaptic complexity during wake as compared to sleep in d. Melanogaster. Sleep. 2011;34 Suppl. [Google Scholar]
- Chklovskii DB, Schikorski T, Stevens CF. Wiring optimization in cortical circuits. Neuron. 2002;34(3):341–347. doi: 10.1016/s0896-6273(02)00679-7. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11988166. [DOI] [PubMed] [Google Scholar]
- Cirelli C. Searching for sleep mutants of drosophila melanogaster. Bioessays. 2003;25(10):940–949. doi: 10.1002/bies.10333. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14505361. [DOI] [PubMed] [Google Scholar]
- Cirelli C. The genetic and molecular regulation of sleep: From fruit flies to humans. Nat Rev Neurosci. 2009;10(8):549–560. doi: 10.1038/nrn2683. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19617891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli C, Bushey D, Hill S, Huber R, Kreber R, Ganetzky B, Tononi G. Reduced sleep in drosophila shaker mutants. Nature. 2005a;434(7037):1087–1092. doi: 10.1038/nature03486. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15858564. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Faraguna U, Tononi G. Changes in brain gene expression after long-term sleep deprivation. J Neurochem. 2006;98(5):1632–1645. doi: 10.1111/j.1471-4159.2006.04058.x. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16923172. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Gutierrez CM, Tononi G. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron. 2004;41(1):35–43. doi: 10.1016/s0896-6273(03)00814-6. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14715133. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Huber R, Gopalakrishnan A, Southard TL, Tononi G. Locus ceruleus control of slow-wave homeostasis. J Neurosci. 2005b;25(18):4503–4511. doi: 10.1523/JNEUROSCI.4845-04.2005. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15872097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli C, Lavaute TM, Tononi G. Sleep and wakefulness modulate gene expression in drosophila. J Neurochem. 2005c;94(5):1411–1419. doi: 10.1111/j.1471-4159.2005.03291.x. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16001966. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Pompeiano M, Tononi G. Neuronal gene expression in the waking state: A role for the locus coeruleus. Science. 1996;274(5290):1211–1215. doi: 10.1126/science.274.5290.1211. Available from: http://proquest.umi.com/pqdweb?SK=3&ScQ=000028298|000000000556484|*&StPt=51&FC=25&RQT=317&SrtM=2. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system. J Neurosci. 2000a;20(24):9187–9194. doi: 10.1523/JNEUROSCI.20-24-09187.2000. Available from: http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/referer? http://www.jneurosci.org/cgi/content/abstract/20/24/9187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Gene expression in the brain across the sleep-waking cycle. Brain Res. 2000b;885(2):303–321. doi: 10.1016/s0006-8993(00)03008-0. Available from: http://www.sciencedirect.com/science?_ob=IssueURL&_tockey=%23TOC%234841%232000%23991149997%23219523%23FLA%23Volume_885,_Issue_2,_Pages_143-324_(8_December_2000)&_auth=y&_acct=C000020958&_version=1&_urlVersion=0&_userid=443835&md5=99b0bf86863c646c13a3445a37c46db4. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Locus ceruleus control of state-dependent gene expression. J Neurosci. 2004;24(23):5410–5419. doi: 10.1523/JNEUROSCI.0949-04.2004. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15190114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Is sleep essential? PLoS Biol. 2008;6(8):e216. doi: 10.1371/journal.pbio.0060216. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18752355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, Weiler IJ, Greenough WT. Abnormal dendritic spines in fragile x knockout mice: Maturation and pruning deficits. Proc Natl Acad Sci U S A. 1997;94(10):5401–5404. doi: 10.1073/pnas.94.10.5401. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9144249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan TM, Siegel RW. Mutational and pharmacological alterations of neuronal membrane function disrupt conditioning in drosophila. J Neurogenet. 1984;1(4):333–344. doi: 10.3109/01677068409107095. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=6100306. [DOI] [PubMed] [Google Scholar]
- Cowan TM, Siegel RW. Drosophila mutations that alter ionic conduction disrupt acquisition and retention of a conditioned odor avoidance response. J Neurogenet. 1986;3(4):187–201. doi: 10.3109/01677068609106849. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2427679. [DOI] [PubMed] [Google Scholar]
