Abstract
Fluorescent proteins and molecules are now widely used to tag and visualize proteins resulting in an improved understanding of protein trafficking, localization, and function. In addition, fluorescent tags have also been used to inactivate protein function in a spatially and temporally-defined manner, using a technique known as fluorophore-assisted light inactivation (FALI) or chromophore-assisted light inactivation (CALI). In this study we tagged the serotonin3 A subunit with the α-bungarotoxin binding sequence (BBS) and subsequently labeled 5-HT3A/BBS receptors with fluorescently conjugated α-bungarotoxin in live cells. We show that 5-HT3A/BBS receptors are constitutively internalized in the absence of an agonist and internalization as well as receptor function are inhibited by fluorescence. The fluorescence-induced disruption of function and internalization was reduced with oxygen radical scavengers suggesting the involvement of reactive oxygen species, implicating the FALI process. Furthermore, these data suggest that intense illumination during live-cell microscopy may result in inadvertent FALI and inhibition of protein trafficking.
Keywords: 5-Hydroxytryptamine type 3 (5-HT3) receptors, Fluorophore assisted light inactivation, Constitutive internalization, Electrophysiology
Introduction
The 5-hydroxytryptamine type 3 (5-HT3) receptor mediates the rapid excitatory currents evoked by serotonin both in the peripheral and central nervous systems (Maricq et al., 1991; Tecott et al., 1993). Currently 5-HT3 antagonists are used clinically to treat irritable bowel syndrome and nausea and emesis during chemotherapy (Hesketh, 2008). Furthermore, 5-HT3 receptor polymorphisms are associated with schizophrenia and bipolar disorder (Niesler et al., 2001). In addition, 5-HT3A receptors may have roles in addiction to alcohol and other drugs of abuse as well as anxiety (Hodge et al., 1993, Olivier et al., 2000 reviewed in Grant, 1995 and McKinzie et al., 2000). Therefore, regulation of 5-HT3A receptors at the cell membrane may play important roles in a number of neural functions, including nausea, anxiety and drug addiction.
The 5-HT3 receptor is a member of the cysteine-loop pentameric ligand gated ion channel (pLGIC) family, which includes the nicotinic-acetylcholine receptor, γ-aminobutyric acid type A (GABAA), and glycine receptors. Nicotinic-acetylcholine and 5-HT3 receptors form cation channels, whereas GABA and glycine receptors form anion channels. Five 5-HT3 isoforms have been described and have been termed 5-HT3A–E (Maricq et al., 1991; Davies et al., 1999; Niesler et al., 2003; Karnovsky et al., 2003). The A subtype can combine to form functional homo-pentameric structures whereas B–E subunits must combine with A subunits to form functional hetero-pentameric receptors (Maricq et al., 1991; Davies et al., 1999; Niesler et al., 2007). Heteromeric 5-HT3AB receptors have larger single channel conductance and are less permeable to Ca2+ compared to 5-HT3A homomeric receptors (Davies et al., 1999). Despite our extensive knowledge of 5-HT3 receptor structure and function, little is known about the cell surface stability and trafficking of this receptor.
The development of fluorescent proteins and strategies for tagging proteins with other fluorescent molecules has made it possible to visualize specific proteins and investigate their localization and trafficking. Furthermore, fluorescent proteins and molecules have been used to specifically inhibit proteins upon the excitation of the fluorophore by light and this technique is known as fluorophore assisted light inactivation (FALI) or chromophore assisted light inactivation (CALI) (Jay, 1988; reviewed in Jacobson et al., 2008). Fluorescent proteins such as enhanced Green Fluorescent Protein (eGFP) (McLean et al., 2009; Rajfur et al., 2002; Tanabe et al., 2005; Vitriol et al., 2007) and synthetic fluorophores like fluorescein or red biarsenical dye (ReAsH) (McLean et al., 2009; Tour et al., 2003; Yan et al., 2006; Guo et al., 2006; Marek and Davis, 2002; Lee et al., 2008) have been used to produce FALI.
FALI is mediated by the actions of singlet oxygen and is dependent on the irradiation energy (Horstkotte et al., 2005; McLean et al., 2009). Singlet oxygen is a reactive oxygen species (ROS) that can cause oxidation of tryptophan, tyrosine, methionine, histidine, and cysteine residues that may result in the cross-linking of residues (reviewed in Davies, 2003). The generation of singlet oxygen is a byproduct of all fluorescence in the presence of oxygen. Due to the close proximity of the fluorophore and the target protein, the singlet oxygen most likely reacts with the target protein (McLean et al., 2009). It has been suggested that FALI is specific to the protein labeled (Jay, 1988; Rajfur et al., 2002; Tanabe et al., 2005; Surrey et al., 1998; Yan et al., 2006; Marek and Davis, 2002; Tour et al., 2003). However, it has also been shown that FALI can lead to collateral damage to non-targeted proteins (Guo et al., 2006; Rahmanzadeh et al., 2007).
In this study, we identified robust constitutive internalization of recombinant 5-HT3A receptors expressed in HEK-293 cells and N1E-115 neuroblastoma cells in the absence of an agonist. Furthermore, we show that receptor internalization and function were attenuated by fluorophore assisted light inactivation.
Results
α-bungarotoxin tagged 5-HT3A receptor construct
5-HT3A receptors were tagged with the binding sequence for α-bungarotoxin allowing us to label surface receptors with fluorescently conjugated α-bungarotoxin in live cells described previously (Sanders and Hawrot, 2004) and used by other groups (Wilkins et al., 2008; Sekine-Aizawa and Huganir, 2004; McCann et al., 2005; Guo et al., 2006). The bungarotoxin binding sequence (BBS) WRYYESSLEPYPD was added to the short extracellular c-terminus of the 5-HT3A receptor by primer addition PCR. The addition of a single BBS yielded receptors that were unable to bind α-bungarotoxin conjugated to Alexa/555 (BTX/555) (data not shown). Repeats of glycine and asparagine were added to extend the BBS further from the membrane and the rest of the pentameric protein structure. Adding 3 or 8 gly-asp repeats before the BBS was not sufficient to allow for detectable BTX/555 binding. However, adding five gly-asp and a second BBS resulted in a construct that showed reliable labeling with BTX/555. A schematic representation of the construct is shown in Fig. 1a. We have previously shown that addition of the α-bungarotoxin pharmatope on the extracellular carboxyl terminus of the 5-HT3A receptor does not alter general receptor function or the agonist concentration– response relationship (Sanghvi et al., 2009). Furthermore, currents mediated by 5-HT3A/BBS receptors were not inhibited in the presence of varying concentrations of non-conjugated BTX (Fig. 1b). Therefore, the addition of the linker sequence and two BBSs did not significantly alter the function of 5-HT3A receptors even in the presence of bound BTX.
Fig. 1.
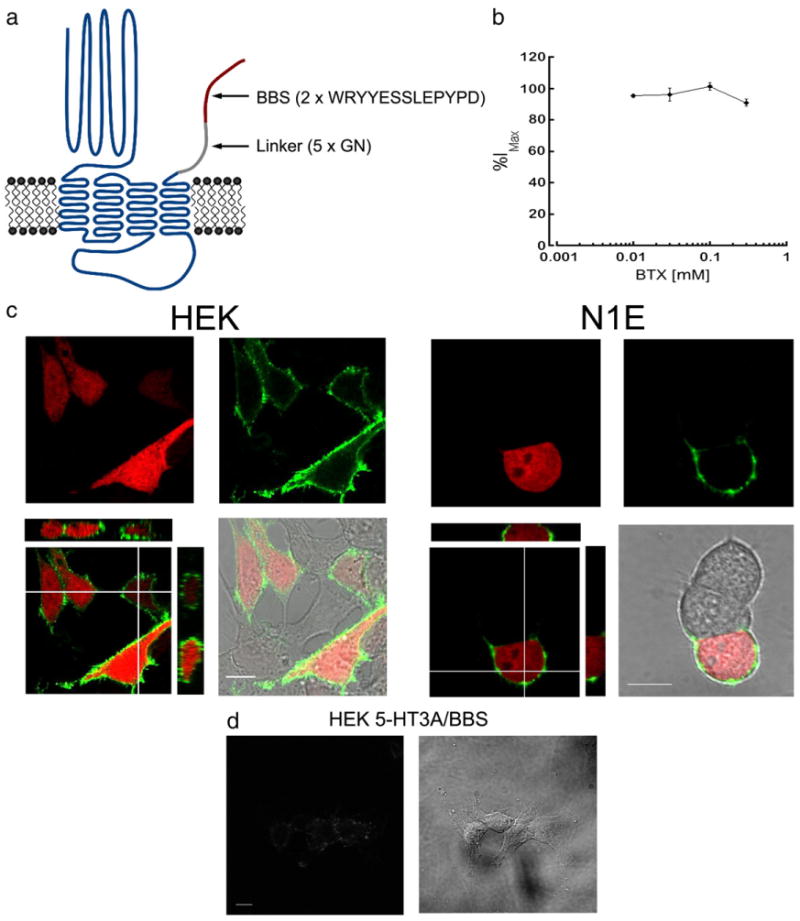
α-bungarotoxin conjugated to Alexa-fluorophores specifically labels surface 5-HT3A/BBS receptors. a) Schematic representation of the 5-HT3A/BBS receptor. b) Whole-cell voltage clamp configuration was obtained with HEK-293 cells stably expressing 5-HT3A/BBS receptors. 5-HT3A/BBS mediated currents were recorded in the absence or presence of varying concentrations of non-conjugated BTX. The peak amplitudes of the currents in the presence of BTX are expressed as a percent of the peak amplitudes in the absence of BTX. c) HEK-293 or N1E-115 cells transiently transfected with 5-HT3A/BBS and pmCherry. Transfected cells identified by the expression of mCherry (red) and 5-HTA/BBS receptors are labeled with BTX/488 (green). Merged images include XY, XZ, and YZ images to show that BTX/488 labeling is consistently at or near the cell surface with intracellularly expressed mCherry. d) HEK-293 cells stably expressing 5-HT3A/BBS receptors incubated in non-conjugated BTX for 30 min at 4 °C prior to incubation with Alexa/488 conjugated BTX for 30 min at 4 °C. Scale bars=10 μm.
Labeling of surface 5-HT3A/BBS receptors
5-HT3A/BBS receptors were expressed in HEK-293, or in N1E-115 neuroblastoma cells. Unlike HEK-293 cells, the N1E-115 cells endogenously express 5-HT3A receptors (Peters and Lambert, 1989) and may express associated proteins that could influence cell surface stability and trafficking. Cells transiently transfected with 5-HT3A/BBS receptors were incubated for 30 min at 4 °C in the presence of 1 μg/ml α-bungarotoxin conjugated to Alexa 488 (BTX/488). The cells were washed, fixed with 4% formaldehyde, and imaged using confocal microscopy. Transfected HEK-293 and N1E-115 cells were identified by the expression of mCherry that was co-transfected with the 5-HT3A/BBS construct. Only transfected cells showed any detectable labeling with BTX/488 and, due to the impermeability of the BTX/488, only surface 5-HT3A/BBS receptors were labeled with BTX/488 (Fig. 1c). The same staining patterns were seen when labeled 5-HT3A/BBS receptors were imaged without fixation (discussed below). Binding of BTX/488 to 5-HT3A/BBS receptors stably expressed in HEK-293 cells could be blocked by pre-incubation with non-conjugated BTX prior to BTX/488 application (Fig. 1d). Therefore, 5-HT3A/BBS surface receptors can be labeled specifically with BTX conjugated to Alexa fluorophores with little to no background labeling.