- Crocker A, Sehgal A. Octopamine regulates sleep in drosophila through protein kinase a-dependent mechanisms. J Neurosci. 2008;28(38):9377–9385. doi: 10.1523/JNEUROSCI.3072-08a.2008. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18799671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker A, Sehgal A. Genetic analysis of sleep. Genes Dev. 2010;24(12):1220–1235. doi: 10.1101/gad.1913110. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20551171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker A, Shahidullah M, Levitan IB, Sehgal A. Identification of a neural circuit that underlies the effects of octopamine on sleep:Wake behavior. Neuron. 2010;65(5):670–681. doi: 10.1016/j.neuron.2010.01.032. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20223202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnecki A, Birtoli B, Ulrich D. Cellular mechanisms of burst firing-mediated long-term depression in rat neocortical pyramidal cells. J Physiol. 2007;578(Pt 2):471–479. doi: 10.1113/jphysiol.2006.123588. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17082228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash MB, Douglas CL, Vyazovskiy VV, Cirelli C, Tononi G. Long-term homeostasis of extracellular glutamate in the rat cerebral cortex across sleep and waking states. J Neurosci. 2009;29(3):620–629. doi: 10.1523/JNEUROSCI.5486-08.2009. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19158289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11(2):114–126. doi: 10.1038/nrn2762. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20046194. [DOI] [PubMed] [Google Scholar]
- Donlea JM, Ramanan N, Shaw PJ. Use-dependent plasticity in clock neurons regulates sleep need in drosophila. Science. 2009;324(5923):105–108. doi: 10.1126/science.1166657. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19342592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas CL, Vyazovskiy V, Southard T, Chiu SY, Messing A, Tononi G, Cirelli C. Sleep in kcna2 knockout mice. BMC Biol. 2007;5:42. doi: 10.1186/1741-7007-5-42. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17925011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y, Jan YN, Byers D, Quinn WG, Benzer S. Dunce, a mutant of drosophila deficient in learning. Proc Natl Acad Sci U S A. 1976;73(5):1684–1688. doi: 10.1073/pnas.73.5.1684. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=818641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE. Synaptic mechanisms for plasticity in neocortex. Annu Rev Neurosci. 2009;32:33–55. doi: 10.1146/annurev.neuro.051508.135516. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19400721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Gutekunst CA, Eberhart DE, Yi H, Warren ST, Hersch SM. Fragile x mental retardation protein: Nucleocytoplasmic shuttling and association with somatodendritic ribosomes. J Neurosci. 1997;17(5):1539–1547. doi: 10.1523/JNEUROSCI.17-05-01539.1997. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9030614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltenyi K, Greenspan RJ, Newport JW. Activation of egfr and erk by rhomboid signaling regulates the consolidation and maintenance of sleep in drosophila. Nat Neurosci. 2007;10(9):1160–1167. doi: 10.1038/nn1957. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17694052. [DOI] [PubMed] [Google Scholar]
- Franken P, Gip P, Hagiwara G, Ruby NF, Heller HC. Changes in brain glycogen after sleep deprivation vary with genotype. Am J Physiol Regul Integr Comp Physiol. 2003;285(2):R413–R419. doi: 10.1152/ajpregu.00668.2002. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12730076. [DOI] [PubMed] [Google Scholar]
- Franken P, Gip P, Hagiwara G, Ruby NF, Heller HC. Glycogen content in the cerebral cortex increases with sleep loss in c57bl/6j mice. Neurosci Lett. 2006;402(1–2):176–179. doi: 10.1016/j.neulet.2006.03.072. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16644123. [DOI] [PubMed] [Google Scholar]
- Ganguly-Fitzgerald I, Donlea J, Shaw PJ. Waking experience affects sleep need in drosophila. Science. 2006;313(5794):1775–1781. doi: 10.1126/science.1130408. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16990546. [DOI] [PubMed] [Google Scholar]
- Gilestro GF, Tononi G, Cirelli C. Widespread changes in synaptic markers as a function of sleep and wakefulness in drosophila. Science. 2009;324(5923):109–112. doi: 10.1126/science.1166673. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19342593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves LA, Hellman K, Veasey S, Blendy JA, Pack AI, Abel T. Genetic evidence for a role of creb in sustained cortical arousal. J Neurophysiol. 2003;23:23. doi: 10.1152/jn.00882.2002. Available from: http://www.ncbi.nlm.nih.gov/htbin-post/Entrez/query?db=m&form=6&dopt=r&uid=12711709. [DOI] [PubMed] [Google Scholar]
- Gronke S, Beller M, Fellert S, Ramakrishnan H, Jackle H, Kuhnlein RP. Control of fat storage by a drosophila pat domain protein. Curr Biol. 2003;13(7):603–606. doi: 10.1016/s0960-9822(03)00175-1. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12676093. [DOI] [PubMed] [Google Scholar]
- Gronke S, Mildner A, Fellert S, Tennagels N, Petry S, Muller G, Jackle H, Kuhnlein RP. Brummer lipase is an evolutionary conserved fat storage regulator in drosophila. Cell Metab. 2005;1(5):323–330. doi: 10.1016/j.cmet.2005.04.003. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16054079. [DOI] [PubMed] [Google Scholar]
- Han KA, Millar NS, Davis RL. A novel octopamine receptor with preferential expression in drosophila mushroom bodies. J Neurosci. 1998;18(10):3650–3658. doi: 10.1523/JNEUROSCI.18-10-03650.1998. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9570796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han PL, Levin LR, Reed RR, Davis RL. Preferential expression of the drosophila rutabaga gene in mushroom bodies, neural centers for learning in insects. Neuron. 1992;9(4):619–627. doi: 10.1016/0896-6273(92)90026-a. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1382471. [DOI] [PubMed] [Google Scholar]
- Hanlon EC, Faraguna U, Vyazovskiy VV, Tononi G, Cirelli C. Effects of skilled training on sleep slow wave activity and cortical gene expression in the rat. Sleep. 2009;32(6):719–729. doi: 10.1093/sleep/32.6.719. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19544747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbison ST, Mackay TF, Anholt RR. Understanding the neurogenetics of sleep: Progress from drosophila. Trends Genet. 2009;25(6):262–269. doi: 10.1016/j.tig.2009.04.003. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19446357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley C. Noradrenergic and locus coeruleus modulation of the perforant path-evoked potential in rat dentate gyrus supports a role for the locus coeruleus in attentional and memorial processes. Prog Brain Res. 1991;88:307–321. doi: 10.1016/s0079-6123(08)63818-2. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1687619. [DOI] [PubMed] [Google Scholar]
- Hendricks JC, Finn SM, Panckeri KA, Chavkin J, Williams JA, Sehgal A, Pack AI. Rest in drosophila is a sleep-like state. Neuron. 2000;25(1):129–138. doi: 10.1016/s0896-6273(00)80877-6. Available from: http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/referer? http://www.neuron.org/cgi/content/full/25/1/129. [DOI] [PubMed] [Google Scholar]
- Hendricks JC, Williams JA, Panckeri K, Kirk D, Tello M, Yin JC, Sehgal A. A non-circadian role for camp signaling and creb activity in drosophila rest homeostasis. Nat Neurosci. 2001;4(11):1108–1115. doi: 10.1038/nn743. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11687816. [DOI] [PubMed] [Google Scholar]
- Hewes RS. The buzz on fly neuronal remodeling. Trends Endocrinol Metab. 2008;19(9):317–323. doi: 10.1016/j.tem.2008.07.008. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18805704. [DOI] [PubMed] [Google Scholar]
- Hinton VJ, Brown WT, Wisniewski K, Rudelli RD. Analysis of neocortex in three males with the fragile x syndrome. Am J Med Genet. 1991;41(3):289–294. doi: 10.1002/ajmg.1320410306. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1724112. [DOI] [PubMed] [Google Scholar]
- Ho KS, Sehgal A. Drosophila melanogaster: An insect model for fundamental studies of sleep. Methods Enzymol. 2005;393:772–793. doi: 10.1016/S0076-6879(05)93041-3. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15817324. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. 2004a. Local sleep and learning. Nature;430(6995):78–81. doi: 10.1038/nature02663. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15184907. [DOI] [PubMed] [Google Scholar]
- Huber R, Hill SL, Holladay C, Biesiadecki M, Tononi G, Cirelli C. Sleep homeostasis in drosophila melanogaster. Sleep. 2004b;27(4):628–639. doi: 10.1093/sleep/27.4.628. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15282997. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Idupulapati M, Gilbert ME, Harris JB, Chakravarti AB, Rogers EJ, Crisostomo RA, Larsen BP, Mehta A, Alcantara CJ, Patel B, Swain RA, Weiler IJ, Oostra BA, Greenough WT. Dendritic spine and dendritic field characteristics of layer v pyramidal neurons in the visual cortex of fragile-x knockout mice. Am J Med Genet. 2002;111(2):140–146. doi: 10.1002/ajmg.10500. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12210340. [DOI] [PubMed] [Google Scholar]
- Ishimoto H, Kitamoto T. The steroid molting hormone ecdysone regulates sleep in adult drosophila melanogaster. Genetics. 2010;185(1):269–281. doi: 10.1534/genetics.110.114587. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20215472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto H, Sakai T, Kitamoto T. Ecdysone signaling regulates the formation of long-term courtship memory in adult drosophila melanogaster. Proc Natl Acad Sci U S A. 2009;106(15):6381–6386. doi: 10.1073/pnas.0810213106. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19342482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner WJ, Crocker A, White BH, Sehgal A. Sleep in drosophila is regulated by adult mushroom bodies. Nature. 2006;441(7094):757–760. doi: 10.1038/nature04811. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16760980. [DOI] [PubMed] [Google Scholar]
- Jones BE. From waking to sleeping: Neuronal and chemical substrates. Trends Pharmacol Sci. 2005;26(11):578–586. doi: 10.1016/j.tips.2005.09.009. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16183137. [DOI] [PubMed] [Google Scholar]