Constitutive internalization of 5-HT3A receptors
The ability to fluorescently label surface 5-HT3A/BBS allows us to monitor the internalization and trafficking of surface receptors. 5-HT3A/BBS receptors were expressed stably in HEK-293 and transiently in N1E cells and surface receptors were labeled with BTX/555 (1 μg/ml). Cells were fixed and imaged (Fig. 2a), or the cells were incubated at 37 °C for 30 min in the absence of an agonist then fixed and imaged. Following 30 min at 37 °C, confocal images show fluorescent puncta that appeared to be inside the cell, and there was very little evidence of cell surface fluorescence (Fig. 2b). Thus, the majority of labeled receptors appear to be internalized during the 30 min incubation period.
Fig. 2.
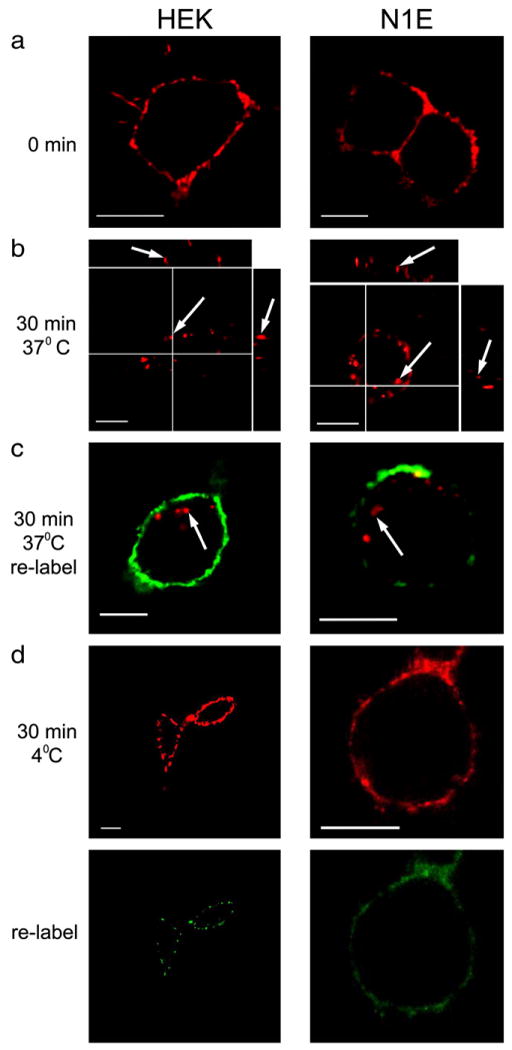
5-HT3A/BBS receptors are constitutively internalized in both HEK-293 and N1E-115 cells. a) HEK-293 and N1E-115 expressing 5-HT3A/BBS receptors incubated with BTX/555 (red label) for 30 min at 4 °C. b) After labeling surface 5-HT3A/BBS receptors, HEK-293 and N1E-115 cells were incubated at 37 °C for 30 min in the absence of an agonist, and subsequently imaged. Arrows indicate internal puncta in XY, XZ, and YZ images. c) Following the internalization of labeled receptors at 37 °C, HEK-293 and N1E-115 cells were incubated in the presence of BTX/488 (green label) for 30 min at 4 °C then fixed and imaged. Arrows indicate previously internalized BTX/555. d) To test the temperature sensitivity of the internalization, surface 5-HT3A/BBS receptors were labeled with BTX/555 (red) in HEK-293 and N1E-115 cells and were incubated at 4 °C for an additional 30 min in the absence of BTX. Following this incubation cells were labeled with BTX/488 (green). Scale bars=10 μm.
Following the internalization of BTX/555 labeled 5-HT3A/BBS at 37 °C, cells were incubated at 4 °C in the presence of BTX/488 (1 μg/ ml) to examine if unlabeled receptors were trafficked to the surface during the 30 min at 37 °C. Fig. 2c shows that the majority of BTX/555-labeled puncta appear to be inside the cell, while BTX/488 labeling is limited to the cell surface. These findings suggest that labeled receptors are internalized over the 30 min period and are replaced by unlabeled receptors. This apparent constitutive internalization of 5-HT3A receptors is temperature sensitive, because surface receptors labeled with BTX/555 remain on the cell surface after 30 min at 4 °C (Fig. 2d top row). Furthermore, if cells are incubated with BTX/488 following the 30 min at 4 °C there is some labeling with the BTX/488 (Fig. 2d bottom row). Similar labeling was seen when cells were labeled with BTX/555, washed, and immediately labeled with BTX/488 (data not shown). Therefore, the labeling of the second fluorophore is most likely due to the unbinding of the first fluorophore that occurs even at low temperatures when internalization is prevented.
We next quantified the internalization rate when surface 5-HT3A/BBS receptors were labeled with BTX/488 and the cells were incubated at 37 °C. Every 5 min for 30 min individual dishes were removed from the incubator and the cell membranes were labeled with WGA/555 (10 μg/ml) for 20 min at 4 °C. Following WGA labeling cells were fixed and imaged. To quantify receptor internalization we measured the BTX/488 fluorescence at the cell membrane and we observed a loss of fluorescence as receptors were internalized (Fig. 3a). To ensure that we were measuring the BTX/488 fluorescence at the cell surface a segmented line was drawn along the WGA/555 fluorescence and the BTX/488 fluorescence was averaged along that line. The majority of surface receptors are internalized in the first 10 min (Fig. 3a).
Fig. 3.
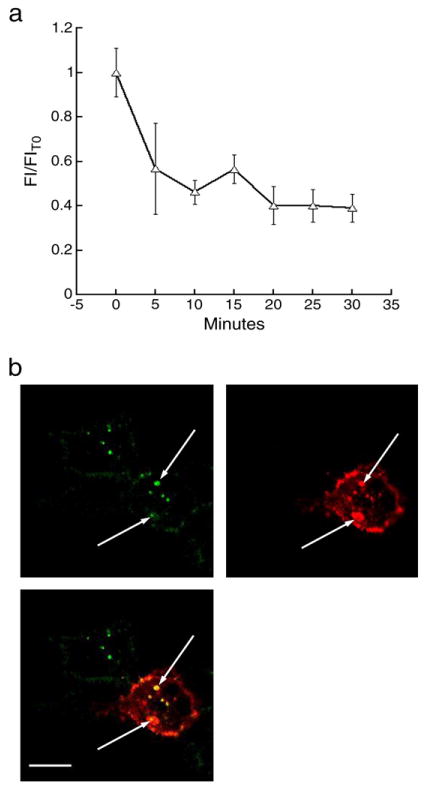
Internalized fluorescent BTX remains associated with 5-HT3A/BBS receptors. a) Surface 5-HT3A/BBS receptors were labeled with BTX/488 and incubated at 37 °C for 30 min. Every 5 min a sample was taken and the cell membranes were labeled with BTX/555 then fixed with 4% formaldehyde. BTX/488 fluorescence was averaged along the WGA/555 fluorescence. b) HEK-293 cells stably expressing 5-HT3A/BBS receptors were transiently transfected with cDNA encoding a 5-HT3A receptor tagged with an HA epitope. Surface receptors were labeled with BTX/488 and then incubated for 30 min at 37 °C. Cells were fixed with 4% formaldehyde for 20 min at 4 °C, permeabilized and incubated with HA antibodies conjugated to Alexa 594 (red label). Arrows indicate co-localized puncta containing both BTX/488 and anti-HA label. Scale bar=10 μm.
HEK-293 cells stably expressing the 5-HT3A/BBS receptors were transiently transfected with 5-HT3A subunits tagged with the hemagglutinin (HA) epitope to determine if the BTX/Alexa remained bound to 5-HT3A/BBS receptors after internalization. Due to the pentameric structure of the 5-HT3A receptors most if not all should contain at least one of each tagged subunit. Surface 5-HT3A/BBS receptors were labeled with BTX/488 and then incubated at 37 °C for 30 min. Cells were fixed, permeabilized and incubated with HA-594 antibodies to label all 5-HT3A/HA subunits. Cells that were transfected with the HA-tagged 5-HT3A receptors showed that every internalized cluster of BTX/488 co-localized with an HA-594 cluster (Fig. 3b), suggesting that the BTX/488 remains bound to the 5-HT3A/BBS receptors after internalization. As expected, there are many anti-HA labeled puncta that are not co-labeled with BTX/488, suggesting the majority of receptors are not on the cell surface at the time of BTX labeling (Fig. 3b).
Fluorescence-induced inactivation of 5-HT3A/BBS receptor internalization
We used live cell microscopy to follow the internalization of labeled surface receptors. 5-HT3A/BBS receptors stably expressed in HEK cells were labeled with BTX/488. Images were collected every minute for 30 min at 37 °C. Fig. 4 shows images collected at 0, 10, 20, and 30 min after labeling, imaged on a wide-field fluorescence microscope with a 100 W Mercury lamp (Fig. 4a) or on a confocal microscope with a 30 mW argon laser (Fig. 4b). The cells imaged on the wide-field or confocal microscope were exposed to ∼488 nm light for approximately 10 s or 4 s, respectively, every minute. Surprisingly, no evidence of receptor/fluorophore internalization was observed after exposure of BTX/488 labeled receptors to ∼488 nm light generated from either a mercury lamp or argon laser. However, surrounding cells not exposed to∼488 nm light during the 30 min had internalized receptors (Figs. 4c and d), suggesting the inhibition of internalization is dependent upon the excitation of the fluorophore. This fluorescence-induced inhibition of internalization was a consistent finding, occurring in the vast majority of cells examined with this protocol. In all experiments (5 wide field and 5 confocal), all labeled cells exposed to fluorescence excitation at our standard intensities (see below) showed little to no evidence of internal fluorescent puncta at the 30 min time point. Measurement of the post-objective power indicated that cells were exposed to 157.5 mW/cm2 ∼488 nm light on the confocal microscope and 9.73 mW/cm2 on the epi-fluorescence microscope. Confocal images required approximately 4 s of light exposure to acquire an image whereas the epi-fluorescence images required approximately 10 s of light per image. Therefore, over the course of the 30 min experiment, cells imaged on the confocal microscope were exposed to the light for a total duration of 120 s compared to about 5 min for cells imaged on the epi-fluorescence microscope. Fluorescence-induced inhibition of 5-HT3A/BBS receptor internalization was not dependent on the wavelength of excitation because the inhibition could also be achieved with α-bungarotoxin conjugated to Alexa-555 (data not shown).