- Jones S, Pfister-Genskow M, Benca RM, Cirelli C. Molecular correlates of sleep and wakefulness in the brain of the white-crowned sparrow. J Neurochem. 2008;105(1):46–62. doi: 10.1111/j.1471-4159.2007.05089.x. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18028333. [DOI] [PubMed] [Google Scholar]
- Keene AC, Duboue ER, Mcdonald DM, Dus M, Suh GS, Waddell S, Blau J. Clock and cycle limit starvation-induced sleep loss in drosophila. Curr Biol. 2010;20(13):1209–1215. doi: 10.1016/j.cub.2010.05.029. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20541409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp N, Bashir ZI. Long-term depression: A cascade of induction and expression mechanisms. Prog Neurobiol. 2001;65(4):339–365. doi: 10.1016/s0301-0082(01)00013-2. [DOI] [PubMed] [Google Scholar]
- Killgore WD. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105–129. doi: 10.1016/B978-0-444-53702-7.00007-5. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21075236. [DOI] [PubMed] [Google Scholar]
- Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163–178. doi: 10.1016/j.smrv.2007.01.002. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17442599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh K, Joiner WJ, Wu MN, Yue Z, Smith CJ, Sehgal A. Identification of sleepless, a sleep-promoting factor. Science. 2008;321(5887):372–376. doi: 10.1126/science.1155942. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18635795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Keene AC, Leung B, Armstrong JD, Waddell S. Sequential use of mushroom body neuron subsets during drosophila odor memory processing. Neuron. 2007;53(1):103–115. doi: 10.1016/j.neuron.2006.11.021. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17196534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl D, Kennedy TE, Barzilai A, Kandel ER. Long-term sensitization training in aplysia leads to an increase in the expression of bip, the major protein chaperon of the er. J Cell Biol. 1992;119(5):1069–1076. doi: 10.1083/jcb.119.5.1069. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1360013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume K, Kume S, Park SK, Hirsh J, Jackson FR. Dopamine is a regulator of arousal in the fruit fly. J Neurosci. 2005;25(32):7377–7384. doi: 10.1523/JNEUROSCI.2048-05.2005. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16093388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesku JA, Vyssotski AL, Martinez-Gonzalez D, Wilzeck C, Rattenborg NC. Local sleep homeostasis in the avian brain: Convergence of sleep function in mammals and birds? Proc Biol Sci. 2011 doi: 10.1098/rspb.2010.2316. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21208955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Yu F, Guo A. Sleep deprivation specifically impairs short-term olfactory memory in drosophila. Sleep. 2009;32(11):1417–1424. doi: 10.1093/sleep/32.11.1417. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19928381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZW, Faraguna U, Cirelli C, Tononi G, Gao XB. Direct evidence for wake-related increases and sleep-related decreases in synaptic strength in rodent cortex. J Neurosci. 2010;30(25):8671–8675. doi: 10.1523/JNEUROSCI.1409-10.2010. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20573912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubenov EV, Siapas AG. Decoupling through synchrony in neuronal circuits with propagation delays. Neuron. 2008;58(1):118–31. doi: 10.1016/j.neuron.2008.01.036. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18400168. [DOI] [PubMed] [Google Scholar]
- Mackiewicz M, Shockley KR, Romer MA, Galante RJ, Zimmerman JE, Naidoo N, Baldwin DA, Jensen ST, Churchill GA, Pack AI. Macromolecule biosynthesis - a key function of sleep. Physiol Genomics. 2007 doi: 10.1152/physiolgenomics.00275.2006. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17698924. [DOI] [PubMed] [Google Scholar]
- Mackiewicz M, Zimmerman JE, Shockley KR, Churchill GA, Pack AI. What are microarrays teaching us about sleep? Trends Mol Med. 2009;15(2):79–87. doi: 10.1016/j.molmed.2008.12.002. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19162550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maret S, Dorsaz S, Gurcel L, Pradervand S, Petit B, Pfister C, Hagenbuchle O, O'hara BF, Franken P, Tafti M. Homer1a is a core brain molecular correlate of sleep loss. Proc Natl Acad Sci U S A. 2007;104(50):20090–20095. doi: 10.1073/pnas.0710131104. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18077435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maret S, Faraguna U, Nelson A, Cirelli C, Tononi G. Sleep and wake modulate spine turnover in the adolescent mouse cortex. Sleep. 2011;34 Suppl. doi: 10.1038/nn.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcbride SM, Choi CH, Wang Y, Liebelt D, Braunstein E, Ferreiro D, Sehgal A, Siwicki KK, Dockendorff TC, Nguyen HT, Mcdonald TV, Jongens TA. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a drosophila model of fragile x syndrome. Neuron. 2005;45(5):753–764. doi: 10.1016/j.neuron.2005.01.038. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15748850. [DOI] [PubMed] [Google Scholar]