Fig. 4.
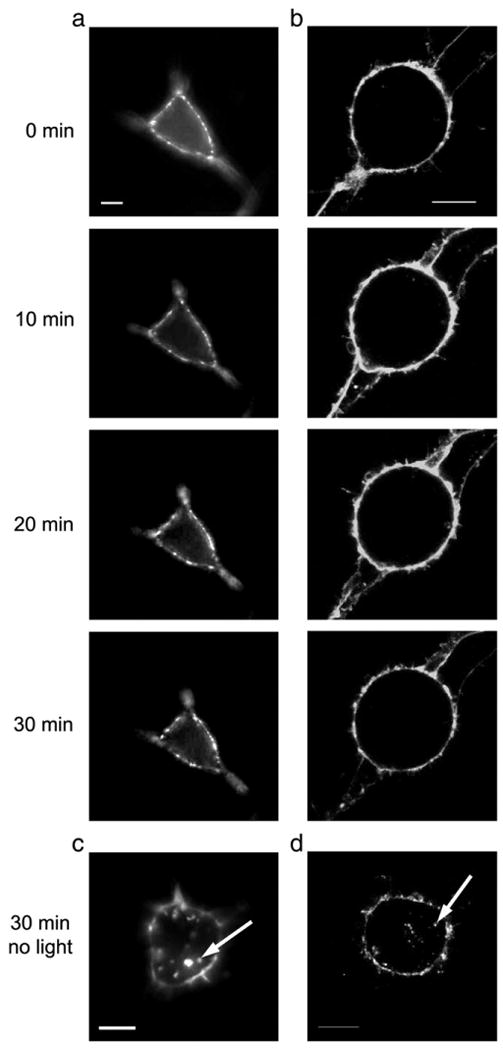
Fluorescent light exposure inhibits the constitutive internalization of 5-HT3A/BBS receptors. BTX/488 labeled surface 5-HT3A/BBS receptors stably expressed in HEK-293 cells were exposed to 9.73 mW/cm2 of ∼488 nm light generated from a 100 W mercury arc lamp (a) or 157.5 mW/cm2 of ∼488 nm light from an argon laser (b) every minute for 30 min at 37 °C. Representative images of cells are shown at 0, 10, 20, and 30 min. After 30 min at 37 °C surrounding cells that were not exposed to light from a mercury arc lamp (c) or an argon laser (d) are shown. Arrows indicate internal puncta. Scale bar=10 μm.
Fluorescence-induced inhibition of receptor internalization was also achieved when labeled surface receptors were imaged with 157.5 mW/cm2 of ∼488 nm light every 5 s for the first 2 min on a confocal microscope then monitored for internalization after 30 min at 37 °C. In confocal imaging experiments, image acquisition required approximately 4 s, and thus the cells were imaged almost continuously for the first 2 min. A representative image of a cell after 2 min of imaging is shown in Fig. 5a (upper image). Following the first 2 min of imaging cells were maintained at 37 °C on the stage and imaged once every 5 min to monitor health and/or movement of the cell. After 30 min a lower magnification image was taken to show the cell that was imaged previously as well as surrounding cells (Fig. 5a bottom image). Unlike the cell imaged for the first 2 min the surrounding cells contain internalized receptors. To quantify the fluorescence-induced inhibition of receptor internalization we counted the number of puncta that were clearly internal and separate from the cell surface in cells that were or were not exposed to excitatory light during the internalization assay. Cells that were not exposed to light contained on average 28 +/−3 internal clusters (18 cells) of varying size, while cells that were exposed to light for 2 min (6 cells) contained on average 2 +/−1 internal clusters (Fig. 5b). To test if the inhibition of internalization was dependent upon the excitation of the fluorophore, surface 5-HT3A receptors were labeled with BTX/488 and exposed to ∼543 nm light (that should not excite this fluorophore) for 2 min (Fig. 5c left image). After 30 min at 37 °C the BTX/488 labeled 5-HT3A receptors appear in intracellular clusters (Fig. 5c right image) suggesting that the fluorescence induced inhibition of internalization is dependent upon the excitation of the specific fluorophore attached to the receptor.
Fig. 5.
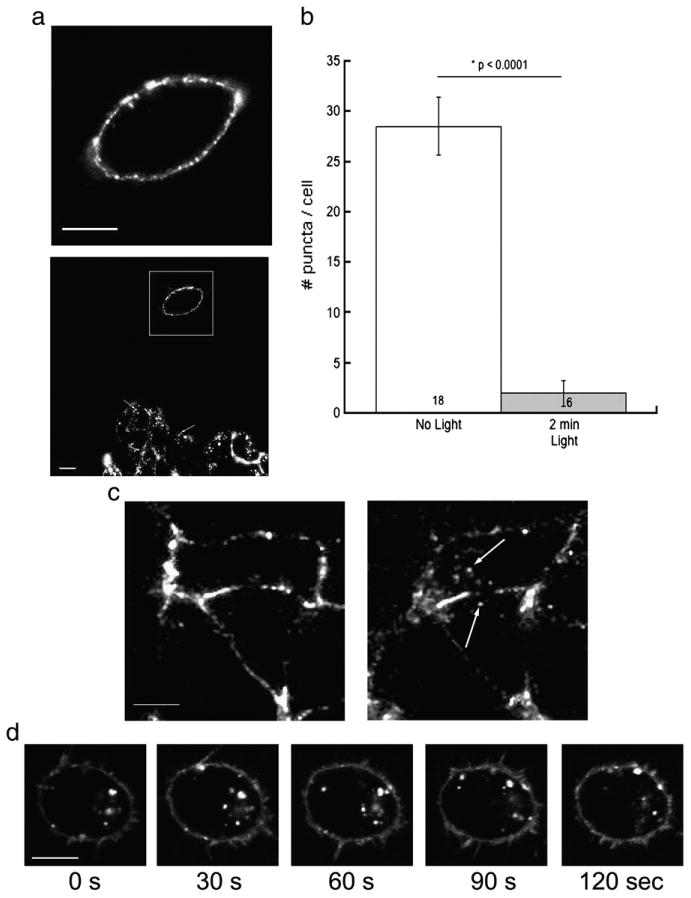
Fluorescence-induced inhibition of internalization but not intracellular movement of labeled receptors. a) Representative confocal image of a HEK-293 cell with labeled surface 5-HT3A/BBS receptors that was exposed to 157.5 mW/cm2 of ∼488 nm light from a 30 mW argon laser every 5 s for 2 min (top image). After 30 min at 37 °C receptor internalization was monitored with reduced magnification to allow visualization of cells examined previously, as well as surrounding cells not previously exposed to light (bottom image). The box indicates the cell imaged for the first 2 min and arrows indicate internal puncta in the unexposed cell. b) The number of internal clusters of BTX/488 that were separate from the cell membrane were counted and averaged per cell. c) Representative confocal image of a HEK-293 cell with labeled surface 5-HT3A/BBS receptors that was exposed to ∼543 nm light for 2 min. After 30 min at 37 °C cells exposed to ∼543 nm light contained internalized receptors. Arrows indicate internalized clusters. d) In HEK-293 cells surface labeled 5-HT3A/BBS receptors were allowed to internalize for 30 min at 37 °C. After 30 min at 37 °C the cells were imaged with 157.5 mW/cm2 of ∼488 nm light for 2 min. Representative images at 30, 60, 90, and 120 s are shown. Note the different positions of the puncta at each time point. Scale bars=10 μm.
We allowed BTX/488 labeled surface receptors to internalize for 30 min at 37 °C in the absence of light and imaged the internalized clusters to determine if general intracellular trafficking of labeled 5-HT3A/BBS receptors is inhibited by fluorescent excitation. Cells were imaged every 5 s for 2 min with 157.5 mW/cm2 of ∼488 nm light. Fig. 5c shows representative images during the 2 min of imaging. The internalized clusters are visible at each time point. However, the locations of these clusters vary at the different time points in the representative images suggesting that the movement of the internalized clusters was not inhibited by the fluorescence (Fig. 5d).
Fluorescence-induced inactivation of 5-HT3A receptor function
We performed voltage clamp electrophysiological recordings using the HEK cell line stably expressing 5-HT3A/BBS receptors to investigate whether exposure to fluorescence affected the function of 5-HT3A/BBS receptors. The whole-cell voltage clamp configuration was used and three responses to 30 μM 5-HT were recorded and the peak amplitudes were averaged to calculate the initial response (I0) at T0 for each cell. Following initial recordings at T0, cells were incubated in the presence or absence of BTX/488 for 20 min at 25 °C during the recording. Following the 20 min incubation cells were exposed again to 30 μM 5-HT three times and the peak amplitudes were averaged to obtain the average response (I) at time point T1. Cells were then incubated for 6 min in the presence or absence of ∼488 nm light. Again following the 6 min incubation cells were exposed to 30 μM 5-HT three times and the evoked currents were averaged to obtain the mean peak current amplitude at time point T2. A schematic representation of the protocol is shown in Fig. 6a. To control for “rundown” of the receptor (i.e. time-dependent loss of current and changes in kinetics due to the whole-cell recording itself) cells were voltage clamped in the whole-cell configuration, and receptor function was assayed by exposure to 30 μM 5-HT at the T0, T1, and T2 time points in the absence of BTX/488 or light. Voltage clamped cells were incubated in the absence of BTX/488 for 20 min, and then exposed to 9.73 mW/cm2 of ∼488 nm light for 6 min with receptor function assayed by exposure to 30 μM 5-HT at the T0, T1, and T0 time points to test if light alone inhibited 5-HT3A/BBS receptors.
Fig. 6.
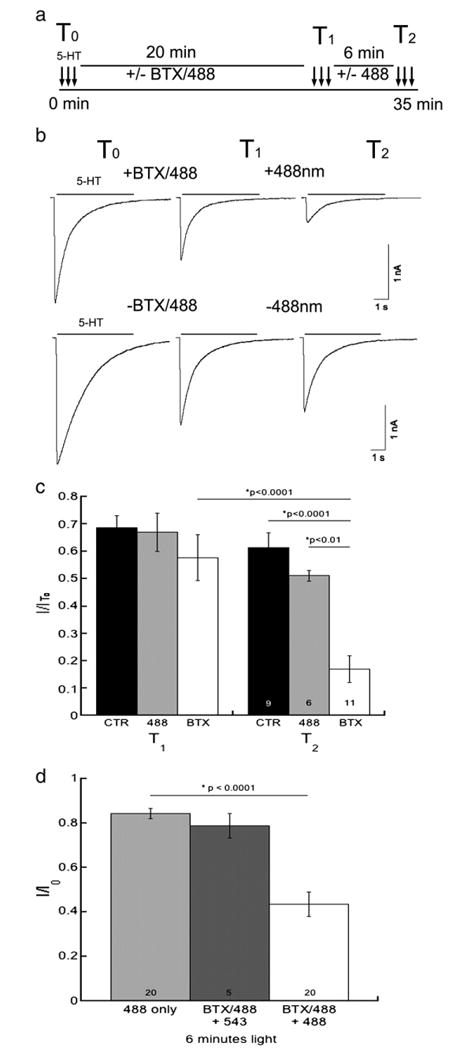
Fluorescent excitation inhibits the function of 5-HT3A/BBS receptors. Whole-cell voltage-clamp electrophysiology was performed on HEK-293 cells stably expressing 5-HT3A/BBS receptors.a)Schematic representation of the protocol used. b) Representative current traces elicited by 30 μM 5-HT at T1, and T2 from a cell exposed to BTX/488 and ∼488 nm light (top traces) or a control cell exposed to neither BTX/488 nor ∼488 nm light (bottom traces). c) Averaged peak current amplitude data expressed as a proportion of the initial peak amplitude (I/I0) at T0 at T1 and T2 for control (CTR), light only (488), and (BTX) cells (n = 6–11). d) HEK cells stably expressing 5-HT3A/BBS receptors were labeled with BTX/488 prior to whole cell voltage clamp electrophysiology. Cells were exposed to 30 μM 5-HT every minute. After a few baseline currents recorded cells were exposed to either ∼488 nm or ∼543 nm light for 6 min. ANOVA analysis with a Tukey HSD post hoc test determined there was a significant reduction in the peak amplitudes of BTX/488 labeled receptors after the exposure to ∼488 nm light for 6 min.