- Misonou H, Trimmer JS. Determinants of voltage-gated potassium channel surface expression and localization in mammalian neurons. Crit Rev Biochem Mol Biol. 2004;39(3):125–145. doi: 10.1080/10409230490475417. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15596548. [DOI] [PubMed] [Google Scholar]
- Mongrain V, Hernandez SA, Pradervand S, Dorsaz S, Curie T, Hagiwara G, Gip P, Heller HC, Franken P. Separating the contribution of glucocorticoids and wakefulness to the molecular and electrophysiological correlates of sleep homeostasis. Sleep. 2010;33(9):1147–1157. doi: 10.1093/sleep/33.9.1147. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20857860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo N, Casiano V, Cater J, Zimmerman J, Pack AI. A role for the molecular chaperone protein bip/grp78 in drosophila sleep homeostasis. Sleep. 2007;30(5):557–565. doi: 10.1093/sleep/30.5.557. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17552370. [DOI] [PubMed] [Google Scholar]
- Nikonova EV, Naidoo N, Zhang L, Romer M, Cater JR, Scharf MT, Galante RJ, Pack AI. Changes in components of energy regulation in mouse cortex with increases in wakefulness. Sleep. 2010;33(7):889–900. doi: 10.1093/sleep/33.7.889. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20614849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7(9):697–709. doi: 10.1038/nrn1970. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16924259. [DOI] [PubMed] [Google Scholar]
- Nitz DA, Van Swinderen B, Tononi G, Greenspan RJ. Electrophysiological correlates of rest and activity in drosophila melanogaster. Curr Biol. 2002;12(22):1934–1940. doi: 10.1016/s0960-9822(02)01300-3. Available from: http://www.ncbi.nlm.nih.gov/htbin-post/Entrez/query?db=m&form=6&dopt=r&uid=12445387. [DOI] [PubMed] [Google Scholar]
- Pan L, Broadie KS. Drosophila fragile x mental retardation protein and metabotropic glutamate receptor a convergently regulate the synaptic ratio of ionotropic glutamate receptor subclasses. J Neurosci. 2007;27(45):12378–12389. doi: 10.1523/JNEUROSCI.2970-07.2007. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17989302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Zhang YQ, Woodruff E, Broadie K. The drosophila fragile x gene negatively regulates neuronal elaboration and synaptic differentiation. Curr Biol. 2004;14(20):1863–1870. doi: 10.1016/j.cub.2004.09.085. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15498496. [DOI] [PubMed] [Google Scholar]
- Parisky KM, Agosto J, Pulver SR, Shang Y, Kuklin E, Hodge JJ, Kang K, Liu X, Garrity PA, Rosbash M, Griffith LC. Pdf cells are a gaba-responsive wake-promoting component of the drosophila sleep circuit. Neuron. 2008;60(4):672–682. doi: 10.1016/j.neuron.2008.10.042. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19038223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Helfrich-Forster C, Lee G, Liu L, Rosbash M, Hall JC. Differential regulation of circadian pacemaker output by separate clock genes in drosophila. Proc Natl Acad Sci U S A. 2000;97(7):3608–3613. doi: 10.1073/pnas.070036197. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10725392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perazzona B, Isabel G, Preat T, Davis RL. The role of camp response element-binding protein in drosophila long-term memory. J Neurosci. 2004;24(40):8823–8828. doi: 10.1523/JNEUROSCI.4542-03.2004. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15470148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschel N, Helfrich-Forster C. Setting the clock - by nature: Circadian rhythm in the fruitfly drosophila melanogaster. FEBS Lett. 2011 doi: 10.1016/j.febslet.2011.02.028. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21354415. [DOI] [PubMed] [Google Scholar]
- Pitman JL, Mcgill JJ, Keegan KP, Allada R. A dynamic role for the mushroom bodies in promoting sleep in drosophila. Nature. 2006;441(7094):753–756. doi: 10.1038/nature04739. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16760979. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Davis RL. Genetic dissection of the learning/memory gene dunce of drosophila melanogaster. Genes Dev. 1993;7(7B):1447–1458. doi: 10.1101/gad.7.7b.1447. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7687228. [DOI] [PubMed] [Google Scholar]