Representative currents elicited by 5-HT3A/BBS receptors in the absence or presence of BTX and light are shown (Fig. 6b). Due to receptor “rundown” after 20 min of whole cell recording in the presence or absence of BTX/488, the peak amplitudes were substantially smaller than at T0 (Fig. 6b). Currents mediated by 5-HT3A/BBS receptors labeled with BTX/488 and exposed to 9.73 mW/cm2 light exhibited a significant decrease in peak amplitude. The decrease in the current amplitude was significantly larger than that produced by rundown or light alone (Fig. 6c). The current peak amplitudes were decreased on average by approximately 30% at T1 for both control and 488 light only cells. The peak amplitudes from cells incubated in the presence of BTX/488 for 20 min were decreased by about 40%. The additional 10% reduction in peak amplitude is consistent with the small concentration independent inhibition shown with un-conjugated BTX in Fig. 1b. Light alone caused no significant reduction in current amplitude. The current peak amplitude of 5-HT3A/BBS receptors labeled with BTX/488 and exposed to ∼488 nm light for 6 min was significantly reduced by approximately 80% relative to the initial current. The current peak amplitude after BTX/488 fluorescence at T2 was significantly different from control (p<0.0001) and light only cells (p<0.01).
Due to the low survival of cells for the entire 35 min recording period, we next examined HEK cells stably expressing 5-HT3A/BBS receptors and labeled with BTX/488 for 20 min at 25 °C prior to obtaining the whole cell configuration. Cells were exposed to 30 μM 5-HT every minute to monitor 5-HT3A/BBS receptor function during the exposure to light for 6 min. Similar to previously shown data, the 5-HT3A/BBS receptor-mediated peak current amplitude was reduced by approximately 20% when exposed to 9.73 mW/cm2 of ∼488 nm light for 6 min in the absence of bound BTX/488 (Fig. 6d). However, when 5-HT3A/BBS receptors were labeled with BTX/488 and exposed to 9.73 mW/cm2 of ∼488 nm for 6 min there was an approximately 60% reduction in the 5-HT3A/BBS receptor mediated peak amplitude (Fig. 6d). 5-HT3A/BBS receptors were labeled with BTX/488 and exposed to ∼543 nm light to test if receptor inhibition was dependent upon the excitation of the fluorophore. Similar to ∼488 nm light alone there was an approximately 20% reduction in 5-HT3A/BBS mediated peak amplitude when receptors were labeled with BTX/488 and exposed to ∼543 nm light for 6 min. These data show that labeling the receptors with BTX/488 and exciting the attached fluorophore can inhibit currents mediated by 5-HT3A/BBS receptors.
Molecular specificity of fluorescence-induced inactivation of 5-HT3A receptors
It has been shown that fluorescence-induced inactivation cannot only inactivate labeled proteins of interest but also adjacent unlabeled proteins (Guo et al., 2006 and Rahmanzadeh et al., 2007). To test for this type of collateral damage we monitored the function of different wild-type receptors co-expressed with BBS tagged receptors labeled with BTX/488. First, we co-expressed transiently wild-type (untagged) 5-HT3A receptors with the metabotropic glutamate receptor 8 (mGluR8) tagged with BBS in HEK-293 cells. Similar to 5-HT3A/BBS receptors, surface mGluR8/BBS receptors were labeled by incubation with BTX/488 for 30 min at 4 °C (Fig. 7a). Cells with BTX/488 labeled mGluR8/BBS receptors were selected for whole-cell patch clamp recordings and cells were exposed to 30 μM 5-HT every minute to monitor the function of 5-HT3A receptors. Alternatively, HEK cells stably expressing 5-HT3A/BBS receptors were transfected with glycine α2 receptors and the pmCherry vector. Cells that expressed mCherry and were labeled with BTX/488 were selected for whole-cell patch clamp recordings. Cells were exposed to 100 μM glycine every minute to monitor the function of the glycine α2 receptors. In both cases a baseline response was established and cells were exposed to 9.73 mW/cm2 of ∼488 nm light generated from a 100 W mercury arc lamp.
Fig. 7.
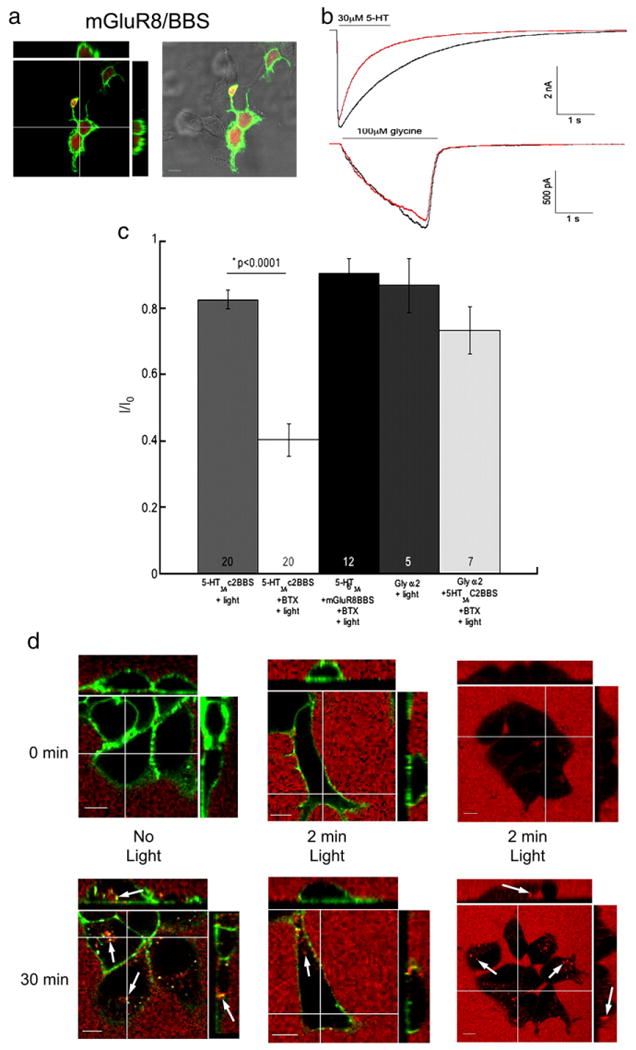
Collateral damage of receptor internalization but not receptor function. HEK-293 cells were transiently transfected with wild-type 5-HT3A, metabotropic glutamate 8 receptors (mGluR8) tagged with the BBS and pmCherry. a) Surface mGluR8 receptors were labeled with BTX/488 and cells were fixed with 4% formaldehyde then imaged. b) Representative traces of currents mediated by 5-HT3A receptors (top traces) and glycine α2 receptors (bottom traces) before (black traces) and after (red traces) BTX/488 labeled mGluR8/BBS or 5-HT3A/BBS receptors respectively were exposed to 9.73 mW/cm2 of ∼488 nm light. c) Averaged cumulative peak amplitudes evoked by either 30 μM 5-HT or 100 μM glycine expressed as a proportion of the initial peak amplitude (I/I0) after 9.73 mW/cm2 of ∼488 nm light from 5-HT3A/BBS, 5-HT3A, or glycine receptors in the presence or absence of increased oxygen. d) Surface 5-HT3A/BBS receptors were labeled with BTX/488 and transferrin/555(red) was added to the medium (top row). Cells were maintained at 37 °C on the microscope and imaged after 30 min (bottom row). Cells with BTX/488 (green) labeled 5-HT3A/BBS receptors were not exposed to light for 2 min (left column) or exposed to ∼488 nm light every 5 s for 2 min (middle column). Cells without BTX/488 were exposed to ∼488 nm light every 5 s for 2 min (right column). Arrows indicate internalized puncta. Scale bar=10 μm.
Representative traces from 5-HT3A and glycine α2 receptors in the presence of BTX/488 labeled mGluR8/BBS or 5-HT3A/BBS receptors, respectively, are shown in Fig. 7b. The initial traces are shown in black and the red traces were recorded following 6 min of 9.73 mW/cm2 ∼488 nm light. There was no significant decrease in the peak amplitude of currents mediated by both receptors after 6 min of ∼488 nm light. The increase in the rate of desensitization of 5-HT3A receptor mediated currents are similar to that observed in control recordings and are not due to fluorescence.
As previously shown, the currents mediated by 5-HT3A/BBS receptors were significantly inhibited when receptors were labeled with BTX/488 and exposed to ∼488 nm light for 6 min compared to those exposed to light alone (Fig. 7c). However, when mGluR8/BBS receptors are labeled with BTX/488 and exposed to ∼488 nm light the 5-HT3A receptor-mediated currents were not significantly decreased, and current amplitude was similar to that under the light alone condition (Fig. 7c). Furthermore, when the 5-HT3A/BBS receptors were labeled and exposed to light for 6 min the function of the glycine α2 receptors was not significantly different compared to that recorded from cells exposed to light alone (Fig. 7c).
We followed the internalization of transferrin conjugated to Alexa/555 to test if the fluorescence-induced inhibition of the internalization of 5-HT3A/BBS receptors also inhibited the internalization of other receptors. HEK cells stably expressing 5-HT3A/BBS receptors were imaged on a confocal microscope in the presence of extracellular transferrin/555 and the accumulation of intracellular transferrin/555 puncta was monitored after 30 min. Fig. 7d shows that in the absence of light, both BTX/488 labeled 5-HT3A/BBS receptors and transferrin/555 are internalized (left column) after 30 min at 37 °C. However, in the presence of BTX/488 bound to the 5-HT3A/BBS receptors the exposure to ∼488 nm light for 2 min noticeably reduced the internalization of both 5-HT3A/BBS receptors and transferrin/555 (Fig. 7d middle column) after 30 min at 37 °C. The internalization of transferrin was not inhibited by 2 min of ∼488 nm light in the absence of BTX/488 (Fig. 7d right column).
Fluorescence-inactivation of 5-HT3A/BBS function and internalization are dependent upon time and intensity of light
Fluorescence-induced inhibition of protein function has been well documented (Jay, 1988; Guo et al., 2006; Rajfur et al., 2002, Tanabe et al., 2005, Surrey et al., 1998; Yan et al., 2006; Marek and Davis, 2002; Tour et al., 2003; McLean et al., 2009; Vitriol et al., 2007; Lee et al., 2008; Horstkotte et al., 2005; McLean et al., 2009), and has been termed FALI or CALI. Furthermore, it has been shown that FALI is dependent on the irradiation energy, which is the product of time and the intensity of the light exposure (Horstkotte et al., 2005; McLean et al., 2009; Guo et al., 2006; Rahmanzadeh et al., 2007). We wanted to compare the fluorescence-induced inhibition that we observed to that of the FALI phenomena. Therefore, we tested if our fluorescence-induced inhibition was dependent upon irradiation energy. To examine if the fluorescence-induced inhibition of 5-HT3A/BBS mediated currents was dependent on the duration of the light exposure, whole-cell voltage clamp recordings were performed with HEK cells stably expressing 5-HT3A/BBS receptors. Currents elicited by 30 μM 5-HT were recorded every minute in the absence or presence of bound BTX/488. Multiple currents were recorded to establish the baseline peak amplitude, cells were then exposed to 9.73 mW/cm2 of ∼488 nm light from a 100 W mercury lamp for 6 min. The peak amplitudes of the currents were measured and expressed as a proportion of the initial current peak amplitude (I0) after 0, 2, 4, or 6 min of ∼488 nm light (Fig. 8a). When surface receptors were labeled with BTX/488 currents elicited by 30 μM 5-HT were reduced by 20% after 2 min of ∼488 nm light. Further exposure to ∼488 nm light for 4 or 6 min decreased the peak amplitude further by 40% and 60%, respectively. A two-way ANOVA analysis determined that there was a significant difference between the time points (p<0.0001), a significant difference between light alone and FALI (p<0.0001), and that there was a significant interaction between the presence or absence of BTX/488 and time of exposure to light (p<0.0001).
Fig. 8.
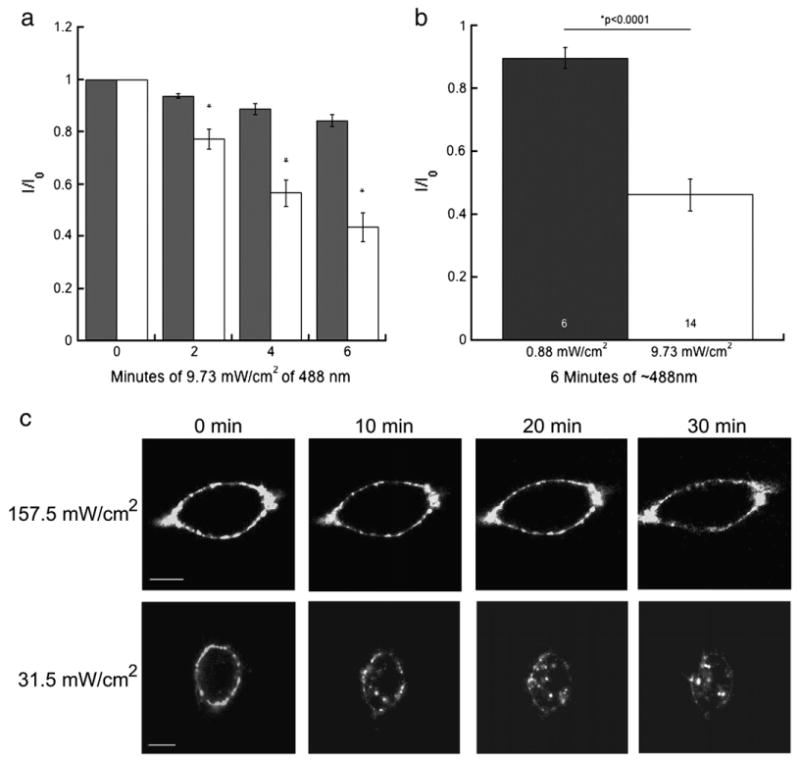
Fluorescence inactivation of 5-HT3A/BBS receptor function and internalization is dependent upon light intensity and duration of exposure. HEK-293 cells stably expressing 5-HT3A/BBS receptors were incubated in the presence or absence of BTX/488. Currents were elicited with 30 μM 5-HT every minute and exposed to 9.73 mW/cm2 of ∼488 nm light from a 100 W mercury arc lamp for 6 min. a) Peak amplitudes expressed as a proportion of the initial current (I0). The * indicates currents of BTX/488 labeled 5-HT3A/BBS receptors that were significantly (p<0.0001) different from currents elicited by cells exposed to light only (n = 14). Two-way ANOVA analysis indicated a significant (p<0.0001) interaction between the duration of light exposure and the presence of the BTX/488. b) The average peak amplitude of BTX/488 labeled 5-HT3A/BBS after 6 min of either 9.73 mW/cm2 or 0.88 mW/cm2 of ∼488 nm light. c) HEK-293 cells stably expressing 5-HT3A/BBS receptors with surface receptors labeled with BTX/488 were imaged every minute with either 157.5 mW/cm2 (top row) or 31.5 mW/cm2 (bottom row) of ∼488 nm light from an argon laser. c) Images of a representative cell at 0, 10, 20, and 30 min. Scale bar=10 μm.
Whole-cell voltage clamp recordings were obtained with cells that had surface 5-HT3A/BBS receptors labeled with BTX/488 and currents induced by 30 μM 5-HT were recorded. Three initial responses were recorded then exposed to ∼488 nm light for 6 min. As previously mentioned cells were exposed to 0.88 mW/cm2 of light or with a neutral density filter that resulted in 9.73 mW/cm2 of ∼488 nm light exposure for 6 min. Fig. 8b shows a significant reduction in the fluorescence-induced inhibition generated from 9.73 mW/cm2 compared to 0.88 mW/cm2 of ∼488 nm light after 6 min. These data suggest that fluorescence-induced inhibition of receptor function is dependent on the presence of the BTX/488, the duration of light exposure, and the intensity of the light.
To determine if the fluorescence-induced inhibition of internalization of 5-HT3A/BBS receptors is also dependent upon the intensity of light, we labeled surface receptors and imaged cells every minute with a confocal microscope. Exposure to 157.5 mW/cm2 of ∼488 nm light resulted in the inhibition of internalization (Fig. 8c top row) as previously shown. However, if we imaged cells with only 31.5 mW/cm2 of ∼488 nm light from the argon laser every minute (Fig. 8c bottom row) we were able to observe the appearance of internalized fluorescent puncta in real time. Similar to the fluorescence-induced inhibition of function, the inhibition of internalization could be prevented by using lower light power. These data highlight the fact that during live-cell fluorescence imaging, the amount of light exposure should always be considered to minimize such inadvertent inactivation of protein internalization. Furthermore, these data are in agreement with previously published work on FALI demonstrating that the inhibition is dependent upon the irradiation energy.
Oxygen radicals mediate FALI of receptor function and internalization
It has been well established that the inhibition of proteins by the FALI process is mediated by reactive oxygen species (ROS). To test if the fluorescence-induced inhibition of the function and internalization of 5-HT3A/BBS receptors are mediated by oxygen radicals we exposed the cells to reactive oxygen scavengers. Currents mediated by labeled 5-HT3A/BBS receptors were recorded once per minute as previously described. However, cells were bathed in oxygen radical scavengers, either 1 mM sodium azide (NaN3 ) or 20 mM mannitol, during the exposure to ∼488 nm light. The peak amplitudes of the currents recorded after 6 min were expressed as a proportion of the initial current (I0) (Fig. 9a). After 6 min of 9.73 mW/cm2 ∼488 nm light, the 5-HT3A/BBS receptor-mediated peak current amplitude was reduced by approximately 28% in the presence of 1 mM sodium azide and by approximately 30% in 20 mM mannitol. Both oxygen radical scavengers significantly reduced the inhibition but were unable to completely prevent the inhibition. Higher concentrations of mannitol (50 mM and 100 mM) directly inhibited currents mediated by 5-HT3A/BBS receptors, and exposure to a higher concentration of sodium azide (10 mM) significantly reduced our ability to obtain a whole cell recording.
Fig. 9.
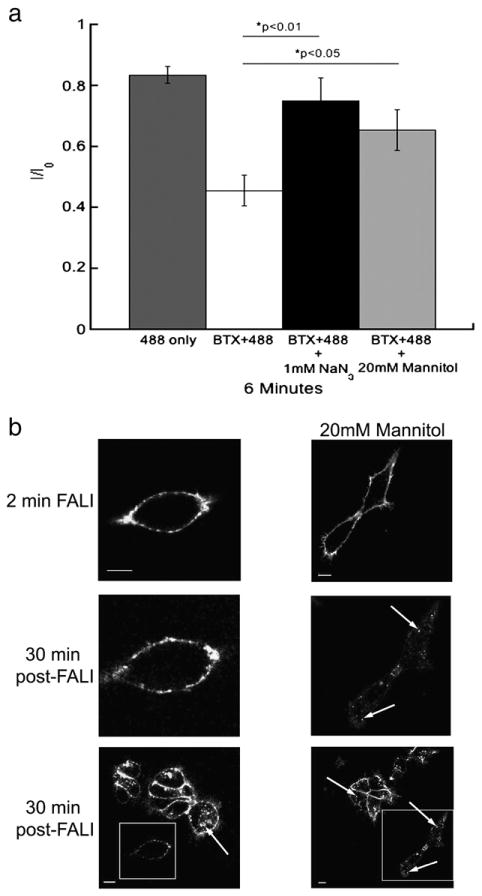
Fluorescence-induced inactivation of internalization and function of 5-HT3A/BBS receptors is reduced by reactive oxygen species scavengers. a) Average peak 5-HT-evoked current amplitudes recorded from HEK-293 cells stably expressing 5-HT3A/BBS receptors expressed as a proportion of the initial current (I0) after 6 min of ∼488 nm. Cells were exposed to 9.73 mW/cm2 of ∼488 nm light from a 100 W mercury arc lamp. Cells were either exposed to light alone (dark gray bars), or were labeled with BTX/488 and exposed to light in the absence of ROS scavengers (white bars). In the presence of 1 mM sodium azide (black bars) or 20 mM mannitol (light gray bars) an ANOVA analysis with a post-hoc Tukey test revealed that both 1 mM sodium azide (p<0.01) and 20 mM mannitol (p<0.05) reduced the fluorescence-induced inhibition of 5-HT3A/BBS receptor function. b) HEK-293 cells stably expressing 5-HT3A/BBS receptors and labeled with BTX/488 were exposed to 157.5 mW/cm2 of ∼488 nm light for 2 min (top row) in the absence (left column) or presence (right column) of 20 mM mannitol. After 30 min at 37 °C the same cell was imaged to monitor receptor internalization (middle row). A lower magnification image was also taken after 30 min at 37 °C to compare to surrounding cells that were not exposed to ∼488 nm light initially (bottom row). Boxes indicate cells exposed to light for 2 min and arrows indicate internal puncta. Scale bars=10 μm.