- Rattenborg N, Martinez-Gonzalez D, Roth Ii T, Pravosudov V. Hippocampal memory consolidation during sleep: A comparison of mammals and birds. Biological Reviews. 2010 doi: 10.1111/j.1469-185X.2010.00165.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restivo L, Ferrari F, Passino E, Sgobio C, Bock J, Oostra BA, Bagni C, Ammassari-Teule M. Enriched environment promotes behavioral and morphological recovery in a mouse model for the fragile x syndrome. Proc Natl Acad Sci U S A. 2005;102(32):11557–11562. doi: 10.1073/pnas.0504984102. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16076950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogero O, Hammerle B, Tejedor FJ. Diverse expression and distribution of shaker potassium channels during the development of the drosophila nervous system. J Neurosci. 1997;17(13):5108–5118. doi: 10.1523/JNEUROSCI.17-13-05108.1997. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9185548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosanova M, Ulrich D. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. J Neurosci. 2005;25(41):9398–9405. doi: 10.1523/JNEUROSCI.2149-05.2005. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16221848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman DL, Behar KL, Hyder F, Shulman RG. In vivo nmr studies of the glutamate neurotransmitter flux and neuroenergetics: Implications for brain function. Annu Rev Physiol. 2003;65:401–427. doi: 10.1146/annurev.physiol.65.092101.142131. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Wenthold RJ. Calnexin and the immunoglobulin binding protein (bip) coimmunoprecipitate with ampa receptors. J Neurochem. 1999;73(3):942–948. doi: 10.1046/j.1471-4159.1999.0730942.x. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10461883. [DOI] [PubMed] [Google Scholar]
- Sakai T, Tamura T, Kitamoto T, Kidokoro Y. A clock gene, period, plays a key role in long-term memory formation in drosophila. Proc Natl Acad Sci U S A. 2004;101(45):16058–16063. doi: 10.1073/pnas.0401472101. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15522971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T. The neural circuit of orexin (hypocretin): Maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8(3):171–181. doi: 10.1038/nrn2092. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17299454. [DOI] [PubMed] [Google Scholar]
- Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68(6):1023–1042. doi: 10.1016/j.neuron.2010.11.032. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21172606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10(3):211–223. doi: 10.1038/nrn2573. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19190638. [DOI] [PubMed] [Google Scholar]
- Schenck A, Van De Bor V, Bardoni B, Giangrande A. Novel features of dfmr1, the drosophila orthologue of the fragile x mental retardation protein. Neurobiol Dis. 2002;11(1):53–63. doi: 10.1006/nbdi.2002.0510. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12460546. [DOI] [PubMed] [Google Scholar]
- Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in drosophila. J Neurosci. 2003;23(33):10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14627633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz TL, Papazian DM, Carretto RC, Jan YN, Jan LY. Immunological characterization of k+ channel components from the shaker locus and differential distribution of splicing variants in drosophila. Neuron. 1990;4(1):119–127. doi: 10.1016/0896-6273(90)90448-o. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2310570. [DOI] [PubMed] [Google Scholar]
- Seol GH, Ziburkus J, Huang S, Song L, Kim IT, Takamiya K, Huganir RL, Lee HK, Kirkwood A. Neuromodulators control the polarity of spike-timing-dependent synaptic plasticity. Neuron. 2007;55(6):919–929. doi: 10.1016/j.neuron.2007.08.013. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17880895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seugnet L, Suzuki Y, Vine L, Gottschalk L, Shaw PJ. D1 receptor activation in the mushroom bodies rescues sleep-loss-induced learning impairments in drosophila. Curr Biol. 2008;18(15):1110–1117. doi: 10.1016/j.cub.2008.07.028. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18674913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P. Awakening to the behavioral analysis of sleep in drosophila. J Biol Rhythms. 2003;18(1):4–11. doi: 10.1177/0748730402239672. Available from: http://www.ncbi.nlm.nih.gov/htbin-post/Entrez/query?db=m&form=6&dopt=r&uid=12568240. [DOI] [PubMed] [Google Scholar]
- Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in drosophila melanogaster. Science. 2000;287(5459):1834–1837. doi: 10.1126/science.287.5459.1834. Available from: http://www.ncbi.nlm.nih.gov/cgibin/Entrez/referer?http://www.sciencemag.org/cgi/content/full/287/5459/1834. [DOI] [PubMed] [Google Scholar]
- Shaw PJ, Tononi G, Greenspan RJ, Robinson DF. Stress response genes protect against lethal effects of sleep deprivation in drosophila. Nature. 2002;417(6886):287–291. doi: 10.1038/417287a. Available from: http://www.ncbi.nlm.nih.gov/htbinpost/Entrez/query?db=m&form=6&dopt=r&uid=12015603. [DOI] [PubMed] [Google Scholar]