The apparent role of reactive oxygen in the fluorescence-induced inactivation, suggested that this effect might be increased by increasing the oxygen present in solution. Thus, we performed additional electrophysiology experiments in which all the solutions were bubbled with 95% oxygen and 5% carbon dioxide both before and during recordings. However, the fluorescence-induced inactivation of BTX/488 labeled 5-HT3A/BBS receptors in the presence of increased oxygen was 64%+/−8% reduction in peak amplitude compared to a 57%+/−5% reduction with normal oxygen levels. We also tested the function of glycine α2 receptors in the presence of BTX/488 labeled 5-HT3A/BBS receptors and in the presence of increased oxygen. Again, the peak amplitude of glycine α2 receptors was only reduced by 9%+/−10% in the presence of BTX/488 labeled 5-HT3A/BBS receptors and increased oxygen.
To investigate the involvement of reactive oxygen species in receptor internalization surface receptors were labeled with BTX/488 and cells were imaged for 2 min with excitation of 157.5 mW/cm2 by an argon laser at 37 °C in the absence or presence of 20 mM mannitol. Receptor internalization was monitored after 30 min (Fig. 9b). Exposure to mannitol significantly reduced the inhibition of internalization of 5-HT3A/BBS receptors, as evidenced by the appearance of fluorescent puncta inside cells following mannitol and light exposure (Fig. 9b). These findings suggest the involvement of reactive oxygen species in the fluorescence-induced inhibition and therefore indicate that the FALI process mediates the inhibition of 5-HT3A/BBS receptor function and internalization.
Discussion
In the present study we were able to tag the 5-HT3A subunit with the α-bungarotoxin pharmatope tag (Sanders and Hawrot, 2004; McCann et al., 2005; Sekine-Aizawa and Huganir, 2004; Wilkins et al., 2008; Guo et al., 2006; Watschinger et al., 2008) on the extracellular carboxyl terminal. Using this method we were able to specifically label surface receptors in live cells with α-bungarotoxin conjugated to Alexa fluorophores. We show that 5-HT3A/BBS receptors are constitutively internalized in the absence of an agonist. Furthermore, we were able to show that fluorophore assisted light inactivation (FALI) inhibits both the function and constitutive internalization of 5-HT3A receptors.
In agreement with previous studies, 5-HT3A receptors expressed in HEK-293 cells were constitutively internalized in the absence of an agonist at 37 °C (Wu et al., 2009; Xiong et al., 2008). We demonstrated that 5-HT3A/BBS receptors are also constitutively internalized in the N1E-115 neuroblastoma cell line that endogenously express 5-HT3A receptors. Therefore, it is unlikely that the constitutive internalization is unique to 5-HT3A receptors in a heterologous system. Constitutive internalization has also been described for GABAA receptors, another member of the cysteine-loop family of receptors, in both HEK-293 cells and primary hippocampal cultures (Herring et al., 2003 and Kittler et al., 2000). Our studies show that the majority of the labeled receptors are internalized after 30 min at 37 °C, which is similar to the internalization rates reported by Herring et al. and Kitler et al. for GABAA receptors.
In initial attempts to visualize constitutive internalization in real time, surface 5-HT3A/BBS receptors were labeled with BTX/488 and imaged by wide-field fluorescence. Cells were exposed to 9.73 mW/cm2 of ∼488 nm light every minute for 30 min at 37 °C. Our data show that the constitutive internalization of labeled surface receptors can be blocked if these receptors are labeled with a fluorophore and that fluorophore is excited by light while on the cell surface. These data provide the first evidence that FALI can inhibit the internalization of tagged proteins. Laser scanning microscopy with 157.5 mW/cm2 ∼488 nm light was used to inhibit the constitutive internalization of BTX/488 labeled 5-HT3A/BBS receptors. The FALI of receptor internalization was specific to the cell imaged because neighboring cells that were not exposed to light during the 30 min imaging period contained internalized clusters of labeled 5-HT3A/BBS receptors. Inhibition of constitutive internalization of 5-HT3A/BBS receptors could also be achieved by exposing the labeled surface receptors to 157.5 mW/cm2 of ∼488 nm light for as little as 2 min. It should be noted that significant photo-bleaching was seen in most cells during FALI, however, it has been suggested that photo-bleaching and FALI are not directly related (Horstkotte et al., 2005 and McLean et al., 2009). If the receptors were labeled with BTX/488 and allowed to internalize at 37 °C for 30 min, then exposed to light, the movement of fluorescent puncta within the cell was not inhibited. This finding suggests that FALI does not inhibit the proteins involved in the trafficking of intracellular vesicles.
FALI has been well documented to inhibit the function of enzymes (Jay, 1988; McLean et al., 2009; Lee et al., 2008) and proteins such as G protein-coupled receptors, motor proteins, cytoskeleton proteins, signal transduction proteins, synaptic vesicle docking and reuptake proteins, gap junction channels, and voltage gated ion channels (Guo et al., 2006; Surrey et al., 1998; Kasprowicz et al., 2008; Rajfur et al., 2002; Vitriol et al., 2007; Yan et al., 2006; Marek and Davis, 2002; Tour et al., 2003). The efficiency of FALI is unpredictable due to differences in the fluorophore used (McLean et al., 2009 and Lee et al., 2008) and the proximity of the fluorophore to the active site of the protein (Beck et al., 2002). In the experiments shown in Fig. 6 where whole cell voltage clamp was established prior to BTX/488-labeling and light exposure, we showed that the currents mediated by 5-HT3A/BBS receptors could be inhibited by 80%. This was most likely an over estimate of the fluorescence induced inhibition due to the deleterious effects of the fluorescence on cell survival. The results presented here are similar to Guo et al., who reported approximately 60% reduction in the mGluR8/BBS modulated calcium currents in the presence of BTX/488 and light. In both studies 100% of the ∼488 nm light generated from a 100 W mercury lamp was used in combination with Alexa fluorophores conjugated to α-bungarotoxin, and in our system we measured the light intensity post objective to be 9.73 mW/cm2. FALI is often achieved using much higher light power such as 2.6 W/cm2 (Surrey et al., 1998) and up to 17 W/cm2 (Tour et al., 2003), however, the duration of exposure in these studies was less than what others have used. Furthermore, the total irradiation energy in our experiments was still less than that applied in previous studies. It is possible that with higher irradiation energies more off target effects could be detected.
It has been suggested that FALI is specific to the protein labeled due to the approximately 40–60 Å half-maximal radius of damage (Beck et al., 2002 and Linden et al., 1992). However, others have shown that FALI can produce collateral damage to other unlabeled proteins (Guo et al., 2006 and Rahmanzadah et al., 2007). We were unable to detect collateral damage to the 5-HT3A wild-type receptors or the glycine α2 receptors when the mGluR8/BBS or 5-HT3A/BBS receptors, respectively, were labeled with BTX/488 and exposed to ∼488 nm light. However, the physical proximity of the labeled receptors and unlabeled receptors is unknown and it could be that they are simply not close enough to one another to allow for actions of the reactive oxygen species. It is also possible that higher light intensities could generate more reactive oxygen species and increase the distance over which damage occurs. The collateral damage reported by others (Guo et al., 2006 and Rahmanzadeh et al., 2007) was achieved with higher light intensities than we used. However, we were able to detect collateral damage to the internalization of transferrin receptors by the FALI of 5-HT3A/BBS receptors. These data suggest that 157.5 mW/cm2 light exposure produced some collateral damage to surrounding proteins or other molecules involved in internalization. It seems unlikely that FALI could affect the intracellular machinery directly due to the extracellular location of the fluorophore. Reactive oxygen species readily react with lipids making it unlikely that the reactive oxygen species would pass through the lipid-bilayer (Greenbaum et al., 2000). It is possible that the internalization of transferrin/555 is more vulnerable to collateral damage due to the low level of endogenously expressed transferrin receptors rather than the over-expressed recombinant proteins. Another possibility is that the majority of the reactive oxygen species do react with the lipid bi-layer causing the cross-linking of lipids, making the lipid bi-layer rigid and unable to form the invaginations necessary for any receptor internalization. Nonetheless, our data suggest that during the FALI process there is clear evidence of collateral damage to the internalization of other proteins and collateral damage should always be considered when using this technique.
As mentioned previously, the conditions that produce FALI of target proteins are somewhat unpredictable. A variety of intensities and durations of light exposure have been used to produce FALI. It has been well documented that both fluorescent synthetic molecules and fluorescent proteins can produce FALI, but it has been shown that fluorescent proteins are less efficient at producing reactive oxygen species (Tour et al., 2003 and Tanabe et al., 2005). This is most likely due to the shielding of the fluorophore by the barrel formation of the fluorescent proteins. Nonetheless, FALI has been shown to be dependent on the irradiation energy (McLean et al., 2009; Guo et al., 2006; Rahmanzadeh et al., 2007). Horstkotte and others have shown that the decay of enzyme function induced by FALI, in the presence of varying concentrations of fluorophore, could be described by a single exponential function (Horstkotte et al., 2005). These data presented here are in general agreement with previous studies with other proteins. There was a significant difference in the peak amplitudes of 5-HT3A/BBS receptors after 2, 4, and 6 min of ∼488 nm light with the BTX/488 bound to the receptor. Furthermore, we show that lower light intensities significantly decreased the FALI of 5-HT3A/BBS receptor function. Similarly, FALI of 5-HT3A/BBS internalization could be completely prevented by imaging the cells with only 31.5 mW/cm2 of ∼488 nm light from an argon laser compared to 157.5 mW/cm2 that resulted in FALI. Overall, our data for both receptor function and internalization are in agreement with previously published data demonstrating that FALI is dependent upon the irradiation energy.
It has been shown that fluorescence in the presence of oxygen can generate reactive oxygen species (reviewed in Schmidt, 2006) and that reactive oxygen species mediate FALI (Tour et al., 2003; Yan et al., 2006; Guo et al., 2006; Lee et al., 2008; Tanabe et al., 2005; (Davies et al., 2003; Bulina et al., 2006). We show that increasing the oxygen in solution did not enhance the FALI effect suggesting that the normal oxygen levels present in solution are sufficient to produce the reactive oxygen species.