- Sheeba V, Gu H, Sharma VK, O'dowd DK, Holmes TC. Circadian- and light-dependent regulation of resting membrane potential and spontaneous action potential firing of drosophila circadian pacemaker neurons. J Neurophysiol. 2008;99(2):976–988. doi: 10.1152/jn.00930.2007. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18077664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Lorbeck MT, Zervos A, Zimmerman J, Elefant F. The histone acetyltransferase elp3 plays in active role in the control of synaptic bouton expansion and sleep in drosophila. J Neurochem. 2010;115(2):493–504. doi: 10.1111/j.1471-4159.2010.06892.x. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20626565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoulakis EM, Kalderon D, Davis RL. Preferential expression in mushroom bodies of the catalytic subunit of protein kinase a and its role in learning and memory. Neuron. 1993;11(2):197–208. doi: 10.1016/0896-6273(93)90178-t. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8352940. [DOI] [PubMed] [Google Scholar]
- Steriade M. The corticothalamic system in sleep. Front Biosci. 2003;8:D878–D899. doi: 10.2741/1043. Available from: http://www.ncbi.nlm.nih.gov/htbin-post/Entrez/query?db=m&form=6&dopt=r&uid=12700074. [DOI] [PubMed] [Google Scholar]
- Terao A, Greco MA, Davis RW, Heller HC, Kilduff TS. Region-specific changes in immediate early gene expression in response to sleep deprivation and recovery sleep in the mouse brain. Neuroscience. 2003a;120(4):1115–1124. doi: 10.1016/s0306-4522(03)00395-6. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12927216. [DOI] [PubMed] [Google Scholar]
- Terao A, Steininger TL, Hyder K, Apte-Deshpande A, Ding J, Rishipathak D, Davis RW, Heller HC, Kilduff TS. Differential increase in the expression of heat shock protein family members during sleep deprivation and during sleep. Neuroscience. 2003b;116(1):187–200. doi: 10.1016/s0306-4522(02)00695-4. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12535952. [DOI] [PubMed] [Google Scholar]
- Terao A, Wisor JP, Peyron C, Apte-Deshpande A, Wurts SW, Edgar DM, Kilduff TS. Gene expression in the rat brain during sleep deprivation and recovery sleep: An affymetrix genechip study. Neuroscience. 2006;137(2):593–605. doi: 10.1016/j.neuroscience.2005.08.059. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16257491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimgan MS, Suzuki Y, Seugnet L, Gottschalk L, Shaw PJ. The perilipin homologue, lipid storage droplet 2, regulates sleep homeostasis and prevents learning impairments following sleep loss. PLoS Biol. 2010;8(8) doi: 10.1371/journal.pbio.1000466. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20824166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinklenberg JA, Segal IS, Guo TZ, Maze M. Analysis of anesthetic action on the potassium channels of the shaker mutant of drosophila. Ann N Y Acad Sci. 1991;625:532–539. doi: 10.1111/j.1749-6632.1991.tb33884.x. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1905502. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep and synaptic homeostasis: A hypothesis. Brain Res Bull. 2003;62(2):143–150. doi: 10.1016/j.brainresbull.2003.09.004. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14638388. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10(1):49–62. doi: 10.1016/j.smrv.2005.05.002. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16376591. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411(6837):583–587. doi: 10.1038/35079077. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11385572. [DOI] [PubMed] [Google Scholar]
- Van Swinderen B, Nitz DA, Greenspan RJ. Uncoupling of brain activity from movement defines arousal states in drosophila. Curr Biol. 2004;14(2):81–87. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=14738728. [PubMed] [Google Scholar]
- Vandenberghe W, Nicoll RA, Bredt DS . Interaction with the unfolded protein response reveals a role for stargazin in biosynthetic ampa receptor transport. J Neurosci. 2005;25(5):1095–1102. doi: 10.1523/JNEUROSCI.3568-04.2005. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15689545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecsey CG, Baillie GS, Jaganath D, Havekes R, Daniels A, Wimmer M, Huang T, Brown KM, Li XY, Descalzi G, Kim SS, Chen T, Shang YZ, Zhuo M, Houslay MD, Abel T. Sleep deprivation impairs camp signalling in the hippocampus. Nature. 2009;461(7267):1122–1125. doi: 10.1038/nature08488. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19847264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11(2):200–208. doi: 10.1038/nn2035. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18204445. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Olcese U, Lazimy YM, Faraguna U, Esser SK, Williams JC, Cirelli C, Tononi G. Cortical firing and sleep homeostasis. Neuron. 2009;63(6):865–878. doi: 10.1016/j.neuron.2009.08.024. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19778514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell S. Dopamine reveals neural circuit mechanisms of fly memory. Trends Neurosci. 2010;33(10):457–464. doi: 10.1016/j.tins.2010.07.001. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20701984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber B, Schaper C, Bushey D, Rohlfs M, Steinfath M, Tononi G, Cirelli C, Scholz J, Bein B. Increased volatile anesthetic requirement in short-sleeping drosophila mutants. Anesthesiology. 2009;110(2):313–316. doi: 10.1097/ALN.0b013e3181942df2. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19164958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werk CM, Chapman CA. Long-term potentiation of polysynaptic responses in layer v of the sensorimotor cortex induced by theta-patterned tetanization in the awake rat. Cereb Cortex. 2003;13(5):500–507. doi: 10.1093/cercor/13.5.500. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12679296. [DOI] [PubMed] [Google Scholar]