Furthermore, we show that FALI of receptor function was significantly reduced in the presence of the oxygen radical scavengers sodium azide and mannitol, which is in agreement with previously published data (Guo et al., 2006; Tour et al., 2003; Tanabe et al., 2005; McLean et al., 2009; Lee et al., 2008; Bulina et al., 2006). The reactive oxygen species generated by FALI of calmodulin have been shown to cause methionine oxidation and cross-linking of residues (Yan et al., 2006). Furthermore, it has been shown that singlet oxygen radicals can lead to damage of Trp, Tyr, His, Met, and Cys residues (Davies, 2003). Thus, it is possible that the inactivation of the function of 5-HT3A/BBS receptors may be due to cross-linking of amino acids resulting in the inability to bind to agonist or to undergo conformational changes necessary for gating. However, the effects of FALI are not catastrophic enough to disrupt the non-covalent binding of α-bungarotoxin to the pharmatope tag. The mechanism by which FALI inhibits internalization of 5-HT3A/BBS receptors remains unclear, but internalization was achieved in the presence of mannitol suggesting the involvement of reactive oxygen species. Taken together our data suggest that 5-HT3A/BBS receptor function as well as internalization can be inhibited by FALI and this inhibition is mediated by reactive oxygen species.
This use of FALI could give insight into 5-HT3A receptor trafficking and function. For example, expressing these recombinant 5-HT3A receptors in neurons and using FALI to inhibit receptor internalization may provide more information about receptor turnover and may reveal receptor roles in neuronal physiology. Furthermore, using FALI in a mouse knock-in model of this receptor may give insight into the role of 5-HT3A neurotransmission in brain circuits such as the mesolimbic reward system or anxiety. However, the effects of overexpression of this construct, as well as potential collateral damage during FALI would have to be considered.
In summary, we have successfully tagged 5-HT3A receptors with the α-bungarotoxin pharmatope and surface receptors could be fluorescently labeled with α-bungarotoxin conjugated to Alexa fluorophores in live cells. Using this method we have demonstrated that 5-HT3A/BBS receptors constitutively internalize in the absence of agonist in HEK-293 cells and the neuroblastoma N1E-115 cell line. Binding of BTX/488 to 5-HT3A/BBS receptors did not inhibit currents mediated by the receptors unless the labeled receptors were exposed to ∼488 nm light. FALI also inhibited the constitutive internalization of 5-HT3A/BBS receptors, and inhibited the internalization of labeled transferrin by the transferrin receptor as well. FALI of both receptor function and internalization could be prevented in the presence of reactive oxygen scavengers or exposure to lower light intensities. Furthermore, our data highlight the fact that inadvertent FALI may occur during live cell microscopy with high intensity light, and care should be taken to avoid this problem when using fluorescent tags to study protein trafficking.
Experimental methods
5-HT3A/BBS construct
The α-bungarotoxin binding sequence was added to the carboxyl terminal of the mouse 5-HT3A by primer addition PCR using the QuickChange system (Stratagene, USA). The stop codon was removed and the first bungarotoxin binding sequence were added using the following forward primer:
5′tggtccatttggcattatacttggagatactacgagagctccctggagccctaccctgactaatctagagggcccgtttaaacc3′
and the following reverse primer:
5′ggtttaaacgggccctctagattagtcagggtagggctccagggagctctcgtagtatctccaagtataatgccaaatggacca3′.
The second bungarotoxin binding sequence and a linker sequence were added with the following forward primer:
5′tggtccatttggcattattctggaaacggaaacggaaacggaaacggaaactggagatactacgagagctccctggagccctaccctgactggagatactacgagagctcc3′
and the following reverse primer:
5′ggagctctcgtagtatctccagtcagggtagggctccagggagctctcgtagtatctccagtttccgtttccgtttccgtttccgtttccagaataatgccaaatggacca3′.
Cell cultures
HEK-293 and N1E-115 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% calf-serum, 1% pen/strep/glutamate supplement (Gibco Invitrogen Carlsbad, California). Cells were transfected with the mouse 5-HT3A/BBS receptor using polyethyleneimine (PEI) (Sigma Aldrich St. Louis, MO) at 150 μM per 1 μg DNA. Cells were incubated for 24 to 48 h after transfection to allow for expression. A HEK-293 clonal population stably expressing the 5-HT3A/BBS receptors was generated by transfecting cells with PEI as described above, and were allowed to express for 48 h. Cells were individualized with 0.25% trypsin, counted, and diluted to 5 cells/ml in DMEM/10%FCS/1% pen/strep/glutamate and geneticin (800 μg/ml). Cells were aliquoted into a 96 well plate at approximately 0.5 cells per well. Wells containing only one cell were incubated at 37 °C with 5% CO2 changing media as needed.
Expression of 5-HT3A/BBS receptors was measured by fluorescent labeling with BTX/Alexa555. Cells were maintained in a humid environment with 5% CO2 at 37 °C. To label the 5-HT3A/BBS receptors, cells were incubated in 1 μg/ml α-bungarotoxin conjugated to an Alexa fluorophore in serum free CO2 independent medium (Gibco Invitrogen Carlsbad, California) at 4 °C for 30 min. Alexa fluorophore conjugated α-bungarotoxin was purchased from Invitrogen (Carlsbad, California).
Microscopy
Confocal images were collected using a Zeiss LSM 510 (Zeiss USA, Thornwood, NY), or a Leica SP2 (Leica USA, Bannockburn, IL) microscope. For green fluorophore excitation, an argon laser was used and a HeNi laser was used for excitation of red fluorophores. For the LSM 510 system an excitation filter of 480/35 and an emission filter of 535/30 for green fluorescence and an excitation filter of 540/25 and an emission filter of 605/55 for red fluorescence were used. The SP2 microscope was equipped with filters to allow excitation at 488 or 543 and the emission filters were set at 505–550 and 550–600 for green and red fluorescence respectively.
During live cell imaging a NevTek heated blower (Williamsville, VA) was used to maintain cells at 37 °C and cells were maintained in serum free CO2-independent medium (Gibco Invitrogen Carlsbad, California). Images were acquired using the LSM imager or the Leica LCS software. Images were collected on both confocal microscopes using a 63× oil immersion objective with a 1.4 NA. Wide-field images were collected with a Zeiss Axiovert 200 (Zeiss USA, Thornwood, NY) inverted microscope equipped with a 32× F-LD, 0.4 NA objective and mercury arc lamp as the fluorescence light source. Excitation filters, 485/25 nm and 540/25 nm, and emission filters, 535/30 nm and 605/55 nm, were used for Alexa/488 and Alexa/555, respectively. Light powers were measured post-objective roughly at the focal plane with an Ophir ORION/PD microprocessor-based laser power meter (Logan, UT). The area of light exposure was measured with a micrometer and the mW/cm2 was calculated by dividing the light power by the area of light exposure at the focal plane.
Surface receptors were quantified by labeling surface receptors with BTX/488 and labeling the surface receptors with WGA/555 (10 μg/ml) (Invitrogen Carlsbad, California). Image acquisition was done with excitation filters, 485/25 nm and 540/25 nm, and emission filters, 535/30 nm and 605/55 nm, were used for Alexa/488 and Alexa/555, respectively. A segmented line was drawn along the cell surface using the WGA fluorescence as a marker in ImageJ software. The pixel intensities of the BTX/488 fluorescence along that line were averaged. This was done for three separate optical slices per cell and those averages were averaged for an entire cell.
Electrophysiology
The whole-cell configuration of the patch-clamp technique was used to record 5-HT3A-mediated currents in voltage-clamped cells. Cells in 35 mm diameter culture dishes were visualized with a Nikon Eclipse TE300 microscope equipped with a Plan Fluor 20× objective with NA of 0.45. Fluorescence was generated using a mercury arc lamp and excitation filter of 480/35, and an emission filter of 535/30 for green fluorescence. Cells were held at −60 mV and continuously washed by superfusion with an extracellular solution (in mM) of NaCl, 150; KCl, 2.5; MgCl2, 2.5; CaCl2, 2.5; glucose, 10; and HEPES, 10; NaOH (pH 7.4). Cells were lifted clear of the dish bottom to facilitate rapid drug application. A local agonist was applied by rapid perfusion exchange using a SF-77B Warner Instruments (Hamden, CT) fast step perfusion system. Pipettes were pulled from thin wall borosilicate glass tubing containing a filament, using a Sutter Flaming-Brown P-97 multi-stage puller. Electrodes were filled with an intracellular solution (in mM) of CsCl, 150; BAPTA 0.2; MgCl2, 1; Mg ATP, 3; HEPES, 10, GTP 0.3, pH 7.2. All chemicals used in the electrophysiological experiments were purchased from Sigma Aldrich (St. Louis, MO).
Currents were amplified and filtered with an Axopatch 200B (Molecular Devices Sunnyvale, CA), low pass filtering at 1 kHz, and digitized at 12.5 kHz using a Digidata 1322A interface (Molecular Devices Sunnywale, CA). Data were collected, displayed, stored and analyzed using pCLAMP8 software. Experiments were performed at room temperature 20–24 °C. Data were analyzed using clampfit 8.2 software. Light power was measured post-objective roughly at the focal plane with an Ophir ORION/PD microprocessor-based laser power meter (Logan, UT).
Statistics
All statistical analyses were performed in KaleidaGraph software (Synergy Software, Reading, PA). Statistical significance was determined by either a one or a two-way ANOVA with a post-hoc Tukey test.
Acknowledgments
We thank Dr. Henry H. Puhl III for the 5-HT3A/HA and the mGluR8/BBS constructs. We thank Dr. Stephen Vogel for use of the light power meter and comments on the manuscript. Thank you to Steve Ikeda for comments on the manuscript. And lastly, we thank Dr. Jennifer Gillette and Dr. Jennifer Lippincott-Schwartz for the use of the microscopes.