- Werk CM, Klein HS, Nesbitt CE, Chapman CA. Long-term depression in the sensorimotor cortex induced by repeated delivery of 10 hz trains in vivo. Neuroscience. 2006;140(1):13–20. doi: 10.1016/j.neuroscience.2006.02.004. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16530972. [DOI] [PubMed] [Google Scholar]
- Wu MN, Joiner WJ, Dean T, Yue Z, Smith CJ, Chen D, Hoshi T, Sehgal A, Koh K. Sleepless, a ly-6/neurotoxin family member, regulates the levels, localization and activity of shaker. Nat Neurosci. 2010;13(1):69–75. doi: 10.1038/nn.2454. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20010822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MN, Koh K, Yue Z, Joiner WJ, Sehgal A. A genetic screen for sleep and circadian mutants reveals mechanisms underlying regulation of sleep in drosophila. Sleep. 2008;31(4):465–472. doi: 10.1093/sleep/31.4.465. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18457233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki D, Horiuchi J, Nakagami Y, Nagano S, Tamura T, Saitoe M. The drosophila dco mutation suppresses age-related memory impairment without affecting lifespan. Nat Neurosci. 2007;10(4):478–484. doi: 10.1038/nn1863. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17322874. [DOI] [PubMed] [Google Scholar]
- Yao WD, Wu CF. Distinct roles of camkii and pka in regulation of firing patterns and k(+) currents in drosophila neurons. J Neurophysiol. 2001;85(4):1384–1394. doi: 10.1152/jn.2001.85.4.1384. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11287463. [DOI] [PubMed] [Google Scholar]
- Yin JC, Del Vecchio M, Zhou H, Tully TA. Creb as a memory modulator: Induced expression of a dcreb2 activator isoform enhances long-term memory in drosophila. Cell. 1995;81(1):107–115. doi: 10.1016/0092-8674(95)90375-5. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7720066. [DOI] [PubMed] [Google Scholar]
- Yu D, Akalal DB, Davis RL. Drosophila alpha/beta mushroom body neurons form a branch-specific, long-term cellular memory trace after spaced olfactory conditioning. Neuron. 2006;52(5):845–855. doi: 10.1016/j.neuron.2006.10.030. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17145505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Keene AC, Srivatsan A, Waddell S, Davis RL. Drosophila dpm neurons form a delayed and branch-specific memory trace after olfactory classical conditioning. Cell. 2005;123(5):945–957. doi: 10.1016/j.cell.2005.09.037. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16325586. [DOI] [PubMed] [Google Scholar]
- Yu D, Ponomarev A, Davis RL. Altered representation of the spatial code for odors after olfactory classical conditioning; memory trace formation by synaptic recruitment. Neuron. 2004;42(3):437–449. doi: 10.1016/s0896-6273(04)00217-x. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15134640. [DOI] [PubMed] [Google Scholar]
- Yuan LL, Chen X. Diversity of potassium channels in neuronal dendrites. Prog Neurobiol. 2006;78(6):374–389. doi: 10.1016/j.pneurobio.2006.03.003. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16716489. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Joiner WJ, Sehgal A. A sleep-promoting role for the drosophila serotonin receptor 1a. Curr Biol. 2006;16(11):1051–1062. doi: 10.1016/j.cub.2006.04.032. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16753559. [DOI] [PubMed] [Google Scholar]
- Zhang S, Yin Y, Lu H, Guo A. Increased dopaminergic signaling impairs aversive olfactory memory retention in drosophila. Biochem Biophys Res Commun. 2008;370(1):82–86. doi: 10.1016/j.bbrc.2008.03.015. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18342622. [DOI] [PubMed] [Google Scholar]
- Zimmerman JE, Naidoo N, Raizen DM, Pack AI. Conservation of sleep: Insights from non-mammalian model systems. Trends Neurosci. 2008;31(7):371–376. doi: 10.1016/j.tins.2008.05.001. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18538867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JE, Rizzo W, Shockley KR, Raizen DM, Naidoo N, Mackiewicz M, Churchill GA, Pack AI. Multiple mechanisms limit the duration of wakefulness in drosophila brain. Physiol Genomics. 2006;27(3):337–350. doi: 10.1152/physiolgenomics.00030.2006. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16954408. [DOI] [PubMed] [Google Scholar]