Abbreviations
- 5-HT3
5-hydroxytryptamine (serotonin) type 3 receptor
- BBS
bungarotoxin binding sequence
- BTX
α-bungarotoxin
- FALI
fluorophore assisted light inactivation
- HA
hemagglutinin
References
- Beck S, Sakurai T, Eustace BK, Beste G, Schier R, Rudert F, Jay DG. Fluorophore-assisted light inactivation: a high-throughput tool for direct target validation of proteins. Proteomics. 2002;2:247–255. doi: 10.1002/1615-9861(200203)2:3<247::aid-prot247>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Bulina ME, Lukyanov KA, Britanova OV, Onichtchouk D, Lukyanov S, Chudakov DM. Chromophore-assisted light inactivation (CALI) using the phototoxic fluorescent protein KillerRed. Nat Protoc. 2006;1:947–953. doi: 10.1038/nprot.2006.89. [DOI] [PubMed] [Google Scholar]
- Davies PA, Pistis M, Hanna MC, Peters JA, Lambert JJ, Hales TG, Kirkness EF. The 5-HT3B subunit is a major determinant of serotonin-receptor function. Nature. 1999;397:359–363. doi: 10.1038/16941. [DOI] [PubMed] [Google Scholar]
- Davies MJ. Singlet oxygen-mediated damage to proteins and its consequences. Biochem Biophys Res Commun. 2003;305:761–770. doi: 10.1016/s0006-291x(03)00817-9. [DOI] [PubMed] [Google Scholar]
- Grant KA. The role of 5-HT3 receptors in drug dependence. Drug Alcohol Depend. 1995;38:155–171. doi: 10.1016/0376-8716(95)01120-n. [DOI] [PubMed] [Google Scholar]
- Greenbaum L, Rothmann C, Lavie R, Malik Z. Green fluorescent protein photobleaching: a model for protein damage by endogenous and exogenous singlet oxygen. Biol Chem. 2000;381:1251–1258. doi: 10.1515/BC.2000.153. [DOI] [PubMed] [Google Scholar]
- Guo J, Chen H, Puhl HL, III, Ikeda SR. Fluorophore-assisted light inactivation produces both targeted and collateral effects on N-type calcium channel modulation in rat sympathetic neurons. J Physiol. 2006;576:477–492. doi: 10.1113/jphysiol.2006.113068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring D, Huang R, Singh M, Robinson LC, Dillon GH, Leidenheimer NJ. Constitutive GABAA receptor endocytosis is dynamin-mediated and dependent on a dileucine AP2 adaptin-binding motif within the beta 2 subunit of the receptor. J Biol Chem. 2003;278:24046–24052. doi: 10.1074/jbc.M301420200. [DOI] [PubMed] [Google Scholar]
- Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358:2482–2494. doi: 10.1056/NEJMra0706547. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Samson HH, Lewis RS, Erickson HL. Specific decreases in ethanol -but not water-reinforced responding produced by the 5-HT3 antagonist ICS 205-930. Alcohol. 1993;10:191–196. doi: 10.1016/0741-8329(93)90034-l. [DOI] [PubMed] [Google Scholar]
- Horstkotte E, Schroder T, Niewohner J, Thiel E, Jay DG, Henning SW. Toward understanding the mechanism of chromophore-assisted laser inactivation—evidence for the primary photochemical steps. Photochem Photobiol. 2005;81:358–366. doi: 10.1562/2004-07-22-RA-240. [DOI] [PubMed] [Google Scholar]
- Jacobson K, Rajfur Z, Vitriol E, Hahn K. Chromophore-assisted laser inactivation in cell biology. Trends Cell Biol. 2008;18:443–450. doi: 10.1016/j.tcb.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay DG. Selective destruction of protein function by chromophore-assisted laser inactivation. Proc Natl Acad Sci USA. 1988;85:5454–5458. doi: 10.1073/pnas.85.15.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky AM, Gotow LF, McKinley DD, Piechan JL, Ruble CL, Mills CJ, Schellin KA, Slightom JL, Fitzgerald LR, Benjamin CW, Roberds SL. A cluster of novel serotonin receptor 3-like genes on human chromosome 3. Gene. 2003;319:137–148. doi: 10.1016/s0378-1119(03)00803-5. [DOI] [PubMed] [Google Scholar]
- Kasprowicz J, Kuenen S, Miskiewicz K, Habets RL, Smitz L, Verstreken P. Inactivation of clathrin heavy chain inhibits synaptic recycling but allows bulk membrane uptake. J Cell Biol. 2008;182:1007–1016. doi: 10.1083/jcb.200804162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Delmas P, Jovanovic JN, Brown DA, Smart TG, Moss SJ. Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J Neurosci. 2000;20:7972–7977. doi: 10.1523/JNEUROSCI.20-21-07972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Yu P, Xiao X, Kodadek T. A general system for evaluating the efficiency of chromophore-assisted light inactivation (CALI) of proteins reveals Ru(II) tris-bipyridyl as an unusually efficient “warhead”. Mol Biosyst. 2008;4:59–65. doi: 10.1039/b712307h. [DOI] [PubMed] [Google Scholar]
- Linden KG, Liao JC, Jay DG. Spatial specificity of chromophore assisted laser inactivation of protein function. Biophys J. 1992;61:956–962. doi: 10.1016/S0006-3495(92)81902-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek KW, Davis GW. Transgenically encoded protein photoinactivation (FlAsH-FALI): acute inactivation of synaptotagmin I. Neuron. 2002;36:805–813. doi: 10.1016/s0896-6273(02)01068-1. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Peterson AS, Brake AJ, Myers RM, Julius D. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science. 1991;254:432–437. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- McCann CM, Bareyre FM, Lichtman JW, Sanes JR. Peptide tags for labeling membrane proteins in live cells with multiple fluorophores. Biotechniques. 2005;38:945–952. doi: 10.2144/05386IT02. [DOI] [PubMed] [Google Scholar]
- McKinzie DL, McBride WJ, Murphy JM, Lumeng L, Li TK. Effects of MDL 72222, a serotonin3 antagonist, on operant responding for ethanol by alcohol-preferring P rats. Alcohol Clin Exp Res. 2000;24:1500–1504. [PubMed] [Google Scholar]
- McLean MA, Rajfur Z, Chen Z, Humphrey D, Yang B, Sligar SG, Jacobson K. Mechanism of chromophore assisted laser inactivation employing fluorescent proteins. Anal Chem. 2009;81:1755–1761. doi: 10.1021/ac801663y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesler B, Weiss B, Fischer C, Nothen MM, Propping P, Bondy B, Rietschel M, Maier W, Albus M, Franzek E, Rappold GA. Serotonin receptor gene HTR3A variants in schizophrenic and bipolar affective patients. Pharmacogenetics. 2001;11:21–27. doi: 10.1097/00008571-200102000-00003. [DOI] [PubMed] [Google Scholar]
- Niesler B, Frank B, Kapeller J, Rappold GA. Cloning, physical mapping and expression analysis of the human 5-HT3 serotonin receptor-like genes HTR3C, HTR3D and HTR3E. Gene. 2003;310:101–111. doi: 10.1016/s0378-1119(03)00503-1. [DOI] [PubMed] [Google Scholar]
- Niesler B, Walstab J, Combrink S, Moller D, Kapeller J, Rietdorf J, Bonisch H, Gothert M, Rappold G, Bruss M. Characterization of the novel human serotonin receptor subunits 5-HT3C, 5-HT3D, and 5-HT3E. Mol Pharmacol. 2007;72:8–17. doi: 10.1124/mol.106.032144. [DOI] [PubMed] [Google Scholar]
- Olivier B, van Wijngaarden I, Soudijn W. 5-HT(3) receptor antagonists and anxiety; a preclinical and clinical review. Eur Neuropsychopharmacol. 2000;10:77–95. doi: 10.1016/s0924-977x(99)00065-6. [DOI] [PubMed] [Google Scholar]
- Peters JA, Lambert JJ. Electrophysiology of 5-HT3 receptors in neuronal cell lines. Trends Pharmacol Sci. 1989;10:172–175. doi: 10.1016/0165-6147(89)90230-7. [DOI] [PubMed] [Google Scholar]
- Rahmanzadeh R, Huttmann G, Gerdes J, Scholzen T. Chromophore-assisted light inactivation of pKi-67 leads to inhibition of ribosomal RNA synthesis. Cell Prolif. 2007;40:422–430. doi: 10.1111/j.1365-2184.2007.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajfur Z, Roy P, Otey C, Romer L, Jacobson K. Dissecting the link between stress fibres and focal adhesions by CALI with EGFP fusion proteins. Nat Cell Biol. 2002;4:286–293. doi: 10.1038/ncb772. [DOI] [PubMed] [Google Scholar]
- Sanders T, Hawrot E. A novel pharmatope tag inserted into the beta4 subunit confers allosteric modulation to neuronal nicotinic receptors. J Biol Chem. 2004;279:51460–51465. doi: 10.1074/jbc.M409533200. [DOI] [PubMed] [Google Scholar]
- Sanghvi M, Hamouda AK, Davis MI, Morton RA, Srivastava S, Pandhare A, Duddempudi PK, Machu TK, Lovinger DM, Cohen JB, Blanton MP. Hydrophobic photolabeling studies identify the lipid-protein interface of the 5-HT3A receptor. Biochemistry. 2009;48:9278–9286. doi: 10.1021/bi901208j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R. Photosensitized generation of singlet oxygen. Photochem Photobiol. 2006;82:1161–1177. doi: 10.1562/2006-03-03-IR-833. [DOI] [PubMed] [Google Scholar]
- Sekine-Aizawa Y, Huganir RL. Imaging of receptor trafficking by using alpha-bungarotoxin-binding-site-tagged receptors. Proc Natl Acad Sci USA. 2004;101:17114–17119. doi: 10.1073/pnas.0407563101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surrey T, Elowitz MB, Wolf PE, Yang F, Nedelec F, Shokat K, Leibler S. Chromophore-assisted light inactivation and self-organization of microtubules and motors. Proc Natl Acad Sci USA. 1998;95:4293–4298. doi: 10.1073/pnas.95.8.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T, Oyamada M, Fujita K, Dai P, Tanaka H, Takamatsu T. Multiphoton excitation-evoked chromophore-assisted laser inactivation using green fluorescent protein. Nat Methods. 2005;2:503–505. doi: 10.1038/nmeth770. [DOI] [PubMed] [Google Scholar]
- Tecott LH, Maricq AV, Julius D. Nervous system distribution of the serotonin 5-HT3 receptor mRNA. Proc Natl Acad Sci USA. 1993;90:1430–1434. doi: 10.1073/pnas.90.4.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tour O, Meijer RM, Zacharias DA, Adams SR, Tsien RY. Genetically targeted chromophore-assisted light inactivation. Nat Biotechnol. 2003;21:1505–1508. doi: 10.1038/nbt914. [DOI] [PubMed] [Google Scholar]
- Vitriol EA, Uetrecht AC, Shen F, Jacobson K, Bear JE. Enhanced EGFP-chromophore-assisted laser inactivation using deficient cells rescued with functional EGFP-fusion proteins. Proc Natl Acad Sci USA. 2007;104:6702–6707. doi: 10.1073/pnas.0701801104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watschinger K, Horak SB, Schulze K, Obermair GJ, Wild C, Koschak A, Sinnegger-Brauns MJ, Tampe R, Striessnig J. Functional properties and modulation of extracellular epitope-tagged Ca(V)2.1 voltage-gated calcium channels. Channels (Austin) 2008;2:461–473. doi: 10.4161/chan.2.6.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins ME, Li X, Smart TG. Tracking cell surface GABAB receptors using an alpha-bungarotoxin tag. J Biol Chem. 2008;283:34745–34752. doi: 10.1074/jbc.M803197200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DF, Othman NA, Sharp D, Mahendra A, Deeb TZ, Hales TG. A conserved cysteine residue in the third transmembrane domain is essential for homomeric 5-HT3 receptor function. J Physiol. 2009;588:603–616. doi: 10.1113/jphysiol.2009.181719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Hosoi M, Koo BN, Zhang L. Anandamide inhibition of 5-HT3A receptors varies with receptor density and desensitization. Mol Pharmacol. 2008;73:314–322. doi: 10.1124/mol.107.039149. [DOI] [PubMed] [Google Scholar]
- Yan P, Xiong Y, Chen B, Negash S, Squier TC, Mayer MU. Fluorophore-assisted light inactivation of calmodulin involves singlet-oxygen mediated cross-linking and methionine oxidation. Biochemistry. 2006;45:4736–4748. doi: 10.1021/bi052395a. [DOI] [PubMed] [Google Scholar]


